Abstract
Background
Epstein–Barr virus (EBV)-associated gastric cancer (EBVaGC) was a unique molecular subtype of gastric cancer (GC). However, the clinicopathological characteristics and prognostic role of EBV infection remains unclear. We aimed to evaluate the clinicopathological features of EBVaGC and its role on prognosis.
Methods
EBV-encoded RNA (EBER) in situ hybridization method was used to evaluate the EBV status in GC. The serum tumor markers AFP, CEA, CA19-9 and CA125 of patients were detected before treatment. HER2 expression and microsatellite instability (MSI) status was evaluated according to established criteria. The relationship between EBV infection and clinicopathological factors as well as its role on prognosis were investigated.
Results
420 patients were enrolled in the study and of 53 patients (12.62%) were identified as EBVaGC. EBVaGC was more common in males (p = 0.001) and related to early T stage (p = 0.045), early TNM stage (p = 0.001) and lower level of serum CEA (p = 0.039). No association could be found between EBV infection and HER2 expression, MSI status and other factors (p all > 0.05). Kaplan–Meier analysis revealed that both the overall survival and disease-free survival of EBVaGC patients were similar to that of EBV-negative GC (EBVnGC) patients (p = 0.309 and p = 0.264, respectively).
Conclusion
EBVaGC was more common in males and in patients with the early T stage and TNM stage as well as patients with lower serum CEA level. Difference in overall survival and disease-free survival between EBVaGC and EBVnGC patients cannot be detected.
Similar content being viewed by others
Introduction
Gastric cancer (GC) is the fifth most common tumor and the third most deadly cancer in the world. In 2018, there were 782,685 deaths because of GC globally [1]. The incidence of GC and mortality varies by region and is highly dependent on diet and Helicobacter pylori infection [2]. Although the survival rate of GC has increased because of the improved treatment, the prognosis of GC patients remains poor [3]. Therefore, significant biomarkers to predict the prognosis of GC patients and to achieve personalized treatment are need.
Epstein–Barr virus (EBV) is a gamma virus and was discovered in Burkitt lymphoma in 1964 [4]. EBV infection can be seen in infectious mononucleosis, nasopharyngeal carcinoma, GC, etc. Generally, EBV infection was seen in about 10% of GC patients [5]. According to The Cancer Genome Atlas (TCGA) research, GC can be classified into four molecular subtypes: chromosomal instable types, genomically stable, microsatellite instable and EBV-positive [6]. EBV-positive performance was defined as a potential EBV infection and monoclonal proliferation of cancer cells. In fact, EBV infection is usually determined by in situ hybridization of EBV-encoded RNA (EBER), which is a reliable method for detecting EBV infection [7]. A meta-analysis in 2016 showed that EBV infection was a risk factor for GC development [8]. Besides, patients with EBV infection presented a unique clinicopathological features, such as high infection rate in males, early stage, etc. [9]. However, the clinicopathological features and prognostic significance of EBV infection for GC patients remains controversial [10, 11].
Therefore, our study will mainly focus on that whether EBV infection can be a prognostic indicator for GC patients. EBER's RNA probe was employed to detect the tissues of GC. The clinicopathological characteristics together with its prognostic value were analyzed in this study.
Materials and methods
Materials
All subjects and experimental protocols have been approved by the Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University in China. All patients have obtained written informed consent and the study was complied with the ethical guidelines in the Declaration of Helsinki.
Patients with gastric adenocarcinoma were included in our study. All of them underwent biopsy or surgery at the First Affiliated Hospital of Sun Yat-sen University, China, from January 2011 to September 2021. The patients included in the study should meet the following criteria: (1) Gastric adenocarcinoma confirmed by histology; (2) Underwent biopsy or gastrectomy; (3) Representative tumor masses, which can be fully evaluated for the presence of EBV. Exclusion criteria include: Chemotherapy, radiotherapy or chemoradiation before surgery.
All tumor samples were histologically classified by senior pathologists on the basis of the World Health Organization (WHO) classification system. The clinicopathological characteristics of patients were obtained by consulting medical archives. The classification and stage of gastric tumors were determined in line with the 8th edition of the International Union for Cancer Control/United States Gastric Cancer Joint Committee. Follow-up of the patient was done every 3 months in the first 2 years after surgery and every 6 months after. The deadline of follow-up was July 2020.
Methods
EBV-encoded RNA (EBER) hybridization in situ
As previously reported in the literature [12], 4 mm sections embedded in paraffin were harvested which compiled with the manufacturer's instructions. EBV-encoded RNA oligonucleotide probes were employed to identify EBV in GC cells by ISH. EBV-positive nasopharyngeal carcinoma specimens confirmed previously were defined as positive controls and slides that were not treated with probes were used as negative controls. Samples with brown staining in tumor nuclei were considered positive. Divide samples into Epstein–Barr virus-associated GC (EBVaGC) and Epstein–Barr virus-negative (EBVnGC) according to the performance of EBER.
Detection of tumor markers
Tumor markers including serum carcinoembryonic antigen (CEA), alpha fetoprotein (AFP), carbohydrate antigen 125 (CA125) and carbohydrate antigen 19-9 (CA19-9) were measured in GC patients before treatment. On the basis of the clinical testing standard, the cut-off values for AFP, CEA, CA19-9 and CA125were 20 ug/mL, 5 ug U/mL, 35 U/mL and 35 U/mL, respectively. A measured value above the cut-offs was considered positive.
HER2 status assessment
HER2 status was evaluated by immunohistochemistry (IHC) and/or in situ hybridization (ISH) assays [13]. According to Hofmann's criteria in GC [14], samples with IHC 3+, or IHC 2+ and HER2 amplification by FISH were defined as HER2 positive and other samples were defined as HER2 negative.
Microsatellite instability status assessment
Patients were randomly selected for Microsatellite instability (MSI) status analysis by clinicians. The MSI status was determined by using IHC or polymerase chain reaction (PCR). As previously reported [15], expression of MMR proteins (PMS2, MSH6, MSH2 and MLH1) was evaluated by IHC. Microsatellite stable (MSS) was determined with expressions of all MMR proteins, and high MSI (MSI-H) was designated with at least one MMR protein in tumor cell nuclei were negative [16].
For PCR-based method, formalin-fixed paraffin-embedded tumor tissues confirmed by pathologist and matched whole blood samples were used for MSI assessment. Briefly, 5 consecutive paraffin-embedded tumor tissues with 10um thickness were harvested and DNA extraction was done with TIANquick FFPE DNA Kit (No. DP330, TIANGEN Company, China) and the matched blood DNA extraction was done with TIANamp Blood DNA Kit (No. DP318, TIANGEN Company, China). As reported in previous studies, PCR based amplification allows the detection of MSI by comparing and measuring the size of amplified DNA fragments from tumors and matched whole blood samples from the same patient by electrophoresis [17], and then PCR amplification was performed for six dinucleotide markers (BAT-25, BAT-26, NR-21, NR-24, NR-27, MONO-27 [18]. The PCR program comprised an initial 3 min at 95 °C followed by 30 cycles of 15 s at 94 °C and 45 s at 60 °C, which were followed by 30 min at 70 °C. MSI-H was designated as at least two markers with instability [16], whereas slides with instability at 1 microsatellite marker and those without instability were defined as MSI-low (MSI-L) and microsatellite stable (MSS) which both were defined as microsatellite stability (MSS) in our study.
Evaluation of clinicopathological characters of EBVaGC
Evaluation was performed following established morphological, histopathological, and immunophenotypic characteristics. Two independent gastrointestinal pathologists who have no idea about the clinical pathological data jointly examined the histological evaluation.
The histological type of gastric adenocarcinoma was classified according to WHO classification, and the GC was staged based on the 8th edition of the International Union for Cancer Control/United States Joint Committee on Gastric Cancer.
Statistical analysis
Cases lost due to follow-up and cases of death due to reasons other than GC were considered censored. Chi-square test or Fisher’s exact test was used to compare categorical variables and evaluate the association between EBV infection and clinicopathological parameters. Log-rank test was used to calculate the difference in survival between subgroups and Kaplan–Meier method was applied to calculate the probability of survival. Cox proportional hazards regression was employed to assess the effect of EBV infection on GC‐specific survival. All statistical analyses were two-sided tests and p < 0.05 were considered statistically significant. SPSS 13.0 (SPSS Inc. Chicago, IL) was employed for all the analysis.
Results
Characteristics of patients
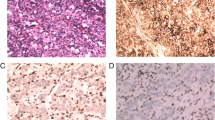
A total of 420 patients (284 males and 136 females) were included in this study. Of the 420 GC patients, 53 cases (12.62%) were EBVaGC patients, with 46 males and 7 females. The Clinicopathological features of all patients were shown in Table 1. All the EBVaGC showed EBER staining in 90% of tumor tissues (Fig. 1).
EBVaGC was more common in males than women (p = 0.001) and related to early T stage (p = 0.045), early TNM stage (p = 0.001) as well as lower serum CEA level (p = 0.039). EBV infection status was not related to age (p = 0.421), tumor location (p = 0.599), histological type (p = 0.190), degree of differentiation (p = 0.146) and tumor size (p = 0.799) (Table 1). Moreover, there were no differences in HER2 expression, MSI status, serum AFP, CA19-9, CA125 levels between EBVaGC and EBVnGC patients (p all > 0.05).
Correlation between EBV infection and overall survival (OS) of patients with gastric cancer
After removing the data of patients who died within a month, 303 patients who underwent gastrectomy from January 2011 to January 2013 were enrolled to analysis the relationship between overall survival (OS) and disease-free survival (DFS) and EBV infection. Baseline characteristics of patients were shown in Additional file 1. The median survival time for EBVaGC patients was 56.5 months, while the median survival time for EBV-negative patients was 59.0 months. However, survival analysis showed no significant difference in OS between EBVaGC and EBVnGC patients (p = 0.309) (Fig. 2). Multivariate analysis showed that TNM stage and tumor size were associated with the prognosis of GC patients (Additional file 2).
Correlation between EBV infection and disease-free survival (DFS) of patients with gastric cancer
Survival analysis showed that DFS of EBVaGC and EBVnGC patients was not statistically different (p = 0.264) (Fig. 3). Univariate analyses and multivariate analyses illustrated that tumor size and TNM stage were all related to DFS, while EBV infection status was not related to DFS (Additional file 3).
Discussion
In recent years, the correlation between EBV infection and the prognosis of GC remains to define. Our study showed no significant difference between the prognosis of EBVaGC and EBVnGC patients regardless of OS or DFS (p all > 0.05). Besides, EBV infection was found to be related to males, early T stage and TNM stage, and serum CEA level, suggesting that EBVaGC has unique clinicopathological features.
In our study, the overall survival of EBVaGC patients was shorter than that of EBVaGC patients (p = 0.306), which was consistent with some previous studies [19, 20]. However, some literatures reported better overall survival in EBVaGC patients [21, 22] while two studies reported that EBV infection was a worse prognostic indicator for patients with EBVaGC [23, 24]. A meta-analysis in 2015 showed that patients with EBV infection had a better prognosis, while accompanied by high heterogeneity, especially in different regions [10]. But one thing to mention is that this study included studies using detection methods of PCR and RNA sequence. However, the prognostic value of EBV infection is still debated as EBER has become the gold standard for detecting EBV [7]. Therefore, larger cases or a high-evidence meta-analysis are needed to unravel the prognostic value of EBV infection in GC patients.
In terms of DFS, no difference was detected between EBVaGC and EBVnGC patients in our study (p = 0.264). Two other recent papers also reported that EBV infection is not related to DFS [25, 26], consistent with our analysis. However, there is also some literature showing better DFS in EBVaGC patients [27, 28]. As GC is highly heterogeneous among individuals, it is necessary to study the gene expression differences between EBVaGC and EBVnGC patients, which may affect prognosis.
In our analysis, the EBV infection rate was 12.62%, close to the 10% infection rate reported in other literature [29, 30], so we can conclude that EBV infection in GC is common. Consistent with most studies [31, 32], we found that EBV-positive expression was more common in male patients (p = 0.001), and a meta-analysis showed the same conclusion [11], which may be related to smoking [33]. One study reported that cigarette smoke extracts could induce EBV reactivation in some EBV-positive cell lines [34], but it lacked high-level evidence. Though the reason for the gender difference remains to discover, the correlation between EBV infection and gender is positive.
As previous studies shown, EBV infection has been significantly correlated with some features, such as gender and tumor site [35]. A meta-analysis in 2020 show that is only correlated with gender and not with other clinical features [11], indicating that EBV infection is not associated with most clinicopathological features. Although many clinical features are controversial, EBVaGC has a unique mechanism, such as DNA methylation microRNAs, which affect carcinogenesis, tumor cell proliferation, apoptosis, etc. [36]. Moreover, EBVaGC has specific immune microenvironment, such as infiltrating immune cells and abundant PD-L1 expression [37,38,39]. These properties of EBVaGC will be the focus of future research.
As reported in previous studies, HER2 expression is usually detectable in EBVaGC [40], and we detected HER2 expression in 3 of 12 (25%) EBVaGC patients. According to Zang et al. [41] and Li et al. [42], HER2 expression is lower in EBVaGC cases than in EBVnGC cases. In accordance with the previous findings [43], our study also found no association between EBV infection and HER2 amplification (p = 0.928), possibly due to the low positive rate of EBV infection and HER2 expression, resulting in insufficient statistical power. The crosstalk between HER2 and EBV signaling pathways may affect gastric carcinogenesis and progression, such as the occurrence and enhancement of the epithelial-mesenchymal transition (EMT) event [44]. However, reports on the crosstalk mechanism are scarce, and more research is needed.
Besides, there was no cross case in EBV-positive and MSI-H molecular subgroups defined by TCGA in our study. EBVaGC and MSI-H GC contain similar epigenetic features, including high levels of DNA methylation in CpG islands, whereas CpG methylation is even more marked in the EBV-positive category than in the MSI class [45], and EBV-positive and MSI-H GCs are considered as mutually exclusive [16, 46]. A possible reason is that the tumor stemness reduce when MLH1 is silenced in EBV-positive GC cell lines [47]. Such exclusivity between EBV infection and MSI status is an interesting research topic, while they are already predictive markers of immunotherapy efficacy [48].
It is well known that the preoperative serum CEA levels and tumor CEA-positivity are positively correlated [49]. Our study found that EBV infection was associated with reduced serum CEA (p = 0.039), in line with a previous report that EBV infection is negatively correlated with CEA expression in tumor tissue [50]. The specific mechanism has not been studied. But there are existing studies showing that EBV interferes with TGF-β signal transduction [51], while TGF-β contributes to the stimulation of CEA transcription in GC cells [52]. Based on this, we infer that EBV could affect CEA expression. How EBV affects CEA production and secretion requires further study.
According to Seung Tae Kim [53], the overall remission rate of metastatic GC patients with EBV infection was 100% if they received anti-PD1 therapy. Previous studies have shown that PD-L1 expression is associated with EBV infection [54, 55] and the efficacy of immune checkpoint inhibitors can be predicted by detecting the status of EBV infection [53]. Therefore, EBV infection maybe not an indicator of prognosis if immunotherapy is not taken.
Conclusions
Our study revealed the clinical and pathological characteristics of EBV-associated GC in south China. EBV infection was not a prognostic indicator for GC patients according to our analysis.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Sitarz R, Skierucha M, Mielko J, Offerhaus GJA, Maciejewski R, Polkowski WP. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res. 2018;10:239–48.
Thrift AP, El-Serag HB. Burden of gastric cancer. Clin Gastroenterol Hepatol. 2020;18(3):534–42.
Epstein MA, Henle G, Achong BG, Barr YM. Morphological and biological studies on a virus in cultured lymphoblasts from Burkitt’s lymphoma. J Exp Med. 1965;121:761–70.
Naseem M, Barzi A, Brezden-Masley C, Puccini A, Berger MD, Tokunaga R, Battaglin F, Soni S, McSkane M, Zhang W, et al. Outlooks on Epstein–Barr virus associated gastric cancer. Cancer Treat Rev. 2018;66:15–22.
Röcken C. Molecular classification of gastric cancer. Expert Rev Mol Diagn. 2017;17(3):293–301.
Shinozaki-Ushiku A, Kunita A, Fukayama M. Update on Epstein–Barr virus and gastric cancer (review). Int J Oncol. 2015;46(4):1421–34.
Bae J-M, Kim EH. Epstein–Barr Virus and gastric cancer risk: a meta-analysis with meta-regression of case-control studies. J Prev Med Public Health. 2016;49(2):97.
Qiao Y-W, Zhao XQ, Liu J, Yang WJ. Clinicopathological features of Epstein–Barr virus-associated gastric carcinoma: a systematic review and meta-analysis. J BUON. 2019;24(3):1092–9.
Liu X, Liu J, Qiu H, Kong P, Chen S, Li W, Zhan Y, Li Y, Chen Y, Zhou Z, et al. Prognostic significance of Epstein–Barr virus infection in gastric cancer: a meta-analysis. BMC Cancer. 2015;15:782.
Pyo JS, Kim NY, Kang DW. Clinicopathological significance of EBV-infected gastric carcinomas: a meta-analysis. Medicina. 2020;56(7):345.
Gulley ML, Tang W. Laboratory assays for Epstein–Barr virus-related disease. J Mol Diagn. 2008;10(4):279–92.
Abrahao-Machado LF, Scapulatempo-Neto C. HER2 testing in gastric cancer: an update. World J Gastroenterol. 2016;22(19):4619–25.
Hofmann M, Stoss O, Shi D, Büttner R, van de Vijver M, Kim W, Ochiai A, Rüschoff J, Henkel T. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52(7):797–805.
Smyth EC, Wotherspoon A, Peckitt C, Gonzalez D, Hulkki-Wilson S, Eltahir Z, Fassan M, Rugge M, Valeri N, Okines A, et al. Mismatch repair deficiency, microsatellite instability, and survival: an exploratory analysis of the medical research council adjuvant gastric infusional chemotherapy (MAGIC) trial. JAMA Oncol. 2017;3(9):1197–203.
Yang N, Wu Y, Jin M, Jia Z, Wang Y, Cao D, Qin L, Wang X, Zheng M, Cao X, et al. Microsatellite instability and Epstein–Barr virus combined with PD-L1 could serve as a potential strategy for predicting the prognosis and efficacy of postoperative chemotherapy in gastric cancer. PeerJ. 2021;9:e11481.
Puliga E, Corso S, Pietrantonio F, Giordano S. Microsatellite instability in GASTRIC CANCER: BETWEEN LIGHTS and shadows. Cancer Treat Rev. 2021;95:102175.
Guan WL, Ma Y, Cui YH, Liu TS, Zhang YQ, Zhou ZW, Xu JY, Yang LQ, Li JY, Sun YT, et al. The impact of mismatch repair status on prognosis of patients with gastric cancer: a multicenter analysis. Front Oncol. 2021;11:712760.
Huang S-C, Ng K-F, Chen K-H, Hsu J-T, Liu K-H, Yeh T-S, Chen T-C. Prognostic factors in Epstein–Barr virus-associated stage I-III gastric carcinoma: implications for a unique type of carcinogenesis. Oncol Rep. 2014;32(2):530–8.
Shibata D, Hawes D, Stemmermann GN, Weiss LM. Epstein–Barr virus-associated gastric adenocarcinoma among Japanese Americans in Hawaii. Cancer Epidemiol Biomarkers Prevent A Publ Am Assoc Cancer Res Cosponsored Am Soc Prevent Oncol. 1993;2(3):213–7.
Beltrán Gárate B, Camara A, Kapsoli Sánchez MDC, Castro Uriol D, Yábar Berrocal A. Impact of the Epstein Barr virus on gastric cancer in Peru. Rev Gastroenterol Peru. 2019;39(4):319–22.
Hewitt LC, Inam IZ, Saito Y, Yoshikawa T, Quaas A, Hoelscher A, Bollschweiler E, Fazzi GE, Melotte V, Langley RE, et al. Epstein–Barr virus and mismatch repair deficiency status differ between oesophageal and gastric cancer: a large multi-centre study. Eur J Cancer. 2018;94:104–14.
Nogueira C, Mota M, Gradiz R, Cipriano MA, Caramelo F, Cruz H, Alarcão A, Oliveira F, Martinho F, et al. Prevalence and characteristics of Epstein–Barr virus-associated gastric carcinomas in Portugal. Infect Agent Cancer. 2017;12:41.
Shen H, Zhong M, Wang W, Liao P, Yin X, Rotroff D, Knepper TC, McLeod HL, Zhou C, Xie S, et al. EBV infection and MSI status significantly influence the clinical outcomes of gastric cancer patients. Clin Chim Acta. 2017;471:216–21.
Ramos M, Pereira MA, Amorim LC, de Mello ES, Faraj SF, Ribeiro U, Hoff PMG, Cecconello I, de Castria TB. Gastric cancer molecular classification and adjuvant therapy: is there a different benefit according to the subtype? J Surg Oncol. 2020;121(5):804–13.
Baek DW, Kang BW, Kim JG. The predictive value of Epstein–Barr virus-positivity in patients undergoing gastrectomy followed by adjuvant chemotherapy. Chonnam Med J. 2018;54(3):173–7.
van Beek J, Zur-Hausen A, Klein-Kranenbarg E, van de Velde CJ, Middeldorp JM, van den Brule AJ, Meijer CJ, Bloemena E. EBV-positive gastric adenocarcinomas: a distinct clinicopathologic entity with a low frequency of lymph node involvement. J Clin Oncol. 2004;22(4):664–70.
Qiu MZ, He CY, Yang DJ, Zhou DL, Zhao BW, Wang XJ, Yang LQ, Lu SX, Wang FH, Xu RH. Observational cohort study of clinical outcome in Epstein–Barr virus associated gastric cancer patients. Ther Adv Med Oncol. 2020;12:1758835920937434.
Chen J-N, He D, Tang F, Shao C-K. Epstein–Barr virus-associated gastric carcinoma: a newly defined entity. J Clin Gastroenterol. 2012;46(4):262–71.
Burgess DE, Woodman CB, Flavell KJ, Rowlands DC, Crocker J, Scott K, Biddulph JP, Young LS, Murray PG. Low prevalence of Epstein–Barr virus in incident gastric adenocarcinomas from the United Kingdom. Br J Cancer. 2002;86(5):702–4.
Murphy G, Pfeiffer R, Camargo MC, Rabkin CS. Meta-analysis shows that prevalence of Epstein–Barr virus-positive gastric cancer differs based on sex and anatomic location. Gastroenterology. 2009;137(3):824–33.
Li S, Du H, Wang Z, Zhou L, Zhao X, Zeng Y. Meta-analysis of the relationship between Epstein–Barr virus infection and clinicopathological features of patients with gastric carcinoma. Sci China Life Sci. 2010;53(4):524–30.
Camargo MC, Koriyama C, Matsuo K, Kim W-H, Herrera-Goepfert R, Liao LM, Yu J, Carrasquilla G, Sung JJY, Alvarado-Cabrero I, et al. Case-case comparison of smoking and alcohol risk associations with Epstein–Barr virus-positive gastric cancer. Int J Cancer. 2014;134(4):948–53.
Suzuki T, Matsuo K, Ito H, Sawaki A, Hirose K, Wakai K, Sato S, Nakamura T, Yamao K, Ueda R, et al. Smoking increases the treatment failure for Helicobacter pylori eradication. Am J Med. 2006;119(3):217–24.
Saito M, Kono K. Landscape of EBV-positive gastric cancer. Gastr Cancer Offic J Int Gastr Cancer Assoc Jpn Gastr Cancer Assoc. 2021;24(5):983–9.
Sun K, Jia K, Lv H, Wang SQ, Wu Y, Lei H, Chen X. EBV-positive gastric cancer: current knowledge and future perspectives. Front Oncol. 2020;10:583463.
Derks S, Liao X, Chiaravalli AM, Xu X, Camargo MC, Solcia E, Sessa F, Fleitas T, Freeman GJ, Rodig SJ, et al. Abundant PD-L1 expression in Epstein–Barr Virus-infected gastric cancers. Oncotarget. 2016;7(22):32925–32.
Ichimura T, Abe H, Morikawa T, Yamashita H, Ishikawa S, Ushiku T, Seto Y, Fukayama M. Low density of CD204-positive M2-type tumor-associated macrophages in Epstein–Barr virus-associated gastric cancer: a clinicopathologic study with digital image analysis. Hum Pathol. 2016;56:74–80.
Kim SY, Park C, Kim HJ, Park J, Hwang J, Kim JI, Choi MG, Kim S, Kim KM, Kang MS. Deregulation of immune response genes in patients with Epstein–Barr virus-associated gastric cancer and outcomes. Gastroenterology. 2015;148(1):137-147.e139.
Gonzalez RS, Messing S, Tu X, McMahon LA, Whitney-Miller CL. Immunohistochemistry as a surrogate for molecular subtyping of gastric adenocarcinoma. Hum Pathol. 2016;56:16–21.
Zhang YW, Zhao XX, Tan C, Zhang ZG, Jiang Y, Chen JN, Wei HB, Xue L, Li HG, Du H, et al. Epstein–Barr virus latent membrane protein 2A suppresses the expression of HER2 via a pathway involving TWIST and YB-1 in Epstein–Barr virus-associated gastric carcinomas. Oncotarget. 2015;6(1):207–20.
Li Z, Lai Y, Sun L, Zhang X, Liu R, Feng G, Zhou L, Jia L, Huang X, Kang Q, et al. PD-L1 expression is associated with massive lymphocyte infiltration and histology in gastric cancer. Hum Pathol. 2016;55:182–9.
Irkkan C, Balci S, Güler Tezel G, Akinci B, Yalcin B, Güler G. Comparison of clinicopathologic parameters and survivals between Epstein–Barr virus-positive and Her2-positive gastric cancers. Appl Immunohistochem Mol Morphol AIMM. 2017;25(9):609–14.
Cyprian FS, Al-Antary N, Al Moustafa AE. HER-2/Epstein–Barr virus crosstalk in human gastric carcinogenesis: a novel concept of oncogene/oncovirus interaction. Cell Adh Migr. 2018;12(1):1–4.
Gulley ML. Genomic assays for Epstein–Barr virus-positive gastric adenocarcinoma. Exp Mol Med. 2015;47(1):e134.
Chang MS, Lee HS, Kim HS, Kim SH, Choi SI, Lee BL, Kim CW, Kim YI, Yang M, Kim WH. Epstein–Barr virus and microsatellite instability in gastric carcinogenesis. J Pathol. 2003;199(4):447–52.
Kim Y, Shin YJ, Wen X, Cho NY, Li M, Kim YJ, Song SH, Kang GH. Alteration in stemness causes exclusivity between Epstein–Barr virus-positivity and microsatellite instability status in gastric cancer. Gastr Cancer Offic J Int Gastr Cancer Assoc Jpn Gastr Cancer Assoc. 2021;24(3):602–10.
Rodriquenz MG, Roviello G, D’Angelo A, Lavacchi D, Roviello F, Polom K. MSI and EBV positive gastric cancer’s subgroups and their link with novel immunotherapy. J Clin Med. 2020;9(5):1427.
Kim DY, Kim HR, Shim JH, Park CS, Kim SK, Kim YJ. Significance of serum and tissue carcinoembryonic antigen for the prognosis of gastric carcinoma patients. J Surg Oncol. 2000;74(3):185–92.
Lee HS, Chang MS, Yang HK, Lee BL, Kim WH. Epstein–Barr virus-positive gastric carcinoma has a distinct protein expression profile in comparison with Epstein–Barr virus-negative carcinoma. Clin Cancer Res Offic J Am Assoc Cancer Res. 2004;10(5):1698–705.
Velapasamy S, Dawson CW, Young LS, Paterson IC, Yap LF. The dynamic roles of TGF-β signalling in EBV-associated cancers. Cancers. 2018;10(8):247.
Han SU, Kwak TH, Her KH, Cho YH, Choi C, Lee HJ, Hong S, Park YS, Kim YS, Kim TA, et al. CEACAM5 and CEACAM6 are major target genes for Smad3-mediated TGF-beta signaling. Oncogene. 2008;27(5):675–83.
Kim ST, Cristescu R, Bass AJ, Kim KM, Odegaard JI, Kim K, Liu XQ, Sher X, Jung H, Lee M, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24(9):1449–58.
Liu X, Choi MG, Kim K, Kim K-M, Kim ST, Park SH, Cristescu R, Peter S, Lee J. High PD-L1 expression in gastric cancer (GC) patients and correlation with molecular features. Pathol Res Pract. 2020;216(4):152881.
Kawazoe A, Kuwata T, Kuboki Y, Shitara K, Nagatsuma AK, Aizawa M, Yoshino T, Doi T, Ohtsu A, Ochiai A. Clinicopathological features of programmed death ligand 1 expression with tumor-infiltrating lymphocyte, mismatch repair, and Epstein–Barr virus status in a large cohort of gastric cancer patients. Gastric Cancer Offic J Int Gastr Cancer Assoc Jpn Gastr Cancer Assoc. 2017;20(3):407–15.
Acknowledgements
Not applicable.
Funding
This study was funded by the National Natural Science Foundation of China under Grant Numbers: 81602049 and 81802342, the Natural Science Foundation of Guangdong Province, China under Grant Number: 2018A030313978 and the Kelin New Star of the First Affiliated Hospital of Sun Yat‐Sen University under Grant Numbers: R08011 and R08010.
Author information
Authors and Affiliations
Contributions
WZ designed this study. LGH and ZZH performed the experiments, interpreted the patient data and drafted the manuscript. LGH and ZZH contributed to data collection and created the figures. WZX and HJH revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All patients have obtained written informed consent and have complied with the ethical guidelines in the Declaration of Helsinki. All subjects and experimental protocols have been approved by the Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University in China.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file1
. Relationship between EBV infection and clinicopathologic characteristics.
Additional file2
. Univariate and multivariate analysis of overall survival-related factors.
Additional file3
. Analysis of univariate and multivariate factors affecting the disease-free survival of patients.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, G., Zhou, Z., Wang, Z. et al. Assessing Epstein–Barr virus in gastric cancer: clinicopathological features and prognostic implications. Infect Agents Cancer 18, 11 (2023). https://doi.org/10.1186/s13027-023-00489-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13027-023-00489-9