Abstract
Background
Thiopurines continue to play an important role in the treatment of inflammatory bowel disease (IBD). It is well known that thiopurines can cause several adverse reactions. Especially, hematopoietic toxicity may lead to severe agranulocytosis. In a previous prospective study, we investigated the relationship between inosine triphosphate pyrophosphatase (ITPA) c.94c > a polymorphism, 6-thioguanine nucleotide (6-TGN) concentration and toxicity.
Methods
To clarify the cause of thiopurine toxicity, we analysed nucleoside disphosphate-linked moiety X-type motif 15 (NUDT15) gene polymorphisms, i.e., R139C, V18I, and V19_V19insGV, and measured 6-mercaptopurines and 6-methylmercaptopurines (6-MMP) using the archived blood samples collected from 49 IBD patients for our previous study.
Results
The ITPA c.94c > a polymorphism was detected in 19 patients (38.7%, all heterozygous). The R139C polymorphism was found in 10 patients (20.4%, 1 homozygous, 9 heterozygous), V18_V19insGV in 7 patients (14.3%, all heterozygous), and V18I in 2 patients (4.08%, all heterozygous). Although R139C was more strongly associated with leukopenia than c.94c > a, there were no significant correlations with 6-TGN and 6-MMP levels, as for c.94c > a. The leukopenia incidence rates for each gene polymorphism were 0% in those with all wild-type genes, 21.4% for c.94c > a only, 42.9% for NUDT15 polymorphism (s) only, and 80.0% for both polymorphisms.
Conclusions
All cases of leukopenia were associated with ITPA c.94c > a and/or polymorphism of NUDT15 and the risk of developing leukopenia was synergistically increased by ITPA and NUDT15 gene polymorphism. However, there was no association between the level of azathioprine metabolites and these polymorphisms.
Similar content being viewed by others
Background
Inflammatory bowel disease (IBD) involves chronic inflammation of the intestinal tract of unknown cause. Although many treatments to induce remission of IBD have been developed, thiopurines continue to play an important role in the treatment of IBD. It is well known that thiopurines can cause several adverse reactions (ARs), such as leukopenia, alopecia, hepatitis, and pancreatitis. In particular, hematopoietic toxicity may lead to severe agranulocytosis.
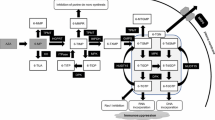
Azathioprine (AZA) is a prodrug of 6-mercaptopurine (6-MP) that is converted to 6-MP in a nonenzymatic pathway in erythrocytes (Fig. 1). 6-MP is then converted to various metabolites by three metabolic enzymes: xanthine oxidase (XO), thiopurine S-methyltransferase (TPMT), and hypoxanthine guanine phosphoribosyl transferase (HGPRT). The activity of TPMT influences the content of 6-thioguanine nucleotide (6-TGN), which increases when activity of the enzyme is reduced due to polymorphism of the gene. Inosine triphosphate pyrophosphatase (ITPase) converts 6-thioinosine triphosphate (6-TITP) to 6-thioinosine monophosphate (6-TIMP), and decreased ITPase activity is thought to lead to accumulation of 6-TITP. In our previous retrospective study, we reported that polymorphism of ITPA is closely related to ARs of thiopurines, especially hematopoietic toxicity, in Japanese patients with IBD [1]. A recent Korean study has revealed that nucleoside diphosphate-linked moiety X-type motif 15 (NUDT15) polymorphism is associated with ARs, in particular, bone marrow toxicity and alopecia [2]. NUDT15, which belongs to the nudix hydrolase enzyme family, converts the thiopurine active metabolites 6-thioguanosine triphosphate (6-TGTP) and 6-thiodeoxyguanosine triphosphate (6-TdGTP) to 6-thioguanosine monophosphate (6-TGMP) and 6-thiodeoxyguanosine monophosphate (6-TdGMP), respectively. Carriers of polymorphism in this gene have lower enzyme activity, and it has been reported that individuals who are homozygous for polymorphism almost certainly develop bone marrow toxicity [3].
Metabolism and transportation of AZA/6MP and its metabolites. XO, xanthine oxidase; TPMT, thiopurine S-methyltransferase; HGPRT, hypoxanthine guanine phosphoribosyl transferase; ITPase, inosine triphosphate pyrophosphatase; IMPDH, inosine monophosphated dehydrogenase; GMPS, guanosine monophosphate synthetase; NUDT15, nucleoside diphosphate-linked moiety X-type motif 15; AZA, azathioprine, 6-MP, 6-mercaptopurine; 6-TUA, 6-thiouric acid; 6-MeMP, 6-methylmercaptopurine; 6-TIMP, 6-thioinosine monophosphate; 6-TIDP, 6-thioinosine diphosphate; 6-TITP, 6-thioinosine triphosphate; 6-MeTIMP, 6-methylthionosine monophosphate; 6-MeTIDP, 6-methylthionosine diphosphate; 6-MeTITP, 6-methylthionosine triphosphate; 6-TXMP, 6-thixanthosine 5’-monophosphate; 6-TGMP, 6-thioguanosine monophosphate; 6-TGDP, 6-thioguanosine diphosphate; 6-TGTP, 6-thioguanosine triphosphate; 6-MeTGMP,; 6-MeTGMP, 6-methylthioguanine monophosphate; 6-MeTGDP, 6-methylthioguanine diphosphate; 6-MeTGTP, 6-methylthioguanine triphosphate; 6-MMPs, 6-methylmercaptopurines; 6-MPs, 6-mercaptopurines; 6-TGNs, 6-thiguanine nucleotide
Nevertheless, NUDT15 polymorphism alone is not sufficient to explain all thiopurine intolerance events, including bone marrow toxicity [4]. Although a higher 6-TGN level is thought to be one of the causes of leukopenia, it has been reported that NUDT15 polymorphism is not related to the content of 6-TGNs [5]. Similarly, our previous prospective study monitoring 6-TGN levels revealed that ITPA polymorphism is associated with the development of myelotoxicity, with no significant difference in 6-TGN level between groups with and without ARs [6]. There was a background that the NUDT15 gene polymorphism had not been reported at the time of our previous prospective study. Therefore, since the relationship between NUDT15 gene polymorphism and thiopurine toxicity has been reported, it is important to analyze the content of intermediate metabolites other than 6-TGN to elucidate the cause of thiopurine toxicity. In this study, we additionally analyzed the NUDT15 gene and measured intermediate metabolites of AZA, namely, 6-mercaptopurines (6-MPs) and 6-methylmercaptopurines (6-MMPs), using archived blood samples from a previous study. We also investigated relationships between AZA metabolites and polymorphism(s) in various genes involved in AZA metabolism, i.e., TPMT, NUDT15 and ITPA.
Methods
Subjects
In this study, we analyzed intermediate metabolites levels and NUDT15 polymorphisms in IBD patients using archived samples from previous research [6]. From March 2008 to June 2011, 49 Japanese IBD patients who received AZA for 52 weeks were enrolled. In Western countries, an AZA dosage of 2–3 mg/kg/day is recommended for the treatment of IBD patients [7]; however, lower dosages (0.6–1.2 mg/kg/day) are used in Japanese individuals because of their relatively heightened sensitivities [8]. In this previous study, the initial AZA dosage was 1.0 mg/kg/day, and whole blood samples were drawn at 0, 1 and 2 weeks and every 4 weeks thereafter until week 52 or the onset of adverse reactions. Blood samples were generally collected in the morning before administration of thiopurine. These blood samples were stored in a -30 °C freezer after the previous study was completed. In our previous study, 48 of 50 patients were observed; we excluded two patients due to cancellation of follow-up because one patient moved and the other complained of hair loss. In the latter case, hair loss occurred immediately after starting the drug, and the patient stopped taking AZA at his own discretion; the degree of hair loss was so mild that it was difficult to diagnose alopecia due to thiopurine. However, improvement in hair volume was objectively confirmed in follow-up. In the present study, we analyzed the archived blood samples of forty-nine patients, including the patient who experienced hair loss. The mean age was 33.8 (range 16–70), and 30 males (61.2%) and 19 females (38.7%) were included. Thirty patients had ulcerative colitis (UC) (61.2%), and 19 had Crohn’s disease (CD) (38.8%).
TPMT, ITPA, NUDT15
For TPMT gene analysis, mutant alleles that reportedly reduce enzyme activity, i.e., *3C and *6, were analyzed by PCR–RFLP; other polymorphism were examined by sequence analysis. For ITPA gene analysis, we screened for the c.94c > a polymorphism, which causes reduced ITPase activity [1, 9]. For NUDT15 gene analysis, we evaluated the R139C, V18I, and V18_V19insGV polymorphism, which cause reduced enzyme activity [10, 11], by PCR amplification and Sanger sequencing. The primer sequences used were based on those reported by Moriyama et al., and those for exon 1 were modified (forward, 5’-GTG GTG CCG AGG TTG GTA AG-3’ and reverse, 5’-ACC TCA CAG ACG AAC TCC CA-3’).
6-TGNs, 6-MMPs, 6-MPs
6-TGN levels in red blood cells (RBCs) were measured using high-performance liquid chromatography (HPLC), as described in our previous study [6, 12, 13]. Liquid chromatography tandem mass spectrometry (LC–MS/MS) was used to assess 6-MMPs and 6-MPs [14]. The average concentration of various metabolites was calculated from the 8th to the 52nd week after the start of administration.
Definition of ARs
In this study, leukopenia was defined as a white blood cell count of 2500/μL or less. Hepatotoxicity was defined as elevated serum alanine aminotransferase and aspartate aminotransferase levels above the upper limit of the normal range.
Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics version 22. The chi-square test was used for cross-tabulation, but Fisher's exact test was applied when cells with less than the minimum expected frequency were detected. The incidence of ARs by each gene was compared with the chi-square test. Comparisons of various averages between groups were examined by the t-test or ANOVA. Logistic regression analyses were employed to evaluated the association between ARs or leukopenia and various risk factors. Statistical significance was declared at the 0.05 level. The chi-square test was used to compare the obtained genotype distribution with the results of previous reports in Japan [15, 16].
Ethical considerations
These experiments were approved by the Ethics Committee of the Jikei University School of Medicine (27–323(8208)) and Niigata University of Pharmacy and Applied Life Sciences (H29-001). For the previous study (19–190 (5121)), we explained the purpose of the study and the methods involved to all participants prior to enrollment, and each individual provided written informed consent.
In conducting additional analysis with an existing sample, we announced the medical research on the bulletin board in front of the outpatient consultation room and asked whether participants wished to be excluded from the study.
Results
TPMT, ITPA, NUDT15
All patients carried the wild-type TPMT gene sequence. The ITPA c.94c > a polymorphism was detected in 19 patients (38.7%, all heterozygous). With respect to the NUDT15 gene, the R139C polymorphism in exon 3 was detected in 10 patients (20.4%, 1 homozygous, 9 heterozygous; allele frequency 0.112); the V18_V19insGV polymorphism in exon 1 was found in 7 patients (14.3%, all heterozygous; allele frequency 0.0714) and the V18I polymorphism in exon 1 in 2 (4.08%, all heterozygous; allele frequency 0.0204). Six of the 7 patients harboring the V18_V19insGV polymorphism also carried the R139C polymorphism, and one showed R139C homozygosity. Wild-type genes were detected in 22 of the 49 patients (44.9%). Fourteen patients (28.6%) carried only the ITPA c.94c > a polymorphism and 8 patients (16.3%) only NUDT15 polymorphism(s); 5 patients (10.2%) carried both ITPA c.94c > a and NUDT15 polymorphisms. There was no significant difference in the allele frequency of each polymorphism compared to previous reports [15, 16] in Japanese (ITPA c.94c > a: p = 0.121, NUDT15: p = 0.400).
ARs
ARs occurred in 15 of the 49 patients (30.6%), with some overlap, including leukopenia in 10 (20.4%), alopecia in 4 (8.2%), agranulocytosis in 1 (2.0%), hepatotoxicity in 1 (2.0%), and eruption in 1 (2.0%) (Table1). These patients consisted of 5 males (33.3%) and 10 females (66.7%). Onset was reported to occur from 7 to 364 days after AZA administration. Seven patients discontinued AZA therapy due to ARs.
The incidence of all ARs tended to be higher in patients with than in those without the ITPA c.94c > a polymorphism, but there was no significant difference (42.1% vs. 23.3%, P = 0.165). In addition, the incidence of all ARs was significantly higher in patients with NUDT15 (P = 0.001) than in patients without (69.2% vs. 16.7%, P = 0.001) NUDT15 gene polymorphism. With respect to leukopenia, the probability was higher in patients with than that in those without the c.94c > a polymorphism (36.8% vs. 10.3%, P = 0.033), as we previously reported. Similar to previous studies, the incidence of leukopenia in patients with NUDT15 polymorphisms was higher than that in those without NUDT15 polymorphisms (53.8% vs 8.3%, P = 0.002). Furthermore, the incidence of leukopenia occurred in the following order: wild-type, c.94c > a only, NUDT15 polymorphism(s) only, and both polymorphisms (0%, 21.4%, 37.5%, and 80.0%, respectively) (Fig. 2). We also confirmed that the number of days until leukopenia developed tended to decrease along the order of both polymorphisms, NUDT15 polymorphism(s) only, and c.94c > a only, but without a significant difference (60.8 ± 50.5, 85.3 ± 47.6, and 158.7 ± 176.234 days, respectively). The incidence of cumulative nonleukopenia based on the various genotypes was higher in the order of wild-type, c.94c > a only, NUDT15 polymorphism(s) only, and both polymorphisms, with significant differences (Fig. 3).
Estimates of cumulative incidence of leukopenia by each gene polymorphism pattern. It was confirmed that the number of days until leukopenia developed tended to decrease in the order of both polymorphisms, only polymorphism(s) of NUDT15, and only ITPA, but there was no statistically significant difference. ITPA, inosine triphosphate pyrophosphatase; NUDT15, nucleoside diphosphate-linked moiety X-type motif 15
The only patient who harbored a homozygous R139C polymorphism developed severe alopecia and agranulocytosis, and this patient also carried the c.94c > a polymorphism.
Similar to our previous report, the percentage of females among patients with ARs was higher than that among patients without ARs (66.7% vs. 26.5%, P = 0.010), and the proportion of females among patients with leukopenia was higher than that among patients without leukopenia (70.0% vs. 30.8%, P = 0.029) (Fig. 4). Moreover, the prevalence of NUDT15 gene polymorphism was significantly higher in females and males (P = 0.049), whereas the prevalence of c.94c > a was not significantly different (P = 0.466).
Gender differences in genetic polymorphisms and adverse events. The percentage of female in patients with adverse reactions was higher than that in patients without leukopenia. However, the polymorphism prevalence of the NUDT15 gene was significantly higher in female. ITPA, inosine triphosphate pyrophosphatase; NUDT15, nucleoside disphosphate-linked moiety X-type motif 15; Adv, Adverse event
Multivariate analysis was performed using ARs as the dependent variable and female sex, ITPA c.94c > a and NUDT15 polymorphism(s) as explanatory variables, and the results showed that NUDT15 polymorphism(s) was an independent factor [OR = 11.1; 95%CI: 2.12–58.1]. When multivariate analysis was performed with leukopenia as the dependent variable, c.94c > a and NUDT15 polymorphism(s) were independent factors [c.94c > a: OR = 16.9; 95%CI: 1.45–197.6, NUDT15 polymorphism(s): OR = 25.5; 95%CI: 2.37–275.0].
Although the number of discontinuation of AZA cases was 3 of 19 (15.8%) among ITPA c.94c > a carriers and 8 of 13 (61.5%) among NUDT15 polymorphism(s) carriers, for the former, all patients who required discontinuation also carried a polymorphism in NUDT15; no carriers of ITPA polymorphism alone required discontinuation. Overall, those who harbored the ITPA polymorphism alone who developed ARs continued taking thiopurines with temporary withdrawal or dosage reduction (Table 1).
Seventeen of the 49 (34.7%) patients did not have the R139C polymorphism but did carry other polymorphisms. All of these patients had a significantly higher incidence of all ARs and leukopenia than those who carried wild-type genes (41.2% vs. 9.1%; p = 0.026, 29.4% vs. 0%; P = 0.011). Survival analysis using the Kaplan–Meier and log rank tests also confirmed significant differences in all ARs and leukopenia (P = 0.024, P = 0.007) (Fig. 5).
6-TGN, 6-MP, and 6-MMP concentrations
The average content of 6-TGN in patients with the c.94c > a polymorphism was generally higher than that in patients carrying wild-type genes, though there was no significant difference between the groups. Despite a strong association between NUDT15 polymorphism(s) and leukopenia, the average 6-TGN level in NUDT15 polymorphism carriers tended to be consistently lower than that of patients with wild-type genes.
No significant correlation between the average 6-MMP concentration and c.94c > a or NUDT15 polymorphism was observed. In addition, 6-MPs were often undetectable, probably because this intermediate is rapidly metabolized by XO, TPMT, and HGPRT and thus unstable. Hene, the average level of 6-MPs was very low compared to that of 6-TGNs and 6-MMPs. Similar to 6-TGNs and 6-MMPs, the average concentration of 6MPs did not differ significantly with genotype (Table 2).
Although a 6-MMP content above 5,700 pmol/8 × 108 RBCs is considered to be related to hepatotoxicity [17], the 6-MMP level in patients with hepatotoxicity was rather low (864 pmol/8 × 108 RBCs at most). In general, patients with higher 6-MMP contents did not exhibit hepatotoxicity (1,640 pmol/8 × 108 RBCs at most).
Discussion
The mechanism by which ARs occur due to thiopurines has remained unclear for many years. In Asians, TPMT polymorphism is not a sufficient factor for myelosuppression. Although several studies have reported that ITPA polymorphism can cause thiopurine-induced myelosuppression, only 36.8% of c.94c > a polymorphism carriers in our previous prospective study exhibited leukopenia [6]. In other words, the ITPA c.94c > a polymorphism has only been demonstrated to be one of several causes. Additionally, a research group in Korea reported on a global discovery, i.e., polymorphism of NUDT15 codon 139, and it was shown that with thiopurine treatment, those homozygous for R139C will likely develop agranulocytosis with hair loss. In particular, the prevalence of the R139C polymorphism is high in Asia, and approximately 1 in 100 Japanese individuals is homozygous. Kakuta Y et al. reported in a multicenter study that NUDT15 R139C is the best pharmacogenetic marker for predicting thiopurine-induced severe ARs, such as agranulocytosis with alopecia totalis, in Japanese patients with IBD [16]. In response to this report, NUDT15 codon 139 analysis was included as part of insurance coverage in Japan in 2019.
The present study confirmed the relationship between NUDT15 polymorphism(s) and leukopenia. Although NUDT15 codon 139 analysis can predict agranulocytosis with alopecia totalis, it cannot predict all other events, such as hepatotoxicity, leukopenia and alopecia, and probably pancreatitis, events that may necessitate discontinuation of the medication. We also confirmed that NUDT15 gene analysis can predict the occurrence of ARs; nonetheless, it should be noted that the NUDT15 gene analysis covered by Japanese insurance only involves R139C. Seventeen of our 49 (34.7%) patients did not carry R139C but did have other polymorphisms. Therefore, even among patients with a ‘wild-type NUDT15 gene’ based on R139C analysis via Japanese insurance practice, 34.7% harbor polymorphisms in exon 1, i.e., V18I, V18_V19insGV, of NUDT15 and/or ITPA c.94c > a. Moreover, all of these patients had a significantly higher incidence of all ARs and leukopenia than patients with wild-type genes. Indeed, 7 of 39 (17.9%) patients in this study without the R139C polymorphism developed some ARs. Thus, some ARs will occur in approximately 20% of patients diagnosed as having a ‘wild-type NUDT15 gene’ based on R139C analysis, forcing them to discontinue thiopurine use, temporarily withdraw from the treatment, or reduce their dose. Of the 5 patients who carried both NUDT15 polymorphism and c.94c > a, 3 (60.0%) required thiopurine discontinuation, and 1 patient (20%) required temporary withdrawal. In contrast, none of the four patients with polymorphisms only in IPTA c.94c > a required discontinuation of thiopurine, although three required dose reduction. Patients with polymorphisms in these genes appear to be less resistant to thiopurine. It should also be noted that those who carry polymorphisms in both ITPA and NUDT15 tend to develop leukopenia in a shorter period of time than those who carry only one of the polymorphisms.
A previous report revealed that homozygous NUDT15 polymorphism has a strong relationship with alopecia totalis. In the present study, the only patient carrying a homozygous polymorphism in NUDT15 (also had heterozygous polymorphism in ITPA) experienced alopecia totalis simultaneously with agranulocytosis. Thus, thiopurine administration should be absolutely avoided when a homozygous NUDT15 polymorphism is detected. On the other hand, of the 4 patients who developed alopecia in this study, one harbored NUDT15 polymorphism alone, one had ITPA polymorphism alone, one had both polymorphisms, and surprisingly, one had no polymorphisms. Therefore, it is unlikely that all drug-induced alopecia can be predicted by NUDT15 gene analysis alone.
Although the prevalence of NUDT15 polymorphisms was significantly higher in females, it is very interesting that even greater significant differences in the incidence of ARs and leukopenia based on sex were observed. Even in cases of a wild-type genotype, the activity of TPMT varies by sex, and it has been reported that activity is low in females [18]. However, the average 6-MMP level, which appears to reflect the activity of TPMT, tended to be higher in females (360.3 ± 299.4 vs. 278.4 ± 131.1, P = 0.200) in this study. Hence, the sex difference with regard to the occurrence of ARs cannot be fully explained by TPMT activity.
In this study, the mean content of 6-TGNs was not related to ARs or polymorphism(s) of NUDT15. NUDT15 converts the thiopurine active metabolite 6-TGTP to the monophosphate thioguanosine nucleotide 6-TGMP. Polymorphisms in the NUDT15 gene are thought to decrease enzyme activity and thus increase levels of 6-TGTP and 6-TdGTP, which are then incorporated into RNA or DNA and cause leukopenia [11]. As the 6-TGN level, which can be measured clinically, is the sum of 6-T(d)GMP, 6-T(d)GDP, and 6-T(d)GTP, it is understandable that NUDT15 polymorphism does not affect the level of 6-TGN. Zhu X et al. reported that neither 6-TGN nor 6-MeTIMP was associated with leukopenia but that when examined separately according to the presence or absence of NUDT15 polymorphism, a correlation between leukopenia and 6-TGN level was detected [19]. In our study, the mean 6-TGN level was calculated at 8 to 52 weeks after the start of azathioprine administration or until discontinuation due to ARs. Comparisons of subgroups were considered inappropriate in our study, as some patients discontinued medication for AR within 4 weeks.
Similar results were obtained for mean concentrations of 6-MMPs and 6-MPs. Accordingly, measurement of 6-TGNs, 6-MMPs, and 6-MPs may not be meaningful for predicting ARs due to thiopurines, as it is technically difficult to measure all metabolites that comprise these metabolites individually. Recently, it has been recommended to specifically measure the active DNA-binding triform of 6-TGN by mass spectrometry. However, the fact that this method was not adopted in our previous study in which samples were collected and analyzed from 2008 to 2011 is also considered to be one of the reasons why no correlation with the NUDT15 gene polymorphism was found.
More recently, there have been numerous reports that ITPA gene polymorphisms do not predict thiopurine toxicity. Even in studies in Asia, where the prevalence of ITPA polymorphisms is known to be high, especially the report from South India by Jena A et al. concluded that ITPA polymorphisms are not predictive of thiopurine-induced leukopenia [20]. As mentioned above, the results of this study suggest that analysis of ITPA gene polymorphism alone is insufficient for predicting thiopurine toxicity. However, carriers of both NUDT15/ITPA polymorphisms are not uncommon in Asia, and both carriers have been shown to be at additively increased risk of thiopurine toxicity. A retrospective study of 1419 Chinese patients with thiopurine-induced myelosuppression also concluded that polymorphism analysis of both the NUDT15 and ITPA genes was recommended [21].
The mechanism by which ITPA gene polymorphisms cause hair loss and leukopenia is not well understood. ITPase is not an enzyme that directly affects the level of 6-T(d)GTP, which inhibits nucleic acid synthesis. Nevertheless, it cannot be ruled out that the decrease in ITPase activity may affect the production of adenosine triphosphate (ATP) and guanosine triphosphate (GTP) by decreasing inosine monophosphate (IMP) in the de novo synthesis system of purine nucleotides, regardless of thiopurine use. IMP dehydrogenase inhibitors such as mycophenolate mofetil (MMF) inhibit the de novo nucleic acid synthesis pathway in which GTP is synthesized from IMP via guanosine monophosphate (GMP). MMF-induced leucopenia has also been reported [22], and it can be understood that the importance of IMP in the de novo nucleic acid synthesis pathway and the ITPA polymorphism have a significant impact on nucleic acid synthesis. One case report described a patient with microscopic polyangiitis who carried a heterozygous c.94c > a polymorphism alone and presented with agranulocytosis immediately after the dose of AZA was increased [10].
The results of this study clearly show that analysis of NUDT15 polymorphisms is effective for predicting the onset of ARs, especially leukopenia. Although the frequency of such serious ARs requiring discontinuation of administration is not high, overconfidence in R139C analysis alone is dangerous, and great care should be taken, especially when starting thiopurine or increasing the dosage during the clinical course.
The limitations of this study are as follows: 6-MPs and 6-MMPs were measured using specimens that had been stored for a long period of time, even though they were stored in an appropriate environment. In this study, we did not specifically measure the 6-TGN triform by mass spectrometry and found no correlation with the NUDT15 polymorphism. Additionally, there was a small number of subjects of a single race, i.e., Japanese. Furthermore, no homozygous ITPA polymorphism carriers were included, and the risk of ITPA polymorphism carriers thus could not be evaluated. It is hoped that these limitations will be addressed in future prospective studies.
Conclusion
All cases of leukopenia were associated with ITPA c.94c > a and/or polymorphism of NUDT15 and the risk of developing leukopenia was synergistically increased by ITPA and NUDT15 gene polymorphisms. However, there was no association between the level of azathioprine metabolites and these polymorphisms. Some ARs will occur in approximately 20% of patients diagnosed as having a ‘wild-type NUDT15 gene’ based on R139C analysis, forcing them to discontinue thiopurine use, temporarily withdraw from the treatment, or reduce their dose and great care should be taken, especially when starting thiopurine or increasing the dosage during the clinical course.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Abbreviations
- XO:
-
Xanthine oxidase
- TPMT:
-
Thiopurine S-methyltransferase
- HGPRT:
-
Hypoxanthine guanine phosphoribosyl transferase
- ITPase:
-
Inosine triphosphate pyrophosphatase
- IMPDH:
-
Inosine monophosphate dehydrogenase
- GMPS:
-
Guanosine monophosphate synthetase
- NUDT15:
-
Nucleoside diphosphate-linked moiety X-type motif 15
- AZA:
-
Azathioprine
- 6-MP:
-
6-Mercaptopurine
- 6-TUA:
-
6-Thiouric acid
- 6-MeMP:
-
6-Methylmercaptopurine
- 6-TIMP:
-
6-Thioinosine monophosphate
- 6-TIDP:
-
6-Thioinosine diphosphate
- 6-TITP:
-
6-Thioinosine triphosphate
- 6-MeTIMP:
-
6-Methylthionosine monophosphate
- 6-MeTIDP:
-
6-Methylthionosine diphosphate
- 6-MeTITP:
-
6-Methylthionosine triphosphate
- 6-TXMP:
-
6-Thixanthosine 5’-monophosphate
- 6-TGMP:
-
6-Thioguanosine monophosphate
- 6-TGDP:
-
6-Thioguanosine diphosphate
- 6-TGTP:
-
6-Thioguanosine triphosphate
- 6-MeTGMP:
-
6-Methylthioguanine monophosphate
- 6-MeTGDP:
-
6-Methylthioguanine diphosphate
- 6-MeTGTP:
-
6-Methylthioguanine triphosphate
- 6-MMPs:
-
6-Methylmercaptopurines
- 6-MPs:
-
6-Mercaptopurines
- 6-TGNs:
-
6-Thiguanine nucleotide
References
Uchiyama K, Nakamura M, Kubota T, Yamane T, Fujise K, Tajiri H. Thioguanine S-methyltransferase and inosine triphosphate pyrophosphohydrolase gene in Japanese patients with inflammatory bowel disease in whom adverse drug reaction were induced by azathioprine/6-mercaptopurine treatment. J Gastroenterol. 2009;44:197–203. https://doi.org/10.1007/s00535-008-2307-1.
Yang SK, Hong M, Baek J, Choi H, Zhao W, Jung Y, et al. A common missense variant in NUDT15 confers susceptibility to thiopurine-induced leukopenia. Nat Genet. 2014;46:1017–20. https://doi.org/10.1038/ng.3060.
Zhu X, Wang X D, Chao K, Zhi M, Zheng H, Ruan H L, et al. NUDT15 polymorphisms are better than thiopurine S-methyltransferase as predictor of risk for thiopurine-induced leukopenia in Chinese patients with Crohn’s disease. Aliment Pharmacol Ther. 2016;44:967–75. https://doi.org/10.1111/apt.13796. Published online Sep 8.
Yan JJ, Landier W, Yang W, Liu C, Hageman L, Cheng C, et al. Inherited NUDT15 variant is a determinant of mercaptopurine intolerance in children with ALL. J Clin Oncol. 2015;33:1235–42. https://doi.org/10.1200/JCO.2014.59.4671.
Asada A, Nishida A, Shioya M, Imaeda H, Inatomi O, Bamba S, et al. NUDT15 R139C-related thiopurine leukocytopenia in mediated by 6-thioguanine nucleotide-independent mechanism in Japanese patients with inflammatory bowel disease. J Gastroenterol. 2016;51:22–9. https://doi.org/10.10007/s00535-015-1142-4.
Odahara S, Uchiyama K, Kubota T, Ito Z, Takami S, Kobayashi H, et al. A prospective study evaluating metabolic capacity of thiopurine and associated adverse reactions in Japanese patients with inflammatory bowel disease. PLoS ONE 2015;10(9):e0137798. https://doi.org/10.1371/journal.pone.0137798. Published online Sep 11.
Lichtenstein GR, Abreu MT, Cohen R, Tremaine W. American Gastroenterological Association Institute medical position statement on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology. 2006;130:935–9. https://doi.org/10.1053/j.gastro.2006.01.047.
Hibi T, Naganuma M, Kitahora T, Kinjyo F, Shimoyama T. Low-dose azathioprine is effective and safe for maintenance of remission in patients with ulcerative colitis. J Gastroenterol. 2003;38:740–6. https://doi.org/10.1007/s00535-003-1139-2.
Appell ML, Berg J, Duley J, Evans E, Kennedy MA, Lennard L, et al. Nomenclature for alleles of the thiopurine methyltransferase gene. Pharmacogenet Genomics. 2013;23:242–8. https://doi.org/10.1097/FPC.0b013e32835f1cc0.
Honda K, Kobayashi A, Niikura T, Hasegawa T, Saito Z, Ito S, et al. Neutropenia related to an azathioprine metabolic disorder induced by an inosine triphosphate pyrophosphohydrolase (ITPA) gene mutation in a patient with PR3-ANCA-positive microscopic polyangiitis. Clin Nephrol. 2018;90(5):363–9. https://doi.org/10.5414/CN109383.
Moriyama T, Nishii R, Perez-Andreu V, Yang W, Klussmann FA, Zhao X, et al. NUDT15 polymorphisms after thiopurine metabolism and hematopoietic toxicity. Nat Genet. 2016;48:367–73. https://doi.org/10.1038/ng.3508.
Lennard L, Maddocks JL. Assay of 6-thioguanine nucleotide, a major metabolite of azathioprine, 6-mercapthopurine and 6-thioguanine, in human red blood cells. J Pharm Pharmacol. 1983;35:15–8. https://doi.org/10.1111/j.2042-7158.1983.tb04255.x.
Pike MG, Franklin CL, Mays DC, Lipsky JJ, Lowry PW, Sandborn WJ. Improved methods for determining concentration of 6-thioguanine nucleotides and 60mercapthoprine nucleotides in blood. J Chromatogr B Biomed Sci Appl. 2001;757:1–9. https://doi.org/10.1016/s0378-4347(00)00513-2.
Motoi Y, Suzuki S, Yamazaki A, Koide M, Ohtaki Y, Uchiyama K, et al. Association of Azathioprine Metabolite Levels and Adverse Reactions in Japanese Patients with Inflammatory Bowel Disease. J Appl Biopharm Pharmacokinet. 2018;6:7–13. https://doi.org/10.20941/2309-4435.2018.06.2.
Tadaka S, Hisinuma E, Komaki S, Motoike IN, Kawashima J, Saigusa D, et al. jMorp updates in 2020: large enhancement of multi-omics data resources on the general Japanese population. Nucleic Acids Res. 2021;8:49(D1):D536-D544. https://doi.org/10.1093/nar/gkaa1034.
Kakuta Y, Kawai Y, Okamoto D, Takagawa T, Ikeya K, Sakuraba H, et al. NUDT15 codon 139 is the best pharmacogenetic marker for predicting thiopurine-induced severe adverse events in Japanese patients with inflammatory bowel disease: a multicenter study. J Gastroenterol. 2018;53(9):1065–78. https://doi.org/10.1007/s00535-018-1486-7.
Dubinsky MC, Lamothe S, Yang HY, Targan SR, Sinnett D, Théorét Y, et al. Pharmacogenomics and Metabolite Measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology. 2000;118:705–13. https://doi.org/10.1016/s0016-5085(00)70140-5.
Karas-Kuzelicki N, Milek M, Mlinaric-Rascan I. MTHDR and TYMS genotypes influence TPMT activity and its differential modulation in males and females. Clin Biochem. 2010;43(1–2):37–42. https://doi.org/10.1016/j.clinbiochem.2009.09.003.
Zhu X, Chao K, Li M, Xie W, Zheng H, Zhang JX, et al. Nucleoside diphosphate-linked moiety X-type motif 15 Ra39C genotypes impact 6-thioguanine nucleotide cut-off levels to predict thiopurine-induced leukopenia in Crohn’s disease patients. World J Gastroenterol. 2019;25:5850–61. https://doi.org/10.3748/wjg.v25.i38.5850.
Jena A, Grover N, Bhatia P, Singh M, Lad D, Prasad KK, et al. IPTA polymorphism do not predict additional risk beyond TPMT and NUDT15 for thiopurine-induced cytopenia in inflammatory bowel disease. Rev Gastroenterol Mex (Engl Ed). 2023;25:S2255–534X(23)00006–3. https://doi.org/10.1016/j.rgmxen.2021.11.017. Online ahead of print.
Chen ZY, Zhu YH, Zhou LY, Shi WQ, Qin Z, Wu B, et al. Association Between Genetic Polymorphisms of Metabolic Enzymes and Azathioprine-Induced Myelosuppression in 1,419 Chinese Patients: A Retrospective Study. Front Pharmacol. 2021;18:12:672769. https://doi.org/10.3389/fphar.2021.672769. eCollection 2021.
Tashkin DP, Roth MD, Clements PJ, Furst DE, Khanna D, Kleerup EC, et al. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir Med. 2016;4(9):708–19. https://doi.org/10.1016/S2213-2600(16)30152-7. (Epub 2016 Jul 25).
Acknowledgements
Not applicable.
Funding
The funder had no role in the study design, data collection, data analysis, or dissemination of the results.
Author information
Authors and Affiliations
Contributions
Shizuka Suzuki and Kan Uchiyama wrote the main manuscript text and prepared Figs. 1, 2, 3 and 4. Shizuka Suzuki and Kan Uchiyama contributed equally to this work. Yutaro Motoi, Yuuki Yoshii, Yukari Inoue, and Takahiro Kubota set up and measured concentrations of AZA metabolite. All of the authors have reviewed the manuscript.
Authors’ information
Not applicable.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
This research was carried out in compliance with the Declaration of Helsinki and the Ethical Guidelines for Medical and Biological Research Involving Human Subjects. This study was approved by the Jikei University Hospital ethics committee (27–323) on April 11th 2016.
All patients who participated in the previous prospective study gave written informed consent in advance for the use of their data and the possibility of additional genetic analyses. If the participant was under the age of 18, informed consent has been obtained from the participant and their parents/guardians. Informed consent was obtained via an opt-out option on a website for this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Suzuki, S., Uchiyama, K., Motoi, Y. et al. Analysis of the NUDT15 gene and metabolites of azathioprine in Japanese patients with inflammatory bowel disease. BMC Gastroenterol 23, 239 (2023). https://doi.org/10.1186/s12876-023-02881-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-023-02881-6