Abstract
Objectives
Nonvalvular atrial fibrillation (NVAF) concomitant with coronary artery disease (CAD) may increase the risk of thromboembolism. Antithrombotic therapy for NVAF patients with percutaneous coronary intervention (PCI) remains contradictory and challenging. This study aimed to assess the safety and efficacy of left atrial appendage closure (LAAC) in a cohort of patients with NVAF and PCI.
Methods
A total of 109 patients undergoing LAAC procedures between March 2017 and December 2020 were categorized into 2 groups, Group I included 36 patients with PCI while group II included 73 patients without. Peri-procedural and long-term complications, as well as ischemia and bleeding events, were retrospectively analyzed.
Results
Group I had more diabetes mellitus (55.6% vs. 26.0%; p = 0.003), higher CHA2DS2-VASc scores (5.44 ± 1.85 vs. 4.22 ± 1.64; p = 0.002) and HAS-BLED scores (3.39 ± 0.93 vs. 2.74 ± 1.05; p = 0.003) compared to Group II. Procedure-related complications within 7 days were similar in both groups (8.3% vs. 8.2%; P = 1.000). Over a median follow-up period of 20.9 months, there were no significant differences between two subgroups with regard to cardiovascular death (2.8% vs. 0%, p = 0.330), stroke/transient ischemic attack (2.8% vs. 5.5%, p = 1.000), major bleeding (0% vs. 2.7%, p = 1.000) and device-related thrombus (8.3% vs. 1.4%, p = 0.104). The observed annualized thromboembolic and major bleeding events determined by Kaplan–Meier analysis decreased by 82.4% and 100% in group I, 55.9% and 75.8% in group II, respectively.
Conclusion
LAAC is a safe and effective option for stroke prevention in NVAF patients with PCI.
Similar content being viewed by others
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia in clinical practice associated with a significantly increased risk of embolic stroke [1]. Oral anticoagulation (OAC) is recommended for stroke prophylaxis in patients with nonvalvular AF (NVAF), but there are still several limitations and side effects in the clinical setting [2,3,4]. Recently, percutaneous left atrial appendage closure (LAAC) has developed as an important therapeutic option for NVAF patients with high thromboembolic risk or relative/absolute contraindications to long-term OAC [5].
It is well known that a high incidence and prevalence of coronary artery disease (CAD) occurred in patients with AF [6,7,8]. AF patients with percutaneous coronary intervention (PCI) always carry high ischemic risk because they have more comorbidities such as diabetes, hypertension, renal insufficiency, and peripheral arterial disease. The optimal antithrombotic regimen remains challenging and needs tailored treatment for these individuals [9]. The combination of dual antiplatelet therapy and oral anticoagulants is associated with high risk of major bleeding, and the optimal use of triple therapy in clinical settings remains controversial.
Currently, LAAC is performed widely for antithrombotic events prevention in NAVF patients with heart failure, chronic kidney disease, and high bleeding risk [10,11,12]. Moreover, in NVAF patients with PCI who may require both OAC and antiplatelet therapy, LAAC may have the potential benefit of reducing the usage of OAC. Whether LAAC is the optimal choice for patients with PCI remains unknown.
In this study, we aimed to evaluate the long-term safety and efficacy of LAAC in NVAF patients with PCI.
Methods
Patients
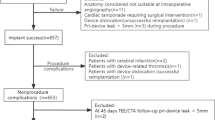
A total of 109 consecutive NVAF patients undergoing successful LAAC between March 2017 to December 2020 in two centers (Shanghai Tongji Hospital and Shanghai East Hospital, Tongji University, Shanghai, China) were enrolled. The indication of LAAC procedure was based on European Heart Rhythm Association/European Association of Percutaneous Cardiovascular Interventions (EHRA/EAPCI) expert consensus statement [13]. The cohort was divided into two groups, patients with PCI (Group I) and those without (Group II). All patients in Group I were diagnosed with chronic coronary syndrome. Transesophageal echocardiography (TEE) or computed tomography angiography (CTA) was performed before LAAC to confirm anatomical characteristics of left atrial appendage (LAA) and rule out LAA thrombus.
LAAC procedure
LAAC was performed under general anesthesia with the guidance of TEE and fluoroscopy. After transseptal puncture and introduction of the delivery system to LAA, intravenous heparin was administered according to the patient’s body weight (100 IU/kg) to maintain an activated clotting time (ACT) at 250–350 s during the procedure. TEE and LAA angiogram were performed to determine the optimal device size and confirm that the device position as well as LAA sealing after the device (either LAmbre™, Lifetech Scientific Corp., Shenzhen, China; Watchman™, BostonScientific, Marlborough, MA, USA or Leftear™, Guangdong Pulse Medical Technology Co., Ltd. Zhuhai, China) was deployed. The implant success was defined as LAA closure with peri-device leak (PDL) < 5 mm under TEE imaging.
Antithrombotic therapy at discharge and follow-up
Postimplant antithrombotic regimen in group I, including triple therapy, the combination of OAC and dual antiplatelet therapy (DAPT), dual therapy, the combination of OAC and single antiplatelet therapy (SAPT) or DAPT, and single OAC, was prescribed according to the interval time between the last PCI and LAAC. In group II, postimplant antithrombotic regimen was based on current guidelines, including warfarin (target international normalized ratio, 2.0–3.0) for 6 weeks, followed by clopidogrel and aspirin for 6 months, and aspirin alone subsequently. Adjustments in antithrombotic therapy during follow-up were based on the physician’s clinical judgment.
TEE or CTA was scheduled at 3 and 6 months post-procedure to discover any peri-device leak or device-related thrombus (DRT). Long-term follow-up was carried out by outpatient visits or telephone interviews to assess survival and complications.
Statistical analysis
Continuous data were described as mean ± standard deviation (SD) and compared by Mann-Whitney U-test or Student’s T-test. Categorical data are described as numbers and percentages and compared by Fisher’s exact test or Chi-square test. Rates of ischemic stroke/transient ischemic attacks (TIA)/peripheral emboli and major bleeding events were calculated as the number of events per 100 patients-year. Predicted risk of annual ischemic stroke/TIA/peripheral emboli and major bleeding events were extrapolated from each patient based on CHA2DS2-VASc and HAS-BLED scores from published risk score literature [14, 15]. P-value < 0.05 was considered significant. SPSS, version 22.0 software was used to manage the data.
Results
Baseline characteristics
The 109 NVAF patients undergoing successful LAAC were enrolled and divided into 2 groups, Group I included 36 patients with PCI while group II included 73 patients without. Baseline characteristics of the study population were presented in (Table 1). Both CHA2DS2-VASc and HAS-BLED scores in group I were higher than those in group II (5.44 ± 1.85 vs. 4.22 ± 1.64, p = 0.002 and 3.39 ± 0.93 vs. 2.74 ± 1.05, p = 0.003 respectively). Diabetes was more prevalent (55.6% vs. 26.0%, P = 0.003) in group I. There were no statistical differences in terms of hypertension, chronic heart failure, previous stroke/TIA, and major bleedings between the two groups. AF pattern, left atrium dimension, left ventricular end-diastolic dimension, and left ventricular ejection fraction (LVEF) did not differ as well.
Abbreviations: TIA: transient ischemic attack; LAD: left atrial diameter; LVEDd: left ventricular end-diastolic dimension; LVEF: left ventricular ejection fraction; LAA: left atrial appendage; PCI: percutaneous coronary intervention; LAAC: left atrial appendage closure.
Procedural data and peri‑procedural complications
Successful implant was achieved in 109 (100%) patients. Procedural parameters were detailed in (Table 2).
In group I, 2 patients suffered pericardial effusions which were managed conservatively within a few days post-procedure and 1 patient suffered hypotension due to vasovagal reactions post-procedure and was recovered by treatment of rehydration. In group II, 1 severe vascular complication and 1 cardiac tamponade requiring pericardiocentesis were documented a few hours after LAAC. There were no significant differences in death, stroke, bleeding, DRT, or pericardial effusion occurrence between the two groups (Table 2).
Abbreviations: TEE: transesophageal echocardiography; LAA: left atrial appendage; DSA: digital subtraction angiography; LAAC: left atrial appendage closure; ACT: activated clotting time; DRT: device-related thrombus.
Antithrombotic medications upon discharge
In group I, 5 patients (13.9%) were discharged with OAC and SAPT, 14 patients (38.9%) with DAPT, 14 patients (38.9%) with single OAC. 2 patients (5.6%) were on triple antithrombotic regimen and 1 patient (2.8%) was discharged without any antithrombotic therapy (Fig. 1).
In group II, the majority of patients (61/73, 83.6%) were prescribed with single OAC, 9 patients (12.3%) with DAPT and 2 patients (2.7%) with SAPT. Only 1 patient (1.4%) had no antithrombotic therapy.
Follow-up results
The mean follow-up period was 20.9 ± 8.63 months. Only 1 cardiovascular death (2.8%) was reported in group I at 1-month post-procedure, which was caused by complications after transcatheter aortic valve implantation (TAVI) procedure. A total of 4 (3.7%) patients were found DRT. In group I, 2 DRT patients had a history of in-stent restenosis (ISR) and were treated with anticoagulant until thrombus resolution on TEE. There were no significant differences involving DRT, heart failure, stroke/TIA, death, bleeding, cancer, PDL, and system embolism between the two groups (Table 3).
Abbreviations: TIA: transient ischemic attack.
Efficacy for prevention of thromboembolic and hemorrhagic events
According to Kaplan-Meier estimation, the observed annualized thromboembolic events including ischemic stroke, TIA, and systemic embolism were decreased by 82.4% in group I and 55.9% in group II respectively, compared to the predicted value (10.8% vs. 8.16%) based on CHA2DS2-VASc score (Fig. 2 A). Meanwhile, compared to the predicted bleeding rate (6.84% vs. 5.79%) based on HAS-BLED score, the observed annualized bleeding rate was reduced by 100% and 75.8% in group I and group II respectively (Fig. 2B).
Discussion
The results of this study have shown that LAAC procedure in AF patients with PCI reduced the risk of thromboembolic events and severe bleeding over a long-term period. Percutaneous LAAC could be a safe and effective option for stroke prevention in this specific population.
The AF patients with PCI carry a high ischemic risk, therefore, short-term OAC with DAPT is recommended after PCI. The increased risk of bleeding involved with triple therapy is deemed to outweigh the benefits of thromboembolic risk reduction [16, 17]. Therefore, during the early period after PCI, when both bleeding and ischemic rates are relatively high, LAAC is expected to be useful in these particular patients. In our study, 6(16.6%) patients were performed LAAC within 3 months after PCI and no adverse cardiac events occurred. Overall, LAAC was successful in all enrolled patients (100%), consistent with 98.3% of National Cardiovascular Data Registry LAAC Registry [18]. There were no statistical differences between the two subgroups in terms of procedure-related complications and long-term outcomes over a 24-months follow-up period, suggesting LAAC appeared to be a viable option for antithrombotic prevention in NVAF patients with PCI.
The main concern is the incidence of thromboembolic and bleeding events in a population of AF with PCI. As expected, our study population had a mean CHA2DS2-VASc score of 5.44 ± 1.85 and HAS-BLED score of 3.39 ± 0.93. We recorded 1 patient in group I but 4 patients in group 2 suffered strokes/TIA. The observed results of both groups were further compared with expected rates of annual thromboembolic events (ischemic stroke, TIA, and peripheral thromboembolism) based on CHA2DS2-VASc score [14], revealing that the risk of thromboembolic events in group I decreased much more dramatically than that in group II (82.4% vs. 55.9%). It is likely explained that NVAF patients with PCI received either inadequate antithrombotic or no anticoagulation therapy due to the high risk of major bleeding and LAAC can prevent even > 99% thrombi from LAA [19]. Additionally, a majority of patients in group I were prescribed with OAC and antiplatelet drugs, particularly in patients who underwent PCI within 1 year, leading to a reduction of coronary and peripheral arterial thrombosis. Bleeding risk remains a clinical concern in NVAF patients receiving OAC and antiplatelet therapy and tailored antithrombotic regimen for individuals is always challenging. In group I, there was no major bleeding except 1 hemorrhagic stroke documented at a 24-months follow-up due to trauma. The actual annualized major bleedings risk of 0% compared favorably to an estimated 6.84% based on HAS-BLED score [15], with a dramatic reduction of 100%. LAAC offers an alternative mechanical approach for NVAF patients with PCI because it allows OAC discontinuation and consequently, leads to a reduced risk of bleeding.
There has been increasing concern about DRT during follow-up after LAAC [20,21,22]. The rate of DRT (3.6%) in this study was comparable to other published data of 4.1% [23] and 3.3% [24]. In group I, 3 patients were found late DRT and 2 of them had a reduced LVEF which was a proven factor associated with DRT formation after LAAC [22]. They were all dissolved with intense anticoagulant treatment without ischemic stroke. Interestingly, in group I, 2 DRT patients had prior ISR. Both ISR and DRT were partly due to poor device endothelialization as a consequence of endothelial dysfunction [25, 26], indicating ISR may hold a potential role in DRT after LAAC. Moreover, these findings support the notion that for the patients undergoing LAAC with ISR history, close follow-up with TEE or CTA and intensive antithrombotic therapy are crucial for DRT prevention.
Our study has important clinical relevance. Drug management of NVAF patients with PCI is complex and challenging because the individualized antithrombotic strategy should be made according to guidelines based on ischemic and bleeding risk [27, 28]. DAPT therapy after LAAC, which is consistent with the drug regimen after PCI, has been recently proven to be feasible and safe [29, 30]. In our study, we found that LAAC is efficient and safe in NVAF patients with PCI, and therefore may be an ideal choice to prevent stroke and other thrombotic complications in this specific high-risk group.
Limitations
The current study has several limitations. First, the sample size was relatively small and it was not a randomized trial. Further larger prospective and randomized controlled studies are needed to confirm the conclusion. Second, considering the relatively small sample size, we did not analyze the differences by the time from the previous PCI which could be extremely meaningful. In the future, by increasing the sample size and modifying entry criteria, we will further study the safety and efficacy of LAAC in AF patients with PCI. Furthermore, the last follow-up using phone contact was prone to subjective bias which might have an impact on the outcome of our study.
Conclusion
LAAC appears to be safe and feasible for NVAF patients with PCI carrying high ischemic and bleeding risk. This observation may provide novel evidence of LAAC application in clinical practice.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author (chenfadong0819@163.com).
Abbreviations
- NVAF:
-
Nonvalvular atrial fibrillation
- CAD:
-
Coronary artery disease
- PCI:
-
Percutaneous coronary intervention
- LAAC:
-
Atrial fibrillation
- OAC:
-
Oral anticoagulation
- EHRA/EAPCI:
-
European Heart Rhythm Association/European Association of Percutaneous Cardiovascular Interventions
- TEE:
-
Transesophageal echocardiography
- CTA:
-
Computed tomography angiography
- DAPT:
-
Dual antiplatelet therapy
- SAPT:
-
Single antiplatelet therapy
- LAA:
-
Left atrial appendage
- ACT:
-
Activated clotting time
- PDL:
-
Peri-device leak
- DRT:
-
Device-related thrombus
- SD:
-
Standard deviation
- TIA:
-
Transient ischemic attacks
- LVEF:
-
Left ventricular ejection fraction
- TAVI:
-
Transcatheter aortic valve implantation
- ISR:
-
In-stent restenosis.
References
Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, Newton-Cheh C, Lubitz SA, Magnani JW, Ellinor PT, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386(9989):154–62.
Kim YH, Shim J, Tsai CT, Wang CC, Vilela G, Muengtaweepongsa S, Kurniawan M, Maskon O, Li Fern H, Nguyen TH, et al. XANAP: A real-world, prospective, observational study of patients treated with rivaroxaban for stroke prevention in atrial fibrillation in Asia. J Arrhythm. 2018;34(4):418–27.
Martinez BK, Sood NA, Bunz TJ, Coleman CI. Effectiveness and Safety of Apixaban, Dabigatran, and Rivaroxaban Versus Warfarin in Frail Patients With Nonvalvular Atrial Fibrillation. J Am Heart Assoc 2018, 7(8).
Sharma M, Cornelius VR, Patel JP, Davies JG, Molokhia M. Efficacy and Harms of Direct Oral Anticoagulants in the Elderly for Stroke Prevention in Atrial Fibrillation and Secondary Prevention of Venous Thromboembolism: Systematic Review and Meta-Analysis. Circulation. 2015;132(3):194–204.
Reddy VY, Gibson DN, Kar S, O’Neill W, Doshi SK, Horton RP, Buchbinder M, Gordon NT, Holmes DR. Post-Approval U.S. Experience With Left Atrial Appendage Closure for Stroke Prevention in Atrial Fibrillation. J Am Coll Cardiol. 2017;69(3):253–61.
Akao M, Chun YH, Wada H, Esato M, Hashimoto T, Abe M, Hasegawa K, Tsuji H, Furuke K, Fushimi AFRI. Current status of clinical background of patients with atrial fibrillation in a community-based survey: The Fushimi AF Registry. J Cardiol. 2013;61(3–4):260–6.
Zhang H, Yang Y, Zhu J, Shao X, Liu Y, Zhao L, Yu P, Zhang H, He Q, Gu X. Baseline characteristics and management of patients with atrial fibrillation/flutter in the emergency department: results of a prospective, multicentre registry in China. Intern Med J. 2014;44(8):742–8.
Zoni-Berisso M, Lercari F, Carazza T, Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol. 2014;6:213–20.
Pilgrim T, Kalesan B, Zanchin T, Pulver C, Jung S, Mattle H, Carrel T, Moschovitis A, Stortecky S, Wenaweser P, et al. Impact of atrial fibrillation on clinical outcomes among patients with coronary artery disease undergoing revascularisation with drug-eluting stents. EuroIntervention: J EuroPCR Collab Working Group Interventional Cardiol Eur Soc Cardiol. 2013;8(9):1061–71.
Fastner C, Brachmann J, Lewalter T, Zeymer U, Sievert H, Borggrefe M, Nienaber CA, Weiß C, Pleger ST, Ince H, et al: Left atrial appendage closure in patients with chronic kidney disease: results from the German multicentre LAARGE registry. Clinical research in cardiology: official journal of the German Cardiac Society 2020.
Fastner C, Brachmann J, Lewalter T, Zeymer U, Sievert H, Borggrefe M, Weiß C, Geist V, Krapivsky A, Käunicke M, et al. Left atrial appendage closure in patients with a reduced left ventricular ejection fraction: results from the multicenter German LAARGE registry. Clin Res cardiology: official J German Cardiac Soc. 2020;109(11):1333–41.
Merella P, Lorenzoni G, Delitala AP, Sechi F, Decandia F, Viola G, Berne P, Deiana G, Mazzone P, Casu G. Left Atrial Appendage Occlusion in High Bleeding Risk Patients. J Interv Cardiol. 2019;2019:6704031.
Meier B, Blaauw Y, Khattab AA, Lewalter T, Sievert H, Tondo C, Glikson M, Document R. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion. Europace. 2014;16(10):1397–416.
Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33(12):1500–10.
Lip GY, Frison L, Halperin JL, Lane DA. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J Am Coll Cardiol. 2011;57(2):173–80.
Lamberts M, Gislason GH, Lip GY, Lassen JF, Olesen JB, Mikkelsen AP, Sørensen R, Køber L, Torp-Pedersen C, Hansen ML. Antiplatelet therapy for stable coronary artery disease in atrial fibrillation patients taking an oral anticoagulant: a nationwide cohort study. Circulation. 2014;129(15):1577–85.
Lamberts M, Olesen JB, Ruwald MH, Hansen CM, Karasoy D, Kristensen SL, Køber L, Torp-Pedersen C, Gislason GH, Hansen ML. Bleeding after initiation of multiple antithrombotic drugs, including triple therapy, in atrial fibrillation patients following myocardial infarction and coronary intervention: a nationwide cohort study. Circulation. 2012;126(10):1185–93.
Freeman JV, Varosy P, Price MJ, Slotwiner D, Kusumoto FM, Rammohan C, Kavinsky CJ, Turi ZG, Akar J, Koutras C, et al. The NCDR Left Atrial Appendage Occlusion Registry. J Am Coll Cardiol. 2020;75(13):1503–18.
Cresti A, García-Fernández MA, Sievert H, Mazzone P, Baratta P, Solari M, Geyer A, De Sensi F, Limbruno U. Prevalence of extra-appendage thrombosis in non-valvular atrial fibrillation and atrial flutter in patients undergoing cardioversion: a large transoesophageal echo study. EuroIntervention: J EuroPCR Collab Working Group Interventional Cardiol Eur Soc Cardiol. 2019;15(3):e225–30.
Dukkipati SR, Kar S, Holmes DR, Doshi SK, Swarup V, Gibson DN, Maini B, Gordon NT, Main ML, Reddy VY. Device-Related Thrombus After Left Atrial Appendage Closure: Incidence, Predictors, and Outcomes. Circulation. 2018;138(9):874–85.
Fauchier L, Cinaud A, Brigadeau F, Lepillier A, Pierre B, Abbey S, Fatemi M, Franceschi F, Guedeney P, Jacon P, et al. Device-Related Thrombosis After Percutaneous Left Atrial Appendage Occlusion for Atrial Fibrillation. J Am Coll Cardiol. 2018;71(14):1528–36.
Yu J, Bai Y, Jiang LS: Device related thrombus after left atrial appendage closure: State of the art. Pacing and clinical electrophysiology: PACE 2020.
Boersma LV, Ince H, Kische S, Pokushalov E, Schmitz T, Schmidt B, Gori T, Meincke F, Protopopov AV, Betts T, et al. Evaluating Real-World Clinical Outcomes in Atrial Fibrillation Patients Receiving the WATCHMAN Left Atrial Appendage Closure Technology: Final 2-Year Outcome Data of the EWOLUTION Trial Focusing on History of Stroke and Hemorrhage. Circulation Arrhythmia and electrophysiology. 2019;12(4):e006841.
Asmarats L, Cruz-González I, Nombela-Franco L, Arzamendi D, Peral V, Nietlispach F, Latib A, Maffeo D, González-Ferreiro R, Rodríguez-Gabella T, et al. Recurrence of Device-Related Thrombus After Percutaneous Left Atrial Appendage Closure. Circulation. 2019;140(17):1441–3.
Sivasambu B, Arbab-Zadeh A, Hays A, Calkins H, Berger RD. Delayed endothelialization of watchman device identified with cardiac CT. J Cardiovasc Electrophys. 2019;30(8):1319–24.
Torrado J, Buckley L, Durán A, Trujillo P, Toldo S, Valle Raleigh J, Abbate A, Biondi-Zoccai G, Guzmán LA. Restenosis, Stent Thrombosis, and Bleeding Complications: Navigating Between Scylla and Charybdis. J Am Coll Cardiol. 2018;71(15):1676–95.
Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, Haeusler KG, Oldgren J, Reinecke H, Roldan-Schilling V, et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39(16):1330–93.
Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, Jüni P, Kastrati A, Kolh P, Mauri L, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS. Eur J cardio-thoracic surgery: official J Eur Association Cardio-thoracic Surg. 2018;53(1):34–78.
Boersma LV, Schmidt B, Betts TR, Sievert H, Tamburino C, Teiger E, Pokushalov E, Kische S, Schmitz T, Stein KM, et al. Implant success and safety of left atrial appendage closure with the WATCHMAN device: peri-procedural outcomes from the EWOLUTION registry. Eur Heart J. 2016;37(31):2465–74.
Søndergaard L, Wong YH, Reddy VY, Boersma LVA, Bergmann MW, Doshi S, Kar S, Sievert H, Wehrenberg S, Stein K, et al. Propensity-Matched Comparison of Oral Anticoagulation Versus Antiplatelet Therapy After Left Atrial Appendage Closure With WATCHMAN. JACC Cardiovasc interventions. 2019;12(11):1055–63.
Acknowledgements
Not applicable.
Funding
The author (s) received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
Fadong Chen conceived and designed this study. Yunan Yu, Jing Xu, Liang Wang, Zi Ye and Zhisong Chen collected the data. Yunan Yu, Jing Xu, Liang Wang performed the statistical analysis and drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
The study was carried out according to the principles of the declaration of Helsinki and was approved by the ethics committees of Shanghai Tongji Hospital and East Hospital. Informed consent was signed by all study participants.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yu, Y., Xu, J., Wang, L. et al. Left atrial appendage closure in nonvalvular atrial fibrillation patients with percutaneous coronary intervention. BMC Cardiovasc Disord 22, 433 (2022). https://doi.org/10.1186/s12872-022-02865-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-022-02865-6