Abstract
Background
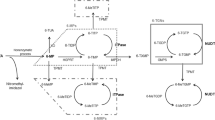
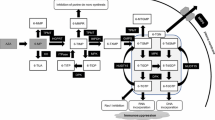
The main cause of azathioprine (AZA)/6-mercaptopurine (6MP)-induced adverse reactions is a reduction in the activities of the metabolizing enzymes thiopurine S-methyltransferase (TPMT) and inosine triphosphate pyrophosphohydrolase (ITPA). Adverse reactions develop at a high frequency in Japanese patients at half the dose required for European and American patients; however, the association with TPMT and ITPA gene polymorphisms in Japanese has not been fully investigated.
Methods
Gene mutations of TPMT and ITPA, the major AZA/6-MP -metabolizing enzymes, were investigated retrospectively in 16 Japanese patients with inflammatory bowel disease (IBD) in whom AZA/6MP treatment induced adverse reactions.
Results
The TPMT gene was found to have a wild-type sequence in all patients, but in the ITPA gene a mutation, 94C>A, was detected at a rate of 50% (8/16), with 83.3% (5/6) occurring in patients with acute bone marrow suppression and 75% (3/4) in those with agranulocytosis. The 94C>A allele frequency was 10 of 32 (0.313; 95% CI, 0.180–0.486). Adverse reactions developed earlier in patients with the 94C>A mutation. However, in half the patients, no gene polymorphism was noted.
Conclusions
It is suggested that the ITPA gene mutation is closely related to the adverse reactions of AZA/6-MP in Japanese patients, and screening for the mutant allele is useful for predicting the most serious adverse reactions, agranulocytosis and acute bone marrow suppression.
Similar content being viewed by others
References
Schwab M, Schaffeler E, Marx C, Fischer C, Lang T, Behrens C, et al. Azathioprine therapy and adverse drug reactions in patients with inflammatory bowel disease: impact of thiopurine S-methyltransferase polymorphism. Pharmacogenetics 2002;12: 429–436.
Ansari A, Hassan C, Dulay J, Marinaki A, Shobowale-Bakre EM, Seed P, et al. Thiopurine methyltransferase activity and the use of azathioprine in inflammatory bowel disease. Aliment Pharmacol Ther 2002;16:1743–1750.
Lennard L. TPMT in the treatment of Crohn’s disease with azathioprine. Gut 2002;51:143–146.
Kubota T, Chiba K. Frequencies of thiopurine S-methyltransferase mutant alleles (TPMT*2, *3A, *3B, *3C) in 151 healthy Japanese subjects and the inheritance of TPMT*3C in the family of a propositus. Br J Clin Pharmacol 2001;51: 475–477.
Ishiguro A, Kubota T, Soya Y, Sasaki H, Yagyu O, Takarada Y, et al. High-throughput detection of multiple genetic polymorphisms influencing drug metabolism with mismatch primers in allele specific polymerase chain reaction. Anal Biochem 2005;337: 256–261.
Marinaki AM, Ansari A, Duley JA, Arenas M, Sumi S, Lewis CM, et al. Adverse drug reactions to azathioprine therapy are associated with polymorphism in the gene encoding inosine triphosphate pyrophosphatase (ITPase). Pharmacogenetics 2004;14: 181–187.
Clarke DA, Philips FS, Sternberg SS, Stock CC, Elion GB, Hitchings H. 6-mercaptopurine: effects in mouse sarcoma 180 and in normal animals. Cancer Res 1953;13:593–604.
Burchenal JH, Murphy ML, Ellison RR, Sykes MP, Tan LA, Leone LA, et al. Clinical evaluation of a new antimetabolite, 6-mercaptopurine, in the treatment of leukemia and allied diseases. Blood 1953;8:965–999.
Lorenzen I, Videbeak A. Treatment of collagen disease with cytostatics. Lancet 1965;18:558–561.
Mason M, Currey HL, Barnes CG, Dunne JF, Hazleman BL, Strickland ID. Azathioprine in rheumatoid arthritis. Br Med J 1969;15:420–422.
Elion GB. Symposium on immunosuppressive drugs. Biochemistry and pharmacology of purine analogues. Fed Proc 1967;26: 898–904.
Brooke BN, Hoffmann DC, Swarbrick ET. Azathioprine for Crohn’s disease. Lancet 1969;2:612–614.
Weinshilboum RM, Sladek SL. Mercaptopurine pharmacogenetics: monogenic inheritance of erythrocyte thiopurine methyltransferase activity. Am J Hum Genet 1980;32:651–662.
Lindqvist M, Skoglund K, Karlgren A, Soederkvist P, Peterson C, Kidhall I, et al. Explaining TPMT genotype/phenotype discrepancy by haplotyping of TPMT*3A and identification of a novel sequence variant, TPMT*23. Pharmacogenet Genomics 2007;17: 891–895.
Kumagai K, Hiyama K, Ishioka S, Sato H, Yamanishi Y, McLeod HL, et al. Allelotype frequency of the thiopurine methyltransferase (TPMT) gene in Japanese. Pharmacogenetics 2001;11: 275–278.
Hiratsuka M, Inoue T, Omori F, Agatsuma Y, Mizugaki M. Genetic analysis of thiopurine methyltransferase polymorphism in a Japanese population. Mutat Res 2000;448:91–95.
Kubota T, Nishida A, Takeuchi K, Takeuchi K, Iida T, Yokota H, et al. Frequency distribution of thiopurine S-methyltransferase activity in red blood cells of a healthy Japanese population. Ther Drug Monit 2004;26:319–321.
Tai HL, Krynetski EY, Yates CR, Loennechen T, Fessing MY, Krynetskaia NF, et al. Thiopurine S-methyltransferase deficiency: two nucleotide transitions define the most prevalent mutant allele associated with loss catalytic activity in Caucasians. Am J Hum Genet 1996;58:694–702.
Hibi T, Naganuma M, Kitahora T, Kinjo F, Shimoyama T. Low dose azathioprine is effective and safe for maintenance of remission in patients with ulcerative colitis. J Gastroenterol 2003;38: 740–746.
McLeod HL, Lin JS, Scott EP, Pui CH, Evans WE. Thiopurine methyltransferase activity in American white subjects and black subjects. Clin Pharmacol Ther 1994;55:15–20.
Lowenthal A, Meyerstein N, Ben-Zvi Z. Thiopurine methyltransferase activity in the Jewish population of Israel. Eur J Clin Pharmacol 2001;57:43–46.
Sumi S, Marinaki AM, Arenas M, Fairbanks L, Shobowale-Bakre E-M, Rees DC, et al. Genetic basis of inosine triphosphohydrolase deficiency. Hum Genet 2002;111:360–367.
Maeda T, Sumi S, Ueta A, Fairbanks L, Shobowale-Bakre E-M, Rees DC, et al. Genetic basis of inosine triphosphate pyrophosphohydrolase deficiency in the Japanese population. Mol Genet Metab 2005;85:271–279.
van Dieren JM, van Vuuren AJ, Kusters JG, Nieuwenhuis EE, Kuipers EJ, van der Woude CJ. ITPA genotyping is not predictive for development of side effects in AZA treated inflammatory bowel disease patients. Gut 2005;54:1664.
Gearry RB, Roberts RL, Barclay ML, Kennedy MA. Lack of association between the ITPA 94C>A polymorphism and adverse effects from azathioprine. Pharmacogenetics 2004;14:779–781.
Lowry PW, Franklin CL, Weaver AL, Szumlanski CL, Mays DC, Loftus EV, et al. Leucopenia resulting from a drug interaction between azathioprine or 6-mercaptopurine and mesalamine, sulphasalazine, or balsalazide. Gut 2001;49:656–664.
de Boer NK, Wong DR, Jharap B, de Graaf P, Hooymans PM, Mulder CJ, et al. Dose-dependent influence of 5-aminosalicylates on thiopurine metabolism. Am J Gastroenterol 2007;102: 2747–2753.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Uchiyama, K., Nakamura, M., Kubota, T. et al. Thiopurine S-methyltransferase and inosine triphosphate pyrophosphohydrolase genes in Japanese patients with inflammatory bowel disease in whom adverse drug reactions were induced by azathioprine/6-mercaptopurine treatment. J Gastroenterol 44, 197–203 (2009). https://doi.org/10.1007/s00535-008-2307-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-008-2307-1