Abstract
Background
A considerable number of patients with colon cancer present with a colonic obstruction. The use of self-expanding metallic stents (SEMS) as a bridge to surgery (BTS) in potential curative patients with left-sided colonic cancer obstruction remains debatable. Therefore, this study aimed to investigate the 5-year oncological outcomes of using a SEMS as a BTS.
Methods
All patients with left-sided malignant colon obstruction who underwent curative surgery with no metastasis upon presentation between March 2009 and May 2013 were retrospectively reviewed and analyzed.
Results
A total of 45 patients were included, 28 patients underwent upfront surgery, and 17 patients had a stent as a bridge to surgery. T4 stage was statistically significantly higher in patients who had a SEMS as a BTS (35.3% vs. 10.7%) (p-value 0.043). The mean duration in days of the SEMS to surgery was 13.76 (SD 10.08). TNM stage 3 was a prognostic factor toward distant metastasis (HR 5.05). When comparing patients who had upfront surgery to those who had a SEMS as a BTS, higher 5-year disease-free survival (75% vs. 72%) and 5-year overall survival (89% vs. 82%) were seen in patients who had upfront surgery. However, both were statistically insignificant.
Conclusion
Using self-expanding metallic stents as a bridge to surgery yields comparable 5-year survival and disease-free survival rates to upfront emergency surgery. The decision to use SEMS versus opting for emergency surgery should be made after careful patient selection and with the assistance of experienced endoscopists.
Trial registration
N/A.
Similar content being viewed by others
Background
Colorectal cancer (CRC) is a significant cause of morbidity and mortality worldwide. It is the third most common cancer and the second most common cause of death globally [1]. The incidence of CRC cancer in Saudi Arabia has increased significantly in the last two decades [2].
13% of patients with CRC present initially with an acute large bowel obstruction [3]. Emergency upfront surgery was the sole intervention for those patients to relieve the obstruction despite its high risk of mortality and morbidity compared to elective surgery due to patients’ poor conditions related to age, malnutrition, sepsis, and inadequate bowel preparation [4, 5]. However, self-expanding metallic stents (SEMS) as a bridge to surgery (BTS) are gaining popularity as an alternative option for colonic decompression prior to surgery [6].
The success rate of endoscopic stenting is reported to be up to 90% [7]. SEMS relieves colonic obstruction, turning an emergency operation into an elective resection, which has a higher primary anastomosis rate, limiting postoperative complications and reducing the rate of stoma creation [8]. Many studies documented a comparable oncological outcome between SEMS as BTS to upfront emergency surgery [9, 10]. In addition, it allows optimization of the patient’s condition and proper tumor staging before definitive intervention [11]. Several authors do not recommend using SEMS as some reports found it to be associated with tumor cell dissemination, tumor perforation, and increased local recurrence rates [12,13,14,15,16,17].
The long-term oncological outcomes of using SEMS as a BTS in potential curative patients with left-sided colonic cancer obstruction remain debatable. We have previously studied the short-term outcomes and complications following SEMS placement in malignant colonic obstruction at our center [18]. This study aimed to investigate the 5-year oncological outcomes of using SEMS as a BTS.
Methodology
After the approval of the Ethical Institutional Review Board (IRB) at King Saud University. We retrospectively reviewed the medical records of all patients with left-sided malignant colon obstruction at King Saud University Medical City (KSUMC), an academic medical institution and a tertiary hospital with 1200 beds in Riyadh, Saudi Arabia.
Patients with left-sided non-metastatic malignant colon obstruction who underwent curative surgery upon presentation between March 2009 and May 2013 were included in this study. Patients managed palliatively, less than 18 years, right-sided colon obstruction, rectal cancer, and benign etiology were excluded. Patients were divided into two groups; those who had an emergency upfront surgery and those who had a SEMS as a BTS.
Management pathway
All patients were assessed and treated initially by the general surgery on-call or acute care team, and the colorectal consultant on-call was involved in the management if needed. The decision of SEMS placement versus upfront surgery was discussed with the gastroenterologist on-call. Patients with hemodynamic instability or generalized peritonitis were not fit for SEMS placement. The following working day, the colorectal team reviews and manages all the cases, in addition to presenting all cases at the tumor board meeting for further management.
The procedure for SEMS placement
For all patients, the endoscopic placement of SEMS was done by senior gastroenterologists. Each patient was evaluated clinically and radiologically to assess the lesion. The colon was prepared with a water-soluble enema prior to the procedure. Uncovered (WallFlex) colonic stents were used, 22 mm in diameter and 60 or 90 mm in length. The stents were placed endoscopically under fluoroscopic guidance. If the scope did not pass through the obstruction, a 0.035-inch guide wire was used to help with stent placement. The patients were monitored prior to, during, and after the procedure. Any adverse events were recorded and addressed.
Statistical analysis
Data were analyzed using Statistical Package for Social Studies (SPSS 22; IBM Corp., New York, NY, USA). Continuous variables were expressed as mean ± standard deviation and median, and Categorical variables were expressed as percentages. The t-test was used for continuous variables with normal distribution, and Mann-Whitney Test was used for continuous variables without normal distribution. The chi-square test and Fisher exact test were used for categorical variables. Cox proportional hazards regression was performed to estimate hazard ratios (HR) (95% CI). Survival curves were estimated by the Kaplan–Meier method. A p-value < 0.05 was considered statistically significant.
Results
Between March 2009 and May 2013, 45 patients with left-sided malignant colon obstruction underwent colonic resection. Of these, 28 patients (62.2%) had an upfront surgery, and 17 (37.8%) had a SEMS as a BTS. Following stent placement, only one patient had an obstruction with impacted stool, and was managed with colonoscopy-mediated irrigation. Total colectomy was done for two patients, one in each group, due to either questionable blood supply or multiple serosal tears in the distended right colon. Worth to mention the total colectomy performed in the SEMS group was for the same patient who had complicated stent stool impaction. The baseline characteristics of both groups are illustrated in Table 1.
Clinical tumor parameters are presented in Table 2. The T2 stage was found only in patients who had upfront surgery; T3 was similar between both groups, whereas the T4 stage was significantly higher in patients who had a SEMS as a BTS (35.3% vs. 10.7%) (p-value 0.043). The mean duration in days of SEMS to curative surgery was 13.76 (± 10.08).
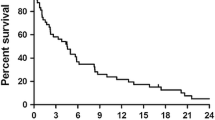
Oncological outcomes are summarized in Table 3. The overall local and distant recurrence rates were 4.44% and 26.67%, respectively. Local recurrence was seen only in the SEMS group, with one patient having a recurrence at the anastomosis site and one at the surgical bed. The overall and disease-free survival rates were 87% and 72%, respectively. When comparing patients who had upfront surgery to those who had a SEMS as a BTS, higher 5-year disease-free survival (75% vs. 71%) and 5-year overall survival (89% vs. 82%) were seen in patients who had upfront surgery. However, both were statistically insignificant, as seen in Figs. 1 and 2.
Prognostic factors toward distant metastasis are shown in Table 4. TNM stage 3 was a risk factor for distant metastasis (p-value 0.025). The univariate regression model tested the significant factors, as shown in Table 5.
Prognostic factors toward survival are displayed in Table 6. The significant factors were tested in the univariate regression model, as shown in Table 5. Survival rate correlated significantly with TNM stages 2 and 3 and distant metastasis. However, all of the factors were insignificant in the multivariant analysis.
Discussion
The use of SEMS in malignant colonic obstruction has gained attention as an alternative decompression intervention prior to curative resection. In many studies, SEMS placement was associated with high clinical success and good short-term outcomes [7,8,9,10]. In spite of this, long-term oncological outcomes remain uncertain. This study presents the 5-year oncological outcomes of using a SEMS as a BTS in left-sided colonic cancer obstruction.
SEMS is a challenging procedure that requires an experienced gastroenterologist as it has a risk of perforation and failed decompression; the placement also depends on the site and length of tumor stenosis [19]. In the literature, some authors reported concerns regarding SEMS, which include a high risk of tumor cell dissemination, distant metastasis, and recurrence [16, 20]. In addition, it has been hypothesized that SEMS induces perineural invasion due to the expanding pressure pushing the obstruction onto the lumen wall, which causes cancer cell invasion to the surrounding vessels and tissues [20]. In this study, the perineural invasion was similar between both groups and lymphovascular invasion was (11.8% vs. 17.9%) in SEMS and upfront surgery, respectively.
A bridging interval between 9 and 14 days is ideal due to concerns about stent-related peritumor inflammation and adhesions, which may increase the technical difficulty of resection [6]. Moreover, a significantly increased risk of disease recurrence in patients with a bridging interval of more than two weeks has been reported [6]. In this study, the mean duration of days between SEMS to surgery was 13.76 (SD 10.08), and (64.7%) of patients had surgery in 14 days or less. We found that the distant recurrence rate was comparable in both groups, with no increased risk in patients with more than 14 days of SEMS to surgery interval (p-value = 0.605).
There have been mixed reports in regard to disease recurrence in the SEMS group compared to upfront surgery. Some studies reported higher recurrence rates in the SEMS group [10, 16, 17]; others reported no differences in local and distant recurrence rates [15, 21]. Our data showed that local recurrence was seen in only 2 patients (11.8%) in the SEMS group, and distant metastasis was seen in 5 (29.4%) vs. 7 (25%) in SEMS and upfront surgery groups, respectively. In addition, positive nodes, male sex, anastomotic dehiscence, and diffuse peritonitis were reported as prognostic factors toward recurrence [22]. The only risk factor for distant metastasis in our patient population was TNM stage 3 (HR 5.05).
Sabbagh et al. reported that the 5-year overall survival rate for left-sided colonic cancer was lower in the SEMS group than in the upfront surgery group (25% vs. 62%) (p-value = 0.003), and the 5-year cancer-specific mortality rate was higher in the SEMS group (48% vs. 21%) (p-value = 0.02) [15]. However, no significant differences between the two groups in 5-year disease-free survival were found [15]. They included TNM stage 4, which was significantly higher in the SEMS group. The increased mortality observed in the SEMS group was likely due to the more advanced stage of the disease. A recent meta-analysis of randomized clinical trials showed no differences in recurrence and 3-year survival rate between the two groups [10]. Our current study only included patients with left-sided colonic obstruction and excluded patients who initially presented with distant metastasis. The 5-year overall survival and disease-free survival for patients who had upfront surgery compared to those who had a SEMS as a BTS were (89% vs. 82%) and (75% vs. 71%), respectively. However, both were statistically insignificant.
This study has a few limitations that should be considered. First, it is a retrospective cohort study, where inherent bias may be present. Second, the sample size is small, which could contribute to the statistical insignificance of different variables. Lastly, a standardized grading system for the severity of the obstruction is lacking, so implementing a scoring system would help categorize the patients appropriately. A prospective, multicentric study with a grading score for the obstruction severity, such as ColoRectal Obstruction Scoring System (CROSS), is recommended to assess the long-term oncological outcome [23].
Conclusion
SEMS as a BTS has emerged as a viable option for patients with left-side colonic cancer obstruction. Our studies have shown that SEMS as a bridge to surgery is associated with comparable outcomes to upfront surgery. However, the optimal management strategy for these patients remains controversial. Therefore, tailored therapy for each patient presenting with left-side colonic cancer obstruction is advised.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- SEMS:
-
Self-expanding metallic stents
- BTS:
-
Bridge to surgery
- CRC:
-
Colorectal cancer
- IRB:
-
Institutional Review Board
- KSUMC:
-
King Saud University Medical City
References
Cancer: World Health Organization. ; 2022 [Available from: https://www.who.int/news-room/fact-sheets/detail/cancer.
Alsanea N, Abduljabbar AS, Alhomoud S, Ashari LH, Hibbert D, Bazarbashi S. Colorectal cancer in Saudi Arabia: incidence, survival, demographics and implications for national policies. Ann Saudi Med. 2015;35(3):196–202.
Jullumstrø E, Wibe A, Lydersen S, Edna TH. Colon cancer incidence, presentation, treatment and outcomes over 25 years. Colorectal Dis. 2011;13(5):512–8.
McArdle CS, Hole DJ. Emergency presentation of colorectal cancer is associated with poor 5-year survival. Br J Surg. 2004;91(5):605–9.
Tanis PJ, Paulino Pereira NR, van Hooft JE, Consten EC, Bemelman WA, Dutch Surgical Colorectal A. Resection of obstructive left-sided Colon cancer at a National Level: a prospective analysis of short-term outcomes in 1,816 patients. Dig Surg. 2015;32(5):317–24.
Lim T, Tham HY, Yaow CYL, Tan IJ, Chan DKH, Farouk R, et al. Early surgery after bridge-to-surgery stenting for malignant bowel obstruction is associated with better oncological outcomes. Surg Endosc. 2021;35(12):7120–30.
Jimenez-Perez J, Casellas J, Garcia-Cano J, Vandervoort J, Garcia-Escribano OR, Barcenilla J, et al. Colonic stenting as a bridge to surgery in malignant large-bowel obstruction: a report from two large multinational registries. Am J Gastroenterol. 2011;106(12):2174–80.
Watt AM, Faragher IG, Griffin TT, Rieger NA, Maddern GJ. Self-expanding metallic stents for relieving malignant colorectal obstruction: a systematic review. Ann Surg. 2007;246(1):24–30.
Jain SR, Yaow CYL, Ng CH, Neo VSQ, Lim F, Foo FJ, et al. Comparison of colonic stents, stomas and resection for obstructive left colon cancer: a meta-analysis. Tech Coloproctol. 2020;24(11):1121–36.
Cirocchi R, Arezzo A, Sapienza P, Crocetti D, Cavaliere D, Solaini L et al. Current status of the self-expandable metal stent as a bridge to surgery versus emergency surgery in Colorectal Cancer: results from an updated systematic review and Meta-analysis of the literature. Med (Kaunas). 2021;57(3).
Saito S, Yoshida S, Isayama H, Matsuzawa T, Kuwai T, Maetani I, et al. A prospective multicenter study on self-expandable metallic stents as a bridge to surgery for malignant colorectal obstruction in Japan: efficacy and safety in 312 patients. Surg Endosc. 2016;30(9):3976–86.
Maruthachalam K, Lash GE, Shenton BK, Horgan AF. Tumour cell dissemination following endoscopic stent insertion. Br J Surg. 2007;94(9):1151–4.
Gorissen KJ, Tuynman JB, Fryer E, Wang L, Uberoi R, Jones OM, et al. Local recurrence after stenting for obstructing left-sided colonic cancer. Br J Surg. 2013;100(13):1805–9.
Kim HJ, Choi GS, Park JS, Park SY, Jun SH. Higher rate of perineural invasion in stent-laparoscopic approach in comparison to emergent open resection for obstructing left-sided colon cancer. Int J Colorectal Dis. 2013;28(3):407–14.
Sabbagh C, Browet F, Diouf M, Cosse C, Brehant O, Bartoli E, et al. Is stenting as “a bridge to surgery” an oncologically safe strategy for the management of acute, left-sided, malignant, colonic obstruction? A comparative study with a propensity score analysis. Ann Surg. 2013;258(1):107–15.
van den Sloothaak DA, Dijkgraaf MG, Fockens P, Tanis PJ, van Hooft JE, et al. Oncological outcome of malignant colonic obstruction in the dutch Stent-In 2 trial. Br J Surg. 2014;101(13):1751–7.
Erichsen R, Horvath-Puho E, Jacobsen JB, Nilsson T, Baron JA, Sorensen HT. Long-term mortality and recurrence after colorectal cancer surgery with preoperative stenting: a danish nationwide cohort study. Endoscopy. 2015;47(6):517–24.
Alkhayal KA, Alshammari SA, Al-Mazrou AM, Almadi MA, Al-Obeed OA, Zubaidi AM, et al. Short-term outcomes after self-expandable metal stent insertion for obstructing colon cancer: a retrospective cohort study. Ann Saudi Med. 2020;40(5):403–7.
Veld JV, Amelung FJ, Borstlap WAA, van Halsema EE, Consten ECJ, Siersema PD, et al. Comparison of Decompressing Stoma vs Stent as a bridge to surgery for left-sided obstructive Colon cancer. JAMA Surg. 2020;155(3):206–15.
Yamashita S, Tanemura M, Sawada G, Moon J, Shimizu Y, Yamaguchi T, et al. Impact of endoscopic stent insertion on detection of viable circulating tumor cells from obstructive colorectal cancer. Oncol Lett. 2018;15(1):400–6.
Hidalgo-Pujol M, Biondo S, Die Trill J, Vigorita V, Paniagua Garcia-Senorans M, Pascual Miguelanez I, et al. Upfront surgery versus self-expanding metallic stent as bridge to surgery in left-sided colonic cancer obstruction: a multicenter observational study. Surgery. 2022;172(1):74–82.
Biondo S, Galvez A, Ramirez E, Frago R, Kreisler E. Emergency surgery for obstructing and perforated colon cancer: patterns of recurrence and prognostic factors. Tech Coloproctol. 2019;23(12):1141–61.
Uehara H, Yamazaki T, Iwaya A, Kameyama H, Komatsu M, Hirai M. Comparison of the oncological outcomes of stenting as a bridge to surgery and surgery alone in stages II to III obstructive colorectal cancer: a retrospective study. Ann Coloproctol. 2022;38(3):235–43.
Acknowledgements
The authors are grateful to the Deanship of Scientific Research, King Saud University, for supporting this study through the Vice Deanship of Scientific Research Chairs. The authors thank Nora Altaweel for her contribution to the data collection.
Funding
The authors declare that they have no funding.
Author information
Authors and Affiliations
Contributions
NH and SS analyzed and interpreted the patient data. AZ and RR were the major contributors to writing the manuscript. All authors read, edited, and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
The project has been approved by the Ethical Institutional Review Board at King Saud University, College of Medicine (No. E- 20-5409). The need for written informed consent was waived by the Ethical Institutional Review Board at King Saud University, College of Medicine due to the retrospective nature of the study. This study was conducted in accordance with the ethical principles of the Declaration of Helsinki for medical research involving human subjects and the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for observational studies.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Alhassan, N.S., AlShammari, S.A., AlRabah, R.N. et al. 5-year oncological outcomes in left-sided malignant colonic obstruction: stent as bridge to surgery. BMC Gastroenterol 23, 262 (2023). https://doi.org/10.1186/s12876-023-02903-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-023-02903-3