Abstract
Background
Hypoalbuminemia is linked to the emergence of cardiovascular events. However, there is an unclear association between serum albumin (ALB) and gender in paroxysmal AF patients. This retrospective study aimed to explore the association between ALB levels and paroxysmal AF by gender in a Chinese population.
Methods
This study included patients with paroxysmal AF who were hospitalized consecutively in China from January 2019 to September 2021. Controls with sinus rhythm and without paroxysmal AF were matched (2:1) to cases by gender and age. Pearson correlation analysis was used to study the correlation between ALB and blood lipid profiles, multivariate regression models were performed to investigate the association between ALB and paroxysmal AF.
Results
There were 305 patients with paroxysmal AF and 610 patients with controls included in this study. Low ALB in male with AF patients were significantly associated with paroxysmal AF (OR = 0.889, 95% CI 0.832–0.950). ALB was positively correlated with triglyceride (TG) (r = 0.212, p < 0.001), total cholesterol (TC) (r = 0.381, p = 0.002), low-density lipoprotein cholesterol (LDL-C) (r = 0.263, p < 0.001), and high-density lipoprotein cholesterol (HDL-C) (r = 0.329, p < 0.001).
Conclusion
Low ALB in male patients is significantly associated with paroxysmal AF in a Chinese population. Monitoring for hypoalbuminemia in men might help reduce the incidence of paroxysmal AF.
Similar content being viewed by others
Introduction
Atrial fibrillation (AF) with a lifetime risk of about 25% represents an increasing public health concern, the most common clinical arrhythmia encountered in clinical practice, currently affecting almost 33 million subjects worldwide accompanied by growing morbidity and mortality especially in developed countries [1, 2]. Stroke and heart failure are serious possible consequences of AF [3], as well as an increased risk of embolism and death [4, 5]. Despite the introduction of therapeutic strategies such as antiarrhythmic drugs, electrical cardioversion, and catheter ablation, it seems that we are still far from highly effective treatment, with only a 66.6% success rate of a single operation for paroxysmal AF [3, 6, 7]. The era of AF prevention has come, and the management of risk factors has become a new paradigm of AF intervention [8]. Although recent guideline hasn’t recommended the use of specific biomarkers for AF [9], it has proved to be a valuable perspective for specific serum biomarkers to be used to understand and manage AF [10].
Serum albumin (ALB) has many biochemical properties [11], including anti-inflammatory, antioxidant, anticoagulant, antiplatelet aggregation, and colloid osmotic effect. Evidence is growing that hypoalbuminemia is considered a modifiable risk factor associated with cardiovascular events [12,13,14], mainly due to malnutrition and inflammation [15,16,17]. Evidence suggests that inflammation and oxidative stress are key to initiating and maintaining AF [18, 19]. Given the anti-inflammatory and antioxidant biochemical properties of serum albumin, we speculate that hypoalbuminemia could act as an important modifiable risk factor for AF [20, 21]. Recently, a prospective cohort study from 12,833 participants in the USA reported that serum ALB was independently inverse related to incident AF in a linear pattern [22]. Another earlier prospective cohort study from 8,870 participants in the Copenhagen City Heart Study (CCHS) also indicated that serum ALB was inversely associated with the risk of AF only in women (HR 0.47, 95% CI 0.28–0.77) [23]. Consequently, it could be speculated that hypoalbuminemia may be involved in the initiation and maintenance of AF; however, it is unclear if the results are the same in the paroxysmal AF population. Moreover, the gender relationship between serum ALB and AF has not been fully explored.
Hereby, in this case–control study, we aim to characterize the association between the ALB levels and paroxysmal AF by gender in 915 patients from China. We hypothesized that low serum ALB is associated with paroxysmal AF, the confirmation of this hypothesis has significant clinical value because serum ALB is adjustable.
Methods
Study population
This study included patients with paroxysmal AF who were hospitalized consecutively from a single center in China between January 2019 and September 2021 in the electronic medical record system. The inclusion criteria for cases were as follows: (1) patients from the community who were hospitalized for less than 21 days, lived a healthy lifestyle through medical history, and a normal nutritional status through the subjective global assessment (SGA) questionnaire. (2) patients aged 28–80 years. (3) Paroxysmal AF was diagnosed by clinicians and clearly documented in the electronic medical record. The exclusion criteria were as follows: (1) cardiac surgery, valvular disease, and heart failure. (2) peripheral vascular disease, prior thromboembolism, and major bleeding. (3) severe hepatic or renal dysfunction on record. (4) malignancy, hyperthyroidism, or gout. (5) pregnant woman. (6) use of uric acid-lowering drugs. Controls were also routine hospital inpatients, most of whom lived in the communities. Controls with sinus rhythm and without paroxysmal AF were matched (2:1) to cases by gender and age. In the current study design, we initially included a total of 2000 patients, including 354 patients with paroxysmal AF, 27 patients were excluded due to abnormal nutritional status based on SGA assessment, and 22 patients met other exclusion criteria. Finally, we included 305 patients with paroxysmal AF and 610 age- and sex-matched controls. This study was based on the principles of the Helsinki Declaration and approved by the Medical Research Ethics Committee of the Affiliated Hospital of Shandong University of Traditional Chinese Medicine. Because the data are anonymized, the Ethics Committee of Affiliated Hospital of Shandong University of Traditional Chinese Medicine (NO.20200512FA62) waived the need for informed consent.
Study variables
Demographic and clinical variables included gender, age, AF comorbidities (coronary heart disease (CHD), hypertension, and diabetes), and laboratory variables (ALB, triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), apolipoprotein A1 (APOA1), serum apolipoprotein B (APOB), lipoprotein (a) [La(a)], serum creatinine (Scr), serum uric acid (SUA), aspartate aminotransferase (AST), and alanine aminotransferase (ALT). These data were obtained during routine hospital procedures and then reviewed by us through the hospital's electronic medical record system. All disease diagnoses were made by professional cardiologists and all serum indicators were determined by hospital laboratories.
Definition and identification of paroxysmal AF
According to the reported guidelines [24], paroxysmal AF was defined as atrial fibrillation that was able to terminate spontaneously or by intervention within 7 days of onset. All patients with paroxysmal AF were identified and diagnosed by specialized clinicians.
Classification of ALB levels and definition of hypoproteinemia
The serum ALB level conversion standard was as follows: 1 g/L = 0.1 g/dL. Levels of serum ALB were classified by tertiles. Serum ALB level in male patients was classified into 3 categories: < 37.9 mg/dL, 37.9–41.7 mg/dL, and > 41.7 mg/dL. Likewise, serum ALB level in female patients was classified into 3 categories: ALB level < 37.7 mg/dL, 37.7–41.1 mg/dL and > 41.1 mg/dL. As previously reported [25], ALB level < 3.5 g/dL was indicated as hypoproteinemia.
Statistical analysis
All analyses were conducted in SPSS software (version 26.0, SPSS Inc., Chicago, IL, USA) and GraphPad Prism software (version 9.0.0). Continuous data were expressed as mean ± standard deviations (SD) or medians and interquartile ranges (IQR) where appropriate. Categorical data were presented as numbers and percentages. Baseline characteristics were compared between groups by Chi-squared tests for categorical variables and T-tests or Mann–Whitney U tests for continuous variables. Serum ALB was modeled as a continuous variable and was divided into tertiles based on sample distribution. The differences among the three groups of variables were analyzed by variance (ANOVA). Pearson correlation analyses were performed to analyze the correlation between ALB and blood lipid profiles. We used multivariable Binary Logistic Regression models to investigate the association between Serum ALB and AF. Model1 has not been adjusted. Model2 adjusted for CHD, hypertension, and diabetes. Model3 adjusted for TG, TC, LDL-C, HDL-C, APOA1, APOB, AST, SCr, and SUA. Model4 further adjusted for all these factors. A P value < 0.05 indicated significance, the tests were 2-sided.
Results
Study Population
Table 1 showed the clinical characteristics of the study population. This study included 305 patients with paroxysmal AF and 610 matched controls without paroxysmal AF. Compared with the control group, ALB levels in male and female patients with paroxysmal AF were significantly lower (p < 0.001). Patients with paroxysmal AF had significantly lower levels of TG, TC, LDL-C, HDL-C, APOA1, and APOB (P < 0.05), and significantly higher levels of AST, Scr, and SUA (P < 0.05). Additionally, the paroxysmal AF group had more patients with CHD, hypertension, diabetes, and hypoproteinemia than the controls (P < 0.05), regardless of gender.
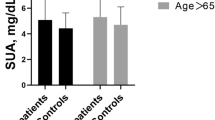
Figure 1 showed the differences in ALB levels by age in patients with paroxysmal AF and controls. Compared with controls, ALB levels in aged ≤ 60 years and aged > 60 years patients with paroxysmal AF were significantly lower (P < 0.001).
Association between ALB and paroxysmal AF
Table 2 showed the association between ALB and paroxysmal AF. After adjustment for all confounding factors, ALB in male patients was significantly associated with paroxysmal AF (odds ratio [OR] 0.889, 95% confidence interval [CI] 0.832–0.950; p < 0.001).
The difference in ALB levels among the comorbidities of paroxysmal AF
Table 3 showed the difference in ALB levels among the comorbidities of paroxysmal AF. The results suggested that there was no significant difference in ALB levels among the comorbidities of paroxysmal AF (p > 0.05), regardless of gender.
Correlation between ALB and blood lipid profiles in patients with paroxysmal AF
Figure 2 showed the correlation between ALB levels and blood lipid profiles in paroxysmal AF patients. The results showed that there was a positive correlation between ALB levels and blood lipid profiles in patients with paroxysmal AF, including TG (r = 0.212, p < 0.001), TC (r = 0.381, p = 0.002), HDL-C (r = 0.329, p < 0.001), and LDL-C (r = 0.263, p < 0.001).
Correlation between ALB levels and blood lipid profiles in paroxysmal AF patients. A Correlation between ALB levels and TG in paroxysmal AF patients (r = 0.212, p < 0.001). B Correlation between ALB levels and TC in paroxysmal AF patients (r = 0.381, p = 0.002). C Correlation between ALB levels and HDL-C in paroxysmal AF patients (r = 0.329, p < 0.001). D Correlation between ALB levels and LDL-C in paroxysmal AF patients (r = 0.263, p < 0.001)
The association between ALB levels and blood lipid profiles in patients with paroxysmal AF by gender
Table 4 showed the subgroup analysis of the association between ALB levels and blood lipid profiles in paroxysmal AF patients. The results indicated that lower ALB in male and female patients with paroxysmal AF had lower TC and LDL-C (p < 0.001). Additionally, lower ALB in male patients with paroxysmal AF had lower TG (p < 0.05) and lower HDL-C in female patients (p < 0.05).
Discussion
To our knowledge, this study is the first to evaluate the association between serum ALB and paroxysmal AF by gender in the Chinese population. Our current finding suggested that low serum low ALB levels in male patients were associated with paroxysmal AF; there was a positive correlation between ALB levels and blood lipid profiles in patients with paroxysmal AF. Nevertheless, further analyses didn’t support a significant difference in ALB levels among the comorbidities of paroxysmal AF. These findings imply that it would be interesting to monitor clinical hypoalbuminemia for clinicians to detect paroxysmal AF. We reported herein an association between low serum ALB levels and paroxysmal AF.
A recent dose–response meta-analysis reported that low serum ALB level was related to an increased risk of AF [26]. Liao et al. [22] conducted a large-scale epidemiological and mendelian randomization (MR) study to evaluate the causal influence of the serum ALB and incident AF; their results demonstrated that although serum ALB was inversely related to the incidence of AF after multiple adjustments, no evidence of a causal relationship between serum ALB levels and AF was found in Mendelian randomization (MR) analysis. Our current study, similar to this study, adjusted for several potential confounders such as CHD, hypertension, diabetes, SCr, SUA, and blood lipid profiles; moreover, the results of our study were supported by this study reporting that serum ALB was inversely associated with AF. Van et al. [27] performed a prospective cohort study to assess the relationship between serum ALB levels and new-onset AF (NOAF) in patients admitted to the ICU; similar to our study, they also analyzed data on hospitalized patients; their main result suggested that low serum ALB was related to the occurrence of NOAF; interestingly, they also found a relationship between low serum ALB land the number of onsets of NOAF; these results are also well in alignment with our main finding. Although these studies imply a close relationship between low serum ALB and AF, paroxysmal AF, which was often not adequately considered. A small retrospective study based on 32 consecutive patients and 32 age- and sex-matched paroxysmal supraventricular tachycardia patients from China reported that paroxysmal AF was associated with decreased serum ALB and hypoalbuminemia was an independent risk factor for paroxysmal AF (P = 0.0129, OR = 0.773) [28]. Compared with our study, the study design method of this study was similar, but our study had a larger sample size; we further analyzed the relationship between serum ALB and gender in patients with paroxysmal AF; meanwhile, we also adjusted for more confounders in the multivariable regression models and analyzed the correlation between serum ALB and blood lipids profiles in patients with paroxysmal AF.
The gender relationship between serum ALB and AF has not been fully explored. A prospective cohort design based on the Copenhagen City Heart Study reported that serum ALB levels only in females were inversely related to the risk of AF (hazard ratio: 0.47, 95% CI 0.28–0.77), which was inconsistent with our current results reporting that low serum low ALB levels in male patients were associated with paroxysmal AF. The main possible reason was the heterogeneity of the study population, it is clear that our study design relied on hospitalized patients in China, not the general population; more importantly, we also reliably differentiated paroxysmal AF from patients with AF; certainly, the influence of several potential unmeasured confounding factors can’t be ruled out. Admittedly, it would be interesting to perform a prospective cohort design to evaluate the association between serum ALB levels and paroxysmal AF in the future.
According to a previous review [29], hypoalbuminemia contributes to the pathological progression of cardiovascular events mainly through inflammation, oxidative stress, and platelet aggregation, as well as pulmonary and myocardial edema. Inflammation and oxidative stress have been considered to be two central mediators of atrial remodeling including electrical remodeling and structural remodeling for AF [30,31,32]. Evidence suggests a close relationship between low serum ALB and inflammatory markers. A previous study based on 4434 patients reported that serum ALB levels were negatively associated with CRP levels (r = -0.311) and white blood cell levels (r = -0.157) [33]. Acute and chronic inflammation affects serum ALB by altering liver protein metabolism and inducing capillary leakage [34,35,36]; monocyte products reduce serum ALB production during inflammation, which might be the underlying mechanisms [37]. Meanwhile, the association between hypoalbuminemia and oxidative stress has also been demonstrated [38]. Moreover, it has been shown that serum ALB has a unique biochemical structure, which forms an extracellular antioxidant defense [39]. Additionally, it must be mentioned that as an important marker of malnutrition, low ALB may also affect the proportional loss of myocardium and electrophysiological stability [28, 40, 41]. In the present study, although we didn’t evaluate the specific nutritional status indexes for hospitalized patients, we designed strict inclusion criteria, that is, patients from the community who were hospitalized for a short period of time had normal nutritional status. Therefore, we believe that it is essential to explore malnutrition-related AF in the future.
In the past, Annoura M et al. [40] reported the “cholesterol paradox”, suggesting that low TC and TG were associated with paroxysmal AF. Likewise, Psaty BM et al. [42, 43] also found the relationship between low blood lipid profiles and AF. Our current results suggesting low blood lipid profiles in paroxysmal AF are supported by this conclusion. Interestingly, we also observed that there was a positive correlation between ALB levels and blood lipid profiles in patients with paroxysmal AF. Even more so, it is possible that lower serum ALB and blood lipid profiles were jointly involved in the pathologic progression of paroxysmal AF. Although we were unable to provide more evidence for this hypothesis, it must be mentioned low HDL-C in our paroxysmal AF patients. Therefore, it could be speculated that the weaker anti-inflammatory and antioxidant properties of HDL-C can’t prevent the formation of AF matrix and some potential risk factors. Nevertheless, further subgroup analysis suggested the different associations between ALB levels and blood lipid profiles in paroxysmal AF by gender. Specifically, lower ALB in male and female patients with paroxysmal AF had lower TC and LDL-C; lower ALB in male patients with paroxysmal AF had lower TG and lower HDL-C in female patients. Gender differences in blood lipids and atrial electrophysiological characteristics [44, 45] might explain our findings.
It is well-known that hypertension, CHD, and diabetes are important risk factors for AF [46]. In our study, there was a higher incidence of hypertension, CHD, and diabetes because our hospitalized patients were from cardiology departments. We adjusted for these confounders as much as possible and analyzed the difference in ALB levels among these comorbidities of paroxysmal AF. Nevertheless, the current results didn’t support a significant difference in ALB levels among the comorbidities of paroxysmal AF. Clearly, this result wasn’t in disagreement with our findings. Certainly, it is necessary to further explore the specific relationship between serum ALB levels and isolated paroxysmal AF.
Study limitation
Several potential study limitations warrant discussion. First, the small study population was recruited retrospectively from a single center in China, which was underrepresented and may have introduced selection bias. Therefore, further multicenter studies with large sample sizes are required. Second, the causal association between ALB levels and paroxysmal AF could not be determined due to the case–control design. Thus, further prospective cohort studies also are required. Third, low ALB, as an acute phase reactant, could be related to long-term inflammation [22]. Several inflammation markers, such as C-reactive protein (CRP) and interleukin-6 (IL-6), weren’t measured in our study at baseline, so we failed to adjust it for inflammation, as well as oxidative stress markers. Fourth, we didn’t collect indicators that quantified the nutritional status of patients, such as body mass index (BMI); nonetheless, we did our best to understand patients' lifestyles by SGA and excluded patients with severely abnormal nutritional status before inclusion. Fifth, due to the limitation of sample size, confounders couldn't be adequately adjusted; many other confounding factors, such as daily exercise, smoking, drinking, medications, and family history weren’t adjusted for in our multivariate regression models. Finally, comorbidities of paroxysmal AF, including hypertension, coronary heart disease, and diabetes mellitus, may interfere with the present results, but the limited sample size makes them impossible to exclude. However, we analyzed the difference in ALB levels among the comorbidities of paroxysmal AF and adjusted for them in our logistic models. It would be interesting to investigate the association between ALB levels and lone AF in the future. Ideally, the findings of this study would be validated in a larger cohort.
Conclusion
In conclusion, our study indicates that low ALB levels in male patients are independently associated with paroxysmal AF. ALB levels are positively associated with TG, TC, HDL-C, and LDL-C. These findings imply that hypoproteinemia and low blood lipid profiles may act synergistically to involve in the pathological development of paroxysmal AF. Moreover, it would be essential to perform a prospective cohort design to investigate the causal relationships between these conditions.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ALB:
-
Albumin
- Paroxysmal AF:
-
Paroxysmal atrial fibrillation
- TG:
-
Triglyceride
- TC:
-
Total cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- HDL-C:
-
High-density lipoprotein cholesterol
- CHD:
-
Coronary heart disease
- APOA1:
-
Apolipoprotein A1
- APOB:
-
Apolipoprotein B
- Lp (a):
-
Lipoprotein (a)
- SUA:
-
Serum uric acid
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- SCr:
-
Serum creatinine
- ORs:
-
Odds ratios
- CI:
-
Confidence interval
- CRP:
-
C-reactive protein
- IL-6:
-
Interleukin-6
- BMI:
-
Body mass index
References
Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fbrillation: the Framingham Heart Study. Circulation. 2004;9:1042–6.
Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 study. Circulation. 2014;129(8):837–47.
Baman JR, Passman RS. Atrial fibrillation. JAMA. 2021;325(21):2218.
Bikdeli B, Abou Ziki MD, Lip GYH. Pulmonary embolism and atrial fibrillation: two sides of the same coin? A systematic review [published correction appears in Semin Thromb Hemost. 2017;43(8):e1]. Semin Thromb Hemost. 2017;43(8):849–63.
Waldmann V, Jouven X, Narayanan K, et al. Association between atrial fibrillation and sudden cardiac death: pathophysiological and epidemiological insights. Circ Res. 2020;127(2):301–9.
Tousoulis D. Biomarkers in atrial fibrillation; from pathophysiology to diagnosis and treatment. Curr Med Chem. 2019;26(5):762–4.
Ganesan AN, Shipp NJ, Brooks AG, et al. Long-term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta-analysis. J Am Heart Assoc. 2013;2(2): e004549.
Hendriks JM, Gallagher C, Middeldorp ME, Lau DH, Sanders P. Risk factor management and atrial fibrillation. Europace. 2021;23(23 Suppl 2):ii52–60.
Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893–962.
Marion DMSV, Lanters EAH, Ramos KS, et al. Evaluating serum heat shock protein levels as novel biomarkers for atrial fibrillation. Cells. 2020;9(9):2105.
Roche M, Rondeau P, Singh NR, Tarnus E, Bourdon E. The antioxidant properties of serum albumin. FEBS Lett. 2008;582(13):1783–7.
Ronit A, Kirkegaard-Klitbo DM, Dohlmann TL, et al. Plasma albumin and incident cardiovascular disease: results from the CGPS and an updated meta-analysis. Arterioscler Thromb Vasc Biol. 2020;40(2):473–82.
Hirata T, Arai Y, Yuasa S, et al. Associations of cardiovascular biomarkers and plasma albumin with exceptional survival to the highest ages. Nat Commun. 2020;11(1):3820.
Arques S. Serum albumin and cardiovascular disease: Does low serum albumin contribute to the emergence and worsening of some cardiovascular diseases? Eur J Intern Med. 2020;80:122–3.
Goldwasser P, Feldman J. Association of serum albumin and mortality risk. J Clin Epidemiol. 1997;50(6):693–703.
Arques S. Human serum albumin in cardiovascular diseases. Eur J Intern Med. 2018;52:8–12.
Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial. 2004;17(6):432–7.
Hu YF, Chen YJ, Lin YJ, Chen SA. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol. 2015;12(4):230–43.
Youn JY, Zhang J, Zhang Y, et al. Oxidative stress in atrial fibrillation: an emerging role of NADPH oxidase. J Mol Cell Cardiol. 2013;62:72–9.
Li J, Solus J, Chen Q, et al. Role of inflammation and oxidative stress in atrial fibrillation. Heart Rhythm. 2010;7(4):438–44.
Zhang YL, Teng F, Han X, et al. Selective blocking of CXCR2 prevents and reverses atrial fibrillation in spontaneously hypertensive rats. J Cell Mol Med. 2020;24(19):11272–82.
Liao LZ, Zhang SZ, Li WD, et al. Serum albumin and atrial fibrillation: insights from epidemiological and mendelian randomization studies. Eur J Epidemiol. 2020;35(2):113–22.
Mukamal KJ, Tolstrup JS, Friberg J, Grønbaek M, Jensen G. Fibrinogen and albumin levels and risk of atrial fibrillation in men and women (the Copenhagen City Heart Study). Am J Cardiol. 2006;98(1):75–81.
Calkins H, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace. 2018;20:e1–160. https://doi.org/10.1093/europace/eux274.
McLean C, Mocanu V, Birch DW, Karmali S, Switzer NJ. Hypoalbuminemia predicts serious complications following elective bariatric surgery. Obes Surg. 2021;31(10):4519–27.
Wang Y, Du P, Xiao Q, et al. Relationship between serum albumin and risk of atrial fibrillation: a dose–response meta-analysis. Front Nutr. 2021;8: 728353.
van Beek DEC, Kuijpers YAM, Königs MHH, van der Horst ICC, Scheeren TWL. Low serum albumin levels and new-onset atrial fibrillation in the ICU: a prospective cohort study. J Crit Care. 2020;56:26–30.
He YM, Yang XJ, Hui J, et al. Low serum albumin levels in patients with paroxysmal atrial fibrillation: What does it mean? Acta Cardiol. 2006;61(3):333–7.
Arques S. Albumine sérique et maladies cardiovasculaires : une revue approfondie de la littérature [Serum albumin and cardiovascular diseases: a comprehensive review of the literature]. Ann Cardiol Angeiol. 2018;67(2):82–90.
Karam BS, Chavez-Moreno A, Koh W, Akar JG, Akar FG. Oxidative stress and inflammation as central mediators of atrial fibrillation in obesity and diabetes. Cardiovasc Diabetol. 2017;16(1):120.
Zakkar M, Ascione R, James AF, Angelini GD, Suleiman MS. Inflammation, oxidative stress and postoperative atrial fibrillation in cardiac surgery. Pharmacol Ther. 2015;154:13–20.
Deng Y, Liu F, Yang X, Xia Y. The key role of uric acid in oxidative stress, inflammation, fibrosis, apoptosis, and immunity in the pathogenesis of atrial fibrillation. Front Cardiovasc Med. 2021;8: 641136.
Sheinenzon A, Shehadeh M, Michelis R, Shaoul E, Ronen O. Serum albumin levels and inflammation. Int J Biol Macromol. 2021;184:857–62.
Levitt DG, Levitt MD. Human serum albumin homeostasis: a new look at the roles of synthesis, catabolism, renal and gastrointestinal excretion, and the clinical value of serum albumin measurements. Int J Gen Med. 2016;9:229–55.
Fleck A, Raines G, Hawker F, et al. Increased vascular permeability: a major cause of hypoalbuminaemia in disease and injury. Lancet. 1985;1(8432):781–4.
Eckart A, Struja T, Kutz A, et al. Relationship of nutritional status, inflammation, and serum albumin levels during acute illness: a prospective study. Am J Med. 2020;133(6):713-722.e7.
Moshage HJ, Janssen JA, Franssen JH, Hafkenscheid JC, Yap SH. Study of the molecular mechanism of decreased liver synthesis of albumin in inflammation. J Clin Invest. 1987;79(6):1635–41.
Saitou T, Watanabe K, Kinoshita H, et al. Hypoalbuminemia is related to endothelial dysfunction resulting from oxidative stress in parturients with preeclampsia. Nagoya J Med Sci. 2021;83(4):741–8.
Sitar ME, Aydin S, Cakatay U. Human serum albumin and its relation with oxidative stress. Clin Lab. 2013;59(9–10):945–52.
Annoura M, Ogawa M, Kumagai K, Zhang B, Saku K, Arakawa K. Cholesterol paradox in patients with paroxysmal atrial fibrillation. Cardiology. 1999;92(1):21–7.
Webb JG, Kiess MC, Chan-Yan CC. Malnutrition and the heart. CMAJ. 1986;135(7):753–8.
Psaty BM, Manolio TA, Kuller LH, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96(7):2455–61.
Li X, Gao L, Wang Z, et al. Lipid profile and incidence of atrial fibrillation: a prospective cohort study in China. Clin Cardiol. 2018;41(3):314–20.
Tse H, Oral H, Pelosi F, Knight B, Strickberger S, Morady F. Effect of gender on atrial electrophysiologic changes induced by rapid atrial pacing and elevation of atrial pressure. J Cardiovasc Electrophysiol. 2001;12(9):986–9.
Lew J, Sanghavi M, Ayers CR, et al. Sex-based differences in cardiometabolic biomarkers. Circulation. 2017;135(6):544–55.
Barriales Alvarez V, Morís de la Tassa C, Sánchez Posada I, et al. Estudio de la etiología y factores de riesgo asociados en una muestra de 300 pacientes con fibrilación auricular [The etiology and associated risk factors in a sample of 300 patients with atrial fibrillation]. Rev Esp Cardiol. 1999;52(6):403–14.
Acknowledgements
I would like to express my special thanks to my partners and our funding agency for the encouragement and support they gave me during this study.
Funding
This study was supported by a study on the Key Research and Development Programs in Shandong Province (Major scientific and technological innovation projects) (NO. 2021SFGC0503). The funding bodies had no role in the research design, data collection, analysis, interpretation, manuscript writing, and submission.
Author information
Authors and Affiliations
Contributions
HJ was the main coordinator of the project and was responsible for the study design. XZ and HJ drafted the manuscript of the present paper. DZ and JT were involved in the supervising of data collection and stratification. XZ and DZ contributed to data assembly and analysis. All authors contributed intellectually to this manuscript and have approved this final version. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval was approved by the Ethics Committee of Affiliated Hospital of Shandong University of Traditional Chinese Medicine. Because the data are anonymized, the Ethics Committee of Affiliated Hospital of Shandong University of Traditional Chinese Medicine (NO.20200512FA62) waived the need for informed consent. All methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhong, X., Jiao, H., Zhao, D. et al. Association between serum albumin levels and paroxysmal atrial fibrillation by gender in a Chinese population: a case–control study. BMC Cardiovasc Disord 22, 387 (2022). https://doi.org/10.1186/s12872-022-02813-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-022-02813-4