Abstract
Introduction
Left atrial appendage closure (LAAC) is a novel treatment for stroke prevention in high-risk patients with non-valvular atrial fibrillation (NVAF). However, the long-term outcomes after LAAC in Chinese NVAF patients are still lacking.
Methods
This was a single-center, bidirectional, nonrandomized registered study. Patients who underwent LAAC implantation from May 2014 to April 2021 in a large Chinese center were enrolled. The primary endpoint was combined all-cause death and stroke.
Results
From May 2014 to April 2021, a total of 673 NVAF patients were enrolled. The overall successful implantation rate was 97.62% (657 of 673). The rate of perioperative adverse events was 1.19% (8 of 673), including 3 cardiac tamponades, 2 ischemic strokes, one device-related thrombus (DRT) and 2 device dislocations. 604 (92.24%) patients completed the follow-up, the median follow-up period was 36.9 months (IQR 24.8–56.5 months). 16 stroke events occurred in 15 patients (one patient suffered from both hemorrhagic and ischemic strokes). 13 patients (2.15%) had ischemic stroke, and the fatal rate was 0.33% (2 of 604). 3 patients (0.15%) suffered from hemorrhagic stroke, and the fatal rate was 0.17% (1 of 604). The overall stroke rate was 0.74% per-year. The combined death and stroke rate was 1.93% per-year. In the multivariate Cox regression analysis, age ≥ 75 (hazard ratio 2.264, 95% CI 1.074–4.772, P = 0.032) and ventricular cardiomyopathy (hazard ratio 2.738, 95% CI 1.060–7.071, P = 0.037) were independent predictors of combined mortality and stroke.
Conclusion
The overall successful implantation rate of LAAC was 97.62% and the rate of perioperative adverse events was 1.19% in this study, and the stroke rate was 0.74% per year during the long-term follow-up. Age ≥ 75 years and ventricular cardiomyopathy were independent predictors of the primary endpoint.
Trial registration This study was retrospectively registered.
Similar content being viewed by others
Introduction
Non-valvular Atrial fibrillation (NVAF) is a common type of cardiac arrhythmia, related with an increased risk of systemic thromboembolism [1, 2]. Previous data has demonstrated that 90% originated from the left atrial appendage (LAA) in NVAF patients [3].
Traditionally, warfarin was a primary treatment protocol of stroke prevention in AF patients. However, it has some disadvantages, such as, high-risk to bleed, susceptible to food and drugs interactions, narrow range of effective doses and frequent need of INR testing. The novel oral anti-coagulants(NOACs) are recommended by most AF guidelines as the first-line stroke prevention medications due to its safety and efficacy [4]. LAA closure (LAAC) has emerged as an alternative for NVAF patients with contraindication for long-term OACs or a high propensity for bleeding or poor drug compliance [5]. The CAP and CAP2 registries [6] showed that LAAC is a safe and effective therapy for stroke prevention in high-risk NVAF patients. The five years meta-analysis of PROTECT and PREVAIL [7] showed that LAAC was non-inferior to warfarin. In long-term follow-up of PRAGUE-17 [8], LAAC remains non-inferior to DOACs in high-risk NVAF patients.
The long-term outcome of LAAC in Chinese AF patients are still lacking. Our bidirectional (prospective, retrospective), single-center study was designed to investigate the long-term outcomes after percutaneous LAAC in patients with NVAF in a registry in mainland China. Periprocedural success and complications were also collected.
Methods
Study population
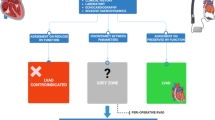
From May 2014 to April 2021, a total of 673 AF patients underwent LAAC were included in our study (retrospectively from 2014 to 2018, prospectively from 2019 to 2021), as shown in Fig. 1. The indications for LAAC operations were as follows: over 18 years of age, presented with paroxysmal or persistent non-valvular AF, CHA2DS2-VASc score ≥ 1, complicated with at least one of the following situations: a high bleeding risk (HAS-BLED score ≥ 3), a contraindication or unwillingness to long-term OACs, or having stroke/TIA despite of regular anticoagulant therapy.
There were exclusion criteria as follows: patients with rheumatic valvular heart disease, mechanical valve replacement, or atrial fibrillation caused by congenital heart disease. The expected lifetime was less than 1 year. Women who are pregnant, lactating or who have a pregnancy plan in the next two years. According to the investigator's judgment, the patients may fail the follow-up.
Baseline clinical characteristics like gender, age, hypertension, diabetes mellitus, coronary artery disease, cardiomyopathy, congenital heart disease, congestive heart failure, ischemic stroke/TIA history, CHA2DS2-VASc score, HAS-BLED score and body mass index (BMI) were recorded for every patient.
The study was approved by the local ethics board. The retrospective data were collected and analyzed anonymously. Informed consent were obtained for all prospectively enrolled patients before the LAAC procedure.
Device implantation operations
The LAAC device was implanted via a septal approach using a catheter-based delivery system. Briefly, the operations were conducted under general anesthesia and tracheal intubation. After the TEE-guided atrial septum puncture, LAA angiography of the right anterior oblique (RAO)at 30° plus caudal (CAU)at 20° was performed for LAA measurements. LAAC devices with suitable diameters were delivered through a catheter-based delivery system and expanded to close the LAA openings. During the procedures, TEE was performed to confirm the LAA closure. Compression ratio of LAAC devices was calculated immediately after the procedures.
In-hospital management and follow-up
After the implantation procedures, patients were transferred to the cardiac care unit (CCU) for anesthesia recovery. A trans-thoracic echo-cardiograph (TTE) was performed at the day of the operation to rule out cardiac effusion or device-related embolism. Then, 4–5 days of observing were completed before the patients discharging.
After the device implantation, patients received warfarin (INR ranges of 2.0–3.0) or NOACs for at least 45 days. 45 days after the procedures, TEE or enhanced left atrial CTA was performed to assess the residual flow, stability of the device, and device-related thrombus-formation. After the closure of LAAs was confirmed, anticoagulants (warfarin/NOACs) were discontinued. Patients then took a combination of Aspirin and Clopidogrel for an additional 4.5 months. After that, patients were prescribed with long-term Aspirin alone.
Definition of endpoints
The primary endpoints of the study were death and stroke rate during the long-term follow-up. The secondary endpoints included TIA, bleeding events and heart failure readmissions during the follow-up. Peri-procedural complications including pericardial tamponade, major-leakage (> 5 mm), device dislocation and device thrombo-embosis were also collected.
Statistical analysis
Data were presented with mean and standard deviation (SD) for continuous variables or with n and percentage for categorical variables. The Kaplan–Meier graph was used to illustrate the long-term cumulative survival rates during the long-term follow-up, computed with Graphpad Prism 9.0 software. Predictors for death and stroke rate were identified by univariate and multivariable Cox regression analysis. Variables with a P < 0.1 in the univariate analysis were incorporated into the multivariable model. All tests were two-tailed, P values of less than 0.05 was considered statistically significant. Statistical analyses were completed with SPSS v.26.0 statistical analysis software packet.
Results
Baseline characteristics
During the period from May 2014 to April 2021, 673 patients who underwent LAAC in our center were included in this study. Of these, 397 (59.0%) were male, and the average age was 66.00 ± 8.9 years. The mean BMI was 26.04 ± 3.75 kg/m2. 5.3% had Ventricular cardiomyopathy, and 32.8% had prior stroke/TIA. 339(50.4%) patients had hypertension, 207 (30.8%) patients had coronary heart disease, and 85 (12.6%) had diabetes mellitus. The average CHA2DS2-VASc score was 2.92 ± 1.67, and the average HAS-BLED score was 1.54 ± 1.08 (Table 1).
Periprocedural complications
Of the 673 patients who underwent LAAC in our study. the implantation attempts were aborted in 11 patients due to unfavorable LAA anatomy (determined by in-procedural angiography). 3 implantation operations were considered unsuccessful due to major peri-device leakages(> 5 mm). Other failure reasons included one device dislocation (the device was successfully snared but re-implantation was not attempted) and one cardiac tamponade requiring surgical intervention and LAA ligation. Ultimately, the successful rate was 97.62% (657 of 673). Of these, 533 (80.76%) were implanted with Watchman devices, 62 (9.38%) were implanted with Lambre devices, 52 (7.88%) were implanted with ACP devices, 12 were implanted with devices of other brands and 1 with Lefort device (Table 2).
The rate of peri-operative adverse events was 1.19% (8 of 673). Pericardial tamponade occurred in 3 patients (one patient had surgical LAA ligation, the other 2 underwent percutaneous pericardial drainage). Peri-procedural cerebral infarction was recorded in 2 patients. One received non-interventional medical treatment and fully recovered. Another one had hemiplegia and suffered from a second stroke 2 months after LAAC, which caused cognitive impairment and worsened limb hemiplegia. Device dislocation occurred in 2 patients. One was successful snared but re-implantation was not attempted. The patient had repeated ischemic stroke attacks (three times) during the long-term follow-up, leaving cognitive impairment and limb hemiplegia. For the other patient, the dislocated LAAC device was successfully snared and a new device was implanted. He completed the follow-up without any events.
Device-related thrombus (DRT) occurred in 1 patient (0.15%). The patient was put on anticoagulation treatment and no cerebrovascular event occurred during the peri-procedure period. However, the thrombosis on the device did not disappear at the 45 days TEE follow-up. Despite of the long-term anticoagulation treatment, the patient had fatal ischemic stroke 4 years after LAAC despite of continued anticoagulation. Pericardial effusion of more than 10 mm occurred in 10 patients (1.49%) all of which had no symptoms and absorbed simultaneously.
Clinical outcomes during follow-up
At the 45-days follow-up, 575 patients received TEE or left atrial CTA. Major leakage or DRT occurred in 13 patients (Table 3). All 13 patients continued anticoagulation and completed the follow-up. 5 patients had major leakage (> 5 mm) around the devices. of whom 3 patients already had major leakage during the procedures. DRT was observed in 8 patients.
The most frequent types implanted were Watchman (80.76%), Lambre (9.38%) and ACP (7.88%). At the 45-days follow-up, the leakage rate was 43% (205/466) for the Watchman devices, 31% (17/54) for ACP devices, and 26% (11/42) for the Lambre devices. The differences were statistically significant (P < 0.05) (Fig. 2). The rate of peri-procedural complications and the rate of DRTs were not different among the three device types.
During a median follow-up of 36.9 months (2018 patient-years). One of the patients with major leakage had ischemic stroke 2 years after the procedure, leaving cognitive impairment and limb hemiplegia. Among the 8 patients with DRT, 2 patients lost follow-up, and 6 patients had no events during the long-term follow-up.
After the exclusion of 47 patients who were lost to follow-up and 22 patients with periprocedural complications or major leakage, complete data were obtained in 604 patients (Fig. 1). The loss rate was 7.76%. The overall death rate was 4.47% (27 of 604). The cardiac death rate was 1.66%(10 of 604), including heart failure 0.99% (6 of 604), and myocardial infarction 0.66% (4 of 604) (Table 4).
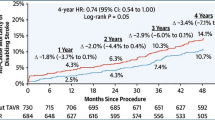
The overall stroke rate was 0.74% per-year. All-cause stroke occurred in 15 patients, including 13 ischemic stroke (2.15%) and 3 hemorrhagic stroke (one patient had one ischemic stroke and one hemorrhagic stroke). The fatality rate of ischemic stroke was 0.33% (2 of 604). The disabling rate of ischemic stroke was 0.99% (6 of 604). The fatality rate of hemorrhagic stroke was 0.17% (1 of 604). The disabling rate of hemorrhagic stroke was 0.17% (1 of 604). The cumulative rate of TIA was 1.66% (10 of 604).
Bleeding occurred in 64 patients, including cerebral bleeding in 3 patients, and minor bleeding in 61 patients (e.g., gastrointestinal bleeding, gingival bleeding, tarry stool, nasal hemorrhage, and all minor bleeding improved after discontinuing antiplatelet or anticoagulant drugs). Heart failure readmission occurred in 58 patients. The Kaplan–Meier graph was used to illustrate the complications on long-term cumulative survival (Fig. 3).
a Kaplan–Meier graph showing long-term cumulative survival from all-cause death and stroke; b Kaplan–Meier graph showing long-term cumulative survival from all-cause stroke; c Kaplan–Meier graph showing long-term cumulative survival from all-cause death; d Kaplan–Meier graph showing long-term cumulative survival from cardiac mortality; e Kaplan–Meier graph showing long-term cumulative survival from ischemic stroke and TIA; f Kaplan–Meier graph showing long-term cumulative survival from cerebral bleeding
The cumulative rate of combined death and stroke was 1.93% per-year. In the univariate Cox regression analysis (Table 5), the history of cardiomyopathy (hazard ratio 2.715, 95% CI 1.137–6.484, P = 0.025) was a predictor of mortality and stroke after LAAC implantation. In the multivariate Cox regression analysis, age ≥ 75 (hazard ratio 2.074, 95% CI 1.003–4.285, P = 0.049) and the history of cardiomyopathy (hazard ratio 2.959, 95% CI 1.231–7.116, P = 0.015) were independent predictors of mortality and stroke after LAAC implantation at long-term follow-up (Fig. 4).
Discussion
The main findings of this study are: (1) LAAC is a safe and effective stroke prevention treatment with satisfactory outcomes in Chinese NVAF population; (2) The rate of combined death and stroke was 1.93% per-year; (3) Age ≥ 75 yr and the history of ventricular cardiomyopathy were independent risk factors for combined death and stroke after LAAC. This long-term followed cohort provides more representational clinical outcomes for NVAF patients from main land Chinese population.
LAAC is an alternative to warfarin or NOACS for lifelong stroke prevention in high-risk patients with NVAF [9]. Especially in elderly NVAF patients with high bleeding risk [10]. The ESC Guidelines recommended that LAAC may be considered for stroke prevention in patients with NVAF and contraindications for long-term anticoagulant treatment (class IIb, level of evidence B) [4].
The LAAC success rate increased from 90.9% in PROTECT to 94.4% in CAP, 95.1% in PREVAIL, 94.8% in CAP2, and reached 98.5% in EWOLUTION [11]. The total successful rate was 97.62% in our study, being comparable to that in the EWOLUTION study Among the 11 patients whose implantation were aborted, the most common reason had been unfavorable anatomy (large opening of the LAAC or mismatch between the LAA and the device). So assessment of the LAA anatomy is crucial for success implantation of LAAC devices. Major leakage(> 5 mm) was observed in 3 patients during the procedures, which were also considered unsuccessful. In another 2 failed patients, one had device dislocation and re-implantation was not attempted; the other had cardiac tamponade requiring surgical intervention.
The rate of perioperative adverse events was about 1.19%, significantly lower than MAUDE database [12] and NCDR registry study [13]. DRT is a fatal complication of LAAC, which is associated with the risk of visceral thromboembolism [14]. Aggressive treatment is required once DRT formation is detected. At 45-day follow-up, 575 patients received TEE or left atrial CTA. A total 8 cases of DRT were detected, among whom 2 patients lost follow-up, and 6 patients had no events during the follow-up. In the previous studies, long-term DRT after 45 days was not uncommon. Boersma et al. [15] reported that there was no statistical relations between DRT and types of anticoagulants post LAAC. Chen et al. [16] reported that NOACs after LAAC appear to be as effective as warfarin in preventing DRT, with lower bleeding rate, during a mean follow-up of 868 days. A single-center study [17] investigated 319 patients who underwent LAAC, TEE follow-up was conducted at intra-procedure, 45 days and 6 months after the index procedures. At 6 months after LAAC, DRT was detected in 14 patients and might be related with peri-device leakage. Sedaghat et al. [18] showed that among 835 patients who completed the TEE follow-up, DRT was detected in 4.1% of patients at 54 days (median) and 91.2% within 3 months. Persistent AF was an independent factor of device thrombosis, and new onset DRT was often detected 3 months after LAAC. Other studies have emphasized the need of TEE to detect DRT more than 6 months after LAAC [19]. Unfortunately, we did not perform long-term TEE follow-up in this study, so the rate of new onset DRT after the termination of oral anticoagulation was unclear. Anyway, considering the overall stroke and TIA rate during the follow-up, we might conjecture that the rate of long term DRT is reasonably low, which could be attributed to active and regular anticoagulation and control of AF.
During a median follow-up of 36.9 months (2018 patient-years) in our study. The rate of death and stroke was 1.93% per-year. Meta-analysis outcomes from the PREVAIL and PROTECT AF trials [7] showed that the all-cause stroke and systemic embolism rate was 1.7% per-year, and the all-cause death was 3.6% per-year in the LAA device group. Data from the Boersma study [15] analyzed 1020 patients during a 2-year follow-up, the stroke rate was 1.3% per-year, the mortality rate was 16.4%. During 3.5 years of follow-up (1,354 patient-years), the mortality rate and all-cause stroke rate in the LAAC arm of PRAGUE-17 trial [8] was 6.23% per-year and 2.08% per-year, respectively. All-cause mortality rates in the CAP and CAP 2 registries [6] were 4.27% and 6.24%, respectively. And all-stroke were 1.48% and 2.25%, respectively. Chiu et al. [20] reported that, during mean 28 months follow-up, the ischemic stroke rate was 1.9% per-year in the Watchman group and 1.4% per-year in the ACP/Amulet group. Compared with aforementioned studies, our study showed comparatively lower mortality and stroke rate. which might be attributed to the fact that our patients had been relatively younger and had lower CHA2DS2-VASc score. Besides, patients with major leakage or DRTs in the procedure and at the 45-day TEE follow-up were excluded from the long-term follow-up analysis, as was shown in Fig. 1.
In multivariate Cox regression analysis, age ≥ 75 (hazard ratio 2.074, 95% CI 1.003–4.285, P = 0.049) and ventricular cardiomyopathy (hazard ratio 2.959, 95% CI 1.231–7.116, P = 0.015) were independent predictors of mortality and stroke after LAAC implantation during the long-term follow-up. Among our ventricular cardiomyopathy patients, 15 are HCM (41.7%). The rate of stroke or death in the HCM patients were particularly high (2 strokes and 2 deaths). While HCM patients are at high risks for stroke and long-term anticoagulation is recommended [21, 22], LAAC does not seem like a good alternative.
One review of HCM management [23] reported that the mortality and stroke rates of HCM patients with AF decreased by 11.6 and 3.5 times, respectively, compared with 2001. For HCM patients with newly-onset symptomatic AF or asymptomatic AF, anticoagulant should be prescribed after evaluating the advantages and disadvantages. Bin-Feng Mo et al. [24] reported that LAAC is safe for primary and secondary stroke prevention in patients with HCM and AF. There were no thromboembolic or death events in HCM patients after LAAC during a mean follow-up of 28.4 months. However, our study showed high stroke and death rate. The patients in our study are predominantly persistent AF, had larger LA and greater LV wall thickness. It seems that in these patients LAAC is not suitable for stroke prevention. Zhang et al. [25] in their study also suggested that LAAC might be unsuitable for AF with ventricular cardiomyopathy because their thrombi were frequently formed in the sites other than LAA.
Limitations
This study is a single-center, non-randomized study, which lacks a control group and is prone to selective bias. In our study, ventricular cardiomyopathy was independently related with post LAAC death and stroke rate during the long term follow-up. However, the enrolled patients with ventricular cardiomyopathy were mainly non-ischemic cardiomyopathy. Ischemic cardiomyopathy had been very rare and thus was not included in the analysis. Our outcome should be interpreted and extrapolated with caution.
Conclusion
The overall successful implantation rate of LAAC was 97.62% and the rate of perioperative adverse events was 1.19% in this study, and the stroke rate was 0.74% per year during the long-term follow-up. Age ≥ 75 years and ventricular cardiomyopathy were independent predictors of the primary endpoint.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- LAAC:
-
Left atrial appendage closure
- NVAF:
-
Non-valvular atrial fibrillation
- DRT:
-
Device-related thrombus
- NOACs:
-
Novel oral anti-coagulants
- BMI:
-
Body mass index
- CCU:
-
Cardiac care unit
- TTE:
-
Trans-thoracic echo-cardiograph
- TEE:
-
Trans-esophageal echocardiography
- TIA:
-
Transient ischemic attacks
- SD:
-
Standard deviation
- DCM:
-
Dilated cardiomyopathy
- HCM:
-
Hypertrophic cardiomyopathy
- PDL:
-
Peri-device leakage
- LAD:
-
Left atrial diameter
- LVEDD:
-
Left ventricular end-diastolic diameter
- Cl:
-
Confidence interval
- HR:
-
Hazard ratio
- LVEF:
-
Left ventricular ejection fraction
References
DeSimone CV, Prakriti BG, Tri J, et al. A review of the relevant embryology, pathohistology, and anatomy of the left atrial appendage for the invasive cardiac electrophysiologist. J Atr Fibrillation. 2015;8:1129.
Watson T, Shantsila E, Lip GY. Mechanisms of thrombogenesis in atrial fibrillation: Virchow’s triad revisited. Lancet. 2009;373:155–66.
Stoddard MF, Dawkins PR, Prince CR, et al. Left atrial appendage thrombus is not uncommon in patients with acute atrial fibrillation and a recent embolic event: a transesophageal echocardiographic study. J Am Coll Cardiol. 1995;25:452–9.
Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–962.
Zweiker D, Sieghartsleitner R, Fiedler L, et al. Indications and Outcome in Patients Undergoing Left Atrial Appendage Closure-The Austrian LAAC Registry. J Clin Med 2020; 9.
Holmes DR, Jr., Reddy V Y, Gordon N T, et al. Long-term safety and efficacy in continued access left atrial appendage closure registries. J Am Coll Cardiol. 2019;74:2878–89.
Reddy VY, Doshi SK, Kar S, et al. 5-Year outcomes after left atrial appendage closure: from the PREVAIL and PROTECT AF trials. J Am Coll Cardiol. 2017;70:2964–75.
Osmancik P, Herman D, Neuzil P, et al. 4-Year outcomes after left atrial appendage closure versus Nonwarfarin oral anticoagulation for atrial fibrillation. J Am Coll Cardiol. 2022;79:1–14.
Reddy VY, Akehurst RL, Gavaghan MB, et al. Cost-effectiveness of left atrial appendage closure for stroke reduction in atrial fibrillation: analysis of pooled, 5-year. Long-Term Data J Am Heart Assoc. 2019;8: e011577.
Yu J, Chen H, Post F, et al. Efficacy and safety of left atrial appendage closure in non-valvular atrial fibrillation in patients over 75 years. Heart Vessels. 2019;34:1858–65.
Boersma LV, Ince H, Kische S, et al. Efficacy and safety of left atrial appendage closure with WATCHMAN in patients with or without contraindication to oral anticoagulation: 1-Year follow-up outcome data of the EWOLUTION trial. Heart Rhythm. 2017;14:1302–8.
Ledesma PA, Uzomah UA, Yu X, et al. MAUDE database analysis of post-approval outcomes following left atrial appendage closure with the watchman device. Am J Cardiol. 2021;152:78–87.
Freeman JV, Varosy P, Price MJ, et al. The NCDR left atrial appendage occlusion registry. J Am Coll Cardiol. 2020;75:1503–18.
Saw J, Nielsen-Kudsk JE, Bergmann M, et al. Antithrombotic therapy and device-related thrombosis following endovascular left atrial appendage closure. JACC Cardiovasc Interv. 2019;12:1067–76.
Boersma LV, Ince H, Kische S, et al. Evaluating real-world clinical outcomes in atrial fibrillation patients receiving the WATCHMAN left atrial appendage closure technology: final 2-year outcome data of the EWOLUTION Trial focusing on history of stroke and hemorrhage. Circ Arrhythm Electrophysiol. 2019;12: e006841.
Chen Y, Zhang Y, Qu L, et al. Short-term non-vitamin K antagonist oral anticoagulants vs. warfarin in preventing device-related thrombosis after left atrial appendage closure. J Thromb Thrombolysis. 2021;52:872–9.
Bai Y, Xue X, Duenninger E, et al. Real-world survival data of device-related thrombus following left atrial appendage closure: 4-year experience from a single center. Heart Vessels. 2019;34:1360–9.
Sedaghat A, Nickenig G, Schrickel JW, et al. Incidence, predictors and outcomes of device-related thrombus after left atrial appendage closure with the WATCHMAN device-Insights from the EWOLUTION real world registry. Catheter Cardiovasc Interv. 2021;97:E1019-e1024.
Sedaghat A, Vij V, Al-Kassou B, et al. Device-related thrombus after left atrial appendage closure: data on thrombus characteristics, treatment strategies, and clinical outcomes from the EUROC-DRT-registry. Circ Cardiovasc Interv. 2021;14: e010195.
Chiu FC, Huang PS, Chen JJ, et al. Long-term outcomes of percutaneous left atrial appendage closure for the prevention of stroke in patients with atrial fibrillation: Asia-Pacific experience. J Formos Med Assoc 2021.
Liu L, Liu Z, Chen X, et al. Thromboembolism in patients with hypertrophic cardiomyopathy. Int J Med Sci. 2021;18:727–35.
Jung H, Sung JH, Yang PS, et al. Stroke risk stratification for atrial fibrillation patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2018;72:2409–11.
Maron BJ, Desai MY, Nishimura RA, et al. Management of hypertrophic cardiomyopathy: JACC State-of-the-Art review. J Am Coll Cardiol. 2022;79:390–414.
Mo BF, Zhang R, Yuan JL, et al. Left atrial appendage closure for primary and secondary stroke prevention in patients with hypertrophic cardiomyopathy and atrial fibrillation: a pilot study. Front Cardiovasc Med. 2021;8: 719755.
Zhang H, Yu M, Xia Y, et al. The differences of atrial thrombus locations and variable response to anticoagulation in nonvalvular atrial fibrillation with ventricular cardiomyopathy. J Arrhythm. 2020;36:1016–22.
Acknowledgements
Not applicable.
Funding
This work was supported by 2019 Wuhan Health Committee Youth Project for Su Xi (WX19Q13), funded by 2018 Wuhan Young & Middle-aged Medical Backbone Training Program, 2020 Wuhan Medical Research Project (WX20C44), and Hubei provincial health and family planning commission joint fund (WJ2018H0043) for Chen Yanhong.
Author information
Authors and Affiliations
Contributions
CC, YHC and XS designed the protocol. CC analyzed the data and drafted the manuscript. CC, YC and LQ collected the data. CC, YHC and XS revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study complied with the Declaration of Helsinki and was approved by the medical ethical committee of Wuhan Asia Heart Hospital, China (No: 2018-YXKY-B017). As this was a retrospective analysis, patients’ informed consent was waived by the institutional review board (The medical ethical committee of Wuhan Asia Heart Hospital, China). Informed consent were obtained for all prospectively enrolled patients before the LAAC procedure.
Consent for publication
Not applicable.
Competing interests
The authors have no conflicts of interest relevant to this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, C., Chen, Y., Qu, L. et al. 3-Year outcomes after left atrial appendage closure in patients with nonvalvular atrial fibrillation: cardiomyopathy related with increased death and stroke rate. BMC Cardiovasc Disord 23, 27 (2023). https://doi.org/10.1186/s12872-023-03054-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-023-03054-9