Abstract
Aim
To evaluate the expression profile of long non-coding RNAs (lncRNAs) in calcific aortic valve disease (CAVD) and explore their potential mechanism of action.
Methods
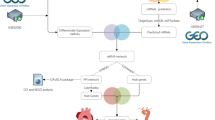
The gene expression profiles (GSE153555, GSE148219, GSE199718) were downloaded from the Gene Expression Omnibus (GEO) database and FastQC was run for quality control checks. After filtering and classifying candidate lncRNAs by differentially expressed genes (DEGs) and weighted co-expression networks (WGCNA) in GSE153555, we predicted the potential cis- or trans-regulatory target genes of differentially expressed lncRNAs (DELs) by using FEELnc and established the competitive endogenous RNA (ceRNA) network by miRanda, more over functional enrichment was analyzed using the ClusterProfiler package in R Bioconductor. The hub cis- or trans-regulatory genes were verified in GSE148219 and GSE199718 respectively.
Results
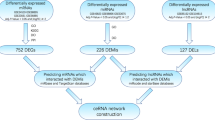
There were 340 up-regulated lncRNAs identified in AS group compared with the control group (|log2Fold Change| ≥ 1.0 and Padj ≤ 0.05), and 460 down-regulated lncRNAs. Based on target gene prediction and co-expression network construction, twelve Long non-coding RNAs (CDKN2B-AS1, AC244453.2, APCDD1L-DT, SLC12A5-AS1, TGFB3, AC243829.4, MIR4435-2HG, FAM225A, BHLHE40-AS1, LINC01614, AL356417.2, LINC01150) were identified as the hub cis- or trans-regulatory genes in the pathogenesis of CAVD which were validated in GSE148219 and GSE19971. Additionally, we found that MIR4435-2HG was the top hub trans-acting lncRNA which also plays a crucial role by ceRNA pattern.
Conclusion
LncRNAs may play an important role in CAVD and may provide a new perspective on the pathogenesis, diagnosis, and treatment of this disease. Further studies are required to illuminate the underlying mechanisms and provide potential therapeutic targets.
Similar content being viewed by others
Introduction
Calcific aortic valve disease (CAVD) is the most common cause of aortic stenosis (AS) and has become an increasing economic and health burden for human populations [1]. Although previous views believed that CAVD was a passive calcium deposition process, recent studies have found that it is an actively regulated process that involves valve endothelial disruption, lipid infiltration, immune cell infiltration, extracellular matrix remodeling, apoptosis, and deranged phospho-calcium metabolism [2]. However, the pathophysiological process of CAVD remains unclear and the treatment strategies for CAVD mainly rely on surgery or transcatheter aortic valve replacement (TAVR). Thus, identification of key genes and pathways is crucial for exploring the molecular mechanisms of CAVD, to find molecular targets for early diagnosis, prevention, and specific treatment of AS.
Non-coding RNAs (ncRNAs), which constitute nearly 98% of the human genome, have always been regarded as junk RNA but were confirmed to participate in various pathophysiological processes [3]. Based on their length, ncRNA can be classified as small ncRNAs and long ncRNAs. LncRNAs are ncRNAs longer than 200 nt which participate in cellular processes at epigenetic, transcriptional, post-transcriptional levels and other modes of gene regulation via chromatin modification、cis- or trans-regulation [4] or competitive endogenous RNAs [5]. Evidence suggests that dysregulated lncRNAs play a vital role in cardiovascular diseases, such as atherosclerosis, myocardial infarction, cardiac fibrosis [6], and CAVD [7,8,9]. However, few studies have focused on the cis- or trans-regulation and ceRNAs regulation of lncRNAs in CAVD.
With the advancements of gene chips, high-throughput sequencing and single-cell sequencing, bioinformatics techniques have become instrumental in studying diseases at the molecular level. In contrast to traditional methods to identify DEGs, the weighted co-expression networks (WGCNA) [10] are used to construct different modules by describing transcriptome expression patterns, which can also elucidate the correlation of mRNA and lncRNA. Multiple bioinformatics methods can provide key genes and pathways in the process of calcification, which will help discover new directions for interventions and therapeutic targets for CAVD.
In this study, DEGs and DELs were first screened based on GSE153555 and the results were validated in GSE148219 and GSE199718. Subsequently, we predicted the potential cis- and trans-regulatory target genes of DELs by using FEELnc software and established the ceRNA network by miRanda. Additionally, integrated bioinformatics analyses, including Principal component analysis (PCA), Weighted correlation network analysis, Gene Ontology term analysis and ROC curve analysis were performed by R version 4.1.3.
Results
Overview of long noncoding RNAs expressed in aortic valve stenosis
To investigate the transcriptome pattern in aortic valves of healthy and aortic stenosis (AS) patients, RNA-seq data were downloaded from GSE153555 [11]. To validate the expression and diagnostic values of hub cis- or trans-regulatory genes in the pathogenesis of CAVD, RNA-seq data of GSE148219 and GSE199718 were downloaded. The sample characteristics of the above datasets are presented in Table 1. In GSE153555, by performing in silico prediction of lncRNAs, we identified 27,960 lncRNA transcripts of 11,996 lncRNA genes. By comparison with GENCODE annotation version 31, we detected 4926 novel lncRNA transcripts, and the average transcript length of the novel lncRNAs (1774 nt) was similar to that of known lncRNAs (1814 nt) and shorter than that of protein-coding genes (2275 nt) (Fig. 1A). At the exonic level, although novel lncRNAs (at an average of 3.72 per transcript) have similar counts of exons to known lncRNAs (at an average of 3.48 per transcript), less than protein-coding genes (at an average of 9.03 per transcript) (Fig. 1B), the average exon length of novel lncRNAs (503 nt) and known lncRNAs (388 nt) were longer than protein-coding genes (252 nt) (Fig. 1C). In the normalized read count expression level (log2TPM), the average values of known lncRNAs, novel lncRNAs, and protein-coding genes were 0.593, 1.35, and 2.09, respectively (Fig. 1D).
Divergent expression patterns of protein-coding genes and lncRNA genes
Principal component analysis (PCA) showed obvious discrimination between the AS and normal groups in both protein-coding genes (Fig. 2A) and lncRNAs (Fig. 2B). By performing differential gene expression analyses for mRNA and lncRNA individually, a total of 1904 protein-coding genes and 800 lncRNA genes were detected to be differentially expressed (|log2Fold Change| ≥ 1.0 and padj ≤ 0.05), with 1119 upregulated, 785 downregulated protein-coding genes (Fig. 2C) and 340 upregulated, 460 downregulated lncRNA genes (Fig. 2D) in the AS group compared with the control group (Table 2, Table S1, Table S2).
To further clarify the functional differences of protein-coding genes between the two groups, we performed Gene Ontology (GO) enrichment analysis. The top enrichment terms of cellular component (CC) ranked by padj showed that the products of both the activated genes and suppressed genes were associated with the extracellular matrix (Fig. 2E, F). The top enrichment terms of Biological Process (BP) for activated genes contribute to extracellular matrix modulation and immune response, and the suppressed genes lead to metabolic dysregulation (Fig. 2E, F), which were reported in previous research [11, 12].
Weighted correlation network analysis showed the key module associated with AS
To identify the hub mRNAs and lncRNAs associated with AS, we constructed an unsigned weighted correlation network analysis (WGCNA) network. Hierarchical clustering analysis was conducted based on unsigned weighted correlation before segmenting according to to set criteria to obtain gene modules and merged modules too close as measured by the correlation of their eigengenes (Fig. 3A). A total of 25 modules were detected, with an average size of 936.4 genes (including protein-coding and lncRNA genes), ranging from 65 to 5,287 genes, whose relationship is shown in a heatmap (Fig. 3B).
By calculating the gene significance (GS) associated with the disease and control groups, the midnightblue module (including 1,194 genes, with 891 protein-coding genes and 303 lncRNA genes) showed the highest correlation to AS (Fig. 3C), which was considered the key functional module.
Considering the expression status of genes in each module, the midnightblue module contained 22.3% of all DEGs (424 in 1904), which was ranked as the highest proportion among all the modules, and was 47.6% of protein-coding genes in the midnightblue module, which was ranked second among all modules (Table 3), indicating that the midnightblue module reflected the expression difference between the two modules. Furthermore, we performed GO enrichment of the midnightblue module and found a strong association with extracellular matrix organization (Fig. 3D), which was similar to GO enrichment of upregulated DEGs (Fig. 2E), indicating the functional representation of the midnightblue module and establishing the importance of the midnightblue module.
Cis-regulation functions of lncRNAs in the key module associated with AS
By analyzing genes in the midnightblue module, we found that 24.1% of lncRNAs were differentially expressed among all 303 lncRNA genes, which was the highest proportion among the modules, and was 9.1% of all DELs (Table 3), which was ranked third among the modules, showing the potential important regulatory function of lncRNAs in this module. Previous studies have shown that lncRNAs can regulate the expression of target genes and participate in functional regulation by cis- or trans-regulation [4].
First, we predicted the potential cis-regulatory target genes of DELs by using FEELnc software, which was used to detect coding genes adjacent to candidate lncRNAs. We obtained 38,364 pairs of colocalized lncRNA genes with the best match protein-coding genes, which included 23 protein-coding genes and 23 lncRNA genes in the midnightblue module. Nine pairs of mRNA‒lncRNA were also detected to be differentially expressed, which were the key cis- regulatory lncRNAs and mRNAs (key cis- lncRNA and cis- mRNA) (Table 4). In particular, the key cis- mRNAs MMP9, CCL3, and TGFB3 are involved in ossification (Fig. 4A). MMP9, which plays a certain role in cardiovascular remodeling [13], is cis- regulated by its antisense lncRNA SLC12A5-AS1. TGFB3, which can drive fibrotic disease pathogenesis [14], is positively regulated by its lncRNA isoforms. CCL3 is involved in inflammation and ossification and is regulated by AC243829.4.
Cis- and trans-regulatory functions of lncRNAs in the midnightblue module
(A) Heat plot of GO enrichment of cis-mRNA in midnightblue module. The black cells in the heat map represent the GO terms that were enriched by cis-mRNAs, while the white cells represent the GO terms that were not enriched by the cis-mRNAs; (B) number of trans-regulated protein-coding genes by lncRNAs in midnightblue module; (C) enrichment score (ES) of GO enrichment of mRNAs trans-regulated by lncRNAs; (D) association of lncRNAs, their coexpressed mRNAs, and GO enrichment terms. Width of edges between GO terms and lncRNAs related to the number of potential mRNAs trans-regulated by lncRNAs enriched in GO terms
Trans-regulation functions of lncRNAs in the key module associated with AS
In addition, lncRNAs can influence the expression of target genes by trans-regulation. By performing coexpression analysis, we detected 192,291 pairs of highly coexpressed mRNA‒lncRNA genes (Pearson’s r > 0.9 and p < 0.01), including 1,163 pairs consisting of 397 mRNAs and 58 lncRNAs in the midnightblue module. Of the 58 coexpressed lncRNAs, several lncRNAs were coexpressed with quantities of protein-coding genes, with MIR4435-2HG having the most numerous coexpressed protein-coding genes at 115 (Fig. 4B). The lncRNAs with the top 5 degrees were considered hub lncRNA genes in the midnightblue module. To further investigate the trans-regulatory functions of those hub-lncRNAs, we performed GO enrichment for their trans-acting protein-coding genes. Hub trans-regulatory genes besides LINC01614 showed a strong relation with extracellular matrix organization (Fig. 4C, D, Supplementary Figure S1) which may work through trans-regulate COL3A1, MMP9, OLFML2B, COL27A1, NPNT etc.. Among lncRNAs associated with extracellular matrix organization, FAM225A, BHLHE40-AS1, and AL356417.2 showed a stronger relationship with ossification and cartilage development, which may lead to calcification by trans-regulate BMP3, RUNX2, CCN1, MMP13, CCN3 etc.. It is noteworthy that MIR4435-2HG may trans-regulate SERPINH1, COL1A1, COL1A2, P3H4, ADAMTS14 etc. to regulate collagen metabolism in CAVD. Trans-acting protein-coding genes of LINC01614 showed less relation to the extracellular matrix but a stronger association with leukocyte proliferation (BCL6, CD28, CD38, IL34, BTN3A1 etc.) and cell junction assembly (TBX5, ICAM5, IRX3, AGRN, NEGR1 etc.) than other genes (Fig. 4C, D, Supplementary Figure S1).
ceRNA network of lncRNAs in the key module associated with AS
Interestingly, we found that MIR4435-2HG, which was the host gene of MIR4435 and was reported to be a key regulatory lncRNA in multiple diseases through the molecular mechanism of competitive endogenous RNA networks [15, 16], was the top hub trans-acting lncRNA, indicating the potential roles of lncRNA‒miRNA interactions in AS pathophysiology. To reveal the ceRNA in AS, we predicted the targets of all human miRNAs at mRNAs and lncRNAs by miRanda, and the mRNAs-lncRNA pairs that had at least 1 common miRNA binding site and were also detected to be highly coexpressed were considered potential ceRNA mRNA‒lncRNA pairs. Finally, we constructed a ceRNA network consisting of 1217 DEGs, 273 DELs, and 2628 miRNAs, including 230 protein-coding genes and 32 lncRNA genes in the midnightblue module. Among all lncRNAs in the midnightblue module, AL589743.7 had the largest number of miRNA binding sites with 1412 miRNAs, while MIR4435-2HG (with 1412 miRNAs), CYTOR (with 1214 miRNAs), FAM225A (with 713 miRNAs), and BHLHE40-AS1 (with 591 miRNAs) also ranked at the top 5 in midnightblue module. Furthermore, FAM225A can regulate 77 mRNAs through the ceRNA mechanism, while MIR4435-2HG, LINC01614, BHLHE40-AS1, and AL356417.2 can regulate the expression of 70, 68, 63, 61 mRNAs in midnightblue module. Combining the relationship between lncRNAs and miRNAs with lncRNAs with mRNAs, MIR4435-2HG had 9,268 potential lncRNA‒miRNA-mRNA axes, ranking the highest in the midnightblue module. To clarify the potential mechanism through miRNA, we performed GO analysis of potential ceRNA target mRNAs of MIR4435-2HG, FAM225A, and BHLHE40-AS1. While MIR4435-2HG showed a relationship with odontogenesis and endocytic vesicles, which are associated with ossification, FAM225A and BHLHE40-AS1 both showed a relationship with collagen-associated extracellular matrix and structural constituents conferring tensile strength (Fig. 5).
ROC curves analysis of hub genes
We constructed ROC curves of each hub cis- or trans-regulatory genes separately and found that their area under ROC curves (AUC) of CDKN2B-AS1, AC244453.2, APCDD1L-DT, SLC12A5-AS1, TGFB3, AC243829.4, MIR4435-2HG, FAM225A, BHLHE40-AS1, LINC01614, AL356417.2, LINC01150 were all higher than 0.7 in GSE148219 (Fig. 6A, Figure S2). In GSE199718, AUC were higher than 0.7 respectively (Fig. 6B, Figure S2), expect for LINC01150 (AUC = 0.575) and CDKN2B-AS1 (AUC = 0.625). These indicate that these twelve genes have a good ability to discriminate between calcified and normal valves.
Materials and methods
Genome-wide RNA-seq data of mRNAs and lncRNAs in AS
The RNA expression dataset of GSE153555 was derived from the study of Greene CL et al. [11], which collected 210.6 Gb data from 10 samples of AS and 10 control samples. Transcriptome sequencing data for GSE199718 and GSE148219 were derived from the study of Cheng S et al. [17]and MacGrogan D et al. [18]. To ensure the reliability of raw reads and suitability for downstream analysis, FastQC was run for quality control checks [19], and the sequences of poor quality were trimmed and filtered by trim_galore 0.6.6 [20] to obtain clean reads. The resulting reads were mapped against the reference genome GRCh38 downloaded from GENCODE by STAR version 2.7.6a [21].
Bioinformatics identification of lncRNAs
Transcripts of 20 samples were assembled individually by StringTie v2.2.1 [22], and then transcripts of all 20 samples were combined by the merge parameter of StringTie into a nonredundant transcript set. Candidate lncRNA transcripts were filtered and classified by the FEElnc [23] pipeline to obtain the high-confidence set of lncRNA transcripts. The high-confidence set of lncRNA transcripts was compared with GENCODE version 31 by gffcompare v0.11.2 [24], and transcripts with class codes of “i”, “j”, “o”, “u”, and “x” were considered as novo transcripts.
Differential expression analysis of mRNAs and lncRNAs
mRNA and lncRNA abundance was quantified using StringTie [22]. The TPM matrix generated by StringTie was used to summarize the lncRNA and mRNA expression levels. Gene differential expression analysis was performed by DESeq2 v1.34.0 [25] in R/Bioconductor (R version 4.1.0). Gene ontology enrichment analysis was performed by clusterProfiler v4.2.0 [26] using the database package org.Hs.eg.db v3.14.0.
WGCNA
The unsigned WGCNA network was constructed by WGCNA v1.71 [10] using an experienced soft power of 6 to obtain gene modules. Modules too close as measured by the correlation of their eigengenes were merged using a cutoff height of 0.20. Gene significances were calculated for the trait of the AS group compared to the healthy control, and the module that had the highest average GS of genes was identified as the key module associated with AS.
Cis- and trans -regulation analysis
FEELnc [23] was used to search for the coding genes near the confirmed lncRNAs (upstream and downstream 10–100 kb), taking the parameter “isBEST = 1” for cis-regulation analysis. Hmisc 4.7-0 was used to calculate the Pearson’s correlation r of lncRNAs and mRNAs, and mRNAs with |r| > 0.9 and p value < 0.01 were viewed as coexpressed with their lncRNAs.
Prediction of miRNA targets
miRNA sequences were retrieved from microrna.org. Miranda v3.3a [27] was used to predict the binding sites of miRNA against the full-length lncRNAs and 3’UTR of mRNAs. The lncRNA-mRNA pairs were predicted to have binding sites for the same miRNA and also to be coexpressed were considered potential ceRNA pairs.
Data visualization
All visualizations were performed in R version 4.1.3. Graphs were plotted using the ggplot2 v3.3.5 package [28] or the preimplemented function “plot” unless otherwise noted. Heatmap was drawn using the pheatmap v1.0.12 package (https://cran.r-project.org/web/packages/ pheatmap/index.html). Network graph was drawn using the igraph v1.3.1 (https://cran.r-project.org/web/packages/igraph/citation.html), tidygraph v1.2.0 (https://cran.r-project.org/web/packages/tidygraph/index.html), and ggraph v2.0.5 packages (https://cran.r-project.org/web/packages/ggraph/index.html).
Discussion
CAVD is the most common valvular heart disease that frequently leads to aortic stenosis and heart failure in developed countries. Up to now, the etiology and pathogenesis of CAVD are still undetermined. In recent years, notable progress can be observed in miRNA-based therapies. Nevertheless, the complex regulatory mechanisms restrict the applications of LncRNA in clinical. Based on their position relative to protein-coding genes, LncRNA can be classified as sense lncRNA, antisense lncRNA, intronic lncRNA, bidirectional lncRNA and intergenic lncRNA. Cis-regulation [29], a form of transcriptional activation and expression regulation of adjacent protein-coding gene mRNAs by lncRNAs (distance lower than 10 kb), make up an important part of the lncRNA regulation network together with trans-regulation [4] and ceRNA [30]. As demonstrated in our results, obvious discrimination can be detected between the AS and normal groups in both protein-coding genes and lncRNAs. Thus, a comprehensive understanding of the lncRNA-mediated network is essential for uncovering the pathophysiological processes of CAVD and identifying potential therapeutic targets.
In this study, we utilized bioinformatics techniques to investigate the potential relationships between lncRNA and mRNA in aortic calcification. Deriving lncRNA function from mRNAs is an important research strategy. To better understand the mechanisms of target mRNAs in DELs, the FEELnc and miRanda were used to build cis- / trans-regulation and lncRNA-miRNA-mRNA regulatory networks. The GO enrichment analysis based on the DEGs and lncRNA-mediated mRNAs between AS and control are mainly associated with the extracellular matrix, immune response and metabolic dysregulation which were consistent with current studies.
As the most important risk factors for cardiovascular disease, metabolic dysregulation especially lipid metabolisms have been expected to become new therapeutic targets for aortic stenosis. Our study showed that the GO enrichment analysis of downregulated DEGs had a tight link with fatty acid and organic acid metabolisms. Although GWAS research from over 114,000 UK Biobank participants did not prove the protection role of circulating polyunsaturated fatty acids in cardiovascular disease [31]. However, Gonzalo Artiach et al. demonstrated that Omega-3 polyunsaturated fatty acids decrease aortic valve disease through the resolvin E1 and chemR23 axis [32]. Therefore, fatty acids, as the substrates for various lipids, may become the novel treatment method for CAVD through alleviation of inflammation progress [33]. CAVD is regarded as an active inflammatory process, similar to atherosclerosis, involving both the adaptive and the innate immune systems [34]. The upregulated DEGs were also enriched in the immune response but the key gene and potentially mediated lncRNAs still need subsequent discovery and verification.
By FEELnc, we predicted the key cis-mRNAs MMP9, CCL3, and TGFB3 which are both classic ossification genes. The research of key cis-lncRNA SLC12A5-AS1, AC243829.4 and TGFB3 mainly concentrated in cancer [35, 36] and immune respone [37]. It is interesting to note that the lncRNA AC243829.4 is the ferroptosis-related lncRNA. Despite still being in its infancy, ferroptosis showed a wide range of perspectives in valve calcification [38, 39].
As research progresses, the ECM is not only considered to be passive mechanical support of the aortic leaflets but also a complex cellular microenvironment that is closely related to the development of CAVD [40]. The ECM of the normal aortic valve is composed of elastin, collagen and proteoglycans which are mainly secreted by aortic valve interstitial cells [41]. Excess provisional extracellular matrix is also regarded as a common factor in Bicuspid Aortic Valve formation [42]. Although the relationship between ECM and non-coding RNA was determined in different diseases [43], few studies have focused on lncRNAs regulatorily dysfunctional ECM in CAVD. As shown in the result, both upregulated DEGs and DELs were strongly association with extracellular matrix organization. By FEELnc, we predicted that the long noncoding gene FAM225A, AL356417.2 and BHLHE40-AS1 can regulate mRNA by trans-regulation function or ceRNA which existing research focuses on cancer and immune disease [44,45,46].
Different from the above ECM regulator lncRNA, GO enrichment for the trans-acting protein-coding genes LINC01614 showed a relationship with phosphate ion transmembrane transport. Calcium phosphate deposition is the characteristic of vascular calcification and its transporters were strictly controlled by eNPP1, 5NT, ENT1, Pit-2 and ANK [47]. Dysregulated phosphate metabolism enhances the osteoblast gene and promotes mineralization [48]. According to existing literature, LINC01614 can promote pancreatic cancer progression by WNT/β‑catenin signaling which is the crucial pathway in aortic valve calcification [49]. While expression of the WNT/β‑catenin signaling pathway can also be against phosphate-induced calcification [50]. Therefore we assume that LINC01614 can regulate phosphate metabolism by trans-regulation through WNT/β‑catenin signaling. However, the hypothesis still needs further experimental verification.
We finally focused our attention on the MIR4435-2HG, also known as LncRNA AWPPH, which ranked the highest in the midnightblue module ceRNA networks and had the most numerous coexpressed protein-coding genes by trans-regulation. As the potential pan-cancer biomarker [51] and hub gene of cardiovascular disease [52, 53], MIR4435-2HG also participates in process of osteogenesis and osteolysis disease [16]. Except for ECM, MIR4435-2HG showed a stronger relationship with collagen metabolism and Wnt-protein binding. Collagen, as the main ingredient of ECM, secretion by dysfunctional cells promotes calcification further by BMPs (bone morphogenetic proteins) and WNT/β‑catenin signaling. Although there is no relevant research, collagen metabolism may be considered as a new therapeutic target for the early treatment of CAVD. Notably, Xiaofang et al. demonstrated that the MIR4435-2HG was significantly increased in plasma samples of periodontitis patients which was remarkably decreased after treatment [54]. To date, several reports have described the relationship between periodontal and aortic calcification [55] or carotid artery calcification [56], but the association between periodontal and CAVD is still controversial. As a result, MIR4435-2HG may become a potential breakthrough in understanding the common pathways between periodontal and aortic calcification. In summary, MIR4435-2HG, which can regulate multiple osteogenesis genes through various pathways, will occupy an important position in the diagnosis and treatment of CAVD.
Conclusion
Via DEGs、DELs and WGCNA, we established an omnifarious lncRNA regulatory network in CAVD and identified 12 hub LncRNA which throughout the pathological process of extracellular matrix, immune response and metabolic dysregulation in CAVD. These crucial genes and networks provide future trends for basic research and new directions for interventions and therapeutic targets. It should be noted that our study only focused on bioinformatics analysis. The next step in future research experiments is required to clarify the result in vitro and in vivo.
Data Availability
The datasets analysed during the current study are available in the NCBI-GEO database (https://www.ncbi.nlm.nih.gov/geo/). The data that support the findings of this study are available from the corresponding author, Guang-Yuan Song or Jie Han, upon reasonable request.
References
Khanji MY, Ricci F, Galusko V, Sekar B, Chahal CAA, Ceriello L, et al. Management of aortic stenosis: a systematic review of clinical practice guidelines and recommendations. Eur Heart J - Qual Care Clin Outcomes. 2021;7:340–53.
Kraler S, Blaser MC, Aikawa E, Camici GG, Lüscher TF. Calcific aortic valve disease: from molecular and cellular mechanisms to medical therapy. Eur Heart J. 2021;:ehab757.
Piovesan A, Antonaros F, Vitale L, Strippoli P, Pelleri MC, Caracausi M. Human protein-coding genes and gene feature statistics in 2019. BMC Res Notes. 2019;12:315.
Yan P, Luo S, Lu JY, Shen X. Cis- and trans-acting lncRNAs in pluripotency and reprogramming. Curr Opin Genet Dev. 2017;46:170–8.
Statello L. Gene regulation by long non-coding RNAs and its biological functions. Nat Rev Mol Cell Biol. 2021;22:96–118.
Lu D, Thum T. RNA-based diagnostic and therapeutic strategies for cardiovascular disease. Nat Rev Cardiol. 2019;16:661–74.
Xie M-Y, Hou L-J. LncRNA MALAT1 aggravates calcific aortic valve disease by sponging miR-195. Int J Cardiol. 2021;333:161.
Chignon A, Argaud D, Boulanger M-C, Mkannez G, Bon-Baret V, Li Z, et al. Genome-wide chromatin contacts of super-enhancer-associated lncRNA identify LINC01013 as a regulator of fibrosis in the aortic valve. PLoS Genet. 2022;18:e1010010.
Hadji F, Boulanger M-C, Guay S-P, Gaudreault N, Amellah S, Mkannez G, et al. Altered DNA methylation of long noncoding RNA H19 in calcific aortic valve Disease promotes mineralization by silencing NOTCH1. Circulation. 2016;134:1848–62.
Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9:559.
Greene CL, Jaatinen KJ, Wang H, Koyano TK, Bilbao MS, Woo YJ. Transcriptional profiling of normal, stenotic, and Regurgitant Human aortic valves. Genes. 2020;11:789.
Ranjbarvaziri S, Kooiker KB, Ellenberger M, Fajardo G, Zhao M, Vander Roest AS, et al. Altered Cardiac Energetics and mitochondrial dysfunction in hypertrophic cardiomyopathy. Circulation. 2021;144:1714–31.
Lindman BR, Clavel M-A, Mathieu P, Iung B, Lancellotti P, Otto CM, et al. Calcific aortic stenosis. Nat Rev Dis Primers. 2016;2:16006.
Sun T, Huang Z, Liang W-C, Yin J, Lin WY, Wu J, et al. TGFβ2 and TGFβ3 isoforms drive fibrotic disease pathogenesis. Sci Transl Med. 2021;13:eabe0407.
Zhang M, Yu X, Zhang Q, Sun Z, He Y, Guo W. MIR4435-2HG: a newly proposed lncRNA in human cancer. Biomed Pharmacother. 2022;150:112971.
Ghasemian M, Rajabibazl M, Sahebi U, Sadeghi S, Maleki R, Hashemnia V, et al. Long non-coding RNA MIR4435-2HG: a key molecule in progression of cancer and non-cancerous disorders. Cancer Cell Int. 2022;22:215.
Chen L, Liu H, Sun C, Pei J, Li J, Li Y, et al. A novel LncRNA SNHG3 promotes osteoblast differentiation through BMP2 upregulation in aortic valve calcification. JACC: Basic to Translational Science. 2022;7:899–914.
MacGrogan D, Martínez-Poveda B, Desvignes J-P, Fernandez-Friera L, Gomez MJ, Gil Vilariño E, et al. Identification of a peripheral blood gene signature predicting aortic valve calcification. Physiol Genom. 2020;52:563–74.
Andrews S, FastQC. A Quality Control Tool for High Throughput Sequence Data [Online]. Available online at: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/. 2010.
Krueger F, James F, Ewels P, Afyounian E, Weinstein M, Schuster-Boeckler B et al. FelixKrueger/TrimGalore: v0.6.10. 2023.
Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21.
Pertea M, Pertea GM, Antonescu CM, Chang T-C, Mendell JT, Salzberg SL. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat Biotechnol. 2015;33:290–5.
Wucher V, Legeai F, Hédan B, Rizk G, Lagoutte L, Leeb T et al. FEELnc: a tool for long non-coding RNA annotation and its application to the dog transcriptome. Nucleic Acids Res. 2017;:gkw1306.
Pertea G, Pertea M. GFF Utilities: GffRead and GffCompare. F1000Res. 2020;9:304.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550.
Yu G, Wang L-G, Han Y, He Q-Y. clusterProfiler: an R Package for comparing Biological Themes among Gene clusters. OMICS. 2012;16:284–7.
Enright AJ, John B, Gaul U, Tuschl T, Sander C, Marks DS. MicroRNA targets in Drosophila. Genome Biol. 2003;5:R1.
Wickham H. ggplot2: Elegant Graphics for Data Analysis. 2nd ed. 2016. Cham: Springer International Publishing: Imprint: Springer; 2016.
Guil S, Esteller M. Cis-acting noncoding RNAs: friends and foes. Nat Struct Mol Biol. 2012;19:1068–75.
Tay Y, Rinn J, Pandolfi PP. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505:344–52.
Borges MC, Haycock PC, Zheng J, Hemani G, Holmes MV, Davey Smith G, et al. Role of circulating polyunsaturated fatty acids on cardiovascular diseases risk: analysis using mendelian randomization and fatty acid genetic association data from over 114,000 UK Biobank participants. BMC Med. 2022;20:210.
Artiach G, Carracedo M, Plunde O, Wheelock CE, Thul S, Sjövall P, et al. Omega-3 polyunsaturated fatty acids decrease aortic valve Disease through the Resolvin E1 and ChemR23 Axis. Circulation. 2020;142:776–89.
Plunde O, Bäck M. Fatty acids and aortic valve stenosis. Kardiol Pol. 2021;:VM/OJS/J/83299.
Bartoli-Leonard F, Zimmer J, Aikawa E. Innate and adaptive immunity: the understudied driving force of heart valve disease. Cardiovascular Res. 2021;:cvab273.
Yao J-M, Zhao J-Y, Lv F-F, Yang X-B, Wang H-J. A potential Nine-lncRNAs signature identification and Nomogram Diagnostic Model establishment for papillary thyroid Cancer. Pathol Oncol Res. 2022;28:1610012.
Wang Z, Chen Z, Guo T, Hou M, Wang J, Guo Y, et al. Identification and Verification of Immune Subtype-Related lncRNAs in Clear Cell Renal Cell Carcinoma. Front Oncol. 2022;12:888502.
Lambert N, Kengne-Ouafo JA, Rissy WM, Diane U, Murithi K, Kimani P et al. Transcriptional Profiles Analysis of COVID-19 and Malaria Patients Reveals Potential Biomarkers in Children. preprint. Bioinformatics; 2022.
Li M, Wang Z-W, Fang L-J, Cheng S-Q, Wang X, Liu N-F. Programmed cell death in atherosclerosis and vascular calcification. Cell Death Dis. 2022;13:467.
Li X-Z, Xiong Z-C, Zhang S-L, Hao Q-Y, Gao M, Wang J-F, et al. Potential ferroptosis key genes in calcific aortic valve disease. Front Cardiovasc Med. 2022;9:916841.
Gomez-Stallons MV, Tretter JT, Hassel K, Gonzalez-Ramos O, Amofa D, Ollberding NJ, et al. Calcification and extracellular matrix dysregulation in human postmortem and surgical aortic valves. Heart. 2019;105:1616–21.
Scott AJ, Simon LR, Hutson HN, Porras AM, Masters KS. Engineering the aortic valve extracellular matrix through stages of development, aging, and disease. J Mol Cell Cardiol. 2021;161:1–8.
Kern CB. Excess Provisional Extracellular Matrix: a common factor in bicuspid aortic valve formation. JCDD. 2021;8:92.
Akbari Dilmaghnai N, Shoorei H, Sharifi G, Mohaqiq M, Majidpoor J, Dinger ME, et al. Non-coding RNAs modulate function of extracellular matrix proteins. Biomed Pharmacother. 2021;136:111240.
Shen M, Duan C, Xie C, Wang H, Li Z, Li B, et al. Identification of key interferon-stimulated genes for indicating the condition of patients with systemic lupus erythematosus. Front Immunol. 2022;13:962393.
Li Z, Li Y, Wang X, Yang Q. Identification of a Six-Immune-Related Long non-coding RNA signature for Predicting Survival and Immune infiltrating status in breast Cancer. Front Genet. 2020;11:680.
DeVaux RS, Ropri AS, Grimm SL, Hall PA, Herrera EO, Chittur SV, et al. Long noncoding RNA BHLHE40-AS1 promotes early breast cancer progression through modulating IL‐6/STAT3 signaling. J Cell Biochem. 2020;121:3465–78.
Villa-Bellosta R. Vascular calcification: key roles of phosphate and pyrophosphate. IJMS. 2021;22:13536.
Bäck M, Michel J-B. From organic and inorganic phosphates to valvular and vascular calcifications. Cardiovascular Res. 2021;117:2016–29.
Chen L-J, Wu L, Wang W, Zhai L-L, Xiang F, Li W-B, et al. Long non–coding RNA 01614 hyperactivates WNT/β–catenin signaling to promote pancreatic cancer progression by suppressing GSK–3β. Int J Oncol. 2022;61:116.
Tian B, Yao L, Sheng Z, Wan P, Qiu X, Wang J, et al. Specific knockdown of WNT8b expression protects against phosphate-induced calcification in vascular smooth muscle cells by inhibiting the Wnt–β‐catenin signaling pathway. J Cell Physiol. 2019;234:3469–77.
Zhong C, Xie Z, Zeng L, Yuan C, Duan S. MIR4435-2HG is a potential Pan-Cancer Biomarker for diagnosis and prognosis. Front Immunol. 2022;13:855078.
Tang T-T, Wang B-Q. Clinical significance of lncRNA-AWPPH in coronary artery diseases. Eur Rev Med Pharmacol Sci. 2020;24:11747–51.
Wang X, Ren L, Chen S, Tao Y, Zhao D, Wu C. Long non-coding RNA MIR4435-2HG/microRNA-125a-5p axis is involved in myocardial ischemic injuries. Bioengineered. 2022;13:10707–20.
Wang X, Ma F, Jia P. LncRNA AWPPH overexpression predicts the recurrence of periodontitis. Biosci Rep. 2019;39:BSR20190636.
Yu Y, Ma J, Li S, Liao M, Xu S, Chen H et al. Association between Periodontitis and aortic calcification: a Cohort Study. Angiology. 2022;:000331972210947.
Wang W, Yang Z, Wang Y, Gao H, Wang Y, Zhang Q. Association between Periodontitis and Carotid Artery calcification: a systematic review and Meta-analysis. Biomed Res Int. 2021;2021:1–9.
Acknowledgements
We appreciated Gene Expression Omnibus database and Y. Joseph Woo et al., Zhe Zheng et al. and de la Pompa JL et al. for providing the original study data. We thank to the reviewers for their careful work and thoughtful suggestions.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 820704041004828).
Author information
Authors and Affiliations
Contributions
Guang-Yuan Song has made substantial contributions to the conception and was major contributors in the writing of the manuscript. Jie Han was engaged for conception and design of the work. Jing Yao designed the draft of the research process. Zhi-Nan Lu was involved in revising the manuscript critically for important intellectual content. Xu-Nan Guo, Jia-Hong Xie, Fang wu, Jing He, Zhao-Lin Fu analyzed the data and also involved in interpretation of data. All authors read and approved the manuscript that is enclosed.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study relied on RNA expression dataset, which are publicly available. All datasets received relevant ethical approval and participant consent; no additional ethical approval was required.
Consent for publication
was obtained from all participants.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Song, GY., Guo, XN., Yao, J. et al. Differential expression profiles and functional analysis of long non-coding RNAs in calcific aortic valve disease. BMC Cardiovasc Disord 23, 326 (2023). https://doi.org/10.1186/s12872-023-03311-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-023-03311-x