Abstract
Background
The research of the sensitivity and specificity point-of-care testing (POCT) of D-dimer as a diagnostic protocol for differential diagnosis of Stanford Type A aortic syndrome (hereafter as TAAS) mimicking ST-elevated myocardial infarction (STEMI) with regular STEMI in the emergency department is limited.
Methods
Full medical information of 32 patients confirmed TAAS and 527 patients confirmed STEMI from January 1st, 2016 to October 1st, 2021 were retrospectively analyzed in Shanghai Tenth People’s Hospital of Tongji University.
Results
The baseline characteristics of two groups of patients were well-balanced post propensity score matching (PSM) analysis, and each group had 32 patients enrolled. Patients in the STEMI group had higher positive cardiac troponin I (cTNI) (0.174 ng/ml vs. 0.055 ng/ml, P = 0.008) results but lower D-dimer (0.365μg/ml vs. 31.50μg/ml, P < 0.001) results than the TAAS group. The D-dimer cutoff value of 2.155μg/ml had the best sensitivity of 100% and specificity of 96.9%, and the positive predictive value (PPV) as well as the negative predictive value (NPV) of the cutoff value were 96.9 and 100%, respectively, in total 64 patients, the area under the curve (AUC) values were 0.998 (95% CI:0.992-1.000, P < 0.001) for the D-dimer. No significant correlation between the D-dimer concentration and the time from symptoms onset to first medical contact in both groups (TAAS group: r = − 0.248, P = 0.170; STEMI group: r = − 0.159, P = 0.383) or significant correlation between D-dimer and creatine clearance (TAAS group: r = − 0.065, P = 0.765; STEMI group: r = 0.222, P = 0.221). The total in-hospital mortality for the patients with TAAS presenting as STEMI was 62.5% (20/32). The mortality rate for TAAS patients confirmed by computed tomography angiography (CTA) was significantly lower (40% vs. 82.4%, P = 0.014) than the mortality rate for TAAS patients confirmed by coronary angiography (CAG) and had a longer average survival time (log-rank = 0.015), less peri-surgical complications especially gastrointestinal hemorrhage (0.00% vs. 55.6%, P < 0.001). CTA diagnosis can reduce the mortality rate by 67.5% (95%CI:0.124-0.850, P = 0.16).
Conclusions
The POCT D-dimer with cut-off 2.155μg/ml would be useful to rule-out TAAS mimicking STEMI from regular STEMI prior to reperfusion therapy. CTA diagnosis is effective in reducing the probability of perioperative complications and lowering perioperative mortality than CAG diagnosis in TAAS patients.
Similar content being viewed by others
Introduction
Stanford type A aortic syndrome (TAAS) extends to the ostium of the coronary artery may lead to acute coronary occlusion, thus leading to acute coronary syndrome (ACS) and mimicking as STEMI in electrocardiography examination. It is a life-threatening acute vascular disease that is not easily detectable because the primary electrocardiographic (ECG) presentation will make physicians ignore the atypical clinical manifestations and lead to misdiagnosis in the Emergency department (ED) [1]. Methods include computed tomography (CT), transesophageal echocardiography (TEE), transthoracic echocardiography (TTE), magnetic resonance imaging (MRI), and point-of-care ultrasound (POCUS) are used to exclude the TAAS in STEMI patients in clinical practice when there is a suspicion [2]. While in ED, TAAS might be ignored and misdiagnosed because definitive imaging may delay the reperfusion time and regular STEMI happens more often. Therefore, it is crucial to find a reliable, fast method to differentiate whether the STEMI is secondary to TAAS and to reduce misdiagnosis. Previous studies have found that D-dimer concentrations can be elevated in TAAS [3], despite its low specificity for the condition, we found that POCT of D-dimer is a simple and efficient method for detecting and discriminating acute aortic syndrome with ST-elevated myocardial infarction in the ED and thus leading to the important clinical decision making.
Here, we retrospectively analyzed the clinical characteristics, lab tests, and outcomes of the confirmed patients diagnosed with STEMI and STEMI secondary to TAAS, hoping this study could provide deep insight into such conditions and offered an effective and fast method to reduce such misdiagnosis and improve the prognosis of these patients.
Materials and methods
Patients
This is a one-center retrospective study. 34 patients who confirmed STEMI secondary to TAAS and 551 STEMI patients in ED enrolled in the study between January 1st, 2017 and October 1st 2021 were evaluated. 24 STEMI patients and 2 TAAS patients were excluded from the study for the lack of the POCT of D-dimer or cardiac infarct biomarker results in the ED.
Stanford type A aortic syndrome was defined as intramural hematoma or aortic dissection involvement of the ascending aorta and aortic arch. Enhanced computed tomography (CTA) or coronary angiography (CAG) confirmed the diagnosis of TAAS.
The diagnosis of STEMI was based on the latest criteria established by the American College of Cardiology [4] and European Society of Cardiology [5], including: (1) chest pain lasting for over 30 min, (2) at least two contiguous leads with ST-segment elevation 2.5 mm in men < 40 years, 2 mm in men over 40 years, or 1.5 mm in women in leads V2–V3 and/or1mm in the other leads, (3) an increase of cardiac biomarker values with at least one value above the 99th percentile of the upper reference limit. And other ECG signs of coronary artery occlusion like De winter syndrome were also included.
Data collection
After approval by the Clinical Ethics Committee (CEC) of the Shanghai Tenth People’s Hospital (application number: SHYS-IEC-5.0/22 K220/P01), two independent physicians retrospectively collected and reviewed the general clinical information, demographics, treatment records, and essential time points according to standards. POCT results, including cTNI, myoglobin (Myo), creatine kinase–myocardial band (CK-MB), brain natriuretic peptide (BNP), D-dimer (DD) were recorded.
The D-dimer assay was performed with the TRIAGE platform assay panels (Alere, San Diego, CA, USA), which comprises a fluorescence immunoassay analyzer and reagents. The D-dimer results were available within 15 minutes after the platform assay panels being placed on the cardiac reader. The following assay results were predefined to be positive on either blood draw: troponin I 0.05 ng/ml or greater, CK-MB 4.3 ng/ml or greater, Myo concentration of 108 ng/ml or greater, BNP 100 pg/ml or greater, and D-dimer 0.6μg/ml or greater. The point cutoffs were based on manufacturer recommendations, with an elevated result defined as any detectable concentration of results.
Statistical analysis
The frequency and percentage of the categorical data were determined. Continuous variables were presented as the 25 and 75% percentiles, median, mean and standard deviation (SD), depending on whether the data were normally distributed. A Mann-Whitney U test, chi-square, the Fischer exact or t-test were used to analyze the difference. A value of p < 0.05 was considered being significant. Propensity score matching (PSM) was used for calculating variables included sex, age, hypertension, coronary heart disease, smoking, diabetes mellitus, Marfan syndrome, previous percutaneous coronary intervention (PCI), previous coronary artery disease, previous myocardial infarction, known aneurysm, and hyperlipidemia to balance heterogeneity in demographics. The 1:1 PSM was applied to create the matched TAAS group and STEMI group with a caliper distance of 0.1. After PSM, the differences between the two groups were compared again using the aforementioned statistical methods.
Receiver operating characteristic (ROC) curves were constructed after calculation of the sensitivity for the matched TAAS group to determine the best cutoff value with a 95% confidence interval (CI) and the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated based on several cut-off values of D-dimer. The Kaplan-Meier estimates of the mortality rates in both groups are reported along with the corresponding hazard ratios (HR) and 95% CIs. All statistical analyses were performed using SPSS Statistics, version 26 (Armonk, NY, USA).
Results
Baseline Characteristics
Detailed demographics are displayed in Table 1. 527 STEMI patients and 32 TAAS patients were finally enrolled in the study from January 1st, 2016 to October 1st, 2021. Male patients accounted mainly in both TAAS and STEMI groups (78.1% vs. 82.0%, P = 0.584). Compared with the STEMI group, TAAS group had similar age (64[59-66] years vs. 66[61-73] years, P = 0.130), similar proportions of smokers (90.6% vs. 90.5%, P = 0.983), of patients with previous history of PCI (12.5% vs. 13.3%, P = 0.899), of patients with hyperlipidemia (56.3% vs. 53.7%, P = 0.779), of patients with hypertension (87.5% vs. 90.7%, P = 0.548), and of patients with coronary heart disease (25.0% vs. 26.6%, P = 0.845), but of more patients with known aortic aneurysm (9.4% vs. 2.8%, P < 0.05) based on the medical history. After 1:1 PSM analysis, each of 32 patients in the TAAS-group and the STEMI-group was extracted, and baseline characteristics were well balanced between the two groups.
Clinical Data
The clinical characteristics of these patients were also summarized in Table 2. Anterior chest pain was the most common initial presenting symptom among the TAAS and STEMI groups (28.1% vs. 30.2%, P = 0.226), subsequent by syncope (25.0% vs. 19.4%, P = 0.435) in PSM analysis. In the TAAS group, 7 (21.9%) patients also suffered from abdominal as well as anterior chest pain, 4 (12.5%) were sent to the hospital for cardiogenic shock and 1 (3.1%) had no other symptoms except abdominal pain. Differences in initial symptoms’ presentations were not significant between the 2 groups, and patients in both groups had normal blood pressure (105 mmHg vs. 105 mmHg, P = 0.543). Even patients in the STEMI group had more heartbeats (78 bpm vs. 60 bpm, P = 0.011) per minute, the result was of no clinical specificity.
The median time of symptoms onset to first medical contact was 85 minutes and 180 minutes and the median first medical contact time to ECG examination time was 2 minutes and 3 minutes in the TAAS group and STEMI group, respectively.
Among the 32 patients in the TAAS group, 20 patients had ST-segments elevation in II, III, AVF leads, and 4 patients combined with level III atrioventricular block. While it made no difference in most types of elevation in the ECG except more patients in the STEMI group could have the V3-V5 leads ST-segments elevation.
Laboratory test and POCT results
All patients enrolled had point-of-care testing. The median time of reports releasing time was 17 minutes in the TAAS group and 26 minutes STEMI group post PSM analysis. In PSM analysis, patients in the STEMI group had higher positive cTNI (0.174 ng/ml vs. 0.055 ng/ml, P = 0.008) results than the TAAS group, but CK-MB (9.35 ng/ml vs. 5.02 ng/ml, P = 0.151) and Myo (106.00 ng/ml vs. 57.16 ng/ml, P = 0.055) made no significant differences between the 2 groups. In the TAAS group, all the patients had elevated D-dimer results (100% vs. 28.1%, P = 0.000). The results of D-dimer (31.500μg/ml vs. 0.365μg/ml, P < 0.001) and LDH (527.5 U/L vs.350.0 U/L, P < 0.05) in TAAS group were higher than the STEMI group. The concentrations of leukocyte count (WBC), platelet (PLT), hemoglobin (Hb), hs-CRP (CRP), and serum creatinine were not significantly different between the two groups (Table 3).
The sensitivities for the total patients of each cutoff value were calculated and used to construct the ROC curves (Fig. 1). The AUC values were 0.998 (95% CI:0.992-1.000, P < 0.001) for the D-dimer, 0.694 (95% CI: 0.561-0.827, P = 0.007) for the LDH, 0.708 (95% CI: 0.581-0.835, P = 0.004) for the cTNT, 0.604 (95% CI: 0.461-0.748, P = 0.150) for the CK-MB, 0.639 (95% CI:0.501-0.777, P = 0.057) for the Myo, 0.529 (95% CI:0.387-0.672, P = 0.682) for the hs-CRP, 0.505 (95% CI:0.362-0.648, P = 0.941) for the WBC.
The D-dimer cutoff value of 2.155μg/ml had the best sensitivity of 100 and 96.9% of specificity, the NPV and PPT of it were 100 and 96.9%, respectively. D-dimer level in the Table 4. showed the cutoff value below 2.15μg/ml exhibited a high NPV, while D-dimer level 2.15μg/ml exhibited a high PPV.
No significant correlation between the D-dimer concentration and the time from symptom onset to first medical contact in both groups (TAAS group: r = − 0.248, P = 0.170; STEMI group: r = − 0.159, P = 0.383, Fig. 2. and Fig. 3.). No significant correlation was found between D-dimer and creatinine clearance (TAAS group: r = − 0.065, P = 0.765; STEMI group: r = 0.222, P = 0.221) as well.
The median time of FMC to CTA confirmation was 41 minutes, while the average time of FMC to CAG was 65.94 ± 3.46 minutes, which is significantly longer than the FMC to CTA confirmation time (p < 0.001).
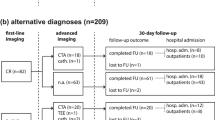
The TAAS group patients were confirmed by the CTA or the digital subtraction angiography (DSA), and the STEMI were confirmed by the DSA. The most common “culprit” artery in regular STEMI patients is right coronary artery (62.5%) followed by the left anterior descending artery which accounts for 31.2%, while in the TAAS group the left main artery is more prone to be affected by the false lumen and the “culprit” artery ECG shows do not coincide with the exact artery affected by the false lumen (Table S1, Fig. S1, Fig. S2 [Supplemental material]).
Clinical outcomes and Mortality rate of the TAAS patients
In the end, 15 patients underwent CTA and confirmed the diagnosis of TAAS. Among them, 11 patients make it to the surgery and 4 died before the operation. 2 died peri-operation for the cardiogenic shock, and 9 patients survived and discharged, and the 30-day in-hospital mortality was 40%.
17 patients were given antiplatelet therapy and underwent urgent CAG without awareness of the D-dimer results. 5 patients died after CAG confirmation TAAS without making to emergency surgery for deteriorating cardiogenic shock and malignant ventricular arrhythmia. 12 patients underwent emergency surgery, but only 3 patients survived and were discharged. Among the 9 patients, one patient died on the 17th-day post-operation due to massive cerebral infarction, 3 patients died perioperatively because of cardiogenic shock and multiple organ failure. 5 patients died from uncontrollable gastrointestinal bleeding within 2-4 weeks post-surgery. The clinical data and the outcomes of all 32 TAAS patients were summarized in Table 5. and Fig. 4 .
The total 30-day in-hospital mortality for the patients with TAAS presenting as STEMI was 62.5% (20/32). The 30-day in-hospital mortality rate for TAAS mimicking STEMI receiving CAG was 82.4% (14/17), the 30-day in-hospital mortality rate for TAAS mimicking STEMI receiving CTA was 40% (6/15) which was significantly lower (P = 0.014). Long-term survival estimates with the use of Kaplan–Meier method after operation for acute type A aortic dissection by diagnostic method. Significant overall difference is observed (P < 0.005 by log-rank test) (Fig. 5).
In the CTA diagnosis group, the average survival time was longer than the CAG diagnosis group with fewer patients who had peri-operation complications, especially gastrointestinal bleeding. CTA diagnosis can reduce the 30-day mortality rate by 67.5% (95% CI:0.124-0.850, P = 0.16) (Table 5).
Discussion
TAAS is rare but a nightmare disease and associated with early mortality rate [6]. Early diagnosis and recognition of the condition is crucial to improve the survival rate. Although the typical patient affected by TAAS can be identified by aortic dissection detection risk score plus D-dimer [7], the patients with atypical features may be misdiagnosed as an uncomplicated regular STEMI [8]. The incidence of acute coronary syndrome secondary to TAAS ranges from 5.7 to 11.3% [9,10,11] due to acute aortic syndrome of the aortic root reaching the coronary ostia. Approximately 2.5% of patients with TAAS present with STEMI [11] on ECG and misdiagnosis at the emergency department has been shown to be 30 to 78% [7, 8] during the initial assessment. In our study, despite the abnormal result of D-dimer, 53.1% (17/32) patients were misdiagnosed as regular STEMI and more cases of TAAS might be discovered if an autopsy was performed to establish the causes for the mortality among the patients with the diagnosis of STEMI or sudden death.
Clearly, any highly suspicious result in physical examination, laboratory test or supplementary examination with a high level of suspicion and awareness is the key to the timely diagnosis for this special group of patients [9] [12] before being misdiagnosed as regular STEMI and undergoing primary percutaneous coronary intervention. Clinical data including age, sex and clinical manifestations were not helpful in predicting TAAS since the patients with TAAS in our study included both male and female with a wide age range, and chest pain and syncope are the most common presenting initial symptoms [6] in both group. Many studies, including our study, also suggest that some patients who had TAAS mimicking regular STEMI share similar risk factors, including hypertension, aging, sex, diabetes mellitus with uncomplicated STEMI patients [1] [11] [13]. Although there are 49–63% of aortic dissection patients have systolic blood pressure ≥ 150 mmHg upon admission and blood hypertension is also being considered a clinical characteristic of acute aortic syndrome, in our study, the two groups had normal blood pressure (105 mmHg vs. 105 mmHg, P = 0.543), thus may lead to overlooking of the rare occurrence of STEMI combined with TAAS when physicians put emphasis on hypertension in TAAS patients. As most physicians agree that ECG with elevated ST-segment and chest pain suffice to diagnose regular STEMI [14] [15], it was certainly possible that some TAAS might have been ignored because of its extremely low odds since over 99% of STEMI is atherosclerotic in nature [16].
CTA scanning is of great value to establish the diagnosis [12]. However, if clinical history and clinical examination may not provide “warning” symptoms, it is very difficult to make the correct and timely differentiate diagnosis, especially in patients with time-sensitive symptoms like cardiogenic shock and malignant ventricular arrhythmia. Emergency CTA is of less value since in primary PCI-capable hospitals, obtaining a CTA imaging could delay the reperfusion therapy.
There was some evidence that aortic dissection may lead to end-organ mal-perfusion syndromes [17] and caused insidious ischemic end-organ complications, which occurred in approximately in one-third of patients [18]. Studies also found that the patients who TAAS underwent CAG who had load dual antiplatelet therapy would have more risk of gastrointestinal hemorrhage [6]. Our research results were consistent with abovementioned results, that TAAS patients confirmed by CAG had more complications peri-operation like gastrointestinal hemorrhage and leading to a higher mortality rate (82.4%) than the published data (23.4–47.7%) from previous studies [1, 19]. As the CAG confirmation time was significantly longer than the CTA confirmation time which may also delay TAAS surgery and increase the risk of mortality.
We found that the rapid bedside D-dimer test gives results in 15 minutes and is useful to screen for TAAS in patients with chest pain combined with ECG ST-segment elevation, and additionally has a D-dimer result of 2.15 μg/ml had the best sensitivity of 100 and 96.9% of specificity.
Serum D-dimer is currently the only clinically proven biological marker available for rapid detection. Contaminated smooth muscle myosin heavy chain [20] and elastin [21] can also be used as biological markers of TAAS with higher specificity, but rapid measurement systems are not clinically available. In general, the fact that D-dimer can be significantly elevated in patients with TAAS is determined by its physiopathological mechanism [22]. And in our study, all patients with TAAS had significantly higher D-dimer elevated over 0.6μg/ml. Additionally, we suspected that the coagulation and fibrinolytic systems are activated immediately after the onset of TAAS, cause no significant correlation between the D-dimer concentration and the time from symptom onset to first medical contact in STEMI group and TAAS group. D-dimer levels have also been reported to correlate with serum creatinine levels [23]. In the present study, there was no correlation between D-dimer levels creatinine clearance in the two groups of patients. Therefore, the cause of elevated D-dimer in patients with TAAS is not impaired renal function, which is an effect of TAAS. Therefore, in the acute phase, a rapid bedside D-dimer test can be used to differentiate between regular STEMI and TAAS combined with STEMI.
Since this special group of STEMI patients has a low incidence and no comprehensive large clinical studies, the management for these patients remains challenging and even controversial. The current guidelines have suggested surgical resection and replacement of the thoracic aorta as the gold standard to treat TAAS [10] [13] [9] [24]. On the other hand, prompt coronary revascularization may do good to the unstable patients, but it can also serve as a double-edge sword and lead to severe complications like refractory hemorrhage peri-operation as in our study. Of course, the management of STEMI caused by TAAS should be individualized. There is no doubt that definitive diagnosis is the most important thing, and up to date, there is no clear guideline to guide the best protocol for distinguishing the diagnosis only the suggestions [25].
Although D-dimer is of high sensitivity and low specificity, and D-dimer concentration tends to be elevated in many other diseases, such as pulmonary embolism [26], deep venous thrombosis [27], cancer [28], atrial fibrillation [29], and congestive heart failure [30]. None of the above diseases are relative contraindications of anti-platelet and reperfusion therapy in STEMI patients, in addition to aortic dissection. If rapid detection shows that the level of D-dimer increases, enhanced CT, transesophageal echocardiography and POCUS are needed to confirm the diagnosis of TAAS before being given antithrombotic therapy. Though the probability of STEMI secondary to TAAS occurrence is very low, patients with more pronounced coronary symptoms and hemodynamic instability may benefit from the fast and accurate diagnosis.
But the authors point out the extreme infrequency, time-sensitive feature, and high mortality rate of STEMI secondary to TAAS in comparison with the total emergency department visits. The prospective systematic approach is difficult to be tested in a clinical situation. Even in the absence of a firm evidence base, the retrospective-based recommendations that the POCT of D-dimer result is important for differential diagnosis between STEMI and TAAS presenting as STEMI are worth consideration. A major strength of the study was to meet the clinical needs in the management of acute TAAS in the emergency department, to minimize time delay in diagnosis and referral of TAAS and increase awareness of the condition and raise the index of suspicion when managing STEMI. The step may only take a few minutes longer, but it may lead to a slightly earlier precise diagnosis and save more lives. To improve on this rather dismal rate, it is worth a try.
Limitations
The current study has several limitations. This was a single-center experience for differentiating diagnosis of the STEMI and STEMI secondary to TAAS, and as its retrospective nature, it had its disadvantages. The generalization of D-dimer cut-off value is limited due to the small size of the sample, further studies are needed to validate new cut-off values and both the sensitivity and specificity of this approach to a real-world emergency department situation are needed to be tested. In addition to its relatively poor speciality patients with higher D-dimer level still required imaging techniques and may lead to negative TAAS diagnosis and delay reperfusion time. Concerning its good sensitivity, the POCT of D-dimer may thus be a useful rule-out tool. To date, many novel biomarkers have been studied, though no biomarker can reliably identify TAAS as they all have some limitations in terms of sensitivity, specificity or consuming more time, the rapid development of biomarkers over the past decade, may soon allow their incorporation into diagnostic algorithms either alone or in combination with imaging techniques, thus providing clinicians with an additional effective tool as a treatment for STEMI patients secondary to TAAS.
Availability of data and materials
The data used to support the finding will not be shared publicly. Researchers can contact the corresponding author for detailed information.
References
Zhan S, Hong S, Shan-Shan L, Chen-Ling Y, Lai W, Dong-Wei S, et al. Misdiagnosis of Aortic Dissection: Experience of 361 Patients. J Clin Hypertension. 2012;14:256–60.
Evangelista A, Isselbacher EM, Bossone E, Gleason TG, Di Eusanio M, Sechtem U, et al. Insights from the international registry of acute aortic dissection: a 20-year experience of collaborative clinical research. Circulation. 2018;137:1846–60.
Fan QK, Wang WW, Zhang ZL, Liu ZJ, Yang J, Zhao GS, et al. Evaluation of D-dimer in the diagnosis of suspected aortic dissection. Clin Chemistry Laboratory Med. 2010;48:1733–7.
Anderson JL. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;127.
Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). European Heart J. 2018;39:119–77.
Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, et al. The International Registry of Acute Aortic Dissection (IRAD). Jama. 2000;283:897.
Nazerian P, Mueller C, De Matos Soeiro A, Leidel BA, SAT S, Giachino F, et al. Diagnostic accuracy of the aortic dissection detection risk score plus D-dimer for acute aortic syndromes the ADvISED prospective multicenter study. Circulation. 2018;137:250–8.
Lovatt S, Wong CW, Schwarz K, Borovac JA, Lo T, Gunning M, et al. Misdiagnosis of aortic dissection: A systematic review of the literature. Am J Emerg Med. 2022;53:16–22.
Kawahito K, Adachi H, Murata SI, Yamaguchi A, Ino T. Coronary Malperfusion Due to Type A Aortic Dissection: Mechanism and Surgical Management. Ann Thoracic Surg. 2003;76:1471–6.
Pêgo-Fernandes P. Management of aortic dissection that involves the right coronary artery. Cardiovascular Surgery. 1999;7:545–8.
Neri E, Toscano T, Papalia U, Frati G, Massetti M, Capannini G, et al. Proximal aortic dissection with coronary malperfusion: Presentation, management, and outcome. J Thoracic Cardiovasc Surg. 2001;121:552–60.
Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: Executive summary: A report of the American college of cardiology foundation/American heart association task force on pra. Circulation. 2010;121:266–369.
Nienaber CA, Clough RE. Management of acute aortic dissection: The Lancet. Elsevier; 2015. p. 800–11.
Zaschke L, Habazettl H, Thurau J, Matschilles C, Göhlich A, Montagner M, et al. Acute type A aortic dissection: Aortic Dissection Detection Risk Score in emergency care – surgical delay because of initial misdiagnosis. Eur Heart J Acute Cardiovascular Care. 2020;9(3_suppl):S40–7.
Salmasi MY, Hartley P, Hussein M, Jarral O, Pepper J, Nienaber C, et al. Diagnosis and management of acute Type-A aortic dissection in emergency departments: Results of a UK national survey. Int J Cardiol. 2020;300, 50(9).
Kohn MA, Kwan E, Gupta M, Tabas JA. Prevalence of acute myocardial infarction and other serious diagnoses in patients presenting to an urban emergency department with chest pain. J Emerg Med. 2005;29:383–90.
Czerny M, Schoenhoff F, Etz C, Englberger L, Khaladj N, Zierer A, et al. The Impact of Pre-Operative Malperfusion on Outcome in Acute Type A Aortic Dissection: Results From the GERAADA Registry. J Am College Cardiol. 2015;65:2628–35.
Bonser RS, Ranasinghe AM, Loubani M, Evans JD, NMA T, Bachet JE, et al. Evidence, lack of evidence, controversy, and debate in the provision and performance of the surgery of acute type A aortic dissection. J Am College Cardiol. 2011;58:2455–74.
DPJ H, Banerjee A, Fairhead JF, Perkins J, Silver LE, Rothwell PM. Population-based study of incidence and outcome of acute aortic dissection and premorbid risk factor control: 10-year results from the oxford vascular study. Circulation. 2013;127:2031–7.
Suzuki T, Katoh H, Watanabe M, Kurabayashi M, Hiramori K, Hori S, et al. Novel biochemical diagnostic method for aortic dissection: Results of a prospective study using an immunoassay of smooth muscle myosin heavy chain. Circulation. 1996;93:1244–9.
Shinohara T, Suzuki K, Okada M, Shigai M, Shimizu M, Maehara T, et al. Soluble elastin fragments in serum are elevated in aortic dissection. J Cardiol. 2004;43:96–7.
ten Cate JW, Timmers H, Becker AE. Coagulopathy in ruptured or dissecting aortic aneurysms. Am J Med. 1975;59:171–6.
Karami-Djurabi R, Klok FA, Kooiman J, Velthuis SI, Nijkeuter M, Huisman MV. D-dimer Testing in Patients with Suspected Pulmonary Embolism and Impaired Renal Function. Am J Med. 2009;122:1050–3.
Neri E, Toscano T, Massetti M, Capannini G, Carone E, Tucci E, et al. Operation for acute type A aortic dissection in octogenarians: Is it justified? J Thoracic Cardiovasc Surg. 2001;121:259–67.
Erbel R, Aboyans V, Boileau C, Bossone E, Di Bartolomeo R, Eggebrecht H, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases. Eur Heart J. 2014;35:2873–926.
Goldhaber SZ, Simons GR, Elliott CG, Haire WD, Toltzis R, Blacklow SC, et al. Quantitative Plasma D-dimer Levels Among Patients Undergoing Pulmonary Angiography for Suspected Pulmonary Embolism. JAMA: The Journal of the American Medical Association. 1993;270:2819–22.
Fattorini A, Crippa L, Vigano D’Angelo S, Pattarini E, D’Angelo A. Risk of deep vein thrombosis recurrence: High negative predictive value of D-dimer performed during oral anticoagulation. Thrombosis and Haemostasis. 2002;88:162–3.
Costantini V, Zacharski LR. Fibrin and cancer. Thrombosis and Haemostasis. 1993;69:406–14.
Kumagai K, Fukunami M, Ohmori M, Kitabatake A, Kamada T, Hoki N. Increased intracardiovascular clotting in patients with chronic atrial fibrillation. J Am College Cardiol. 1990;16:377–80.
Jafri SM, Ozawa T, Mammen E, Levine TB, Johnson C, Goldstein S. Platelet function, thrombin and fibrinolytic activity in patients with heart failure. Eur Heart J. 1993;14:205–12.
Acknowledgements
The authors thank the patients, staff involved in the research for the cooperation.
Funding
None.
Author information
Authors and Affiliations
Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki of 1975, as revised in 2000, and was approved by the ethics committee for medical research at Shanghai tenth People’s Hospital of Tongji University. The subjects or their legal representatives were fully aware of the nature of the study and agreed to participate and sign the informed consent approved by the ethics committees.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chang, X., Yao, J. & Xu, Y. The point-of-care D-dimer test provides a fast and accurate differential diagnosis of Stanford Type A aortic syndrome and ST-elevated myocardial infarction in emergencies. BMC Cardiovasc Disord 22, 556 (2022). https://doi.org/10.1186/s12872-022-02925-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-022-02925-x