Abstract
Background
The Systemic Immune-Inflammation Index (SII), a novel marker of inflammation based on neutrophil, platelet, and lymphocyte counts, has demonstrated potential prognostic value in patients undergoing percutaneous coronary intervention (PCI). Our aim was to assess the correlation between the SII and major adverse cardiovascular events following percutaneous coronary intervention.
Methods
We searched PubMed, Web of Science, Embase, and The Cochrane Library from inception to November 20, 2023, for cohort studies investigating the association between SII and the occurrence of MACEs after PCI. Statistical analysis was performed using Revman 5.3, with risk ratios (RRs) and 95% confidence intervals (CIs) as relevant parameters.
Results
In our analysis, we incorporated a total of 8 studies involving 11,117 participants. Our findings revealed that a high SII is independently linked to a increased risk of MACEs in PCI patients (RR: 2.08,95%CI: 1.87–2.32, I2 = 42%, p < 0.00001). Additionally, we demonstrated the prognostic value of SII in all-cause mortality, heart failure, and non-fatal myocardial infarction.
Conclusions
Elevated SII may serve as a potential predictor for subsequent occurrence of MACEs in patients undergoing PCI.
Trial registration
Our protocol was registered in PROSPERO (registration number: CRD42024499676).
Similar content being viewed by others
Introduction
Coronary artery atherosclerotic disease is recognized as a primary contributor to illnesses and mortality in the elderly population [1], with a mortality rate constituting around 30% of total deaths [2]. Among them, acute coronary syndrome(ACS) is regarded as the primary subtype of the disease. With the rising burden of ischemic heart disease, percutaneous coronary intervention (PCI) has emerged as a primary therapeutic approach for acute coronary syndrome [3]. Despite the continuous breakthroughs in modern PCI technology, drug-eluting stents, and antiplatelet therapy, many patients still face various cardiovascular complications after undergoing PCI treatment [4, 5] such as cardiogenic shock [6], all-cause mortality [7], non-fatal myocardial infarction [8], non-fatal stroke [9] and repeat revascularization [10], among other adverse cardiovascular events. Such a scenario has the potential to significantly jeopardize the future survival and quality of life of patients. Hence, it is of paramount importance to identify patients actively undergoing PCI treatment, yet still at a heightened risk of adverse cardiovascular events.
Atherosclerosis represents a chronic inflammatory vascular disease with systemic implications [11, 12]. In recent years, Evidence from clinical practice supports the role of the neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as predictors of prognosis in cardiovascular disease. Hu et al. introduced the Systemic Immune-Inflammation Index (SII) in 2014, a comprehensive inflammatory assessment tool calculated as SII = (neutrophil × platelet) / lymphocyte [13]. This index determines the immune and inflammatory status by comprehensively evaluating neutrophil, platelet, and lymphocyte counts obtained from routine complete blood cell analysis. Currently, SII has been confirmed as an independent prognostic factor for various cancers [13,14,15], and research has found that SII also has a good predictive role in cardiovascular diseases [16]. Further studies indicate that, in predicting cardiovascular disease outcomes, SII may have better prognostic value compared to NLR and PLR [17]. Yang et al.'s research revealed an independent association between the SII and the occurrence of major adverse cardiovascular events in patients with Coronary Artery Disease (CAD) following coronary artery intervention [16]. Faysal Saylik et al. found that SII can effectively predict the occurrence of major adverse cardiovascular events (MACEs) in patients with ST-segment elevation myocardial infarction (STEMI) after undergoing PCI treatment [18]. However, there is currently a lack of comprehensive systematic analysis regarding the relationship between SII and MACEs after PCI treatment. Therefore, we conducted a meta-analysis to thoroughly investigate the relationship between SII and MACEs after PCI treatment by integrating current research findings, aiming to provide guidance for future research and clinical practice.
Methods
Search strategy
Adhering to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, our systematic review and meta-analysis were conducted [19]. Our protocol was registered in PROSPERO (registration number: CRD42024499676). Up to November 20, 2023, articles from four English databases (PubMed, Embase, Web of Science, and The Cochrane Library) were retrieved, with language restrictions. using keywords including "systemic immune-inflammation index", "SII", "coronary artery disease", "myocardial infarction", "acute coronary syndrome", "percutaneous coronary intervention", "Percutaneous transluminal coronary angioplasty", "STEMI", "NSTEMI", "PCI", "PTCA", "AMI", "ACS" and "major adverse cardiovascular and cerebrovascular events". Furthermore, manual searches were conducted, involving the examination of reference lists from prior systematic reviews and meta-analyses, to pinpoint relevant articles for in-depth analysis.
Study selection
Independently, two investigators (ZCY and LMH) evaluated the methodological quality of the included studies. If discrepancies were identified, we recorded and negotiated with the third investigators (LL) to resolve the differences. The inclusion criteria for this study were: (1) Study type: retrospective or prospective cohort studies; (2) Study population: patients undergoing PCI; (3) The primary outcome, defined as a composite of cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, repeat revascularization, and heart failure, is MACEs; (4) Secondary outcome measures encompass all-cause mortality, myocardial infarction, non-fatal stroke, heart failure, and repeat revascularization.
Exclusion: (1) Excluded from the analysis were cross-sectional studies, reviews, preclinical investigations, and studies not aligned with the meta-analysis objectives; (2) Animal experiments, conference papers, case reports, and duplicate publications were excluded; (3) Studies that did not provide outcome indicators for MACEs after SII grouping were excluded.
Data extraction and quality assessment
Initially, duplicate articles were excluded, and the remaining retrieved papers underwent independent screening by two researchers. Through the review of titles and abstracts and the application of consistent inclusion and exclusion criteria, articles meeting the criteria underwent a meticulous screening process. Following a thorough full-text analysis, articles with insufficient information in their abstracts were scrutinized. Any discrepancies were resolved through discussions or negotiations, often requiring the input of a third researcher.
The collected data encompassed: (1) Author's name, publication year, and country of origin; (2) Study design characteristics; (3) Patient attributes, encompassing diagnosis, sample size, age, and gender distribution; (4) SII index analysis approach; (5) Duration of follow-up; (6) Outcomes of adverse events. The quality assessment employed the Newcastle–Ottawa Scale (NOS), evaluating cohort study quality based on three criteria: group selection, group comparability, and outcome determination. Scores on the NOS range from 1 to 9 stars. Those with a NOS score of 6 were considered to be of high quality [20].
Statistical analysis
In the statistical analysis, the risk ratio (RR) and its corresponding 95% confidence interval (CI) served as the standard measurements to assess the correlation between SII and the risk of adverse events in PCI patients. For studies analyzing SII as a categorical variable, we extracted data on major adverse cardiovascular events from the highest and lowest SII groups for statistical analysis. To demonstrate the potential independent association between SII and MACEs occurrence rate in PCI patients, we only extracted and combined RR data from the most extensively adjusted multivariate analysis models. To assess heterogeneity among the included cohort studies, we utilized Cochrane's Q test and calculated the I2 statistic [21], Acknowledging significant heterogeneity when I2 > 50%, the synthesis of risk ratio data was performed using a random-effects model. This model was selected for its broader applicability in accommodating potential heterogeneity among the included studies [22]. Sensitivity analysis, systematically excluding one individual study at a time, was conducted to assess result stability [23]. Statistical significance was set at P < 0.05. Assessment of potential publication bias involved a visual examination of funnel plot symmetry and the application of Egger's test [24]. Analysis was performed using RevMan software (version 5.1; Cochrane Collaboration, Oxford, UK).
Results
Study selection and study characteristics
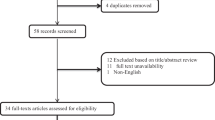
From PubMed, Embase, Web of Science, and The Cochrane database, a total of 604 records were obtained. By manual retrieval, two more articles were added, resulting in a total of 8 studies that met the eligibility criteria for analysis based on inclusion and exclusion criteria [16, 18, 25,26,27,28,29,30]. Figure 1 presents the flowchart outlining the process of study selection and the reasons for exclusion after a full-text examination. Initially, 324 duplicate publications were removed using reference management software (EndNote X7). Subsequently, 152 articles were excluded due to animal experiments, case reports, reviews, or summaries. Then, 57 publications were identified for full-text review. After further screening, 8 cohort studies, including 11,117 participants, were used for subsequent meta-analysis. The participants had an average/median age spanning from 56.93 to 75.47 years. The conducted studies were published in two regions: Turkey and China. The cutoff values for SII were determined using ROC analysis, the Youden index, tertiles, and quartiles. Table 1 offers a comprehensive summary of the characteristics of the included studies. Six studies scored between 7 and 8 on the NOS scale, Signifying a reduced bias risk. Two studies received a score of 6, primarily due to an increased bias risk resulting from insufficient comparability caused by unaddressed confounding factors (Table 2).
Major adverse cardiovascular events
A total of 8 observational studies were included, comprehensively analyzing data from 11,117 participants to determine the relationship between SII and MACEs during follow-up periods ranging from 1 year to 3.1 years. Compared to the lowest SII group, the highest SII group had a significantly higher risk of MACEs after PCI. The summary results of the fixed-effect model showed that the risk of MACEs after PCI in the highest SII group was 2.08 times that of the lowest group (RR: 2.08, 95% CI: 1.87–2.32, I2 = 42%, p < 0.00001) (Fig. 2).
Single adverse event
Three studies reported an association between SII and all-cause mortality (RR: 4.71, 95% CI: 2.75–8.08, I2 = 76%, p < 0.00001) (Fig. 3a). Four studies reported an association between SII and non-fatal myocardial infarction (RR: 1.84, 95% CI: 1.36–2.48, I2 = 51%, p < 0.0001) (Fig. 3b). Three studies reported an association between SII and heart failure (RR: 1.61, 95% CI: 1.39–1.86, I2 = 21%, p < 0.00001) (Fig. 3c). An association between SII and non-fatal stroke was reported in three studies (RR: 2.34, 95% CI: 0.64–8.51, I2 = 93%, p = 0.20) (Fig. 3d). Four studies reported an association between SII and repeat revascularization (RR: 1.19, 95% CI: 0.78–1.83, I2 = 89%, p = 0.41) (Fig. 3e).
Forest plot for the associations between SII and different cardiovascular adverse events in patients with PCI: a Forest plot for the associations between SII and all-cause mortality in patients with PCI: b Forest plot for the associations between SII and non-fatal MI in patients with PCI: c Forest plot for the associations between SII and heart failure in patients with PCI: d Forest plot for the associations between SII and non-fatal stroke in patients with PCI: e Forest plot for the associations between SII and repeat revascularization in patients with PCI
Sensitivity analysis and publication bias
Sensitivity analysis of the main outcome indicators showed that the heterogeneity mainly stemmed from the study by Ya-Ling Yang [16]. After excluding this study, the heterogeneity decreased to 0 (Fig. 4) (RR: 2.35, 95% CI: 2.03–2.73, I2 = 0%, p < 0.00001). Upon analyzing the included literature, we found that the study population in the study by Ya-Ling Yang had a hypertension prevalence of 87%, much higher than in the other included studies. Furthermore, the population selected in this study included stable coronary artery disease patients, which may have led to the occurrence of heterogeneity.
Publication bias
Due to the small number of included studies (n < 10), this study cannot perform publication bias and subgroup analysis according to established guidelines.
Discussion
In this meta-analysis, we included 8 cohort studies, primarily focusing on the relationship between SII and the risk of adverse cardiovascular events after undergoing PCI. The results of the study indicate that patients in the high SII group have a higher risk of experiencing MACEs after undergoing PCI compared to those in the low SII group (RR: 2.08, 95% CI: 1.87–2.32, I2 = 42%, p < 0.00001). Additionally, we also demonstrated the association between high SII and the occurrence of all-cause mortality, heart failure, and non-fatal myocardial infarction after undergoing PCI. The findings of this study suggest that SII can serve as an indicator for identifying high-risk populations after undergoing PCI treatment.
Our study indicates a correlation between high SII and the risk of MACEs after PCI. Although PCI is a therapeutic measure, it further exacerbates the inflammatory response in patients' bodies. Bibek et al. found that the pre-treatment inflammation level in PCI patients is closely related to short-term and long-term complications [31], and SII reflects the level of inflammation in the body to some extent. Initially, SII was used to predict tumor progression and adverse survival outcomes in different types of malignancies [32, 33]. These findings prompted researchers to further explore the role of SII in the cardiovascular field. Ma et al. conducted a large cross-sectional study involving 15,905 patients, and the results showed that higher SII values may be associated with a higher incidence of coronary heart disease [34, 35]. Dziedzic et al. found an association between SII and the incidence rate of acute coronary syndrome [36]. Liu et al. found a positive correlation between SII and the severity of coronary artery stenosis [37]. Lütfi et al.'s study also demonstrated that SII can effectively predict in-hospital and long-term mortality rates in STEMI patients [28]. The above studies may partially explain the potential association between higher SII levels in PCI patients and increased subsequent MACE risk. From a pathophysiological perspective, SII is a new indicator of systemic inflammation based on neutrophil, platelet, and lymphocyte counts. Neutrophils are the most abundant subtype of white blood cells in the circulation. Neutrophils enhance monocyte adhesion and transform into atherosclerotic plaques, releasing myeloperoxidase, NADPH oxidase, lipoxygenase, and neutrophil extracellular traps (NETs), thereby promoting endothelial dysfunction and vascular wall degeneration [38, 39]. Higher platelet counts reflect destructive inflammatory processes in the body [40], and activated platelets promote thrombosis by secreting thromboxane A2 and adenosine diphosphate [41]. Multiple studies have confirmed that increased platelet activity in PCI patients is associated with an increased risk of short-term and long-term MACEs [41,42,43]. CD4 + T lymphocytes belong to the regulatory arm of the immune system, playing a role in controlling immune responses and reducing myocardial damage in vivo [44]. Current research has confirmed that an increased NLR before PCI treatment is an independent predictor of three-year mortality rate and MACEs in patients [45]. Higher PLR has also been proven to be a powerful predictor of adverse cardiovascular events [46,47,48]. Compared to PLR and NLR, SII can more comprehensively and balancedly reflect human immune and inflammatory responses [49]. Erdoğan et al. found that SII is a more predictive inflammatory marker than NLR and PLR [50]. Additionally, Candemir M et al. found that compared to NLR and PLR, SII can better predict the severity of coronary artery lesions [51].
Currently, in clinical practice, Gensini score and SYNTAX score are commonly used to assess the risk of short-term and long-term adverse cardiovascular events in patients undergoing PCI [52, 53]. SII is closely related to the above two scores. Huang et al. found a positive correlation between SII and Gensini score [54]. Demet Ozkaramanli Gur et al. also confirmed a positive correlation between SII and SYNTAX [55]. Some researchers have begun to combine SII with other relevant indicators to enhance its predictive value. For example, results from Wang et al. [56] showed that combining SII with GRACE score can more accurately predict the occurrence of short-term MACEs after PCI in STEMI patients. Additionally, Zhu et al. found that high SII and high CHA2DS2-VASC score are risk factors for CI-AKI, and their combination can improve the accuracy of predicting CI-AKI in ACS patients undergoing PCI [57]. Therefore, in the future, clinicians can develop individualized diagnosis, treatment, and prevention strategies based on the SII value of patients before undergoing PCI, especially for high-risk patients.
There are still some limitations in this study. Firstly, current studies on the association between SII and PCI risk have used different SII cutoff values, so standardization of SII is needed before its widespread use. Secondly, limited by the fact that all included studies were retrospective and single-center, and the number of included studies was small, we were unable to perform publication bias tests, which may lead to inherent clinical heterogeneity. Lastly, the included studies were only conducted in China and Turkey, so caution is needed when applying the results to other regions or populations. Therefore, in the future, we hope for more randomized controlled trials with larger samples from different regions to validate the applicability of our conclusions.
Conclusions
In conclusion, current cohort studies suggest that elevated SII may serve as a potential predictor for subsequent occurrence of MACEs in patients undergoing PCI.
Availability of data and materials
The data used to support the findings of this study are included within the article.
Abbreviations
- SII:
-
Systemic Immune-Inflammation Index
- ACS:
-
Acute coronary syndrome
- PCI:
-
Percutaneous coronary intervention
- NLR:
-
Neutrophil-to-lymphocyte ratio
- PLR:
-
Platelet-to-lymphocyte ratio
- CAD:
-
Coronary Artery Disease
- MACEs:
-
Major adverse cardiovascular events
- NOS:
-
The Newcastle–Ottawa Scale
- RR:
-
Risk ratio
- CI:
-
Confidence interval
- DM:
-
Diabetes
- HTN:
-
Hypertension
- HPL:
-
Hyperlipidemia
- STEMI:
-
STsegment elevation myocardial infarction
- NSTEMI:
-
Non-ST-segment elevation myocardial infarction
References
Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, et al. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67–e492.
Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362(23):2155–65.
Zheng X, Curtis JP, Hu S, Wang Y, Yang Y, Masoudi FA, et al. Coronary catheterization and percutaneous coronary intervention in China: 10-year results from the China PEACE-retrospective CathPCI Study. JAMA Intern Med. 2016;176(4):512–21.
Ren H, Zhao L, Liu Y, Tan Z, Luo G, Deng X. The high-sensitivity C-reactive protein to prealbumin ratio predicts adverse cardiovascular events after ST-Elevation myocardial infarction. Heart Surg Forum. 2021;24(1):E153–7.
Gragnano F, Cao D, Pirondini L, Franzone A, Kim HS, von Scheidt M, et al. P2Y(12) inhibitor or aspirin monotherapy for secondary prevention of coronary events. J Am Coll Cardiol. 2023;82(2):89–105.
Henry TD, Tomey MI, Tamis-Holland JE, Thiele H, Rao SV, Menon V, et al. Invasive management of acute myocardial infarction complicated by cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2021;143(15):e815–29.
Docherty KF, Ferreira JP, Sharma A, Girerd N, Gregson J, Duarte K, et al. Predictors of sudden cardiac death in high-risk patients following a myocardial infarction. Eur J Heart Fail. 2020;22(5):848–55.
Arai R, Okumura Y, Murata N, Fukamachi D, Honda S, Nishihira K, et al. Prevalence and impact of polyvascular disease in patients with acute myocardial infarction in the contemporary era of percutaneous coronary intervention- insights from the Japan Acute Myocardial Infarction Registry (JAMIR). Circ J. 2023. Online ahead of print.
Shimono H, Tokushige A, Kanda D, Ohno A, Hayashi M, Fukuyado M, et al. Association of preoperative clinical frailty and clinical outcomes in elderly patients with stable coronary artery disease after percutaneous coronary intervention. Heart Vessels. 2023;38(10):1205–17.
Jonik S, Kageyama S, Ninomiya K, Onuma Y, Kochman J, Grabowski M, et al. Five-year outcomes in patients with multivessel coronary artery disease undergoing surgery or percutaneous intervention. Sci Rep. 2024;14(1):3218.
Libby P. The changing landscape of atherosclerosis. Nature. 2021;592(7855):524–33.
Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011;17(11):1410–22.
Hu B, Yang X-R, Xu Y, Sun Y-F, Sun C, Guo W, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20(23):6212–22.
Yang R, Chang Q, Meng X, Gao N, Wang W. Prognostic value of Systemic immune-inflammation index in cancer: a meta-analysis. J Cancer. 2018;9(18):3295–302.
Zhong J-H, Huang D-H, Chen Z-Y. Prognostic role of systemic immune-inflammation index in solid tumors: a systematic review and meta-analysis. Oncotarget. 2017;8(43):75381–8.
Yang Y-L, Wu C-H, Hsu P-F, Chen S-C, Huang S-S, Chan WL, et al. Systemic immune-inflammation index (SII) predicted clinical outcome in patients with coronary artery disease. Eur J Clin Invest. 2020;50(5):e13230.
Geng Y, Shao Y, Zhu D, Zheng X, Zhou Q, Zhou W, et al. Systemic immune-inflammation index predicts prognosis of patients with esophageal squamous cell carcinoma: a propensity score-matched analysis. Sci Rep. 2016;6:39482.
Saylik F, Akbulut T. Systemic immune-inflammation index predicts major cardiovascular adverse events in patients with ST-segment elevated myocardial infarction. Arq Bras Cardiol. 2022;119(1):14–22.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
Lo CK-L, Mertz D, Loeb MJBmrm. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14:1–5.
Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58.
Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JPT, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10(10):ED000142.
Patsopoulos NA, Evangelou E, Ioannidis JPA. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol. 2008;37(5):1148–57.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical Research ed). 1997;315(7109):629–34.
Gur DO, Efe MM, Alpsoy S, Akyüz A, Uslu N, Çelikkol A, et al. Systemic immune-inflammatory index as a determinant of atherosclerotic burden and high-risk patients with acute coronary syndromes. Arq Bras Cardiol. 2022;119(3):382–90.
Shi S, Kong S, Ni W, Lu Y, Li J, Huang Y, et al. Association of the systemic immune-inflammation index with outcomes in acute coronary syndrome patients with chronic kidney disease. J Inflamm Res. 2023;16:1343–56.
Demirci G, Şahin AA, Aktemur T, Demir AR, Çetin İ, Hüseyin K, et al. Relationship between systemic immune-inflammation index and no-reflow in percutaneous coronary intervention for saphenous grafts. Biomark Med. 2023;17(8):427–35.
Öcal L, Keskin M, Cerşit S, Eren H, Özgün Çakmak E, Karagöz A, et al. Systemic immune-inflammation index predicts in-hospital and long-term outcomes in patients with ST-segment elevation myocardial infarction. Coron Artery Dis. 2022;33(4):251–60.
Fan W, Zhang Y, Gao X, Liu Y, Shi F, Liu J, et al. The prognostic value of a derived neutrophil-lymphocyte ratio in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Clin Appl Thromb Hemost. 2021;27:10760296211034580.
Wei X, Zhang Z, Wei J, Luo C. Association of systemic immune inflammation index and system inflammation response index with clinical risk of acute myocardial infarction. Front Cardiovasc Med. 2023;10:1248655.
Bibek S-B, Xie Y, Gao J-J, Wang Z, Wang J-F, Geng D-F. Role of pre-procedural C-reactive protein level in the prediction of major adverse cardiac events in patients undergoing percutaneous coronary intervention: a meta-analysisof longitudinal studies. Inflammation. 2015;38(1):159–69.
Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20(23):6212–22.
Yang R, Chang Q, Meng X, Gao N, Wang W. Prognostic value of systemic immune-inflammation index in cancer: a meta-analysis. J Cancer. 2018;9(18):3295–302.
Ma J, Li K. Systemic immune-inflammation index is associated with coronary heart disease: a cross-sectional study of NHANES 2009–2018. Front Cardiovasc Med. 2023;10:1199433.
Xing Q, Zhao X, Xie L, Chen X, Wang Y, Xie Y. Advances in non-pharmacological management of Parkinson's disease complicated with blood pressure abnormalities. J Physiol Pharmacol. 2023;74(4):369–75
Dziedzic EA, Gąsior JS, Tuzimek A, Paleczny J, Junka A, Dąbrowski M, et al. Investigation of the associations of novel inflammatory biomarkers-Systemic Inflammatory Index (SII) and Systemic Inflammatory Response Index (SIRI)-with the severity of coronary artery disease and acute coronary syndrome occurrence. Int J Mol Sci. 2022;23(17):9553.
Liu Y, Ye T, Chen L, Jin T, Sheng Y, Wu G, et al. Systemic immune-inflammation index predicts the severity of coronary stenosis in patients with coronary heart disease. Coron Artery Dis. 2021;32(8):715–20.
Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339(6116):161–6.
Döring Y, Soehnlein O, Weber C. Neutrophil extracellular traps in atherosclerosis and atherothrombosis. Circ Res. 2017;120(4):736–43.
Li L, Ma Y, Geng X-B, Tan Z, Wang J-H, Cui C, et al. Platelet-to-lymphocyte ratio relates to poor prognosis in elderly patients with acute myocardial infarction. Aging Clin Exp Res. 2021;33(3):619–24.
Alexopoulos D, Xenogiannis I, Vlachakis P, Tantry U, Gurbel PA. Peri-procedural platelet reactivity in percutaneous coronary intervention. Thromb Haemost. 2018;118(7):1131–40.
Parodi G, Marcucci R, Valenti R, Gori AM, Migliorini A, Giusti B, et al. High residual platelet reactivity after clopidogrel loading and long-term cardiovascular events among patients with acute coronary syndromes undergoing PCI. JAMA. 2011;306(11):1215–23.
Price MJ, Angiolillo DJ, Teirstein PS, Lillie E, Manoukian SV, Berger PB, et al. Platelet reactivity and cardiovascular outcomes after percutaneous coronary intervention: a time-dependent analysis of the Gauging Responsiveness with a VerifyNow P2Y12 assay: Impact on Thrombosis and Safety (GRAVITAS) trial. Circulation. 2011;124(10):1132–7.
Chen C, Cong BL, Wang M, Abdullah M, Wang XL, Zhang YH, et al. Neutrophil to lymphocyte ratio as a predictor of myocardial damage and cardiac dysfunction in acute coronary syndrome patients. Integr Med Res. 2018;7(2):192–9.
Sen N, Afsar B, Ozcan F, Buyukkaya E, Isleyen A, Akcay AB, et al. The neutrophil to lymphocyte ratio was associated with impaired myocardial perfusion and long term adverse outcome in patients with ST-elevated myocardial infarction undergoing primary coronary intervention. Atherosclerosis. 2013;228(1):203–10.
Park J-S, Seo K-W, Choi B-J, Choi S-Y, Yoon M-H, Hwang G-S, et al. Importance of prognostic value of neutrophil to lymphocyte ratio in patients with ST-elevation myocardial infarction. Medicine. 2018;97(48): e13471.
Tamhane UU, Aneja S, Montgomery D, Rogers E-K, Eagle KA, Gurm HS. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol. 2008;102(6):653–7.
Kurtul A, Yarlioglues M, Murat SN, Ergun G, Duran M, Kasapkara HA, et al. Usefulness of the platelet-to-lymphocyte ratio in predicting angiographic reflow after primary percutaneous coronary intervention in patients with acute ST-segment elevation myocardial infarction. Am J Cardiol. 2014;114(3):342–7.
Fest J, Ruiter R, Ikram MA, Voortman T, van Eijck CHJ, Stricker BH. Reference values for white blood-cell-based inflammatory markers in the Rotterdam Study: a population-based prospective cohort study. Sci Rep. 2018;8(1):10566.
Erdoğan M, Erdöl MA, Öztürk S, Durmaz T. Systemic immune-inflammation index is a novel marker to predict functionally significant coronary artery stenosis. Biomark Med. 2020;14(16):1553–61.
Candemir M, Kiziltunç E, Nurkoç S, Şahinarslan A. Relationship between Systemic Immune-Inflammation Index (SII) and the severity of stable coronary artery disease. Angiology. 2021;72(6):575–81.
Wang K-Y, Zheng Y-Y, Wu T-T, Ma Y-T, Xie X. Predictive value of Gensini score in the long-term outcomes of patients with coronary artery disease who underwent PCI. Front Cardiovasc Med. 2022;8:778615.
Windecker S, Kolh P, Alfonso F, Collet J-P, Cremer J, Falk V, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization. Kardiol Pol. 2014;72(12):1253–379.
Huang J, Zhang Q, Wang R, Ji H, Chen Y, Quan X, et al. Systemic immune-inflammatory index predicts clinical outcomes for elderly patients with acute myocardial infarction receiving percutaneous coronary intervention. Med Sci Monit. 2019;25:9690–701.
Gur DO, Efe MM, Alpsoy S, Akyüz A, Uslu N, Çelikkol A, et al. Systemic immune-inflammatory index as a determinant of atherosclerotic burden and high-risk patients with acute coronary syndromes. Arq Bras Cardiol. 2022;119(3):382–90.
Wang J, Zhang F, Liu L, Gao M, Song X, Li Y, et al. Prognostic value of GRACE risk score combined with systemic immune-inflammation index in patients with ST-segment elevation myocardial infarction after percutaneous coronary intervention. Angiology. 2023:33197231213674. Online ahead of print.
Zhu Y, Qiu H, Wang Z, Shen G, Li W. Predictive value of systemic immune-inflammatory index combined with CHA2DS2-VASC score for contrast-induced acute kidney injury in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Int Urol Nephrol. 2023;55(11):2897–903.
Acknowledgements
Not applicable.
Funding
This research was funded by grants from Sichuan Science and Technology Program (2022YFS0610), Luzhou Municipal People's Government—Southwest Medical University Science and Technology Strategic Cooperation (2021LZXNYD-J33), Hejiang People's Hospital—Southwest Medical University Science and Technology Strategic Cooperation Project (2021HJXNYD13) and Gulin County People's Hospital—Affiliated Hospital of Southwest Medical University Science and Technology strategic Cooperation (2022GLXNYDFY13) and 2022-N-01–33 project of China International Medical Foundation.
Author information
Authors and Affiliations
Contributions
Minghao Li, Ling Liu, and Yi Zhong conducted the initial literature search. Xie Yulei and Li Deng created the figures. The manuscript was prepared by Chunyu Zhang. The manuscript was edited by Bin Liao, and revised by Jian Feng and Lu Yu. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This review was conducted according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines. The study was registered with PROSPERO under registration number CRD42024499676.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, C., Li, M., Liu, L. et al. Systemic immune-inflammation index as a novel predictor of major adverse cardiovascular events in patients undergoing percutaneous coronary intervention: a meta-analysis of cohort studies. BMC Cardiovasc Disord 24, 189 (2024). https://doi.org/10.1186/s12872-024-03849-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-024-03849-4