Abstract
Background
Health care providers have reported low knowledge, skill, and confidence for discussing movement behaviours (i.e., physical activity, sedentary behaviour, and sleep), which may be improved with the use of tools to guide movement behaviour discussions in their practice. Past reviews have examined the psychometric properties, scoring, and behavioural outcomes of physical activity discussion tools. However, the features, perceptions, and effectiveness of discussion tools for physical activity, sedentary behaviour, and/or sleep have not yet been synthesized. The aim of this review was to report and appraise tools for movement behaviour discussions between health care providers and adults 18 + years in a primary care context within Canada or analogous countries.
Methods
An integrated knowledge translation approach guided this review, whereby a working group of experts in medicine, knowledge translation, communications, kinesiology, and health promotion was engaged from research question formation to interpretation of findings. Three search approaches were used (i.e., peer-reviewed, grey literature, and forward searches) to identify studies reporting on perceptions and/or effectiveness of tools for physical activity, sedentary behaviour, and/or sleep. The quality of included studies was assessed using the Mixed Methods Appraisal Tool.
Results
In total, 135 studies reporting on 61 tools (i.e., 51 on physical activity, one on sleep, and nine combining two movement behaviours) met inclusion criteria. Included tools served the purposes of assessment (n = 57), counselling (n = 50), prescription (n = 18), and/or referral (n = 12) of one or more movement behaviour. Most tools were used or intended for use by physicians, followed by nurses/nurse practitioners (n = 11), and adults accessing care (n = 10). Most tools were also used or intended to be used with adults without chronic conditions aged 18–64 years (n = 34), followed by adults with chronic conditions (n = 18). The quality of the 116 studies that evaluated tool effectiveness varied.
Conclusions
Many tools were positively perceived and were deemed effective at enhancing knowledge of, confidence for, ability in, and frequency of movement behaviour discussions. Future tools should guide discussions of all movement behaviours in an integrated manner in line with the 24-Hour Movement Guidelines. Practically, this review offers seven evidence-based recommendations that may guide future tool development and implementation.
Similar content being viewed by others
Introduction
The Canadian 24-Hour Movement Guidelines for Adults (24HMG) offer integrated recommendations on physical activity (PA), sedentary behaviour, and sleep for adults [1]. Uniquely, these guidelines emphasize a 24-h movement paradigm, which characterizes optimal patterns of, and interactions between, PA, sedentary behaviour, and sleep [2, 3]. This paradigm has been gaining traction nationally and internationally, such as with health guidelines for some chronic diseases [4]. In a 24-h paradigm, it is recommended to trade sedentary behaviour for light PA or moderate-to-vigorous PA (MVPA), while preserving sufficient sleep [1].Footnote 1 Regrettably, general population adults are largely non-adherent to movement behaviours [5] and are incurring increased morbidity and mortality risk as a result [6].
Health care providers are well-positioned to promote healthy PA, sedentary behaviour, and sleep as they are in semi-frequent contact with general population adults [7] and are deemed reliable sources of health information [8]. Indeed, 74% of Canadian adults met with their provider at least once in 2018 [9]. Moreover, health care providers are appropriate disseminators and implementers of movement behaviour guidelines as primary care covers a spectrum of services devoted to the improvement of health outcomes [10, 11]. In Canada and other developed, high-income countries, primary care is commonly delivered by physicians, nurses, and nurse practitioners [12, 13]; however, family health teams may involve other providers, including pharmacists [12, 14, 15], dietitians [12, 16], psychologists [15], registered psychotherapists [17], and social workers [10, 12], who perform key roles in movement behaviour promotion.
Providers can engage in several actions to promote sufficient sleep, PA, and reduce sedentary behaviour among adults accessing care. For the present study, the terminology “adults accessing care” is used in place of “patients” as it is more inclusive and disaffirms a power dynamic with providers [18]. When engaging in discussions with adults accessing care, providers can assess current movement behaviours, counsel on behaviour change strategies, prescribe targets for behaviour change, and refer to other professionals or programs for follow-up [19, 20]. However, providers have reported low knowledge, skill, confidence, and motivation for movement behaviour conversations as well as barriers to movement behaviour promotion, such as lack of remuneration [21,22,23,24]. Notably, materials and strategies to support providers have been identified as facilitators to implementing movement behaviour promotion practices [25].
Tools that guide movement behaviour discussions between providers and adults accessing care have been developed and used since the early 2000s (e.g., [26, 27]). Overwhelmingly, these tools have focused on PA, while only some have focused on sleep and few have focused on sedentary behaviour only in combination with PA. New tools have emerged over recent years (e.g., [28]), showing a continued and growing interest in the field. Nevertheless, these single-behaviour tools are limited in their utility to inform discussions on integrated movement behaviours in line with the 24HMG. Thus, considering tools that guide discussions on all three movement behaviours between providers and individuals accessing care is important and timely. Notably, this work could inform the development of new, integrated tools or the refinement of existing tools to promote healthy movement behaviours.
Rationale
Two systematic reviews [29, 30] and one literature review [31] have examined the psychometric properties, scoring, and behavioural outcomes of PA discussion tools. However, these reviews have captured neither all previously available tools nor tools that have emerged since 2017. Furthermore, tools for sedentary behaviour or sleep discussions for health promotion have not been reviewed. Finally, reviews have not considered whether tools are theory-based or stem from public health guidelines. Implementation efforts grounded in behaviour change theory may procure greater success [32]. Likewise, public health guidelines are developed via systematic review and expert appraisal [33] and their implementation success is strategically monitored and supported by multidisciplinary teams [34]. Lastly, the perceptions of providers and individuals accessing care regarding the utility of movement behaviour discussion tools have not been reviewed, which could inform more practically relevant and useful tools. A scoping review was deemed necessary to build upon the evidence base by capturing a broader range of features and outcomes for a larger number of tools on PA, sedentary behaviour, and sleep for health promotion discussions in primary care.
Objectives
The purpose of this scoping review was to report on and appraise tools that guide movement behaviour discussions between health care providers (i.e., physicians, nurses, nurse practitioners, pharmacists, dietitians, psychologists, registered psychotherapists, and social workers) and adults 18 + years accessing care in a primary care context within Canada or analogous countries (i.e., English-speaking, developed, high-income).
The research questions (RQs) were: (1) What tools are available to guide discussions on assessment, counselling, prescription, and/or referral for PA, sedentary behaviour, and/or sleep for chronic disease prevention and health promotion among adults 18 + years, and what are their features? (2) What are the positive and/or negative perceptions of health care providers and adults accessing care toward the potential and/or actual utility of these tools in clinical interactions? and (3) Has the use of these tools enhanced (i) providers’ knowledge, ability, confidence for, and/or frequency of assessing, counselling, prescribing, and/or referring or (ii) levels of PA, sedentary behaviour, and/or sleep among adults accessing care?
Methods
Engaging knowledge partners bridges the gap between research and non-research audiences to enhance the applicability, clarity, awareness, and dissemination of review findings [35]. Therefore, an integrated knowledge translation approach [36, 37] was chosen to guide this review. A working group of experts (i.e., academic professionals, health professionals, and representatives of organizations related to the topic of study) were personally invited to be involved from research question formation to interpretation of findings. Working group involvement transpired over three 90-min structured online meetings led by TLM and email correspondence (June-October, 2021). Scoping review methodology was chosen as we aimed to broadly appraise the characteristics of movement behaviour discussion tools where much of the research is emergent and not amenable to examining effectiveness alone [38]. Established guidance for scoping review methodology [39] and quality of reporting on knowledge partner engagement in reviews [40, 41] were followed. An a priori review protocol was registered in Open Science Framework on July 23, 2021 [42] that, like this paper, noted conflicts of interest. Findings are reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR [43]). The PRISMA-ScR checklist is shown in Additional File 1. Ethics approval was not required for this review.
Eligibility criteria
Eligible studies reporting on, and sufficiently describing, a tool for assessment, counselling, prescription, and/or referral of PA, sedentary behaviour, and/or sleep used or intended for use among adults 18 + years with or without a chronic disease(s) accessing primary care were considered. Primary care settings were defined as the first point of contact for adults accessing care [11]. Studies must have been published in Canada or a similar country (i.e., English-speaking, developed, high-income) in 2000 or later to be eligible for inclusion. All study designs were eligible, including those reported in conference proceedings, except for scoping reviews, systematic reviews, and meta-analyses. Studies and tools not available in English were excluded and those implemented in a low-to-middle income country were not considered due to the dissimilarity of their health care context compared to that of high-income countries [44].
Search strategy
Recommendations by Levac and colleagues [45] informed the search strategy. A professional librarian (ARW) developed and carried out the peer-reviewed (MedLine [Ovid], EMBASE [Ovid], PsycINFO [Ovid], CINAHL [Ebsco], Cochrane Controlled Trials Register [Ovid], and Google Scholar [Publish or Perish [46]) and grey literature (Thesis & Dissertations-ProQuest Dissertations Online, Web of Science, Canadian Electronic Library-Canadian Public Policy Collection and Canadian Health Research Collection) searches in June 2021. In addition, forward searches of all studies deemed eligible for full-text screening were performed by TLM in August 2021 to ensure all related references would be captured. Forward searching has been found to be a highly effective method for reviews where concepts are challenging to retrieve using subject headings or keywords [47, 48]. Finally, review authors were asked to check their personal libraries to identify additional, potentially relevant peer-reviewed publications. Additional File 2 contains the full search strategy.
Study selection
Deduplication of studies occurred in Covidence [49]. Two reviewers (TLM and EF) independently screened all titles and abstracts from the peer-reviewed and grey literature searches. Title and abstract screening of forward search results was performed by TLM. Full-texts deemed as potentially relevant were downloaded and independently screened by the two reviewers. Discrepancies regarding inclusion at the title and abstract and full-text phases were resolved by discussion between the two reviewers, and in consultation with a third reviewer (JRT) when necessary.
Data extraction
The data extraction table was independently piloted by four researchers (TLM, EF, and two research assistants, LK and MK). All four researchers attempted extraction of three papers and met to reach consensus on the table’s utility for addressing all RQs. Subsequently, the four researchers each extracted one-quarter of included studies prior to auditing another one-quarter (e.g., TLM audited LK, and vice versa). Data was extracted per: study characteristics (i.e., author(s), title, year, study design, country, and participant characteristics), tool characteristics (i.e., RQ1: name, purpose(s), description, format, population(s) used by and served, and guideline and/or theoretical basis), tool perception outcomes as per a modified coding framework by Neudorf and colleagues [50] (i.e., RQ2: satisfaction, content, efficiency, navigation, understandability, usability, visibility, and workflow), and tool effectiveness outcomes (i.e., RQ3: knowledge, ability, confidence, frequency of assessment/counselling/prescription/referral, movement behaviours, and outcomes not stated in RQ3). Neudorf and colleagues’ [50] framework was modified to include an eighth component—tool satisfaction—per the recommendation of a recent systematic review [29]. Applying this framework may help capture a broader understanding of why tools may or may not have been positively rated or effective in practice. Included tools were classified into five formats: paper-based, integrated into the Electronic Medical Record (EMR), electronic-based (i.e., tablet, website, online portal, software/program), mobile-based (i.e., app, text messaging), or pedometer-based.
Data synthesis
Following data extraction, discussion with the working group was sought to interpret the results. Narrative synthesis of studies reporting on outcomes across all RQs was performed [51] to corroborate positive perceptions of tools with their features and effectiveness outcomes. Study results pointing toward the future improvement of tools were also synthesized. Synthesis of studies examining outcomes across all RQs involved reporting the same language and statistical data used by the authors of the primary studies. Results are presented across four supplementary files and one table included herein: Additional Files 3–5 report tool characteristics (RQ1), including a description of each tool, and are organized alphabetically by tool name; Additional File 6 reports all studies addressing RQ2 and 3 outcomes, organized alphabetically by tool name, then chronologically by study year within each tool; Table 1 shows the links between tool features, perceptions, and outcomes and evidence-based recommendations for future tool development.
Quality assessment
The methodological quality of included studies answering RQ3 (i.e., tool effectiveness) was appraised by TLM, EF, LK, and MK using the Mixed Methods Appraisal Tool (MMAT [107]). The MMAT includes five unique rating criteria for each of five study designs (i.e., randomized controlled trials, non-randomized designs, quantitative descriptive designs, qualitative research, and mixed methods designs). MMAT scores ranged from 0 to 5 out of a possible 5 (see rightmost column in Additional File 6); however, only scores of similar study designs should be compared [108].
Results
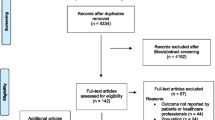
Peer-reviewed and grey literature searches identified 8 292 studies. After de-duplication, 5 298 studies remained. Following title and abstract screening, 155 studies were carried forward to full-text screening. Forward searching and authors’ searching of personal libraries identified another 71 and 4 studies, respectively, resulting in a total of 230 full-texts. In total, 135 studies (i.e., peer-reviewed and grey literature searches, n = 77; forward searches, n = 55; researchers files, n = 3) were included in data synthesis (Fig. 1).
Tool characteristics
Sixty-one tools were identified, with several tools including more than one purpose, movement behaviour, format, and/or population. Of the 61 tools, 57 were for the purpose of assessment, 50 were for counselling, 18 were for prescription, and 12 were for referral. Assessment was rarely performed in isolation; only five tools were for assessment alone. Counselling was performed once in isolation [77]. Prescription and referral were not performed alone in any included tool. Nine tools that integrated two movement behaviours were identified. Fifty-one tools focused on PA, one focused on sleep, and nine focused on multiple behaviours (i.e., seven on PA/sedentary behaviour, two on PA/sleep). Most tools were paper-based (including PDF; n = 33), followed by EMR-based (n = 18), electronic-based (e.g., software, website, tablet; n = 17), pedometer-based (n = 6), mobile-based (e.g., app, text messaging; n = 2). Most tools were used or intended for use by physicians (n = 49), followed by nurses/nurse practitioners (n = 11), adults accessing care (n = 10), dietitians (n = 3), pharmacists (n = 3), and psychologists/social workers (n = 1). Many tools were used or intended to be used with adults without chronic conditions aged 18–64 years (n = 34) and adults with chronic conditions (n = 18); less were used or intended to be used with adults with overweight or obesity specifically (n = 10), adults 65 + years (n = 5), adults who smoke (n = 1), and veterans (n = 1).
Perceptions of tools
Positive perceptions regarding all RQ2 outcomes except navigation were reported for four of the seven PA/sedentary behaviour tools. For example, providers felt the functions of the EMR PA Tool were easy to use, worthwhile, and should be permanently integrated into the EMR [109]. Further, negative perceptions for two RQ2 outcomes (i.e., navigation and visibility) were shared for one tool: nurses reported overlooking certain functional elements (i.e., buttons within individual charts [84]) in the Interactive Tool for Self-management through LIfestyle FEedback! (It’s LiFe!) tool and felt that navigation was too complex and laden with technical issues [84, 110]. Mixed perceptions on five RQ2 outcomes (i.e., satisfaction, content, efficiency, usability, and workflow) were reported for two tools. For instance, adults accessing care valued providers’ use of the Paper-Based Decision Tool; however, providers did not seem to recognize that the tool was valued and found it “impossible to use in everyday practice”[58 p7]. Only one of the two PA/sleep tools reported RQ2 outcomes. Positive perceptions of usability were reported for EMR-based the Integrated Wellness Tool (IWT), wherein adults and providers stated that the tool was easy to use [111]. However, mixed perceptions regarding users’ satisfaction and workflow were also given for the IWT. Most adults reported that the IWT would aid their provider in better understanding their health; however, providers stated that the tool did not offer any new information [111].
Of the 51 PA tools, positive perceptions regarding all RQ2 outcomes (i.e., satisfaction, content, efficiency, navigation, usability, understandability, visibility, and workflow) were reported for 30 tools. For example, physicians and adults accessing care reported that the EMR-based Electronic Case-finding and Help Assessment (eCHAT) tool was acceptable, easy to use and understand [66], and facilitated discussions of PA that otherwise may not have been initiated [67]. However, negative perceptions covering all RQ2 outcomes except satisfaction (i.e., only positive or mixed satisfaction was reported) were given for 22 tools. For instance, the 5A’s Team Tools offer a toolkit of provider counselling and prescription tools and shared decision-making resources in both paper and electronic formats, but these were perceived as “too long” by providers [112] and certain elements (i.e., mnemonics) were not seen as useful for communicating with adults accessing care [87]. Finally, there were mixed perceptions covering all RQ2 outcomes across 19 tools. One example is the Computer-Based Counselling System, an electronic tool, where some adults accessing care felt that the informational videos were relatable to them, but others felt they were not applicable to adults who are employed [113].
Positive perceptions of satisfaction were given for the one sleep tool. Most adults accessing care responded positively about the paper-based Sleep Health Materials tool and stated they would recommend it to a friend [14].
Tool effectiveness
Among the PA/sedentary behaviour tools, It’s LiFe! was positively associated with nurses’ knowledge and ability [110] and increases in adults’ PA behaviour [110, 114, 115] while the Electronic Medical Prescription in Portugal was positively associated with frequency of PA/sedentary behaviour discussions [96]. Alternatively, the EMR PA Tool was negatively associated with physicians’ knowledge [109], and the Paper-Based Decision Tool was associated with decreases in sedentary behaviour but also PA [76]. Moreover, three tools showed mixed associations with knowledge [110] and frequency of counselling and prescription [110, 115, 116]. For example, the Rapid Assessment Disuse Index (RADI) was used to provide general advice to reduce sedentary time in 10% of adults accessing care; however, no adults received a written plan to reduce sedentary behaviour and only 2% were given specific strategies to target sedentary behaviour change [116]. One PA/sleep tool, the IWT, was not associated with any changes in physicians’ ability to provide counselling [111].
Thirty-eight of the 51 PA tools were positively associated with all RQ3 outcomes over time: increases in providers’ knowledge (n = 5), ability (n = 4), confidence (n = 5), and frequency (n = 8) of PA assessment, counselling, prescription, and/or referral, and PA behaviour (n = 16) among adults accessing care. For example, Activity Counselling Trial, a 3–4 min paper-based tool, was reported to improve providers’ perceived ability to counsel on PA [68] and significantly increase self-reported PA in adults accessing care compared to baseline [69]. Effect sizes of PA tools that were successful at increasing PA were only reported in two studies, with one showing large effect sizes [117] and the other showing small effect sizes [61]. Alternatively, eight tools showed negative associations across all 5 RQ3 outcomes except PA behaviour. For instance, in reference to using the PA Screen in EMR tool, providers mentioned low knowledge surrounding PA guidelines or exercise programs [28]. Five tools were not associated with any changes in confidence and frequency in PA discussions or PA behaviour. For example, the paper-based and EMR-integrated Physical Activity Vital Sign was not associated with changes in providers’ confidence to give PA advice and prescriptions from pre- to post-intervention; however, levels of confidence for giving PA advice were already high at baseline (89–94% [104]).
The one sleep tool (Sleep Health Materials tool) was associated with increases in adults’ sleep knowledge and confidence in managing sleep problems and showed positive associations with pharmacists’ sleep knowledge and frequency of counselling [14]. Effectiveness outcomes that were reported in included studies but not listed in RQ3 are included in Additional File 6.
Evidence-based recommendations for future tool development
Recommendations and their supporting studies are presented in Table 1.
Quality assessment
One-hundred-sixteen studies evaluated the effectiveness of tools. Of the 21 qualitative studies, quality ranged from 0–5 out of a possible 5. The most common reasons for lower scores were findings not derived from data (n = 2 [70, 118]), interpretation not substantiated by data (n = 2 [70, 118]), and lack of coherence between data sources, collection, and analysis (n = 2 [70, 118]). Of the 35 RCTs, study quality ranged from 0–5. Lower scores resulted from incomplete outcome data (n = 22 [57, 58, 68, 69, 71, 72, 78, 85, 88, 89, 97, 117, 119,120,121,122,123,124,125,126,127,128]), outcome assessors not blinded (n = 24 [58,59,60, 68, 69, 71, 72, 75, 78, 88, 97, 98, 119, 124,125,126,127,128,129,130,131,132,133,134]), and lack of adherence to the intervention (n = 18 [58,59,60, 68, 79, 80, 85, 88, 89, 114, 117, 120,121,122,123, 128, 129, 133]).
Of the 15 non-randomized designs, study quality varied from 0–5. Common reasons for lower scores included incomplete outcome data (n = 13 [54, 61, 62, 69, 73, 81, 90, 99, 135,136,137,138,139]) and no accounting of confounders (n = 14 [54, 61, 62, 73, 81, 90, 99, 100, 135,136,137,138,139,140]).
Of the 34 descriptive studies, quality ranged from 0–5. Lower scores were primarily due to sample not representative of the target population (n = 11 [55, 63, 66, 113, 139, 141,142,143,144,145,146]) and high risk of non-response bias (n = 24 [55, 63, 64, 66, 69, 74, 91, 101, 113, 139, 142,143,144,145,146,147,148,149,150,151,152,153,154,155]).
Of the 11 mixed methods studies, quality varied from 2–5. Lower scores were due to inconsistency between quantitative and qualitative results (n = 6 [61, 104, 110, 115, 156, 157]) and quantitative and qualitative components not adhering to the quality criteria of their method (n = 5 [84, 115, 156,157,158]). The full MMAT ratings are presented in Additional File 7.
Discussion
This scoping review aimed to comprehensively report and appraise tools for PA, sedentary behaviour, and/or sleep discussions between health care providers and adults (18 + years) accessing care in clinical settings in Canada and analogous countries. Findings indicated that a vast number of tools to guide discussions on PA, sedentary behaviour, and sleep have been developed and implemented to varying degrees in clinical settings. The 61 tools showcased a broader picture of PA discussion tools than did previous reviews in this area [29,30,31] including several sedentary behaviour and sleep discussion tools that had not been previously synthesized.
Providers and adults accessing care held positive perceptions toward many (58.8%) of the included PA tools and nearly three-quarters (74.5%) were positively associated with effectiveness outcomes. These results are promising as the positively rated tools in this review can provide insight as to what specific features (e.g., length of the tool, supportive language) can be borrowed from in emerging tools or carried forward in future iterations of existing tools. Conversely, insight from studies reporting negative or mixed perceptions and effectiveness outcomes of PA tools can also be gleaned to make strategic improvements to the design and functionality of tools moving forward. For instance, many negative perceptions of included PA tools pertained to their efficiency and workflow [90, 152, 159]. It is no surprise that providers face unrelenting time constraints and workload [160], but these findings highlight the need to develop movement behaviour discussion tools that can be embedded seamlessly into a clinical encounter. Additionally, negative associations with providers’ knowledge, confidence, ability, and frequency were present for some PA tools. A systematic review found that the greatest barriers to PA counselling reported by physicians were a lack of knowledge, training, and resources [23]. However, only one tool in the present review was associated with decreases in PA (specifically MVPA) among adults accessing care [76]. This suggests that while providers may not feel knowledgeable, confident, or skilled in engaging in PA discussions, the mere act of facilitating PA discussions through a tool appears to positively influence the PA levels of adults accessing their care [114, 119, 127].
Only four tools did not cover PA assessment. That most tools included an assessment of PA in addition to counselling, prescription, and/or referral is consistent with the sequence of actions that providers enact to promote PA, as reported in the literature [19, 20]. Assessing PA is the first step to providing effective discussions of PA behaviour change and this was evidenced by many tools where data from the PA assessment step was used to prompt and support PA counselling and/or prescription later in the consultation (e.g., [70, 96, 161]).
Only one tool focused solely on sleep for health promotion purposes. Unsurprisingly, it was more detailed than the two combined PA and sleep tools, including information on sleep duration and on sleep environment and lifestyle considerations, such as sleep quality and sleep hygiene [14]. Notably, during screening, many tools were excluded given their focus on sleep disorders. The absence of sleep disorders is not always indicative of healthy sleep patterns; healthy sleep is comprised of sufficient duration and quality, suitable timing, and a lack of sleep disorders [162]. In contrast to the predominant focus in clinical practice on diagnosing and treating disordered sleep [163], discussions on healthy sleep behaviours should be approached whether or not an individual presents with a sleep disorder. This evidence suggests there is a need to develop more tools for assessment, counselling, prescription and referral for sleep behaviour change and health promotion in clinical settings.
Given the growing body of literature noting the differences between sedentary behaviour and inactivity [164, 165], tools focusing on increasing PA and decreasing sedentary behaviour have begun to emerge. Of the seven PA/sedentary behaviour, six were published in the last 10 years [26, 76, 96, 109, 114, 116]. Some perceptions and outcomes of the PA/sedentary behaviour tools were mixed or negative; however, three tools were positively related to enhancements in providers’ knowledge, ability [110], and frequency discussing movement behaviours [96], and adults’ sedentary behaviour [76]. The Paper-Based Decision Tool [76] was also associated with small reductions in MVPA. It is unclear what may have caused this drop in PA, though perhaps, in an effort to conserve their energy to reduce their sedentary behaviour, participants in the study may have simultaneously traded some MVPA for lighter-intensity PA. Given this study was published before the 24HMG for Adults were released [1], providers were unlikely counselling participants on the integrated relationships between PA, sedentary behaviour, and sleep. Therefore, future tools combining movement behaviour recommendations should promote trading sedentary time for light PA without decreasing MVPA (or with increasing MVPA where possible), while preserving sufficient sleep. Further, as a low number of sedentary behaviour tools have been evaluated, more research is needed to establish best practices for developing tools that target healthy sedentary behaviour habits overall, and in the context of a 24-h movement paradigm.
There are several explanations for these mixed results. One could be that sedentary behaviour remains an unfamiliar topic for some providers and adults accessing care, leading to fewer instances where sedentary behaviour discussions are initiated in clinical settings. For instance, breaking up sedentary time is known to have health benefits [1]; however, the 24HMG lack suggestions on how often sedentary time should be interrupted (e.g., every 30 min, every 60 min, etc.), therefore providers may feel unsure what practical recommendations to make. Indeed, providers have reported limited knowledge about sedentary behaviour counselling and a desire for education on the topic [24]. Another reason is that sedentary behaviour can be difficult to quantify in absence of objective measures (e.g., accelerometers), thus adults may misperceive how much sedentary time they are engaging in. Low awareness of sedentary behaviour guidelines in the general population has also been described [166]. Two recent reviews [167, 168] have emphasized that establishing knowledge, awareness, and positive attitudes should precede efforts to improve self-efficacy, intentions, and actual performance of a given behaviour. Thus, it is logical that the included sedentary behaviour tools were associated with varying degrees of confidence, ability, and frequency of use.
User perceptions and effectiveness outcomes were only reported for four of the nine multi-behaviour tools (3 PA/sedentary behaviour tools: EMR PA Tool, It’s LiFe!, Paper-based Decision Tool; 1 PA/sleep tool: IWT). Similar to the sleep-only tool, the studies reporting on PA/sleep tools did not evaluate sleep behaviour. Further, studies for only two of the PA/sedentary behaviour tools (RADI; Paper-based Decision Tool) evaluated for changes in sedentary behaviour. Thus, gaps exist on whether discussions on PA and sleep were more successful than discussions on PA/sedentary behaviour tools or what features of multi-behaviour tools may make them successful or not. In comparison, user perceptions and changes in PA were assessed in many of the PA-only tools, which allowed us to synthesize what features may have influenced their success (e.g., theoretical basis, low time to administer) into our recommendations for future movement behaviour tools in Table 1. Future studies implementing sedentary behaviour and sleep health promotion tools should strive to measure and evaluate changes in sedentary time (i.e., occupational and recreational screen time, non-screen-based sedentary time [169]) and sleep behaviour (i.e., duration, quality, and timing [162]) in adults accessing care to ascertain why sedentary behaviour and sleep tools may or may not influence behaviour change. Finally, tool implementation should also involve a process evaluation to gauge whether tools are being used as intended.
Practice implications
All tools but one [14] focused on the assessment, counselling, prescription and/or referral of PA, which was in line with our expectation that the vast majority of movement behaviour discussion tools would target only PA. Given that the 24HMG [1] were relatively new at the time our searches were run, and that we included studies published since 2000, it is understandable that the integration of PA, sedentary behaviour, and sleep was not reflected in any tool. Unexpectedly, we discovered nine tools that integrated two of the three movement behaviours (see Additional Files 3 and 4). Targeting the integration of PA, sedentary behaviour, and sleep in clinical discussions can present opportunities to broach health promotion discussions for multiple movement behaviours concurrently, augmenting the potential impact of a single discussion. Discussing one movement behaviour can open the door to conversations about how changes in one behaviour can influence changes in the other two behaviours within a 24-h day [2, 3]. For instance, adults can be advised to replace high sedentary time with more PA or more sleep, and both avenues will help the individual achieve more favourable health outcomes [1]. However, a person-centered approach that respects adults’ individual needs and preferences should be used, as movement behaviour discussions will likely be different for people of different ability levels, with or without chronic conditions [170]. Moving forward, researchers could investigate how shared decision making in discussions about PA, sedentary behaviour, and sleep in clinical settings influences the health outcomes of adults accessing care of all abilities and health statuses.
Several tools were accompanied by training and resources [14, 82, 104]. In one study, 45% of adults accessing care found that the take-home printouts for the Patient-centered Assessment and Counselling for Exercise (PACE +) tool helped them change their PA behaviour [82]. Another study found that providers who received a single training session on the PAVS tool were significantly more likely to assess, counsel, and prescribe PA compared to providers who did not receive training [104]. Accompanying training resources, such as user guides or workshops that target knowledge, confidence, and skills may help users digest the content of tools and improve their effectiveness and feasibility in practice. When translating movement behaviour tools into primary care, available PA, sedentary behaviour, and sleep interventions (e.g., community-based programs, workplace interventions, movement/sleep studies) are other resources to consider. While challenging, establishing clear referral pathways to support adults in achieving healthy movement behaviours beyond the primary care discussion will be necessary.
A small number of tools included device-based measures of movement behaviours, such as apps, accelerometers, or pedometers. Increasingly, devices have made movement behaviour data readily available, which may increase adults’ awareness of movement behaviour deficits and interest in interventions to improve them. Ideally, appropriate data management systems should be used or developed to store, and possibly integrate, data across movement behaviours to better inform providers’ movement behaviour advice.
Based on our findings, we offer seven recommendations for future movement behaviour discussion tool development (Table 1) in an effort to close the abovementioned gaps. Following these recommendations is advisable as it may inform the development, refinement, and/or implementation of discussion tools that are well-received by their intended audiences and more effective at guiding discussions that integrate PA, sedentary behaviour, and sleep.
Strengths and limitations
This scoping review contributes to the literature by investigating the characteristics, perceptions, and effectiveness of tools for PA, sedentary behaviour, and sleep discussions in primary care settings. The integrated knowledge translation approach [36, 37] allowed us to engage relevant knowledge partners who were invested in the review topic to increase the applicability and uptake of our findings. Moreover, we used a modified coding framework [50] to categorize our RQ2 outcomes, which we found valuable for capturing a range of perceptions about tools. Future reviews or qualitative research could similarly use this framework to structure their coding of study outcomes or transcripts, respectively. Importantly, this scoping review informed a list of recommendations that researchers and health care providers, including ourselves, can use to guide the development of evidence-based, positively-valued, and effective health promotion tools that integrate all movement behaviours.
Despite our rigorous search strategy, it is possible that some relevant studies were missed. For instance, studies published in languages other than English were excluded; however, a recent study suggested that the results of 59 Cochrane reviews did not significantly change when non-English studies were excluded [171]. Moreover, several English studies were excluded as they reported on tools published in languages other than English, which was one of our exclusion criteria. Nevertheless, this did not limit the comprehensiveness of our scoping review as 135 studies spanning 61 tools were retrieved and synthesized, which is greater in scope than previous reviews [29,30,31]. Finally, our eligible health care provider populations did not include qualified exercise professionals (QEPs; e.g., Kinesiologists), who are another ideal group to promote movement behaviours as it is relevant to their scope of practice and they have the requisite education and training [172]. Future research is warranted to review movement behaviour discussion tools that are used, or intended for use, among QEPs to ascertain whether tool characteristics or the effectiveness of movement behaviour discussions differ in this context compared to clinical settings. In some settings, QEPs may already be integrated within the health care team [173].
Conclusions
Clinical discussion tools have the potential to enhance the promotion of movement behaviours between providers and individuals who access care. In this scoping review, we identified a large number of studies, with 51 focusing on PA, one focusing on sleep, and nine combining two movement behaviours. Many tools were positively perceived and effective at enhancing knowledge of, confidence for, ability in, and frequency of movement behaviour discussions, and most were used or intended for use among physicians and adults without chronic conditions aged 18–64 years. However, to fill remaining gaps in knowledge and practice, tools should be designed to guide discussions of all three movement behaviours in an integrated manner and researchers and providers should consider using our list of seven evidence-based recommendations to inform future tool development and refinement.
The following studies included in this review were not referenced in this manuscript but are referenced in Additional Files 3, 4, 5 and 6: [174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199].
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Notes
The 24-Hour Movement Guidelines recommends that adults ages 18–64 years preserve 7–9 h of good-quality sleep per day, limit sedentary behaviour to ≤ 8 h per day (including ≤ 3 h of recreational screen time per day), obtain ≥ 3 h of light physical activity per day, and achieve ≥ 150 min of moderate-to-vigorous physical activity per week (including ≥ 2 days of strength training). For adults 65 + years, the sleep recommendations are 7–8 h of good quality sleep per day and the physical activity recommendations also include balance training [1].
Abbreviations
- 24HMG:
-
24-Hour Movement Guidelines
- eCHAT:
-
Electronic Case-finding and Help Assessment tool
- EMR:
-
Electronic medical record
- It’s LiFe!:
-
LIfestyle FEedback! Tool
- IWT:
-
Integrated Wellness Tool
- MMAT:
-
Mixed Methods Appraisal Tool
- MVPA:
-
Moderate-to-vigorous physical activity
- PA:
-
Physical activity
- PACE + :
-
Patient-centered Assessment and Counselling for Exercise
- PRISMA-ScR:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews
- QEP:
-
Qualified Exercise Professional
- RADI:
-
Rapid Assessment Disuse Index
- RQ:
-
Research question
References
Ross R, Chaput J-P, Giangregorio LM, et al. Canadian 24-Hour Movement Guidelines for adults aged 18–64 years and adults aged 65 years or older: An integration of physical activity, sedentary behaviour, and sleep. Appl Physiol Nutr Metab. 2020;45:S57–102. https://doi.org/10.1139/apnm-2020-0467.
Rosenberger ME, Fulton JE, Buman MP, Troiano RP, Grandner MA, Buchner DM, et al. The 24-Hour Activity Cycle: A New Paradigm for Physical Activity. Med Sci Sports Exerc. 2019;51:454–64. https://doi.org/10.1249/MSS.0000000000001811.
Tremblay MS, Ross R. How should we move for health? The case for the 24-hour movement paradigm. Can Med Assoc J. 2020;192:E1728–9. https://doi.org/10.1503/cmaj.202345.
Davies MJ, Aroda VR, Collins BS, Gabbay RA, Green J, Maruthur NM, et al. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022;45(11):2753–86.
Statistics Canada. 2021. Household population meeting/not meeting the 2020 Canadian physical activity guidelines. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310082101. Accessed 22 Oct, 2021.
Chastin S, McGregor D, Palarea-Albaladejo J, Diaz KM, Hagströmer M, Hallal PC, et al. Joint association between accelerometry-measured daily combination of time spent in physical activity, sedentary behaviour and sleep and all-cause mortality: A pooled analysis of six prospective cohorts using compositional analysis. British Journal of Sports Medicine; 2021:1–10. https://doi.org/10.1136/bjsports-2020-102345
Stanford FC, Durkin MW, Stallworth JR, Powell CK, Poston MB, Blair SN. Factors that influence physicians’ and medical students’ confidence in counseling patients about physical activity. J Prim Prev. 2014;35:193–201. https://doi.org/10.1007/s10935-014-0345-4.
Wattanapisit A, Tuangratananon T, Thanamee S. Physical activity counseling in primary care and family medicine residency training: A systematic review. BMC Med Educ. 2018;18:1–7. https://doi.org/10.1186/s12909-018-1268-1.
Michas F. 2018. Percentage of Canadians that visited their primary care physician a select number of times per year as of 2018. Available from: https://www.statista.com/statistics/891832/doctors-visits-among-canadians/. Accessed 16 Jun, 2021.
Ashcroft R, McMillan C, Ambrose-Miller W, McKee R, Brown JB. The emerging role of social work in primary health care: A survey of social workers in Ontario family health teams. Heal Soc Work. 2018;43:109–17. https://doi.org/10.1093/hsw/hly003.
Government of Canada. 2012. About primary health care. https://www.canada.ca/en/health-canada/services/primary-health-care/about-primary-health-care.html. Accessed 22 Oct, 2021.
Lobelo F, de Quevedo IG. The Evidence in Support of Physicians and Health Care Providers as Physical Activity Role Models. Am J Lifestyle Med. 2016;10:36–52. https://doi.org/10.1177/1559827613520120.
Tulloch H, Fortier M, Hogg W. Physical activity counseling in primary care: Who has and who should be counseling? Patient Educ Couns. 2006;64:6–20. https://doi.org/10.1016/j.pec.2005.10.010.
Fuller JM, Wong KK, Krass I, Grunstein R, Saini B. Sleep disorders screening, sleep health awareness, and patient follow-up by community pharmacists in Australia. Patient Educ Couns. 2011;83:325–35. https://doi.org/10.1016/j.pec.2011.05.004.
Meaklim H, Jackson ML, Bartlett D, Saini B, Falloon K, Junge M, et al. Sleep education for healthcare providers: Addressing deficient sleep in Australia and New Zealand. Sleep Heal. 2020;6:636–50. https://doi.org/10.1016/j.sleh.2020.01.012.
Dietitians of Canada. Dietitians in primary health care : A pan-Canadian environmental scan. 2018. https://www.dietitians.ca/DietitiansOfCanada/media/Documents/Resources/2018-Executive-Summary-Dietitians-in-Primary-Health-Care-A-Pan-Canadian-Environmental-Scan.pdf. Accessed 8 Oct, 2021.
Kates N, McPherson-Doe C, George L. Integrating mental health services within primary care settings: The Hamilton family health team. J Ambul Care Manage. 2011;34:174–82. https://doi.org/10.1097/JAC.0b013e31820f6435.
BC Centre for Disease Control. 2020. BCCDC COVID-19 Language Guide: Guidelines for inclusive language for written and digital content. http://www.bccdc.ca/Health-Info-Site/Documents/Language-guide.pdf. Accessed 10 Aug, 2021.
Fowles JR, O’Brien MW, Solmundson K, Oh PI, Shields CA. Exercise is medicine Canada physical activity counselling and exercise prescription training improves counselling, prescription, and referral practices among physicians across Canada. Appl Physiol Nutr Metab. 2018;43:535–9. https://doi.org/10.1139/apnm-2017-0763.
McFadden T, Fortier M, Sweet SN, Tomasone JR, McGinn R, Levac BM. Canadian medical students’ perceived motivation, confidence and frequency recommending physical activity. Prev Med Reports; 2019(15):100898; https://doi.org/10.1016/j.pmedr.2019.100898
Baillot A, Baillargeon JP, Pare A, Poder TG, Brown C, Langlois MF. Physical activity assessment and counseling in Quebec family medicine groups Évaluation de l ’ activité physique et counseling dans des groupes de médecine de famille au Québec. Can Fam Physician. 2018;64:234–41 (PMID: 29760272).
Brown CA, Wielandt P, Wilson D, Jones A, Crick K. Healthcare providers’ knowledge of disordered sleep, sleep assessment tools, and nonpharmacological sleep interventions for persons living with dementia: A national survey. Sleep Disord. 2014;2014:1–9. https://doi.org/10.1155/2014/286274.
Hébert ET, Caughy MO, Shuval K. Primary care providers’ perceptions of physical activity counselling in a clinical setting: A systematic review. Br J Sports Med. 2012;46:625–31. https://doi.org/10.1136/bjsports-2011-090734.
Huntington J, Dwyer JJM, Shama S, Brauer P. Registered dietitians’ beliefs and behaviours related to counselling patients on physical activity and sedentary behaviour from a theory of planned behaviour perspective. BMC Nutr. 2020;6:1–13. https://doi.org/10.1186/s40795-020-00392-1.
Huijg JM, Gebhardt WA, Verheijden MW, van der Zouwe N, de Vries JD, Middelkoop BJC, et al. Factors influencing primary health care professionals’ physical activity promotion behaviors: A systematic review. Int J Behav Med. 2015;22:32–50. https://doi.org/10.1007/s12529-014-9398-2.
Frémont P, Fortier M, Frankovich RJ. Exercise prescription and referral tool to facilitate brief advice to adults in primary care. Can Fam Physician. 2014;60:1120 2-e591 2 (PMID: 25500602).
Gans KM, Ross E, Barner CW, Wylie-rosett J, Mcmurray J, Eaton C. REAP and WAVE: New tools to rapidly assess/discuss nutrition. J Nutr. 2003;133:556S-562S (PMID: 35400010393867).
Clark RE, Milligan J, Ashe MC, Faulkner G, Canfield C, Funnell L, et al. A patient-oriented approach to the development of a primary care physical activity screen for embedding into electronic medical records. Appl Physiol Nutr Metab. 2020;8:1–8. https://doi.org/10.1139/apnm-2020-0356.
Golightly YM, Allen KD, Ambrose KR, Stiller JL, Evenson KR, Voisin C, et al. Physical activity as a vital sign: A systematic review. Prev Chronic Dis. 2017;14:E123 (PMID: 29191260).
Smith TO, McKenna MC, Salter C, Hardeman W, Richardson K, Hillsdon M, et al. A systematic review of the physical activity assessment tools used in primary care. Fam Pract. 2017;34:384–91 (PMID: 28334801).
Glasgow RE, Ory MG, Klesges LM, Cifuentes M, Fernald DH, Green LA. Practical and relevant self-report measures of patient health behaviors for primary care research. Ann Fam Med. 2005;3:73–81 (PMID: 15671195).
Nilsen P. Making sense of implementation theories, models and frameworks. Implement Sci. 2015;10:53 (PMID: 25895742).
National Academy of Sciences. 2011. Clinical practice guidelines we can trust - Institute of Medicine. Graham R, Mancher M, Miller Wolman D, Greenfield S, Steinberg E, editors. The National Academies Press. 266 pp. http://www.nap.edu/catalog.php?record_id=13058%0Ahttps://www.awmf.org/fileadmin/user_upload/Leitlinien/International/IOM_CPG_lang_2011.pdf. Accessed 10 Oct, 2021.
Tomasone JR, Flood SM, Latimer-Cheung AE, Faulkner G, Duggan M, Jones R, et al. Knowledge translation of the Canadian 24-Hour Movement Guidelines for Adults aged 18–64 years and Adults aged 65 years or older: A collaborative movement guideline knowledge translation process. Appl Physiol Nutr Metab. 2020;45:S103–24. https://doi.org/10.1139/apnm-2020-0251.
Keown K, Van Eerd D, Irvin E. Stakeholder engagement opportunities in systematic reviews: Knowledge transfer for policy and practice. 2008;28:67–72. doi: https://doi.org/10.1002/chp
Nguyen T, Graham ID, Mrklas KJ, Bowen S, Cargo M, Estabrooks CA, et al. How does integrated knowledge translation (IKT) compare to other collaborative research approaches to generating and translating knowledge? Learning from experts in the field. Heal Res Policy Syst. 2020;18:1–20 (PMID: 32228692).
Kothari A, McCutcheon C, Graham ID. Defining integrated knowledge translation and moving forward: A response to recent commentaries. Int J Heal Policy Manag. 2017;6:299–300. https://doi.org/10.15171/ijhpm.2017.15.
Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18:1–7 (PMID: 30453902).
Peters MD, Godfrey CM, McInerney P, Soares CB, Khalil H, Parker D. 2015. The Joanna Briggs Institute reviewers’ manual 2015: Methodology for JBI scoping reviews. Joanna Briggs Institute. http://joannabriggs.org/assets/docs/sumari/ReviewersManual_Mixed-Methods-Review-Methods-2014-ch1.pdf. Accessed 10 Jun, 2021.
Feldmann J, Puhan MA, Mütsch M. Characteristics of stakeholder involvement in systematic and rapid reviews: A methodological review in the area of health services research. BMJ Open. 2019;9:1–11 (PMID: 31420378).
Pollock A, Campbell P, Struthers C, Synnot A, Nunn J, Hill S, et al. Stakeholder involvement in systematic reviews: A scoping review. Syst Rev. 2018;7:1–26 (PMID: 30474560).
Morgan TL, Faught E, Ross-White A, Fortier MS, Jain R, McFadden T, et al. Tools to guide clinical discussions on physical activity, sedentary behaviour, and / or sleep for health promotion between health care providers and individuals who access care: Protocol for a scoping review. Open Science Framework; 2021. 1–17. Available from: https://osf.io/ztxe9/
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann Intern Med. 2018;169:467–73 (PMID: 30178033).
Mounier-Jack S, Mayhew SH, Mays N. Integrated care: Learning between high-income, and low- and middle-income country health systems. Health Policy Plan. 2017;32:iv6-12 (PMID: 29194541).
Levac D, Colquhoun H, O’Brien KK. Scoping studies: Advancing the methodology. Implement Sci. 2010;5:1–9 (PMID: 20854677).
Harzing AW. Publish or Perish. 2007. https://harzing.com/resources/publish-or-perish. Accessed 16 Jun, 2021.
Linder SK, Kamath GR, Pratt GF, Saraykar SS, Volk RJ. Citation searches are more sensitive than keyword searches to identify studies using specific measurement instruments. J Clin Epidemiol. 2015;68:412–7. https://doi.org/10.1016/j.jclinepi.2014.10.008.
Cooper C, Lovell R, Husk K, Booth A, Garside R. Supplementary search methods were more effective and offered better value than bibliographic database searching: A case study from public health and environmental enhancement. Res Synth Methods. 2017;9:195–223 (PMID: 29193834).
Veritas Health Innovation. Covidence systematic review software. Melbourne, Australia; www.covidence.org.
Neudorf B, Giangregorio L, Morita P. Insights from an usability review of an electronic medical record–integrated physical activity counseling tool for primary care. Ergon Des Q Hum Factors Appl; 2020. https://doi.org/10.1177/1064804620982140
Verbeek J, Ruotsalainen J, Hoving JL. Synthesizing study results in a systematic review. Scand J Work Environ Heal. 2012;38:282–90 (PMID: 22015561).
Sopcak N, Aguilar C, O’Brien MA, Nykiforuk C, Aubrey-Bassler K, Cullen R, et al. Implementation of the BETTER 2 program: A qualitative study exploring barriers and facilitators of a novel way to improve chronic disease prevention and screening in primary care. Implement Sci. 2016;11:158 (PMID: 27906041).
Verwey R, Van Der Weegen S, Spreeuwenberg M, Tange H, Van Der Weijden T, De Witte L. Upgrading physical activity counselling in primary care in the Netherlands. BMC Fam Pract. 2016;31:344–54 (PMID: 25539787).
Heath GW, Kolade VO, Haynes JW. Exercise is MedicineTM: A pilot study linking primary care with community physical activity support. Prev Med Reports. 2015;2:492–7. https://doi.org/10.1016/j.pmedr.2015.06.004.
Hamlin MJ, Yule E, Elliot CA, Stoner L, Kathiravel Y. Long-term effectiveness of the New Zealand Green Prescription primary health care exercise initiative. Public Health. 2016;140:102–8. https://doi.org/10.1016/j.puhe.2016.07.014.
Patel A, Schofield GM, Kolt GS, Keogh JWL. General practitioners’ views and experiences of counselling for physical activity through the New Zealand green prescription program. BMC Fam Pract. 2011;12:119.
Petrella RJ, Koval JJ, Cunningham DA, Paterson DH. Can primary care doctors prescribe exercise to improve fitness? The Step Test Exercise Prescription (STEP) project. Am J Prev Med. 2003;24:316–22 (PMID: 12726869).
Petrella RJ, Lattanzio CN, Shapiro S, Overend T. Improving aerobic fitness in older adults: Effects of a physician-based exercise counseling and prescription program. Can Fam Physician. 2010;56:e191-200 (PMID: 20463260).
Agarwal P, Kithulegoda N, Bouck Z, Bosiak B, Birnbaum I, Reddeman L, et al. Feasibility of an electronic health tool to promote physical activity in primary care: Pilot cluster randomized controlled trial. J Med Internet Res. 2020;22:e15424 (PMID: 32130122).
Petrella RJ, Wight D. An office-based instrument for exercise counseling and prescription in primary care. Arch Fam Med. 2000;9:339–44 (PMID: 32142507).
Knight E, Stuckey MI, Petrella RJ. Health promotion through primary care: Enhancing self-management with activity prescription and mhealth. Phys Sportsmed. 2014;42:90–9 (PMID: 25295771).
Knight E, Stuckey MI, Petrella RJ. Prescribing physical activity through primary care: Does activity intensity matter? Phys Sportsmed. 2014;42:78–9 (PMID: 25419886).
Aittasalo M, Miilunpalo S, Ståhl T, Kukkonen-Harjula K. From innovation to practice: Initiation, implementation and evaluation of a physician-based physical activity promotion programme in Finland. Health Promot Int. 2007;22:19–27 (PMID: 17135327).
Knight E, Petrella RJ. Prescribing physical activity for healthy aging: Longitudinal follow-up and mixed method analysis of a primary care intervention. Phys Sportsmed. 2014;42:30–8 (PMID: 25419886).
Thornton JS, Frémont P, Khan K, Poirier P, Fowles J, Wells GD, et al. Physical activity prescription: a critical opportunity to address a modifiable risk factor for the prevention and management of chronic disease: a position statement by the Canadian Academy of Sport and Exercise Medicine. Br J Sports Med. 2016;50:1109–14. https://doi.org/10.1136/bjsports-2016-096291.
Goodyear-Smith F, Warren J, Elley CR. The eCHAT program to facilitate healthy changes in New Zealand primary care. J Am Board Fam Med. 2013;26:177–82 (PMID: 23471931).
Elley CR, Dawes D, Dawes M, Price M, Goodyear-Smith HDF. Screening for lifestyle and mental health risk factors in the waiting room: Feasibility study of the Case-finding Health Assessment Tool. Can Fam Physician. 2014;60:e527–34 (PMID: 25551137).
Albright CL, Cohen S, Gibbons L, Miller S, Marcus B, Sallis J, et al. Incorporating physical activity advice into primary care: Physician-delivered advice within the activity counseling trial. Am J Prev Med. 2000;18:225–34 (PMID: 10722989).
Simons-Morton DG, Blair SN, King AC, Morgan TM, Applegate WB, O’Toole M, et al. Effects of physical activity counseling in primary care. The Activity Counseling Trial: A randomized controlled trial. J Am Med Assoc. 2001;286:677–87. https://doi.org/10.4082/kjfm.19.0113.
Sciamanna CN, Marcus BH, Goldstein MG, Lawrence K, Swartz S, Bock B, et al. Feasebility of incorporating computer-tailored health behaviour communications in primary care settings. Inform Prim Care. 2004;12:40–8 (PMID: 15140352).
King AC, Marcus B, Ahn D, Dunn AL, Rejeski WJ, Sallis JF, et al. Identifying subgroups that succeed or fail with three levels of physical activity intervention: The activity counseling trial. Heal Psychol. 2006;25:336–47 (PMID: 16719605).
Anderson RT, King A, Stewart AL, Camacho F, Rejeski WJ. Physical activity counseling in primary care and patient well-being: Do patients benefit? Ann Behav Med. 2005;30:146–54 (PMID: 16173911).
Grant RW, Schmittdiel JA, Neugebauer RS, Uratsu CS, Phn RN, Sternfeld B. Exercise as a Vital Sign: A Quasi-Experimental Analysis of a Health System Intervention to Collect Patient-Reported Exercise Levels. J Gen Intern Med. 2013;29:341–8. https://doi.org/10.1007/s11606-013-2693-9.
Liu I-L, Moy M, Estrada E, Rippberger E, Nguyen H. An, “Exercise Vital Sign” is a valid proxy measure of physical activity in COPD in routine clinical care. Transl J Am Coll Sport Med. 2017;2:148. https://doi.org/10.1249/TJX.0000000000000049.
Ryu B, Kim N, Heo E, Yoo S, Lee K, Hwang H, et al. Impact of an electronic health record-integrated personal health record on patient participation in health care: Development and randomized controlled trial of myhealthkeeper. J Med Internet Res. 2017;19:1–12 (PMID: 29217503).
Cupples ME, Cole JA, Hart ND, Heron N, McKinley MC, Tully MA. Shared decision-making (SHARE-D) for healthy behaviour change: A feasibility study in general practice. BJGP Open; 2018;1–12. https://doi.org/10.3399/bjgpopen18X101517
Burr K, Roberson KB, Onsomu EO, Yancu CN, Pritchard R. Evaluating Ten Top Tips (10TT): Brief Dietary and physical activity counseling in rural overweight and obese adults. Fam Community Heal. 2020;43:106–17 (PMID: 32079967).
Bolognesi M, Nigg CR, Massarini M, Lippke S. Reducing obesity indicators through brief physical activity counseling (PACE) in Italian primary care settings. Ann Behav Med. 2006;31:179–85 (PMID: 16542133).
Osunlana AM, Asselin J, Anderson R, Ogunleye AA, Cave A, Sharma AM, et al. 5As Team obesity intervention in primary care: Development and evaluation of shared decision-making weight management tools. Clin Obes. 2015;5:219–25 (PMID: 26129630).
Campbell-Scherer DL, Asselin J, Osunlana AM, Ogunleye AA, Fielding S, Anderson R, et al. Changing provider behaviour to increase nurse visits for obesity in family practice: the 5As Team randomized controlled trial. C Open. 2019;7:E371–8. https://doi.org/10.9778/cmajo.20180165.
Sassano NW. The Food and Physical Activity Habit Inventory: A new tool to change patient behavior. Journal of Allergy and Clinical Immunology. University of Wyoming; 2004; https://doi.org/10.1016/j.jaci.2012.05.050
Prochaska JJ, Zabinski MF, Calfas KJ, Sallis JF, Patrick K. PACE+. Interactive communication technology for behavior change in clinical settings. Am J Prev Med. 2000;19:127–31 (PMID: 10913904).
Asselin J, Salami E, Osunlana AM, Ogunleye AA, Cave A, Johnson JA, et al. Impact of the 5As Team study on clinical practice in primary care obesity management: a qualitative study. C Open. 2017;5:E322–9. https://doi.org/10.9778/cmajo.20160090.
Verwey R, van der Weegen S, Tange H, Spreeuwenberg M, Van Der Weijden T, De Witte L. Get moving: The practice nurse is watching you! A case study of the user-centred design process and testing of a web-based coaching system to stimulate the physical activity of chronically ill patients in primary care. Inform Prim Care. 2012;20:289–98 (PMID: 23890341).
Abu-Saad K, Murad H, Barid R, Olmer L, Ziv A, Younis-Zeidan N, et al. Development and efficacy of an electronic, culturally adapted lifestyle counseling tool for improving diabetes-related dietary knowledge: Randomized controlled trial among ethnic minority adults with type 2 diabetes mellitus. J Med Internet Res. 2019;21:e13674 (PMID: 31621640).
Mishuris RG, Yoder J, Wilson D, Mann D. Integrating data from an online diabetes prevention program into an electronic health record and clinical workflow, a design phase usability study. BMC Med Inform Decis Mak. 2016;16:1–13. https://doi.org/10.1186/s12911-016-0328-x.
Noël G, Luig T, Heatherington M, Campbell-Scherer D. Developing tools to support patients and healthcare providers when in conversation about obesity. Inf Des J. 2018;24:131–50. https://doi.org/10.1075/idj.00004.noe.
Plaete J, De Bourdeaudhuij I, Verloigne M, Crombez G. Acceptability, feasibility and effectiveness of an eHealth behaviour intervention using self-regulation: “MyPlan.” Patient Educ Couns. 2015;98:1617–24. https://doi.org/10.1016/j.pec.2015.07.014.
Pears S, Bijker M, Morton K, Vasconcelos J, Parker RA, Westgate K, et al. A randomised controlled trial of three very brief interventions for physical activity in primary care. BMC Public Health. 2016;16:1–13 (PMID: 27716297).
Matoff-Stepp S. Findings and recommendations from the interim evaluation of the bright futures for women’s health and wellness physical activity and healthy eating tools. Health Promot Pract. 2012;13:55–62 (PMID: 21300858).
Murphy MP, Coke L, Staffileno BA, Robinson JD, Tillotson R. Improving cardiovascular health of underserved populations in the community with Life’s Simple 7. J Am Assoc Nurse Pract. 2015;27:615–23 (PMID: 25776437).
Neubeck L, Coorey G, Peiris D, Mulley J, Heeley E, Hersch F, et al. Development of an integrated e-health tool for people with, or at high risk of, cardiovascular disease: The Consumer Navigation of Electronic Cardiovascular Tools (CONNECT) web application. Int J Med Inform. 2016;96:24–37. https://doi.org/10.1016/j.ijmedinf.2016.01.009.
Faught E, Walters AJ, Latimer-Cheung AE, Faulkner G, Jones R, Duggan M, et al. Optimal messaging of the Canadian 24-Hour Movement Guidelines for Adults aged 18–64 years and Adults aged 65 years and older. Appl Physiol Nutr Metab. 2020;45:S125–50 (PMID: 33054338).
Walters AJ. An exploration and experimental test of a generic messaging approach to the Canadian 24-Hour Movement Guidelines for Adults. Queen’s University; 2020.
Patel A, Kolt GS, Keogh JWL, Schofield GM. The green prescription and older adults: What do general practitioners see as barriers? J Prim Health Care. 2012;4:320–7 (PMID: 23205382).
Mendes R, Silva MN, Silva CS, Marques A, Godinho C, Tomás R, et al. Physical activity promotion tools in the portuguese primary health care: An implementation research. Int J Environ Res Public Health. 2020;17:815 (PMID: 32012974).
Calfas KJ, Sallis JF, Zabinski MF, Wilfley DE, Rupp J, Prochaska JJ, et al. Preliminary evaluation of a multicomponent program for nutrition and physical activity change in primary care: PACE+ for adults. Prev Med (Baltim). 2002;34:153–61. https://doi.org/10.1006/pmed.2001.0964.
Norris SL, Grothaus LC, Buchner DM, Pratt M. Effectiveness of physician-based assessment and counseling for exercise in a staff model HMO. Prev Med (Baltim). 2000;30:513–23 (PMID: 10901494).
Porter S, Vilshanskaya O. Moving patients toward a more active lifestyle: the GP physical activity project in South Eastern Sydney area health service. Heal Promot J Aust. 2002;13:178–83.
Spink KS, Reeder B, Chad K, Wilson K, Nickel D. Examining physician counselling to promote the adoption of physical activity. Can J Public Heal. 2008;99:26–30. https://doi.org/10.1007/978-3-030-01557-2_2.
Gonzalez-Viana A, Violan Fors M, Castell Abat C, Rubinat Masot M, Oliveras L, Garcia-Gil J, et al. Promoting physical activity through primary health care: The case of Catalonia. BMC Public Health. 2018;18:1–17 (PMID: 30075720).
Bertozzi N, Bakken E, Bolognesi M, Castoldi F, Massarini M, Galvani C, et al. Promoting physical activity in overweight and obese patients: counseling in primary care from Italy (Cesena, 2002–2003). Sport Sci Health. 2004;1:25–30 (PMID: 15697004).
Harris MF, Hobbs C, Powell Davies G, Simpson S, Bernard D, Stubbs A. Implementation of a SNAP intervention in two divisions of general practice: A feasibility study. Med J Aust. 2005;183:S54–8 (PMID: 16296953).
Birchfield NR. Implementing the Exercise is MedicineTM solution: A process evaluation conducted in a university-based healthcare system. Arizona State University; 2019.
Sopcak N, Fernandes C, O’Brien MA, Ofosu D, Wong M, Wong T, et al. What is a prevention visit? A qualitative study of a structured approach to prevention and screening – the BETTER WISE project. BMC Fam Pract. 2021;22:1–11 (PMID: 34275453).
Carey S, Silvis M, Potiaumpai M, Schmitz K, Butts J. Assessing readiness and buy-in of clinical stakeholders in implementation of a physical activity vital sign. In: 2021 American Medical Society for Sports Medicine Oral Research Poster Presentations, Volume 31, Number 2. 2021. p. 207.
Hong QN, Pluye P, Fàbregues S, Bartlett G, Boardman F, Cargo M, et al. Mixed Methods Appraisal Tool (MMAT), version 2018. 2018. Registration of copyright (#1148552), Canadian Intellectual Property Office, Industry Canada. Accessed 12 Aug, 2021.
Truong LK, Mosewich AD, Holt CJ, Le CY, Miciak M, Whittaker JL. Psychological, social and contextual factors across recovery stages following a sport-related knee injury: A scoping review. Br J Sports Med. 2020;54:1149–56 (PMID: 32060141).
Dedier JJ, Wright JA, Heeren T, Friedman RH. EMR-based physical activity counseling and referral in primary care: An evaluation study. Proceedings of the 37th Annual Meeting of the Society of General Internal Medicine; 2014; San Diego, California, USA. S85.
Verwey R, van der Weegen S, Spreeuwenberg M, Tange H, van der Weijden T, de Witte L. Process evaluation of physical activity counselling with and without the use of mobile technology: A mixed methods study. Int J Nurs Stud. 2016;53:3–16 (PMID: 26518108).
Foucher-Urcuyo J, Longworth D, Roizen M, Hu B, Rothberg MB. Patient-entered wellness data and tailored electronic recommendations increase preventive care. J Am 5-Board Fam Med. 2017;30:350–61.
Ogunleye A, Osunlana A, Asselin J, Cave A, Sharma AM, Campbell-Scherer DL. The 5As team intervention: Bridging the knowledge gap in obesity management among primary care practitioners. BMC Res Notes. 2015;8:1–13 (PMID: 26695407).
Becker A, Herzberg D, Marsden N, Thomanek S, Jung H, Leonhardt C. A new computer-based counselling system for the promotion of physical activity in patients with chronic diseases-Results from a pilot study. Patient Educ Couns. 2011;83:195–202. https://doi.org/10.1016/j.pec.2010.05.024.
van der Weegen S, Verwey R, Spreeuwenberg M, Tange H, Van Der Weijden T, De Witte L. It’s LiFe! Mobile and web-based monitoring and feedback tool embedded in primary care increases physical activity: A cluster randomized controlled trial. J Med Internet Res. 2015;17:e184 (PMID: 26209025).
Verwey R, van der Weegen S, Spreeuwenberg M, Tange H, van der Weijden T, de Witte L. A pilot study of a tool to stimulate physical activity in patients with COPD or type 2 diabetes in primary care. J Telemed Telecare. 2014;20:29–34 (PMID: 24414397).
Shuval K, DiPietro L, Sugg Skinner C, Barlow CE, Morrow J, Goldsteen R, et al. “Sedentary behaviour counselling”: The next step in lifestyle counselling in primary care; Pilot findings from the Rapid Assessment Disuse Index (RADI) study. Br J Sports Med. 2014;48:1451–5 (PMID: 22976910).
Patel A, Keogh JWL, Kolt GS, Schofield GM. The long-term effects of a primary care physical activity intervention on mental health in low-active, community-dwelling older adults. Aging Ment Heal. 2013;17:766–72 (PMID: 23547971).
Lin JJ, Mann DM. Application of persuasion and health behavior theories for behavior change counseling: Design of the ADAPT (Avoiding Diabetes Thru Action Plan Targeting) program. Patient Educ Couns. 2012;88:460–6. https://doi.org/10.1016/j.pec.2012.06.017.
Katz DL, Shuval K, Comerford BP, Faridi Z, Njike VY. Impact of an educational intervention on internal medicine residents ’ physical activity counselling: The Pressure System Model. J Eval Clin Pract. 2008;14:294–9. https://doi.org/10.1111/j.1365-2753.2007.00853.x.
Mehring M, Haag M, Linde K, Wagenpfeil S, Frensch F, Blome J, et al. Effects of a general practice guided web-based weight reduction program - Results of a cluster-randomized controlled trial. BMC Fam Pract. 2013;14:16 (PMID: 23981507).
Kolt GS, Schofield GM, Kerse N, Garrett N, Ashton T, Patel A. Healthy steps trial: Pedometer-based advice and physical activity for low-active older adults. Ann Fam Med. 2012;10:206–12 (PMID: 22585884).
Elley CR, Garrett S, Rose SB, O’Dea D, Lawton BA, Moyes SA, et al. Cost-effectiveness of exercise on prescription with telephone support among women in general practice over 2 years. Br J Sports Med. 2011;45:1223–9 (PMID: 21081641).
Lawton BA, Rose SB, Elley CR, Dowell AC, Fenton A, Moyes SA. Exercise on prescription for women aged 40–74 recruited through primary care: Two year randomised controlled trial. Br J Sports Med. 2009;43:120–3 (PMID: 19204077).
Kerse N, Elley CR, Robinson E, Arroll B. Is physical activity counseling effective for older people? A cluster randomized, controlled trial in primary care. J Am Geriatr Soc. 2005;53:1951–6 (PMID: 16274377).
Viglione C, Bouwman D, Rahman N, Fang Y, Beasley JM, Sherman S, et al. A technology-assisted health coaching intervention vs. enhanced usual care for Primary Care-Based Obesity Treatment: A randomized controlled trial. BMC Obes. 2019;6:4 (PMID: 30766686).
Mann DM, Palmisano J, Lin JJ. A pilot randomized trial of technology-assisted goal setting to improve physical activity among primary care patients with prediabetes. Prev Med Reports. 2016;4:107–12. https://doi.org/10.1016/j.pmedr.2016.05.012.
ter Bogt NCW, Milder IEJ, Bemelmans WJE, Beltman FW, Broer J, Smit AJ, et al. Changes in lifestyle habits after counselling by nurse practitioners: 1-year results of the Groningen Overweight and Lifestyle study. Public Health Nutr. 2011;14:995–1000 (PMID: 21272417).
ter Bogt NCW, Bemelmans WJE, Beltman FW, Broer J, Smit AJ, van der Meer K. Preventing Weight Gain by Lifestyle Intervention in a General Practice Setting. Arch Intern Med. 2011;171:306–13. https://doi.org/10.1001/archinternmed.2011.22.
Hardeman W, Mitchell J, Pears S, Van Emmenis M, Theil F, Gc VS, et al. Evaluation of a very brief pedometer-based physical activity intervention delivered in NHS Health Checks in England: The VBI randomised controlled trial. PLoS Med. 2020;17:e1003046 (PMID: 32142507).
van Sluijs EMF, van Poppel MNM, Twisk JWR, Chin A Paw MJ, Calfas KJ, Van Mechelen W. Effect of a tailored physical activity intervention delivered in general practice settings: Results of a randomized controlled trial. Am J Public Health. 2005;95:1825–31 (PMID: 16186461).
van Sluijs EMF, van Poppel MNM, Twisk JWR, Brug J, Van Mechelen W. The positive effect on determinants of physical activity of a tailored, general practice-based physical activity intervention. Health Educ Re. 2005;20:345–56 (PMID: 15479705).
Redfern J, Coorey G, Mulley J, Scaria A, Neubeck L, Hafiz N, et al. A digital health intervention for cardiovascular disease management in primary care (CONNECT) randomized controlled trial. NPJ Digit Med; 2020(3). https://doi.org/10.1038/s41746-020-00325-z
Parekh S, King D, Boyle FM, Vandelanotte C. Randomized controlled trial of a computer-tailored multiple health behaviour intervention in general practice: 12-month follow-up results. Int J Behav Nutr Phys Act. 2014;11:1–10 (PMID: 24646165).
Nanchahal K, Townsend J, Letley L, Haslam D, Wellings K, Haines A. Weight-management interventions in primary care: A pilot randomised controlled trial. Br J Gen Pract. 2009;59:349–55. https://doi.org/10.3399/bjgp09X420617.
Aittasalo M, Kukkonen-Harjula K, Toropainen E, Rinne M, Tokola K, Vasankari T. Developing physical activity counselling in primary care through participatory action approach. BMC Fam Pract. 2016;17:1–15 (PMID: 27716068).
Shuval K, Sahar L, Gabriel KP, Knell G, Weinstein G, Gal TG, et al. Sedentary behavior, physical inactivity, and metabolic syndrome: Pilot findings from the rapid assessment disuse index study. J Phys Act Heal. 2020;17:1042–6 (PMID: 32908021).
VanDenToorn ML. Physical activity assessment and intervention among adult patients at a community health center. Grand Valley State University; 2016.
Lv N, Xiao L, Simmons ML, Rosas LG, Chan A, Entwistle M. Personalized hypertension management using patient-generated health data integrated with electronic health records (EMPOWER-H): Six-month pre-post study. J Med Internet Res. 2017;19:e311 (PMID: 28928111).
Barnes AS. Prescribing pedometers in a safety-net health system: Pilot and feasibility study results. Denver: 36th Annual Meeting of the Society of General Internal Medicine; 2013. (PMID: 23625304).
Meriwether RA, McMahon PM, Islam N, Steinmann WC. Physical activity assessment: Validation of a clinical assessment tool. Am J Prev Med. 2006;31:484–91 (PMID: 17169709).
Dalziel K, Segal L, Elley CR. Cost utility analysis of physical activity counselling in general practice. Aust N Z J Public Health. 2006;30:57–63 (PMID: 16502953).
Leung W, Ashton T, Kolt GS, Schofield GM, Garrett N, Kerse N, et al. Cost-effectiveness of pedometer-based versus time-based green prescriptions: the healthy steps study. Aust J Prim Care. 2012;18:204–11 (PMID: 23069363).
Ball TJ, Joy EA, Goh TL, Hannon JC, Gren LH, Shaw JM. Validity of two brief primary care physical activity questionnaires with accelerometry in clinic staff. Prim Health Care Res Dev. 2015;16:100–8 (PMID: 24472569).
Topolski TD, LoGerfo J, Patrick DL, Williams B, Walwick J, Patrick MB. The Rapid Assessment of Physical Activity (RAPA) among older adults. Prev Chronic Dis. 2006;3:1–8 (PMID: 16978493).
Yamane SS, De Gagne JC, Riggs A, Kimberly GD, Holye M. Assessment of a patient-centered initiative to improve hypertension management for adults with comorbid type 2 diabetes at a free clinic in the rural south. Nurs Forum. 2020;55:348–55 (PMID: 32034778).
Patel A, Schofield GM, Kolt GS, Keogh JWL. Perceived barriers, benefits, and motives for physical activity: Two primary-care physical activity prescription programs. J Aging Phys Act. 2013;21:85–99 (PMID: 22832475).
Sinclair KM, Hamlin MJ. Self-reported health benefits in patients recruited into New Zealand’s “Green Prescription” primary health care program. Southeast Asian J Trop Med Public Health. 2007;38:1158–67 (PMID: 18613560).
Ahmad S, Harris T, Limb E, Kerry S, Victor C, Ekelund U, et al. Evaluation of reliability and validity of the General Practice Physical Activity Questionnaire (GPPAQ) in 60–74 year old primary care patients Service organization, utilization, and delivery of care. BMC Fam Pract. 2015;16:1–9 (PMID: 26329981).
Ball TJ, Joy EA, Gren LH, Cunningham R, Shaw JM. Predictive validity of an adult physical activity “Vital Sign” recorded in electronic health records. J Phys Act Heal. 2016;13:403–8 (PMID: 26445164).
Greenwood JLJ, Joy EA, Stanford JB. The physical activity vital sign: A primary care tool to guide counseling for obesity. J Phys Act Heal. 2010;7:571–6 (PMID: 20864751).
Aubrey-Bassler K, Fernandes C, Penney C, Cullen R, Meaney C, Sopcak N, et al. The effectiveness of a proven chronic disease prevention and screening intervention in diverse and remote primary care settings: An implementation study on the BETTER 2 Program. BJGP Open. 2019;3:1–11. https://doi.org/10.3399/bjgpopen19X101656.
Hessler DM, Fisher L, Bowyer V, Dickinson LM, Jortberg BT, Kwan B, et al. Self-management support for chronic disease in primary care: Frequency of patient self-management problems and patient reported priorities, and alignment with ultimate behavior goal selection. BMC Fam Pract. 2019;20:1–10 (PMID: 31464589).
Resnick B, Ory MG, Hora K, Rogers ME, Page P, Chodzko-Zajko W, et al. The Exercise Assessment and Screening for You (EASY) Tool: Application in the Oldest Old Population. Am J Lifestyle Med. 2008;2:432–40 (PMID: 18483443).
Eakin EG, Brown WJ, Marshall AL, Mummery K, Larsen E. Physical activity promotion in primary care: Bridging the gap between research and practice. Am J Prev Med. 2004;27:297–303 (PMID: 15488359).
Kuntz JL, Young DR, Saelens BE, Frank LD, Meenan RT, Dickerson JF, et al. Validity of the Exercise Vital Sign tool to assess physical activity. Am J Prev Med. 2021;60:866–72. https://doi.org/10.1016/j.amepre.2021.01.012.
Carlfjord S, Johansson K, Bendtsen P, Nilsen P, Andersson A. Staff perspectives on the use of a computer-based concept for lifestyle intervention implemented in primary health care. Health Educ J. 2010;69:246–56. https://doi.org/10.1177/0017896910364883.
Flocke SA, Gordon LE, Pomiecko GL. Evaluation of a community health promotion resource for primary care practices. Am J Prev Med. 2006;30:243–51 (PMID: 16476641).
Aittasalo M, Miilunpalo S, Kukkonen-Harjula K, Pasanen M. A randomized intervention of physical activity promotion and patient self-monitoring in primary health care. Prev Med (Baltim). 2006;42:40–6 (PMID: 16297442).
Dickfos M, King D, Parekh S, Boyle FM, Vandelanotte C. General practitioners’ perceptions of and involvement in health behaviour change: can computer-tailored interventions help? Prim Health Care Res Dev. 2015;16:316–21 (PMID: 25391284).
Albert FA, Crowe MJ, Malau-Aduli AEO, Malau-Aduli BS. Physical activity promotion: A systematic review of the perceptions of healthcare professionals. Int J Environ Res Public Health. 2020;17:1–36 (PMID: 32570715).
Minian N, Lingam M, Moineddin R, Thorpe KE, Veldhuizen S, Dragonetti R, et al. Impact of a web-based clinical decision support system to assist practitioners in addressing physical activity and/or healthy eating for smoking cessation treatment: Protocol for a hybrid type i randomized controlled trial. JMIR Res Protoc. 2020;9:1–11. https://doi.org/10.2196/19157.
Chaput JP, Dutil C, Featherstone R, Ross R, Giangregorio L, Saunders TJ, et al. Sleep duration and health in adults: an overview of systematic reviews. Appl Physiol Nutr Metab. 2020;45:S218–31 (PMID: 33054337).
Rains JC. Sleep and migraine: Assessment and treatment of comorbid sleep disorders. Headache. 2018;58:1074–91 (PMID: 30095163).
Peterson MJ, Pieper CF, Sloane R, Crowley GM, Cowper A, Mcconnell ES, et al. Differences between completely physically inactive and low active older men and their responses to an exercise intervention: The Veterans LIFE study. Heal Aging Res. 2017;4:1–12. https://doi.org/10.12715/har.2015.4.36.Differences.
van der Ploeg HP, Hillsdon M. Is sedentary behaviour just physical inactivity by another name? Int J Behav Nutr Phys Act. 2017;14:1–8 (PMID: 29058587).
LeBlanc AG, Berry T, Deshpande S, Duggan M, Faulkner G, Latimer-Cheung AE, et al. Knowledge and awareness of Canadian physical activity and sedentary behaviour guidelines: A synthesis of existing evidence. Appl Physiol Nutr Metab. 2015;40:716–24. https://doi.org/10.1139/apnm-2014-0464.
Morgan TL, Romani C, Ross-White A, Latimer-Cheung AE, Tomasone JR. Dissemination and implementation strategies for physical activity guidelines among adults 1 with disability, chronic conditions, and pregnancy: A systematic scoping review. Accepted: BMC Public Health; 2022.
Tomasone JR, Kauffeldt KD, Morgan TL, Magor KW, Latimer-Cheung AE, Faulkner G, et al. Dissemination and implementation of national physical activity, sedentary behaviour, and/or sleep guidelines among community-dwelling adults aged 18 years and older: a systematic scoping review and suggestions for future reporting and research. Appl Physiol Nutr Metab. 2020;45:S258–83. https://doi.org/10.1139/apnm-2020-0251.
Saunders TJ, McIsaac T, Douillette K, et al. Sedentary behaviour and health in adults: an overview of systematic reviews. Appl Physiol Nutr Metab. 2020;45(10 Suppl 2):S197–217. https://doi.org/10.1139/apnm-2020-0272.
O’Connor A, Tugwell P, Wells G, Elmslie T, Jolly E, Hollingworth G. A decision aid for women considering hormone therapy after menopause: Deci- sion support framework and evaluation. Patient Educ Couns. 1998;33(3):267–79.
Nussbaumer-Streit B, Klerings I, Dobrescu AI, Persad E, Stevens A, Garritty C, et al. Excluding non-English publications from evidence-syntheses did not change conclusions: a meta-epidemiological study. J Clin Epidemiol. 2020;118:42–54. https://doi.org/10.1016/j.jclinepi.2019.10.011.
O’Brien MW, Bray NW, Kivell MJ, Fowles J. A scoping review of exercise referral schemes involving qualified exercise professionals in primary health care. Appl Physiol Nutr Metab. 2021;46:1007–18.
Fortier M, Tulloch H, Hogg W. A good fit: Integrating physical activity counselors into family practice. Can Fam Physician. 2006;52:942–4 (PMID: 17273491).
Carlfjord S, Nilsen P, Leijon M, Andersson A, Johansson K, Bendtsen P. Computerized lifestyle intervention in routine primary health care: Evaluation of usage on provider and responder levels. Patient Educ Couns. 2009;75:238–43 (PMID: 19046844).
Mann DM, Lin JJ. Increasing efficacy of primary care-based counseling for diabetes prevention: Rationale and design of the ADAPT (Avoiding Diabetes Thru Action Plan Targeting) trial. Implement Sci. 2012;7:6 (PMID: 22269066).
Chrimes D, Kitos NR, Kushniruk A, Mann DM. Usability testing of Avoiding Diabetes Thru Action Plan Targeting (ADAPT) decision support for integrating care-based counseling of pre-diabetes in an electronic health record. Int J Med Inform. 2014;83:636–47. https://doi.org/10.1016/j.ijmedinf.2014.05.002.
Manca DP, Campbell-Scherer D, Aubrey-Bassler K, Kandola K, Aguilar C, Baxter J, et al. Developing clinical decision tools to implement chronic disease prevention and screening in primary care: The BETTER 2 program (building on existing tools to improve chronic disease prevention and screening in primary care). Implement Sci. 2015;10:1–10 (PMID: 26238338).
Sopcak N, Aguilar C, Nykiforuk CIJ, O’Brien MA, Aubrey-Bassler K, Cullen RM, et al. Patients’ perspectives on BETTER 2 prevention and screening: Qualitative findings from Newfoundland & Labrador. BJGP Open. 2017;1:1–9. https://doi.org/10.3399/bjgpopen17X101037.
Sturgiss EA, Douglas K. A collaborative process for developing a weight management toolkit for general practitioners in Australia-an intervention development study using the Knowledge To Action framework. Pilot Feasibility Stud. 2016;2:1–8. https://doi.org/10.1186/s40814-016-0060-4.
Carlfjord S, Lindberg M, Andersson A. Staff perceptions of addressing lifestyle in primary health care: A qualitative evaluation 2 years after the introduction of a lifestyle intervention tool. BMC Fam Pract. 2012;13:1–8 (PMID: 23052150).
Coleman KJ, Ngor E, Reynolds K, Quinn VP, Koebnick C, Young DR, et al. Initial validation of an exercise “vital sign” in electronic medical records. Med Sci Sports Exerc. 2012;44:2071–6 (PMID: 22688832).
Lucini D, Pagani M. Exercise prescription to foster health and well-being: A behavioral approach to transform barriers into opportunities. Int J Environ Res Public Health. 2021;18:1–22 (PMID: 33499284).
Jacobs E, Tamayo M, Rosenbauer J, Schulze MB, Kuss O, Rathmann W. Protocol of a cluster randomized trial to investigate the impact of a type 2 diabetes risk prediction model on change in physical activity in primary care 11 Medical and Health Sciences 1117 Public Health and Health Services 11 Medical and Health Sciences. BMC Endocr Disord. 2018;18:1–13 (PMID: 30326888).
Mateo KF, Berner NB, Ricci NL, Seekaew P, Sikerwar S, Tenner C, et al. Development of a 5As-based technology-assisted weight management intervention for veterans in primary care. BMC Health Serv Res. 2018;18:1–14 (PMID: 29378584).
Patel A, Schofield GM, Kolt GS, Keogh JWL. Older adults’ evaluations of the standard and modified pedometer-based green prescription. J Prim Health Care. 2020;12:41–8. https://doi.org/10.1071/HC19007.
Albert JR. What are the experiences of Māori with the green prescription service? Auckland University of Technology; 2020
Foraker RE, Shoben AB, Lopetegui MA, Lai AM, Payne PRO, Kelley M, et al. Assessment of Life’s Simple 7TM in the primary care setting: The Stroke Prevention in Healthcare Delivery EnviRonmEnts (SPHERE) study. Contemp Clin Trials. 2014;38:182–9. https://doi.org/10.1016/j.cct.2014.03.007.
Fransen GAJ, Hiddink GJ, Koelen MA, van Dis SJ, Drenthen AJM, van Binsbergen JJ, et al. The development of a minimal intervention strategy to address overweight and obesity in adult primary care patients in The Netherlands. Fam Pract. 2008;25:112–5 (PMID: 18978011).
Plaete J, Crombez G, DeSmet A, Deveugele M, Verloigne M, De Bourdeaudhuij I. What do general practitioners think about an online self-regulation programme for health promotion? Focus group interviews BMC Fam Pract. 2015;16:1–11 (PMID: 25608851).
Plaete J, De Bourdeaudhuij I, Verloigne M, Oenema A, Crombez G. A self-regulation eHealth intervention to increase healthy behavior through general practice: Protocol and systematic development. JMIR Res Protoc. 2015;4:e141. https://doi.org/10.2196/resprot.4835.
Broström A, Pakpour AH, Ulander M, Nilsen P. Development and psychometric evaluation of the Swedish propensity to achieve healthy lifestyle scale in patients with hypertension. J Clin Nurs. 2018;27:4040–9 (PMID: 29776007).
Ball TJ, Joy EA, Gren LH, Shaw JM. Concurrent validity of a self-reported Physical Activity “Vital Sign” questionnaire with adult primary care patients. Prev Chronic Dis. 2016;13:1–10 (PMID: 26851335).
Laws RA, Chan BC, Williams AM, Davies GP, Jayasinghe UW, Fanaian M, et al. An efficacy trial of brief lifestyle intervention delivered by generalist community nurses (CN SNAP trial). BMC Nurs. 2010;9:4.
Barte JCM, ter Bogt NCW, Beltman FW, van der Meer K, Bemelmans WJE. Process Evaluation of a Lifestyle Intervention in Primary Care: Implementation Issues and the Participants’ Satisfaction of the GOAL Study. Heal Educ Behav. 2012;39:564–73 (PMID: 22020404).
Knight E, Stuckey MI, Petrella RJ. Validation of the step test and exercise prescription tool for adults. Can J Diabetes. 2014;38:164–71. https://doi.org/10.1016/j.jcjd.2014.03.007.
Diaz VA, Mainous AG, Gavin JK, Player MS, Wright RU. Use of a tablet-based risk assessment program to improve health counseling and patient–provider relationships in a federally qualified health center. Am J Med Qual. 2016;31:434–40 (PMID: 25995332).
Saunders JT, Pastors JG. Practical tips on lifestyle management of type 2 diabetes for the busy clinician. Curr Diab Rep. 2008;8:353–60 (PMID: 18778583).
Shuval K, Kohl HW, Bernstein I, Cheng D, Gabriel KP, Barlow CE, et al. Sedentary behaviour and physical inactivity assessment in primary care: The rapid assessment disuse index (RADI) study. Br J Sports Med. 2014;48:250–5 (PMID: 24144532).
Resnick B, Ory MG, Hora K, Rogers ME, Page P, Bolin JN, et al. A proposal for a new screening paradigm and tool called Exercise Assessment and Screening for You (EASY). J Aging Phys Act. 2008;16:215–33.
Acknowledgements
The authors would like to thank Lauren Konikoff and Marlee Konikoff for their contributions to the data extraction and auditing phases of the study and Jensen Pletch for her contributions to data charting.
Funding
This work was funded by the Public Health Agency of Canada (grant number 1920-HQ-000004) and supported by the Canadian Society for Exercise Physiology. The funding body played no role in the design of the study and collection, analysis, interpretation of data, and in writing the manuscript. Working group members received no financial compensation for their involvement in this review.
Author information
Authors and Affiliations
Contributions
TLM wrote the review protocol and original manuscript draft and tabulated the findings. ARW conducted the peer-reviewed literature and grey literature searches. TLM conducted the forward searches. TLM and EF performed title and abstract screening, full-text screening, data extraction, and auditing. MSF, MD, RJ, KNL, AL, KM, TM, and JRT contributed to study conceptualization and interpretation of the findings and read, provided feedback on, and approved the review protocol and final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The opinions and conclusions expressed are the writers’ own and are not those of the Canadian Medical Association. ARW receives consultancy fees from ProQuest LLC. The authors have no other competing interests, financial or other.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
PRISMA-ScR checklist.
Additional file 2:
Search strategy.
Additional file 3:
Physical activity tools (n = 51).
Additional file 4:
Sleep tools (n = 1).
Additional file 5:
Multi-behaviour tools (n = 9).
Additional file 6:
Perceptions and effectiveness outcomes of included discussion tools.
Additional file 7:
Mixed Methods Appraisal Tool (MMAT) Quality Assessment Ratings.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Morgan, T.L., Faught, E., Ross-White, A. et al. Tools to guide clinical discussions on physical activity, sedentary behaviour, and/or sleep for health promotion between primary care providers and adults accessing care: a scoping review. BMC Prim. Care 24, 140 (2023). https://doi.org/10.1186/s12875-023-02091-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12875-023-02091-9