Abstract
Background
Epstein–Barr virus (EBV)-positive gastric carcinoma (GC) is defined by the proliferation of GC cells with EBV infection. The co-existence of EBV-positive and -negative components in a single GC is rare. We report a case of GC with the co-existence of EBV-positive and EBV-negative components, in which we performed—for the first time—various molecular analyses to elucidate their histogenesis.
Case presentation
An 81-year-old man was diagnosed with GC based on the results of endoscopy and a pathological examination of the biopsy specimen. Systemic chemotherapy was performed, since lymph node and lung metastases were diagnosed based on computed tomography. Total gastrectomy and lymph node dissection were performed after chemotherapy, after confirming that the size of the metastatic lymph nodes had decreased and that the lung metastasis had disappeared. Grossly, a type 3 tumor was located in the middle posterior part of the stomach body. At the cut section, the tumor consisted of a white and solid part on the anal side of the tumor and a flat and elevated part on the oral side. Histologically, the former part consisted of GC with lymphoid stroma and the latter part was composed of poorly differentiated adenocarcinoma without prominent lymphocytic infiltration. The two histopathological components were clearly separated from each other. On EBV-encoded small RNA (EBER)-in situ hybridization (ISH), the part with the lymphoid stroma component was positive, while the other part was negative. Immunohistochemistry revealed that both components showed the overexpression of p53. Sequencing of TP53 using DNA extracted from the two components was conducted, and revealed different patterns. Targeted next generation sequencing revealed MYC amplification in the EBV-positive component of the tumor and HER2 amplification in the EBV-negative part. Immunohistochemistry revealed that the EBV-positive part was C-MYC( +)/HER2(−) and the EBV-negative part was C-MYC(−)/HER2( +). Correspondingly, chromogenic ISH and dual-color ISH showed amplification of C-MYC and no amplification of HER2 in the EBV-positive part, and no amplification of C-MYC and amplification of HER2 in the EBV-negative part.
Conclusion
We presented a case of collision of two different GCs composed of EBER-ISH ( +)/C-MYC ( +) and EBER-ISH (−)/HER2 ( +) cells.
Similar content being viewed by others
Background
Epstein–Barr virus (EBV)-positive gastric carcinoma (GC) is a histological and molecular subtype that is defined by the proliferation of GC cells infected with EBV, demonstrated by EBV-encoded small RNA (EBER)-in situ hybridization (ISH) [1, 2]. EBV-positive GCs account for approximately 10% of GC cases worldwide and have a number of characteristic clinicopathological features, including predominance among males, proximal location in the stomach, lymphoepithelioma-like histology, and a favorable prognosis [1]. Synchronous multiple EBV-positive and -negative GCs are rare, and the co-existence of EBV-positive and -negative components in one GC is rarer [3,4,5]. To the best of our knowledge, only three cases of gastric cancer with such features have been reported. Among them, only one case of GC with EBV-positive and -negative components was analyzed by p53 immunohistochemistry to investigate its molecular characteristics, which indicated the collision of EBV-positive and -negative components; the other cases were not molecularly analyzed [6,7,8].
We herein present another case of GC with the co-existence of EBV-positive and -negative components and we performed various molecular analyses (TP53 sequencing, targeted next generation sequencing, and HER2 and C-MYC ISH) of the EBV-positive and -negative components in order to clarify their histogenesis.
Case presentation
An 81-year-old Japanese man complained of hematemesis and visited a doctor. He was referred to our hospital with the diagnosis of gastric cancer. Since he had not been seen by a doctor before, there are no special notes in his medical history, including the presence or absence of H. pylori infection. Esophagogastroduodenoscopy revealed an ulcer with irregular edges at the middle posterior part of the stomach body. Gastric poorly differentiated adenocarcinoma was diagnosed based on the pathological examination of the biopsy specimen. After making a radiological diagnosis of lymph node and lung metastasis, based on computed tomography findings, the patient was treated with 4 courses of tegafur gimeracil oteracil (S-1) therapy (80 mg/day for 4 weeks with a 2-week rest). After chemotherapy, total gastrectomy and lymph node dissection were performed for the treatment of gastric cancer after confirming that the size of the lymph nodes metastases had decreased and that the lung metastasis had disappeared.
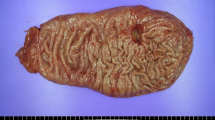
Grossly, a type 3 tumor of 83 × 50 mm in size was located in the middle posterior part of the stomach body (Fig. 1). At the cut section, the tumor consisted of a white and solid part on the anal side of the tumor and flat and an elevated part on the oral side (Fig. 2a).
The gross appearance of the gastric tumor. A mucosal view of the total gastrectomy specimen, which was opened along the greater curvature, with the resection margin of the duodenum on the left (a) and the gastroesophageal junction on the right (b). A type 3 tumor of 83 × 50 mm in size was located in the middle posterior part of the stomach body
Histological findings of the tumor. a Loupe view of the tumor. Hematoxylin and eosin staining (H&E). The EBV-positive and -negative components are outlined by solid and dotted curves, respectively. b A high-power view of the EBV-positive part of the tumor, marked with boxed area in a. Poorly differentiated adenocarcinoma with dense lymphocytic infiltration, gastric carcinoma with lymphoid stroma was seen (H&E, ×200). c A high-power view of the EBV-negative part of the tumor, marked with boxed area in a. Poorly differentiated adenocarcinoma without prominent lymphocytic infiltration was seen (H&E, ×200). d A low-power view of the border between the two histopathological components marked with boxed area in a. The two components were clearly separated from each other (H&E, ×100)
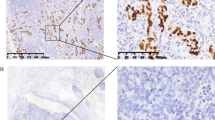
Histologically, the white and solid part of the tumor consisted of poorly differentiated adenocarcinoma with dense lymphocytic infiltration, GC with lymphoid stroma (Fig. 2b). In contrast, the flat and elevated part was composed of poorly differentiated adenocarcinoma without prominent lymphocytic infiltration (Fig. 2c). These two histopathological components were clearly separated from each other (Fig. 2d). The tumor cells invaded up to the serosa in the gastric carcinoma with lymphoid stroma component. Lymphatic and venous invasion as well as lymph node metastasis were detected, all of which showed gastric carcinoma with lymphoid stroma histology. On EBER-ISH, the GC with lymphoid stroma component was positive, while the other component was negative (Fig. 2a, 3a–c). On EBER-ISH, tumor cells in the metastatic lymph nodes were positive.
Findings of EBV-encoded small RNA (EBER) in situ hybridization (ISH) and immunohistochemistry. Loupe views of a EBER-ISH, d p53, g C-MYC, and j HER2. On EBER-ISH, the gastric carcinoma with a lymphoid stroma component was positive (b), while the other component was negative (c). Immunohistochemical staining revealed that both the EBV-positive (e) and EBV-negative (f) components showed the overexpression of p53. The EBV-positive component of the tumor was diffusely positive for C-MYC (h), while the EBV-negative component was negative for C-MYC (i). The EBV-positive component of the tumor was negative for HER2 (k), while the EBV-negative component was positive for HER2 (l)
Since both the EBV-positive and -negative components immunohistochemically showed the overexpression of p53 (Fig. 3d–f), exons 5–9 of TP53 were sequenced using DNA extracted from formalin-fixed paraffin-embedded sections from these two components. The EBV-negative component showed a C→T transition at nucleotide position 477 (c. 477C>T) in exon 5, which gave rise to a synonymous mutation. In contrast, the EBV-positive component showed no mutations at this nucleotide position (Fig. 4). In this study, non-synonymous mutations were not detected in exons 5–9 of TP53 in either component. These observations are in line with previous results from The Cancer Genome Atlas project, which showed that TP53 was less frequently mutated in EBV-positive GC [2]. However, it is possible that there may be mutations outside the region that was examined in this study or that mutations could not be detected in DNA extracted from EBV-positive GC because of the large number of lymphocytes and the small percentage of cancer cells.
Targeted next generation sequencing (Oncomine™ Target Test, Thermo Fisher Scientific, Carlsbad, CA), which did not contain a TP53 test, was performed using DNA and RNA extracted from the EBV-positive component and the EBV-negative component, which revealed MYC amplification in the former and ERBB2 (HER2) amplification in the latter.
Immunohistochemically, the EBV-positive component of the tumor was diffusely positive for C-MYC and negative for HER2, while the EBV-negative part was positive for HER2 and negative for C-MYC (Fig. 3g–l). Chromogenic in situ hybridization (CISH) showed high C-MYC amplification in the EBV-positive component (Fig. 5a) and no amplification in the EBV-negative part. On dual-color in situ hybridization (DISH) for HER2, the HER2/chromosome 17 (Chr17) signal count ratio was 3.9 in the EBV-negative component, which was scored as “amplified” (Fig. 5b). In contrast, the HER2/Chr17 signal count ratio was 1.4 and the average number of HER2 signals per cell was 2.8 in the EBV-positive part, which was scored as “not amplified”.
Findings of chromogenic in situ hybridization (ISH). a C-MYC ISH showed C-MYC gene amplification in the EBV-positive part (×400). b HER2 dual-color ISH showed HER2 gene amplification in the EBV-negative part with a HER2/chromosome 17 (Chr17) ratio of 3.9 (×400). Black and red signals represent HER2 and Chr17, respectively
Discussion and conclusions
The co-existence of EBV-positive and -negative components in one GC is extremely rare; only one case in the relevant English literature and two cases in the relevant Japanese literature have been reported to date [6,7,8]. Assuming that the histogenesis of such GCs involves the collision of EBV-positive and -negative cancer components, its rarity could be attributed to rarity of co-existing EBV-positive and -negative gastric cancers in a single patient. In fact, in 24 patients with synchronous or metachronous multiple GCs, at least one of which was EBV-positive, the GCs in were all EBV-positive in 15 of 24 cases (62.5%) [3,4,5, 9, 10]. This percentage is high, considering that EBV-positive GC accounts for only approximately 10% of all GC cases. A high prevalence of EBV-positive GC was reported in synchronous and metachronous multiple gastric cancers and the high frequency would be explained by the acceleration of EBV-associated carcinogenesis by the background mucosa of EBV-positive GC [9, 11].
Regarding the co-existence of EBV-positive and -negative components in a single GC, another possible pathway of histogenesis is the infection or disappearance of EBV in the middle to late steps of GC carcinogenesis. However, this is unlikely, as several studies on EBV-associated gastric carcinogenesis have suggested that EBV infection occurs in the early carcinogenesis of EBV-positive GC, leading to clonal and whole infection in EBV-positive GC [1, 12].
The histological and molecular pathological findings in the present case indicate that it developed via the former pathway, with the nature of the collision demonstrated as follows. (1) The tumor was composed of two histologically different components without any apparent transition between them. (2) The sequencing of exons 5–9 on TP53 using DNA extracted from the two components showed different patterns. (3) The two components had different patterns of C-MYC and HER2 amplification.
In this case, the EBV-positive component showed the overexpression of C-MYC and the EBV-negative component showed the overexpression of HER2, which corresponded to the gene amplification patterns; however, the causal relationship between these gene amplifications or the overexpression of their proteins and EBV-positive GCs has not been clarified to date [2, 13,14,15,16].
Helicobactor pylori (H. pylori) has long been known to play a major role in gastric carcinogenesis, and the possibility of H. pylori involvement should be considered in this case. We therefore subjected a surgical specimen to Giemsa staining and H. pylori immunohistochemistry. H. pylori infection was not detected in the non-cancerous mucosa of the stomach.
The frequency of nodal metastasis is lower in EBV-positive GCs than in EBV-negative GCs [1, 17, 18]; however, for deeply invasive GCs, the frequency of lymph node metastasis is fairly high, even in EBV-positive GCs [17]. In the present case, the EBV-positive component invaded up to the serosa, while the EBV-negative component invaded up to the subserosa; only the EBV-positive component involved the lymph nodes. It was conceivable that the EBV-positive component, which invaded more deeply than EBV-negative component, metastasized to the lymph nodes.
EBV-positive GCs show a high response rate to immune checkpoint inhibitors [19, 20], and trastuzumab-based chemotherapy is a standard treatment for HER2-positive GC. Thus, in the present case, in which the tumor was composed of EBER-ISH ( +)/HER2 (−) and EBER-ISH (−)/HER2 ( +) components, the response to immune checkpoint inhibitors or trastuzumab-based chemotherapy would be unpredictable. Such unpredictability of the therapeutic effect might be challenge when using molecularly targeted therapy in the treatment of cases with the collision of gastric cancers with different molecular characteristics.
In conclusion, we presented a rare case of collision of two GCs composed of EBER-ISH ( +)/C-MYC ( +) and EBER-ISH (−)/HER2 ( +) cells. This is the first report to describe the analysis of a gastric collision tumor, composed of EBV-positive and -negative components, by targeted next generation sequencing and CISH.
Availability of data and materials
Additional information is available from the corresponding author on reasonable request from the editor.
Abbreviations
- EBV:
-
Epstein–Barr virus
- GC:
-
Gastric carcinoma
- EBER:
-
EBV-encoded small RNA
- ISH:
-
In situ hybridization
- CISH:
-
Chromogenic in situ hybridization
- DISH:
-
Dual-color in situ hybridization
- Ch17:
-
Chromosome 17
References
Fukayama M, Abe H, Kunita A, Shinozaki-Ushiku A, Matsusaka K, Ushiku T, et al. Thirty years of Epstein–Barr virus-associated gastric carcinoma. Virchows Arch. 2020;476(3):353–65.
Bass AJ, Thorsson V, Shmulevich I, Reynolds SM, Miller M, Bernard B, et al. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513(7517):202–9.
Matsunou H, Konishi F, Hori H, Ikeda T, Sasaki K, Hirose Y, et al. Characteristics of Epstein–Barr virus-associated gastric carcinoma with lymphoid stroma in Japan. Cancer. 1996;77(10):1998–2004.
Tokunaga M, Land CE, Uemura Y, Tokudome T, Tanaka S, Sato E. Epstein–Barr virus in gastric carcinoma. Am J Pathol. 1993;143(5):1250–4.
Shibata D, Hawes D, Stemmermann GN, Weiss LM. Epstein–Barr virus-associated gastric adenocarcinoma among Japanese Americans in Hawaii. Cancer Epidemiol Biomarkers Prev. 1993;2(3):213–7.
Matsuda I, Kan K, Doi S, Motoki Y, Onodera M, Hirota S. A case of gastric cancer with heterogeneous components of EB virus (+)/TP53 (+) and EB virus (−)/TP53 (−). Int J Clin Exp Pathol. 2015;8(9):11766–71.
Okada A, Arai T, Saeki S, Okada Y, Aizu K, Hayakawa S. Gastric collision tumor of adenocarcinoma and Epstein–Barr virus-related carcinoma-a case report-. J Japan Surg Assoc. 2010;71(6):1513–7 ((In Japanese)).
Aoyama H, Kurumiya Y, Sekoguchi E, Kobayashi S, Kiriyama M, Oiwa T. Collision tumor involving gastric carcinoma with lymphoid stroma and moderately differentiated adenocarcinoma. J Japan Surg Assoc. 2015;76(12):2971–6 ((In Japanese)).
Kaizaki Y, Hosokawa O, Sakurai S, Fukayama M. Epstein–Barr virus-associated gastric carcinoma in the remnant stomach: de novo and metachronous gastric remnant carcinoma. J Gastroenterol. 2005;40(6):570–7.
Liu S, Zhao Z, Han L, Liu S, Luo B. Epstein–Barr virus infection in gastric remnant carcinoma and recurrent gastric carcinoma in qingdao of Northern China. PLoS ONE. 2016;11:e0148342.
Nishikawa J, Iizasa H, Yoshiyama H, Shimokuri K, Kobayashi Y, Sasaki S, et al. Clinical importance of Epstein–Barr virus-associated gastric cancer. Cancers. 2018;10(6):167.
Takada K. Epstein-Barr virus and gastric carcinoma. Mol Pathol. 2000;53(5):255–61.
Luo B, Wang Y, Wang XF, Gao Y, Huang BH, Zhao P. Correlation of Epstein–Barr virus and its encoded proteins with Helicobacter pylori and expression of c-met and c-myc in gastric carcinoma. World J Gastroenterol. 2006;12(12):1842–8.
Zhu S, Sun P, Zhang Y, Yan L, Luo B. Expression of c-myc and PCNA in Epstein–Barr virus-associated gastric carcinoma. Exp Ther Med. 2013;5(4):1030–4.
Irkkan C, Balci S, Güler Tezel G, Akinci B, Yalcin B, Güler G. Comparison of clinicopathologic parameters and survivals between Epstein–Barr virus-positive and Her2-positive gastric cancers. Appl Immunohistochem Mol Morphol. 2017;25(9):609–14.
Lima VP, de Lima MAP, André AR, Ferreira MVP, Barros MAP, Rabenhorst SHB. H pylori (CagA) and Epstein–Barr virus infection in gastric carcinomas: correlation with p53 mutation and c-Myc, Bcl-2 and Bax expression. World J Gastroenterol. 2008;14(6):884–91.
Tokunaga M, Land CE. Epstein–Barr virus involvement in gastric cancer: biomarker for lymph node metastasis. Cancer Epidemiol Biomarkers Prev. 1998;7(5):449–50.
Cheng Y, Zhou X, Xu K, Huang J, Huang Q. Very low risk of lymph node metastasis in Epstein–Barr virus-associated early gastric carcinoma with lymphoid stroma. BMC Gastroenterol. 2020;20(20):273.
Togasaki K, Sukawa Y, Kanai T, Takaishi H. Clinical efficacy of immune checkpoint inhibitors in the treatment of unresectable advanced or recurrent gastric cancer: an evidence-based review of therapies. Onco Targets Ther. 2018;11:8239–50.
Kim ST, Cristescu R, Bass AJ, Kim KM, Odegaard JI, Kim K, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24(9):1449–58.
Acknowledgements
Not applicable.
Funding
This work was supported by the Japan Society for the Promotion of Science 19K07454 (http://www.jsps.go.jp/english/) and the Smoking Research Foundation (http://www.srf.or.jp/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
[KM]1 and AG wrote the paper. MS and [KK]2 performed the surgery of this case and administered treatment. MS and KS collected samples and clinical data. YK performed immunohistochemical staining. [KM]1, YK and HN performed ISH. DM performed targeted next generation sequencing and analyzed the data. [KM]2, DM, [KK]1, MU, YI and MY contributed to some discussion. All authors read and approved the manuscript. [KM/KK]1 and [KM/KK]2 correspond to the names in order of the author list.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by Ethics Committee of Fukushima Medical University (number 2847; Fukushima, Japan). Informed consent was obtained from the patient by an opt-out option on our hospital website.
Consent for publication
The patient gave his written consent for the publication of his personal or clinical details along with any identifying images included in this case report.
Competing interests
The research on DM was partly supported by Takara Bio Inc.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Miyabe, K., Saito, M., Koyama, K. et al. Collision of Epstein–Barr virus-positive and -negative gastric cancer, diagnosed by molecular analysis: a case report. BMC Gastroenterol 21, 97 (2021). https://doi.org/10.1186/s12876-021-01683-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-021-01683-y