Abstract
Background
Gastric cancer is characterized by high invasiveness, heterogeneity, and late diagnosis, leading to high incidence and mortality rates. It is a significant public health concern globally. Early prevention is crucial in reducing the occurrence of gastric cancer, and dietary prevention, particularly focusing on carotenoids, has been considered a convenient and effective approach. However, the association between carotenoid intake and gastric cancer incidence remains controversial.
Methods
A systematic search was conducted in PubMed, Ovid Embase, Web of Science, and Cochrane databases from inception to January 5, 2023. Two reviewers independently screened search results, extracted relevant data, and evaluated study quality. Statistical analysis was performed using the "metan" command in STATA 16 software. Random-effects or fixed-effects models were chosen based on the magnitude of heterogeneity among studies.
Results
This study included a total of 35 publications, consisting of 23 case–control studies and 12 cohort studies. Meta-analysis of case–control studies showed that alpha-carotene (OR = 0.71, 95% CI: 0.55–0.92), beta-carotene (OR = 0.62, 95% CI: 0.53–0.72), and lutein (OR = 0.82, 95% CI: 0.69–0.97) significantly reduced the risk of gastric cancer, while beta-cryptoxanthin (OR = 0.88, 95% CI: 0.75–1.04) and lycopene (OR = 0.86, 95% CI: 0.73–1.00) showed no significant correlation. Meta-analysis of cohort studies indicated no significant associations between any of the five carotenoids and gastric cancer incidence (alpha-carotene: RR = 0.81, 95% CI: 0.54–1.23; beta-carotene: RR = 0.86, 95% CI: 0.64–1.16; beta-cryptoxanthin: RR = 0.86, 95% CI: 0.64–1.16; lutein: RR = 0.94, 95% CI: 0.69–1.29; lycopene: RR = 0.89, 95% CI: 0.69–1.14).
Conclusions
The relationship between carotenoids and gastric cancer incidence may vary depending on the type of study conducted. Considering that evidence from cohort studies is generally considered stronger than evidence from case–control studies, and high-quality randomized controlled trials show no significant association between carotenoids and gastric cancer incidence, current evidence does not support the supplementation of carotenoids for gastric cancer prevention. Further targeted research is needed to explore the association between the two.
Similar content being viewed by others
Introduction
Gastric cancer is a heterogeneous and multifactorial disease characterized by high invasiveness. It ranks fifth in terms of incidence and fourth in terms of mortality among all cancers, with over 1 million new cases and nearly 800,000 deaths recorded in 2020 alone. It is a significant public health problem worldwide [1, 2]. Up to 80% of gastric cancer patients exhibit no apparent symptoms in the early stages, and diagnosis often occurs after metastasis has already taken place [3]. Despite significant variations, the 5-year survival rate for gastric cancer remains low and is below 30% in most countries [4, 5]. Therefore, early prevention has become a crucial public health strategy to reduce the incidence and mortality of gastric cancer.
Early prevention of gastric cancer primarily focuses on primary and secondary prevention strategies such as exploring risk and protective factors, improving dietary and lifestyle habits, early screening, and treatment [6]. Among these, the correlation between dietary factors and gastric cancer incidence has been extensively studied. The National Cancer Institute in the United States suggests that the intake of fruits and vegetables can prevent the onset of gastric cancer, while the consumption of grilled, salt-preserved, and smoked foods may contribute to its progression [7]. Most research studies have also indicated that an adequate intake of fruits and vegetables significantly reduces the risk of gastric cancer [8, 9]. Further studies have revealed that carotenoids and other antioxidant substances found in vegetables and fruits play a protective role against gastric cancer [10, 11].
Carotenoids are natural colored pigments widely distributed in nature and are known for their antioxidant and anti-inflammatory properties. There are more than 700 types of carotenoids, including alpha-carotene, beta-carotene, beta-cryptoxanthin, lutein, and lycopene [12, 13]. However, not all carotenoids are capable of reducing the risk of gastric cancer, and there is controversy regarding whether carotenoids can reduce the risk of gastric cancer. Yuan et al., based on the results of a large cohort study involving 18,224 individuals, found a significant correlation between alpha-carotene, beta-carotene, and lycopene levels and a decreased risk of gastric cancer. However, they did not find a significant correlation between serum levels of beta-cryptoxanthin and lutein and the occurrence of gastric cancer [14]. Additionally, Knekt et al. did not find a negative correlation between beta-carotene and gastric cancer incidence in their study [15]. Varis et al. also found that even after supplementing with beta-carotene for five years, the risk of gastric cancer in elderly men did not significantly decrease [16].
In summary, there are conflicting results regarding the relationship between carotenoids and the occurrence of gastric cancer. The role of carotenoids in reducing the risk of gastric cancer remains uncertain. Therefore, this study aims to comprehensively collect relevant research, systematically summarize and analyze the relationship between carotenoids and gastric cancer, in order to provide dietary guidance for future gastric cancer prevention.
Methods
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [17]. The review protocol has been registered with PROSPERO, number CRD42022344012 (https://www.crd.york.ac.uk/prospero/).
Inclusion and exclusion criteria
Patients & diseases
Patients with gastric cancer who were diagnosed by histological examination.
Intervention (exposure)
Intake of carotenoids (β-carotene, α-carotene, lycopene, other carotenoids) or blood (plasma or serum) levels of carotenoids. Because the incidence of gastric cancer varies greatly among different age and gender populations, we only included studies that adjusted for age and gender as confounding factors [3, 18]. Other confounding factors were not limited.
Outcome
Gastric cancer.
Type of study
Cohort studies, case–control studies, and cross-sectional studies.
Exclusion criteria
Animal studies, cell research was excluded. Reviews, case reports, opinion articles, conference abstracts, and non‐published data were also excluded.
Data sources and searches
Systematic searches were conducted in the PubMed, Ovid-Embase, The Cochrane Library, and Web of Science databases, with the search cutoff date set at January 5, 2023. Additionally, the reference lists of included studies were also searched. A combination of free text terms and subject headings was used in the search approach. The search terms and search strategy were as follows: (stomach cancer OR gastric cancer OR gastric carcinoma OR stomach neoplasms OR gastric neoplasms OR stomach carcinoma) AND (lutein OR carotenoids OR carotene OR carotae OR lycopene). Please refer to Additional file 1: Table 1 for the detailed search strategy.
Data extraction and risk of bias assessment
According to the inclusion and exclusion criteria mentioned above, literature screening and data extraction were performed by two trained researchers. The extracted contents included: 1) Basic information of the included studies: authors, publication year, country, study type, sample size, age, data collection method, gastric cancer diagnostic criteria, data analysis methods, follow-up time, and adjusted confounding factors. 2) Exposure factors: types of carotenoids. 3) Key elements of bias risk assessment.
Based on the Newcastle–Ottawa Scale (NOS), two trained researchers independently assessed the inherent risk of bias in the included studies from three aspects including selection of study population, comparability between groups, and outcome measurement [19]. Scores of 0–3, 4–6, and 7–9 were classified as low, moderate, and high quality, respectively.
Statistical analysis
Statistical analysis was performed using STATA 16 software. The results were presented as odds ratios (OR) or risk ratios (RR) with corresponding 95% confidence intervals (95% CI). Heterogeneity among the included studies was assessed using the chi-square test with a significance level of α = 0.05, and the magnitude of heterogeneity was determined based on the I2 value. A P-value > 0.05 indicated that the heterogeneity among the study results was not statistically significant, and a fixed-effect model was used for the meta-analysis. Conversely, if the heterogeneity was found to be statistically significant after further analysis of its sources, a random-effects model was used for the meta-analysis. Additionally, subgroup analyses were conducted based on the types of carotenoids and study design.
Results
Literature screening results
A total of 2,385 relevant articles were initially retrieved. After excluding duplicates, articles that did not involve carotenoids as the exposure factor or non-gastric cancer patients (such as precancerous lesions, atrophic gastritis, etc.), and studies that did not meet the appropriate study design (such as reviews, conference abstracts, case reports), 35 studies were ultimately included. The results of the literature screening are summarized in Fig. 1.
Basic information and risk of bias assessment of included studies
Among the 35 included studies, there were 23 case–control studies [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42] and 12 cohort studies [14, 16, 43,44,45,46,47,48,49,50,51,52]. In the cohort studies, the follow-up time ranged from 5.2 years [51] to 17 years [45], and the sample sizes ranged from 3,123 [43] to 82,002 [48]. The sample sizes of the case–control studies ranged from 223 [25] to 2,747 [29]. The age of the patients ranged from 19 to 89 years, with 9 studies not reporting the age of the patients. All studies used questionnaire surveys to assess exposure factors and gastric cancer was confirmed through histological examination. Multiple logistic regression analysis was conducted in all studies, adjusting for age and gender as confounding factors. The 35 included studies investigated the correlation between five types of carotenoids (alpha-carotene, beta-carotene, beta-cryptoxanthin, lutein, and lycopene) and the risk of gastric cancer. Please refer to Additional file 1: Table 2 for details.
The NOS scores of all 35 studies were ≥ 7, with 19 studies scoring 8 and 17 studies scoring 7, indicating a relatively high quality of included studies. However, the scores for confounding variables and measurement of exposure were relatively low. Please refer to Additional file 1: Table 3 for details.
Meta-analysis results
Alpha-carotene
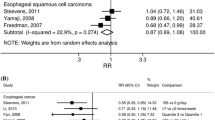
A total of 9 case–control studies and 6 cohort studies reported on the correlation between alpha-carotene and the occurrence of gastric cancer. Due to the statistical heterogeneity test resulting in a P-value < 0.05, a random-effects model was used for the meta-analysis. The meta-analysis results based on case–control studies showed that intake of alpha-carotene significantly reduced the risk of gastric cancer (OR = 0.71, 95% CI: 0.55–0.92). However, the meta-analysis results based on cohort studies showed no significant correlation between intake of alpha-carotene and a reduced risk of gastric cancer (RR = 0.81, 95% CI: 0.54–1.23). Please refer to Fig. 2 for further details.
Beta-carotene
A total of 21 case–control studies and 11 cohort studies reported on the correlation between beta-carotene and gastric cancer. Due to the statistical heterogeneity test resulting in a P-value < 0.05, a random-effects model was used for the meta-analysis. The meta-analysis results based on case–control studies showed that intake of beta-carotene significantly reduced the risk of gastric cancer (OR = 0.62, 95% CI: 0.53–0.72). However, the meta-analysis results based on cohort studies showed no significant correlation between intake of beta-carotene and a decreased risk of gastric cancer (RR = 0.86, 95% CI: 0.64–1.16). Please refer to Fig. 2 for more details.
Beta-cryptoxanthin
A total of 8 case–control studies and 4 cohort studies reported on the correlation between beta-cryptoxanthin and gastric cancer. Due to the statistical heterogeneity test resulting in a P-value > 0.05, a fixed-effect model was used for the meta-analysis. The meta-analysis results based on both case–control studies and cohort studies showed no significant correlation between intake of beta-cryptoxanthin and a reduced risk of gastric cancer (case–control studies: OR = 0.88, 95% CI: 0.75–1.04; cohort studies: RR = 0.95, 95% CI: 0.73–1.25). Please refer to Fig. 3 for more details.
Lutein
A total of 9 case–control studies and 3 cohort studies reported on the correlation between Lutein and the occurrence of gastric cancer. Due to the statistical heterogeneity test resulting in a P-value > 0.05, a fixed-effect model was used for the meta-analysis. The meta-analysis results based on case–control studies showed that intake of Lutein significantly reduced the risk of gastric cancer (OR = 0.82, 95% CI: 0.69–0.97). However, the meta-analysis results based on cohort studies showed no significant correlation between intake of Lutein and the occurrence of gastric cancer (RR = 0.94, 95% CI: 0.69–1.29). Please refer to Fig. 3 for more details.
Lycopene
A total of 9 case–control studies and 6 cohort studies reported on the correlation between Lycopene and gastric cancer. Due to the statistical heterogeneity test resulting in a P-value > 0.05, a fixed-effect model was used for the meta-analysis. The meta-analysis results based on both case–control studies and cohort studies showed no significant correlation between intake of Lycopene and the occurrence of gastric cancer (case–control studies: OR = 0.86, 95% CI: 0.73–1.00; cohort studies: RR = 0.89, 95% CI: 0.69–1.14). Please refer to Fig. 3 for more details.
Sensitivity analysis and publication bias of beta-carotene
After conducting sensitivity analysis on beta-carotene, we found that the combined values and confidence intervals did not change direction when excluding any specific study, indicating the stability of the meta-analysis results (Fig. 4). Additionally, funnel plots were created based on different study types. The generally symmetrical funnel plots suggest a lower likelihood of publication bias in the current studies (Figs. 5 and 6).
Discussion
Carotenoids are powerful antioxidants that can interact with reactive oxygen species through oxidation, reduction, hydrogen atom extraction, or addition reactions, reducing oxidative damage to tissue cells [53, 54]. They can also lower the risk of gastric cancer by inhibiting cell proliferation, inducing apoptosis, affecting cell communication, and enhancing immune function [55,56,57,58]. Among them, lycopene has been found to inhibit the growth of gastric cancer cells, arrest the cell cycle, induce late-stage apoptosis/necrosis, and decrease mitochondrial membrane potential [59]. Lycopene consists of 89.45% carbon and 10.51% hydrogen, with 11 linear conjugated bonds and 2 non-conjugated double bonds [60]. This unique structure provides strong antioxidant activity, with an effect that is 100 times greater than α-tocopherol and more than twice that of β-carotene [61, 62]. In normal cells, it downregulates inflammation, protects DNA, RNA, and lipids from oxidative damage, and prevents genomic mutations that may lead to cancer [63, 64]. Research has also found that lycopene inhibits the proliferation of Helicobacter pylori-infected gastric adenocarcinoma cells by reducing ROS levels and inhibiting Jak1/Stat3 activation, Wnt/β-catenin signaling, and oncogene expression [64]. Other carotenoids, including α-carotene, β-carotene, lutein, and β-cryptoxanthin, can also influence the development of gastric cancer. For example, β-carotene exhibits anticancer activity by reducing ROS production mediated by NADPH oxidase, activating NF-κB, and regulating the expression of TRAF1 and TRAF2 genes controlled by NF-κB, while inhibiting excessive proliferation of AGS cells [65]. β-cryptoxanthin demonstrates anti-proliferative activity by reducing cell viability, migration, and inducing G0/G1 arrest [66, 67]. Lutein increases the translocation of NADPH oxidase subunit p47Phox to the cell membrane, enhances ROS levels, promotes NADPH oxidase activity, and increases NF-κB activity and apoptotic indices in AGS cells, such as Bax, caspase-3 cleavage, and DNA fragmentation [68]. However, according to the World Cancer Research Fund, not all carotenoids are beneficial for health. Studies have shown that high-dose supplementation of beta-carotene can increase the risk of lung cancer [69, 70]. The effects of carotenoid-containing food supplements on other types of cancer have been considered limited and controversial [71, 72]. In fact, recent research suggests that carotenoids have a dual role in both antioxidant and pro-oxidant activities, making it complex to determine their role in cancer development [71, 73].
Previous case–control studies exploring the association between carotenoids and gastric cancer incidence have indicated that higher intake of carotenoids significantly reduces the risk of gastric cancer even after adjusting for potential confounding factors such as gender, smoking, and Helicobacter pylori infection [28]. However, the results of a randomized, double-blind, placebo-controlled clinical trial involving 22,071 healthy males demonstrated that 12 years of continuous beta-carotene supplementation had no beneficial or harmful effects on reducing the incidence of gastric cancer [74]. These findings are consistent with our research results. Our meta-analysis based on cohort studies revealed no significant correlation between the five types of carotenoids included in the analysis and the occurrence of gastric cancer. In addition, our meta-analysis based on case–control studies showed no significant correlation between beta-cryptoxanthin and lycopene and gastric cancer incidence, while alpha-carotene, beta-carotene, and lutein were negatively associated with gastric cancer incidence. However, it should be noted that except for beta-carotene, the odds ratio values for alpha-carotene and lutein were close to 1, suggesting that their association with gastric cancer occurrence is limited.
Interestingly, when comparing the results of meta-analyses from different study designs, we found that study design appears to have a significant impact on the research outcomes. In contrast to the results of meta-analyses based on case–control studies, the meta-analysis based on cohort studies showed that none of the five carotenoids included in the analysis could reduce the risk of gastric cancer. This is similar to the findings of Friedenreich et al., who found that the correlation between increased intake of vegetables and fruits and reduced cancer risk was only supported by case–control studies, with weaker evidence provided by cohort studies [75]. In fact, prospective cohort studies involve grouping participants based on their exposure characteristics before the occurrence of the outcomes of interest. This minimizes the impact of baseline characteristics, dietary recall, or selection/participation bias. On the other hand, case–control studies are prone to recall bias and selection bias, which can lead to biased results. Therefore, evidence from cohort studies is generally considered stronger than evidence from case–control studies [76,77,78]. Furthermore, it is challenging to establish a clear protective effect of a specific compound in the development of chronic diseases within a complex diet influenced by many other risk factors. In such cases, conflicting results may also arise [79, 80]. Although the studies we included adjusted for age and gender as confounding factors, there is still a possibility of inadequate control for other confounding factors. Additionally, there are variations in the confounding factors across each study, which may lead to biases in the estimation of risks. Moreover, there are significant differences in the quartiles of carotenoid intake across different studies, which can also affect the calculation of effect sizes. Some studies suggest that inconsistent results may be due to variations in consumption levels of phytochemicals, regional differences, dietary and lifestyle variations, sample size limitations, and data acquisition methods [81,82,83].
Strengths and limitations
Strengths
1) We only included studies that adjusted for age and gender as confounding factors, minimizing the impact of important confounding factors on the results. 2) We conducted subgroup analyses based on study type and types of carotenoids, resulting in more accurate and representative results. 3) Based on the NOS scoring, we found that the included 35 studies had high quality of evidence, ensuring the reliability of the meta-analysis results. Additionally, we performed publication bias detection and sensitivity analyses to ensure the stability of the meta-analysis results.
Limitations
1) Different subtypes of gastric cancer may have varying sensitivities to carotenoids [50]. However, due to the lack of reporting on specific types and stages of gastric cancer in the included studies, we were unable to conduct further subgroup analyses. 2) We only included English-language literature, which may introduce language bias. 3) We did not search for gray literature and conference abstracts, which could potentially introduce publication bias.
Conclusions
The association between carotenoids and gastric cancer incidence is influenced by the type of study design. Meta-analysis based on case–control studies showed that alpha-carotene, beta-carotene, and lutein can reduce the risk of gastric cancer, while beta-cryptoxanthin and lycopene do not. On the other hand, meta-analysis based on cohort studies showed that there is no significant correlation between different carotenoids and the occurrence of gastric cancer. Considering the evidence from cohort studies is generally stronger than case–control studies, and high-quality randomized controlled trials also show no significant association between carotenoids and gastric cancer, the existing evidence does not support the intake of carotenoids to reduce the risk of gastric cancer. Further high-quality and well-designed experimental studies are needed to investigate the relationship between carotenoids and gastric cancer incidence.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses
- AOR:
-
Adjusted odds ratio
- NOS:
-
Newcastle–ottawa scale
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–49. https://doi.org/10.3322/caac.21660. Epub 2021/02/05. Cited in: Pubmed; PMID 33538338.
Wong MCS, Huang J, Chan PSF, Choi P, Lao XQ, Chan SM, Teoh A, Liang P. Global Incidence and Mortality of Gastric Cancer, 1980–2018. JAMA Netw Open. 2021;4(7):e2118457. https://doi.org/10.1001/jamanetworkopen.2021.18457. Epub 2021/07/27. Cited in: Pubmed; PMID 34309666.
Lyons K, Le LC, Pham YT, Borron C, Park JY, Tran CTD, Tran TV, Tran HT, Vu KT, Do CD, Pelucchi C, La Vecchia C, Zgibor J, Boffetta P, Luu HN. Gastric cancer: epidemiology, biology, and prevention: a mini review. Eur J Cancer Prev. 2019;28(5):397–412. https://doi.org/10.1097/CEJ.0000000000000480. Epub 2019/08/07. Cited in: Pubmed; PMID 31386635.
Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Niksic M, Bonaventure A, Valkov M, Johnson CJ, Esteve J, Ogunbiyi OJ, Azevedo ESG, Chen WQ, Eser S, Engholm G, Stiller CA, Monnereau A, Woods RR, Visser O, Lim GH, Aitken J, Weir HK, Coleman MP, Group CW. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–75. https://doi.org/10.1016/S0140-6736(17)33326-3. Epub 2018/02/06. Cited in: Pubmed; PMID 29395269.
Arnold M, Rutherford MJ, Bardot A, Ferlay J, Andersson TM, Myklebust TA, Tervonen H, Thursfield V, Ransom D, Shack L, Woods RR, Turner D, Leonfellner S, Ryan S, Saint-Jacques N, De P, McClure C, Ramanakumar AV, Stuart-Panko H, Engholm G, Walsh PM, Jackson C, Vernon S, Morgan E, Gavin A, Morrison DS, Huws DW, Porter G, Butler J, Bryant H, Currow DC, Hiom S, Parkin DM, Sasieni P, Lambert PC, Moller B, Soerjomataram I, Bray F. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995–2014 (ICBP SURVMARK-2): a population-based study. Lancet Oncol. 2019;20(11):1493–505. https://doi.org/10.1016/S1470-2045(19)30456-5. Epub 2019/09/16. Cited in: Pubmed; PMID 31521509.
Yang L, Ying X, Liu S, Lyu G, Xu Z, Zhang X, Li H, Li Q, Wang N, Ji J. Gastric cancer: Epidemiology, risk factors and prevention strategies. Chin J Cancer Res. 2020;32(6):695–704. https://doi.org/10.21147/j.issn.1000-9604.2020.06.03. Epub 2021/01/16. Cited in: Pubmed; PMID 33446993.
Kim J, Cho YA, Choi WJ, Jeong SH. Gene-diet interactions in gastric cancer risk: a systematic review. World J Gastroenterol. 2014;20(28):9600–10. https://doi.org/10.3748/wjg.v20.i28.9600. Epub 2014/07/30. Cited in: Pubmed; PMID 25071358.
Buckland G, Travier N, Huerta JM, Bueno-de-Mesquita HB, Siersema PD, Skeie G, Weiderpass E, Engeset D, Ericson U, Ohlsson B, Agudo A, Romieu I, Ferrari P, Freisling H, Colorado-Yohar S, Li K, Kaaks R, Pala V, Cross AJ, Riboli E, Trichopoulou A, Lagiou P, Bamia C, Boutron-Ruault MC, Fagherazzi G, Dartois L, May AM, Peeters PH, Panico S, Johansson M, Wallner B, Palli D, Key TJ, Khaw KT, Ardanaz E, Overvad K, Tjonneland A, Dorronsoro M, Sanchez MJ, Quiros JR, Naccarati A, Tumino R, Boeing H, Gonzalez CA. Healthy lifestyle index and risk of gastric adenocarcinoma in the EPIC cohort study. Int J Cancer. 2015;137(3):598–606. https://doi.org/10.1002/ijc.29411.
Du S, Li Y, Su Z, Shi X, Johnson NL, Li P, Zhang Y, Zhang Q, Wen L, Li K, Chen Y, Zhang X, Fei Y, Ding X. Index-based dietary patterns in relation to gastric cancer risk: a systematic review and meta-analysis. Br J Nutr. 2020;123(9):964–74. https://doi.org/10.1017/S0007114519002976. Epub 2019/11/27. Cited in: Pubmed; PMID 31767045.
Terry P, Lagergren J, Hansen H, Wolk A, Nyren O. Fruit and vegetable consumption in the prevention of oesophageal and cardia cancers. Eur J Cancer Prev. 2001;10(4):365–9. https://doi.org/10.1097/00008469-200108000-00010. Epub 2001/09/06. Cited in: Pubmed; PMID 11535879.
Freedman ND, Subar AF, Hollenbeck AR, Leitzmann MF, Schatzkin A, Abnet CC. Fruit and vegetable intake and gastric cancer risk in a large United States prospective cohort study. Cancer Causes Control. 2008;19(5):459–67. https://doi.org/10.1007/s10552-007-9107-4. Epub 2008/01/02. Cited in: Pubmed; PMID 18166992.
Yabuzaki J. Carotenoids Database: structures, chemical fingerprints and distribution among organisms. Database (Oxford). 2017;2017(1). doi:https://doi.org/10.1093/database/bax004. Epub 2017/04/04. Cited in: Pubmed; PMID 28365725.
Jorgensen K, Skibsted LH. Carotenoid scavenging of radicals. Effect of carotenoid structure and oxygen partial pressure on antioxidative activity. Z Lebensm Unters Forsch. 1993;196(5):423–9. https://doi.org/10.1007/BF01190806. Epub 1993/05/01. Cited in: Pubmed; PMID 8511974.
Yuan JM, Ross RK, Gao YT, Qu YH, Chu XD, Yu MC. Prediagnostic levels of serum micronutrients in relation to risk of gastric cancer in Shanghai. China Cancer Epidemiol Biomarkers Prev. 2004;13(11 Pt 1):1772–80 Epub 2004/11/10. Cited in: Pubmed; PMID 15533906.
Knekt P, Aromaa A, Maatela J, Aaran RK, Nikkari T, Hakama M, Hakulinen T, Peto R, Teppo L. Serum vitamin A and subsequent risk of cancer: cancer incidence follow-up of the Finnish Mobile Clinic Health Examination Survey. Am J Epidemiol. 1990;132(5):857–70. https://doi.org/10.1093/oxfordjournals.aje.a115728. Epub 1990/11/01. Cited in: Pubmed; PMID 2239900.
Varis K, Taylor PR, Sipponen P, Samloff IM, Heinonen OP, Albanes D, Harkonen M, Huttunen JK, Laxen F, Virtamo J. Gastric cancer and premalignant lesions in atrophic gastritis: a controlled trial on the effect of supplementation with alpha-tocopherol and beta-carotene. The Helsinki Gastritis Study Group Scand J Gastroenterol. 1998;33(3):294–300. https://doi.org/10.1080/00365529850170892. Epub 1998/04/21. Cited in: Pubmed; PMID 9548624.
Moher D, Liberati A, Tetzlaff J, Altman DG, Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9, W64. https://doi.org/10.7326/0003-4819-151-4-200908180-00135. Epub 2009/07/23. Cited in: Pubmed; PMID 19622511.
Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int J Mol Sci. 2020;21(11). doi:https://doi.org/10.3390/ijms21114012. Epub 2020/06/10. Cited in: Pubmed; PMID 32512697.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. https://doi.org/10.1007/s10654-010-9491-z. Epub 2010/07/24. Cited in: Pubmed; PMID 20652370.
Abnet CC, Qiao YL, Dawsey SM, Buckman DW, Yang CS, Blot WJ, Dong ZW, Taylor PR, Mark SD. Prospective study of serum retinol, beta-carotene, beta-cryptoxanthin, and lutein/zeaxanthin and esophageal and gastric cancers in China. Cancer Causes Control. 2003;14(7):645–55. https://doi.org/10.1023/a:1025619608851. Epub 2003/10/25. Cited in: Pubmed; PMID 14575362.
Buiatti E, Palli D, Decarli A, Amadori D, Avellini C, Bianchi S, Bonaguri C, Cipriani F, Cocco P, Giacosa A, et al. A case-control study of gastric cancer and diet in Italy: II. Association with nutrients Int J Cancer. 1990;45(5):896–901. https://doi.org/10.1002/ijc.2910450520. Epub 1990/05/15. Cited in: Pubmed; PMID 2335393.
De Stefani E, Boffetta P, Brennan P, Deneo-Pellegrini H, Carzoglio JC, Ronco A, Mendilaharsu M. Dietary carotenoids and risk of gastric cancer: a case-control study in Uruguay. Eur J Cancer Prev. 2000;9(5):329–34. https://doi.org/10.1097/00008469-200010000-00007. Epub 2000/11/15. Cited in: Pubmed; PMID 11075886.
Ekstrom AM, Serafini M, Nyren O, Hansson LE, Ye W, Wolk A. Dietary antioxidant intake and the risk of cardia cancer and noncardia cancer of the intestinal and diffuse types: a population-based case-control study in Sweden. Int J Cancer. 2000;87(1):133–40 Epub 2000/06/22. Cited in: Pubmed; PMID 10861464.
Garcia-Closas R, Gonzalez CA, Agudo A, Riboli E. Intake of specific carotenoids and flavonoids and the risk of gastric cancer in Spain. Cancer Causes Control. 1999;10(1):71–5. https://doi.org/10.1023/a:1008867108960. Epub 1999/05/20. Cited in: Pubmed; PMID 10334645.
Harrison LE, Zhang ZF, Karpeh MS, Sun M, Kurtz RC. The role of dietary factors in the intestinal and diffuse histologic subtypes of gastric adenocarcinoma: a case-control study in the U.S. Cancer. 1997;80(6):1021–8 Epub 1997/09/26. Cited in: Pubmed; PMID 9305701.
Jenab M, Riboli E, Ferrari P, Friesen M, Sabate J, Norat T, Slimani N, Tjonneland A, Olsen A, Overvad K, Boutron-Ruault MC, Clavel-Chapelon F, Boeing H, Schulz M, Linseisen J, Nagel G, Trichopoulou A, Naska A, Oikonomou E, Berrino F, Panico S, Palli D, Sacerdote C, Tumino R, Peeters PH, Numans ME, Bueno-de-Mesquita HB, Buchner FL, Lund E, Pera G, Chirlaque MD, Sanchez MJ, Arriola L, Barricarte A, Quiros JR, Johansson I, Johansson A, Berglund G, Bingham S, Khaw KT, Allen N, Key T, Carneiro F, Save V, Del Giudice G, Plebani M, Kaaks R, Gonzalez CA. Plasma and dietary carotenoid, retinol and tocopherol levels and the risk of gastric adenocarcinomas in the European prospective investigation into cancer and nutrition. Br J Cancer. 2006;95(3):406–15. https://doi.org/10.1038/sj.bjc.6603266. Epub 2006/07/13. Cited in: Pubmed; PMID 16832408.
Kim HJ, Kim MK, Chang WK, Choi HS, Choi BY, Lee SS. Effect of nutrient intake and Helicobacter pylori infection on gastric cancer in Korea: a case-control study. Nutr Cancer. 2005;52(2):138–46. https://doi.org/10.1207/s15327914nc5202_4. Epub 2005/10/06. Cited in: Pubmed; PMID 16201845.
Kim JH, Lee J, Choi IJ, Kim YI, Kwon O, Kim H, Kim J. Dietary Carotenoids Intake and the Risk of Gastric Cancer: A Case-Control Study in Korea. Nutrients. 2018 Aug 7;10(8). Epub 2018/08/09. doi:https://doi.org/10.3390/nu10081031. Cited in: Pubmed; PMID 30087311.
La Vecchia C, Ferraroni M, D’Avanzo B, Decarli A, Franceschi S. Selected micronutrient intake and the risk of gastric cancer. Cancer Epidemiol Biomarkers Prev. 1994;3(5):393–8 Epub 1994/07/01. Cited in: Pubmed; PMID 7920206.
Lissowska J, Gail MH, Pee D, Groves FD, Sobin LH, Nasierowska-Guttmejer A, Sygnowska E, Zatonski W, Blot WJ, Chow WH. Diet and stomach cancer risk in Warsaw. Poland Nutr Cancer. 2004;48(2):149–59. https://doi.org/10.1207/s15327914nc4802_4. Epub 2004/07/03. Cited in: Pubmed; PMID 15231449.
Mayne ST, Risch HA, Dubrow R, Chow WH, Gammon MD, Vaughan TL, Farrow DC, Schoenberg JB, Stanford JL, Ahsan H, West AB, Rotterdam H, Blot WJ, Fraumeni JF Jr. Nutrient intake and risk of subtypes of esophageal and gastric cancer. Cancer Epidemiol Biomarkers Prev. 2001;10(10):1055–62 Epub 2001/10/06. Cited in: Pubmed; PMID 11588131.
Munoz SE, Ferraroni M, La Vecchia C, Decarli A. Gastric cancer risk factors in subjects with family history. Cancer Epidemiol Biomarkers Prev. 1997;6(2):137–40 Epub 1997/02/01. Cited in: Pubmed; PMID 9037565.
Nomura AM, Hankin JH, Kolonel LN, Wilkens LR, Goodman MT, Stemmermann GN. Case-control study of diet and other risk factors for gastric cancer in Hawaii (United States). Cancer Causes Control. 2003;14(6):547–58. https://doi.org/10.1023/a:1024887411846. Epub 2003/09/02. Cited in: Pubmed; PMID 12948286.
Palli D, Decarli A, Cipriani F, Forman D, Amadori D, Avellini C, Giacosa A, Manca P, Russo A, Salkeld RM, et al. Plasma pepsinogens, nutrients, and diet in areas of Italy at varying gastric cancer risk. Cancer Epidemiol Biomarkers Prev. 1991;1(1):45–50 Epub 1991/11/01. Cited in: Pubmed; PMID 1845168.
Palli D, Russo A, Decarli A. Dietary patterns, nutrient intake and gastric cancer in a high-risk area of Italy. Cancer Causes Control. 2001;12(2):163–72. https://doi.org/10.1023/a:1008970310963. Epub 2001/03/15. Cited in: Pubmed; PMID 11246845.
Palli D, Russo A, Ottini L, Masala G, Saieva C, Amorosi A, Cama A, D’Amico C, Falchetti M, Palmirotta R, Decarli A, Mariani Costantini R, Fraumeni JF Jr. Red meat, family history, and increased risk of gastric cancer with microsatellite instability. Cancer Res. 2001;61(14):5415–9 Epub 2001/07/17. Cited in: Pubmed; PMID 11454685.
Pelucchi C, Tramacere I, Bertuccio P, Tavani A, Negri E, La Vecchia C. Dietary intake of selected micronutrients and gastric cancer risk: an Italian case-control study. Ann Oncol. 2009;20(1):160–5. https://doi.org/10.1093/annonc/mdn536. Epub 2008/08/02. Cited in: Pubmed; PMID 18669867.
Persson C, Sasazuki S, Inoue M, Kurahashi N, Iwasaki M, Miura T, Ye W, Tsugane S, Group JS. Plasma levels of carotenoids, retinol and tocopherol and the risk of gastric cancer in Japan: a nested case-control study. Carcinogenesis. 2008;29(5):1042–8. https://doi.org/10.1093/carcin/bgn072. Epub 2008/03/15. Cited in: Pubmed; PMID 18339681.
Qiu JL, Chen K, Zheng JN, Wang JY, Zhang LJ, Sui LM. Nutritional factors and gastric cancer in Zhoushan Islands, China. World J Gastroenterol. 2005;11(28):4311–6. https://doi.org/10.3748/wjg.v11.i28.4311. Epub 2005/07/23. Cited in: Pubmed; PMID 16038026.
Sengngam K, Hoc TH, Hang DV, Tran Ngoan L. Trans-Lycopene and beta-Cryptoxanthin Intake and Stomach Cancer in Vietnamese Men: A Pilot Case-Control Study. Asian Pac J Cancer Prev. 2022;23(3):861–5. https://doi.org/10.31557/APJCP.2022.23.3.861. Epub 2022/03/30. Cited in: Pubmed; PMID 35345357.
Terry P, Lagergren J, Ye W, Nyren O, Wolk A. Antioxidants and cancers of the esophagus and gastric cardia. Int J Cancer. 2000;87(5):750–4 Epub 2000/08/05. Cited in: Pubmed; PMID 10925371.
Zhang ZF, Kurtz RC, Yu GP, Sun M, Gargon N, Karpeh M Jr, Fein JS, Harlap S. Adenocarcinomas of the esophagus and gastric cardia: the role of diet. Nutr Cancer. 1997;27(3):298–309. https://doi.org/10.1080/01635589709514541. Epub 1997/01/01. Cited in: Pubmed; PMID 9101561.
Botterweck AA, van den Brandt PA, Goldbohm RA. Vitamins, carotenoids, dietary fiber, and the risk of gastric carcinoma: results from a prospective study after 6.3 years of follow-up. Cancer. 2000;88(4):737–48 Epub 2000/02/19. Cited in: Pubmed; PMID 10679641.
Egnell M, Fassier P, Lecuyer L, Gonzalez R, Zelek L, Vasson MP, Hercberg S, Latino-Martel P, Galan P, Druesne-Pecollo N, Deschasaux M, Touvier M. Antioxidant intake from diet and supplements and risk of digestive cancers in middle-aged adults: results from the prospective NutriNet-Sante cohort. Br J Nutr. 2017;118(7):541–9. https://doi.org/10.1017/S0007114517002392. Epub 2017/09/21. Cited in: Pubmed; PMID 28927476.
Eichholzer M, Stahelin HB, Gey KF, Ludin E, Bernasconi F. Prediction of male cancer mortality by plasma levels of interacting vitamins: 17-year follow-up of the prospective Basel study. Int J Cancer. 1996;66(2):145–50. https://doi.org/10.1002/(SICI)1097-0215(19960410)66:2<145::AID-IJC1>3.0.CO;2-2. Epub 1996/04/10. Cited in: Pubmed; PMID 8603802.
Ito Y, Kurata M, Hioki R, Suzuki K, Ochiai J, Aoki K. Cancer mortality and serum levels of carotenoids, retinol, and tocopherol: a population-based follow-up study of inhabitants of a rural area of Japan. Asian Pac J Cancer Prev. 2005;6(1):10–5 Epub 2005/03/23. Cited in: Pubmed; PMID 15780024.
Ito Y, Suzuki K, Ishii J, Hishida H, Tamakoshi A, Hamajima N, Aoki K. A population-based follow-up study on mortality from cancer or cardiovascular disease and serum carotenoids, retinol and tocopherols in Japanese inhabitants. Asian Pac J Cancer Prev. 2006;7(4):533–46 Epub 2007/01/26. Cited in: Pubmed; PMID 17250424.
Larsson SC, Bergkvist L, Naslund I, Rutegard J, Wolk A. Vitamin A, retinol, and carotenoids and the risk of gastric cancer: a prospective cohort study. Am J Clin Nutr. 2007;85(2):497–503. https://doi.org/10.1093/ajcn/85.2.497. Epub 2007/02/08. Cited in: Pubmed; PMID 17284749.
Malila N, Taylor PR, Virtanen MJ, Korhonen P, Huttunen JK, Albanes D, Virtamo J. Effects of alpha-tocopherol and beta-carotene supplementation on gastric cancer incidence in male smokers (ATBC Study, Finland). Cancer Causes Control. 2002;13(7):617–23. https://doi.org/10.1023/a:1019556227014. Epub 2002/09/26. Cited in: Pubmed; PMID 12296509.
Nouraie M, Pietinen P, Kamangar F, Dawsey SM, Abnet CC, Albanes D, Virtamo J, Taylor PR. Fruits, vegetables, and antioxidants and risk of gastric cancer among male smokers. Cancer Epidemiol Biomarkers Prev. 2005;14(9):2087–92. https://doi.org/10.1158/1055-9965.EPI-05-0038. Epub 2005/09/21. Cited in: Pubmed; PMID 16172214.
Wang GQ, Dawsey SM, Li JY, Taylor PR, Li B, Blot WJ, Weinstein WM, Liu FS, Lewin KJ, Wang H, et al. Effects of vitamin/mineral supplementation on the prevalence of histological dysplasia and early cancer of the esophagus and stomach: results from the General Population Trial in Linxian. China Cancer Epidemiol Biomarkers Prev. 1994;3(2):161–6 Epub 1994/03/01. Cited in: Pubmed; PMID 8049638.
Zheng W, Sellers TA, Doyle TJ, Kushi LH, Potter JD, Folsom AR. Retinol, antioxidant vitamins, and cancers of the upper digestive tract in a prospective cohort study of postmenopausal women. Am J Epidemiol. 1995;142(9):955–60. https://doi.org/10.1093/oxfordjournals.aje.a117743. Epub 1995/11/01. Cited in: Pubmed; PMID 7572976.
Lei CS, Hou YC, Pai MH, Lin MT, Yeh SL. Effects of quercetin combined with anticancer drugs on metastasis-associated factors of gastric cancer cells: in vitro and in vivo studies. J Nutr Biochem. 2018;51:105–13. https://doi.org/10.1016/j.jnutbio.2017.09.011. Epub 2017/11/11. Cited in: Pubmed; PMID 29125991.
Nowak R, Nowacka-Jechalke N, Juda M, Malm A. The preliminary study of prebiotic potential of Polish wild mushroom polysaccharides: the stimulation effect on Lactobacillus strains growth. Eur J Nutr. 2018;57(4):1511–21. https://doi.org/10.1007/s00394-017-1436-9. Epub 2017/03/30. Cited in: Pubmed; PMID 28353071.
Velmurugan B, Nagini S. Combination chemoprevention of experimental gastric carcinogenesis by s-allylcysteine and lycopene: modulatory effects on glutathione redox cycle antioxidants. J Med Food. 2005;8(4):494–501. https://doi.org/10.1089/jmf.2005.8.494. eng. Epub 2005/12/29. Cited in: Pubmed; PMID 16379561.
Liu C, Russell RM, Wang XD. Lycopene supplementation prevents smoke-induced changes in p53, p53 phosphorylation, cell proliferation, and apoptosis in the gastric mucosa of ferrets. J Nutr. 2006;136(1):106–11. https://doi.org/10.1093/jn/136.1.106. eng. Epub 2005/12/21. Cited in: Pubmed; PMID 16365067.
Krinsky NI, Johnson EJ. Carotenoid actions and their relation to health and disease. Molecular aspects of medicine. 2005 Dec;26(6):459–516. eng. Epub 2005/11/29. doi:https://doi.org/10.1016/j.mam.2005.10.001. Cited in: Pubmed; PMID 16309738.
Kelley DS, Bendich A. Essential nutrients and immunologic functions. Am J Clin Nutr. 1996;63(6):994s–6s. https://doi.org/10.1093/ajcn/63.6.994. Epub 1996/06/01. Cited in: Pubmed; PMID 8644700.
Zhou Y, Fu R, Yang M, Liu W, Tong Z. Lycopene suppresses gastric cancer cell growth without affecting normal gastric epithelial cells. J Nutr Biochem. 2023;116:109313. https://doi.org/10.1016/j.jnutbio.2023.109313. Epub 2023/03/06. Cited in: Pubmed; PMID 36871837.
Shi J, Le Maguer M. Lycopene in tomatoes: chemical and physical properties affected by food processing. Critical reviews in food science and nutrition. 2000;40(1):1–42. https://doi.org/10.1080/10408690091189275. Epub 2000/02/16. Cited in: Pubmed; PMID 10674200.
Di Mascio P, Murphy ME, Sies H. Antioxidant defense systems: the role of carotenoids, tocopherols, and thiols. Am J Clin Nutr. 1991;53(1 Suppl):194s–200s eng. Epub 1991/01/01. Cited in: Pubmed; PMID 1985387.
Paiva SA, Russell RM. Beta-carotene and other carotenoids as antioxidants. Journal of the American College of Nutrition. 1999;18(5):426–33. https://doi.org/10.1080/07315724.1999.10718880. eng. Epub 1999/10/08. Cited in: Pubmed; PMID 10511324.
Kelkel M, Schumacher M, Dicato M, Diederich M. Antioxidant and anti-proliferative properties of lycopene. Free radical research. 2011;45(8):925–40. https://doi.org/10.3109/10715762.2011.564168. eng. Epub 2011/05/28. Cited in: Pubmed; PMID 21615277.
Park B, Lim JW, Kim H. Lycopene treatment inhibits activation of Jak1/Stat3 and Wnt/β-catenin signaling and attenuates hyperproliferation in gastric epithelial cells. Nutrition research (New York, NY). 2019;70:70–81. https://doi.org/10.1016/j.nutres.2018.07.010. eng. Epub 2018/08/14. Cited in: Pubmed; PMID 30098838.
Park Y, Lee H, Lim JW, Kim H. Inhibitory Effect of β-Carotene on Helicobacter pylori-Induced TRAF Expression and Hyper-Proliferation in Gastric Epithelial Cells. Antioxidants (Basel). 2019;8(12). https://doi.org/10.3390/antiox8120637. Epub 2019/12/15.Cited in: Pubmed; PMID 31835889.
Gao M, Dang F, Deng C. β-Cryptoxanthin induced anti-proliferation and apoptosis by G0/G1 arrest and AMPK signal inactivation in gastric cancer. Eur J Pharmacol. 2019;859:172528. https://doi.org/10.1016/j.ejphar.2019.172528. Epub 2019/07/10. Cited in: Pubmed; PMID 31288004.
Wu C, Han L, Riaz H, Wang S, Cai K, Yang L. The chemopreventive effect of β-cryptoxanthin from mandarin on human stomach cells (BGC-823). Food chemistry. 2013;136(3–4):1122–9. https://doi.org/10.1016/j.foodchem.2012.09.073. eng. Epub 2012/12/01. Cited in: Pubmed; PMID 23194503.
Eom JW, Lim JW, Kim H. Lutein Induces Reactive Oxygen Species-Mediated Apoptosis in Gastric Cancer AGS Cells via NADPH Oxidase Activation. Molecules (Basel, Switzerland). 2023;28(3). https://doi.org/10.3390/molecules28031178. Epub 2023/02/12.Cited in: Pubmed; PMID 36770846.
Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL Jr, Valanis B, Williams JH Jr, Barnhart S, Cherniack MG, Brodkin CA, Hammar S. Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. J Natl Cancer Inst. 1996;88(21):1550–9. https://doi.org/10.1093/jnci/88.21.1550. Epub 1996/11/06. Cited in: Pubmed; PMID 8901853.
Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Valanis B, Williams JH, Barnhart S, Hammar S. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334(18):1150–5. https://doi.org/10.1056/NEJM199605023341802. Epub 1996/05/02. Cited in: Pubmed; PMID 8602180.
Russo GL, Moccia S, Russo M, Spagnuolo C. Redox regulation by carotenoids: Evidence and conflicts for their application in cancer. Biochem Pharmacol. 2021;194:114838. https://doi.org/10.1016/j.bcp.2021.114838. Epub 2021/11/15. Cited in: Pubmed; PMID 34774845.
Virtamo J, Pietinen P, Huttunen JK, Korhonen P, Malila N, Virtanen MJ, Albanes D, Taylor PR, Albert P, Group AS. Incidence of cancer and mortality following alpha-tocopherol and beta-carotene supplementation: a postintervention follow-up. JAMA. 2003;290(4):476–85. https://doi.org/10.1001/jama.290.4.476. Epub 2003/07/24. Cited in: Pubmed; PMID 12876090.
Shin J, Song MH, Oh JW, Keum YS, Saini RK. Pro-Oxidant Actions of Carotenoids in Triggering Apoptosis of Cancer Cells: A Review of Emerging Evidence. Antioxidants (Basel). 2020 Jun 17;9(6). https://doi.org/10.3390/antiox9060532. Epub 2020/06/21. Cited in: Pubmed; PMID 32560478.
Hennekens CH, Buring JE, Manson JE, Stampfer M, Rosner B, Cook NR, Belanger C, LaMotte F, Gaziano JM, Ridker PM, Willett W, Peto R. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. 1996;334(18):1145–9. https://doi.org/10.1056/NEJM199605023341801. Epub 1996/05/02. Cited in: Pubmed; PMID 8602179.
Friedenreich CM, Howe GR, Miller AB. The effect of recall bias on the association of calorie-providing nutrients and breast cancer. Epidemiology. 1991;2(6):424–9. https://doi.org/10.1097/00001648-199111000-00006. Epub 1991/11/01. Cited in: Pubmed; PMID 1790194.
Musa-Veloso K, Card JW, Wong AW, Cooper DA. Influence of observational study design on the interpretation of cancer risk reduction by carotenoids. Nutr Rev. 2009;67(9):527–45. https://doi.org/10.1111/j.1753-4887.2009.00225.x. Epub 2009/08/26. Cited in: Pubmed; PMID 19703260.
Li P, Zhang H, Chen J, Shi Y, Cai J, Yang J, Wu Y. Association between dietary antioxidant vitamins intake/blood level and risk of gastric cancer. Int J Cancer. 2014;135(6):1444–53. https://doi.org/10.1002/ijc.28777. Epub 2014/02/11. Cited in: Pubmed; PMID 24510802.
Suzuki KJ, Nakaji S, Tokunaga S, Shimoyama T, Umeda T, Sugawara K. Confounding by dietary factors in case-control studies on the efficacy of cancer screening in Japan. Eur J Epidemiol. 2005;20(1):73–8. https://doi.org/10.1007/s10654-004-0872-z. Epub 2005/03/11. Cited in: Pubmed; PMID 15756907.
Kavanaugh CJ, Trumbo PR, Ellwood KC. The U.S. Food and Drug Administration’s evidence-based review for qualified health claims: tomatoes, lycopene, and cancer. J Natl Cancer Inst. 2007;99(14):1074–85. https://doi.org/10.1093/jnci/djm037. Epub 2007/07/12. Cited in: Pubmed; PMID 17623802.
Lin J, Cook NR, Albert C, Zaharris E, Gaziano JM, Van Denburgh M, Buring JE, Manson JE. Vitamins C and E and beta carotene supplementation and cancer risk: a randomized controlled trial. J Natl Cancer Inst. 2009;101(1):14–23. https://doi.org/10.1093/jnci/djn438. Epub 2009/01/01. Cited in: Pubmed; PMID 19116389.
Ko KP, Park SK, Park B, Yang JJ, Cho LY, Kang C, Kim CS, Gwack J, Shin A, Kim Y, Kim J, Yang HK, Kang D, Chang SH, Shin HR, Yoo KY. Isoflavones from phytoestrogens and gastric cancer risk: a nested case-control study within the Korean Multicenter Cancer Cohort. Cancer Epidemiol Biomarkers Prev. 2010;19(5):1292–300. https://doi.org/10.1158/1055-9965.EPI-09-1004. Epub 2010/05/08. Cited in: Pubmed; PMID 20447921.
Mariani S, Lionetto L, Cavallari M, Tubaro A, Rasio D, De Nunzio C, Hong GM, Borro M, Simmaco M. Low prostate concentration of lycopene is associated with development of prostate cancer in patients with high-grade prostatic intraepithelial neoplasia. Int J Mol Sci. 2014;15(1):1433–40. https://doi.org/10.3390/ijms15011433. eng. Epub 2014/01/24. Cited in: Pubmed; PMID 24451130.
van Breemen RB, Sharifi R, Viana M, Pajkovic N, Zhu D, Yuan L, Yang Y, Bowen PE, Stacewicz-Sapuntzakis M. Antioxidant effects of lycopene in African American men with prostate cancer or benign prostate hyperplasia: a randomized, controlled trial. Cancer prevention research (Philadelphia, Pa). 2011;4(5):711–8. https://doi.org/10.1158/1940-6207.Capr-10-0288. eng. Epub 2011/03/25. Cited in: Pubmed; PMID 21430075.
Acknowledgements
Not applicable.
Funding
We acknowledge the financial support from National Natural Science Foundation of China (No. 82060800), Gansu Provincial Science Foundation for Young Scholars of China (No. 21JR1RA149, 21JR11RA114, 20JR10RA759), and Cuiying Scientific and Technological Innovation Program of Lanzhou University Second Hospital (No. CY2022-QN-A15).
Author information
Authors and Affiliations
Contributions
Xuan Ren completed the project design, manuscript revision and review, Wei Han completed the data collection, analysis and manuscript writing, and Wei Zhang completed the data extraction and analysis.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Table 1. Literature search strategy. Table 2. Basic information of included studies. Table 3. Risk of bias assessment results.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Han, W., Zhang, W. & Ren, X. Not all carotenoids can reduce the risk of gastric cancer: a systematic review with meta-analysis. BMC Gastroenterol 24, 51 (2024). https://doi.org/10.1186/s12876-024-03139-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-024-03139-5