Abstract
Background
Hyperglycaemia is known to result in oxidative stress tissue injury and dysfunction. Interestingly, studies have reported hepatic and renal oxidative stress injury during prediabetes; however, any injury to the myocardium during prediabetes has not been investigated. Hence this study aims to assess changes in the myocardial tissue in an HFHC diet-induced model of prediabetes.
Methods
Male Sprague Dawley rats were randomly grouped into non-prediabetes and prediabetes (n = 6 in each group) and consumed a standard rat chow or fed a high-fat-high-carbohydrate diet respectively for a 20-week prediabetes induction period. Post induction, prediabetes was confirmed using the ADA criteria. Aldose reductase, NADH oxidase 1, superoxide dismutase, glutathione peroxide, cardiac troponins were analysed in cardiac tissue homogenate using specific ELISA kits. Lipid peroxidation was estimated by determining the concentration of malondialdehyde in the heart tissue homogenate according to the previously described protocol. Myocardial tissue sections were stained with H&E stain and analysed using Leica microsystem. All data were expressed as means ± SEM. Statistical comparisons were performed with Graph Pad instat Software using the Student's two-sided t-test. Pearson correlation coefficient was calculated to assess the association. Value of p < 0.05 was considered statistically significant.
Results
The prediabetes group showed a markedly high oxidative stress as indicated by significantly increased NADH oxidase 1 and malondialdehyde while superoxide dismutase and glutathione peroxide were decreased compared to non-prediabetes group. There was no statistical difference between cardiac troponin I and T in the non-prediabetes and prediabetes groups. Cardiac troponins had a weak positive association with glycated haemoglobin.
Conclusion
The findings of this study demonstrate that prediabetes is associated with myocardial injury through oxidative stress. Future studies are to investigate cardiac contractile function and include more cardiac biomarkers.
Similar content being viewed by others
Background
Prediabetes is a state of intermediate hyperglycaemia with glucose levels above normal but below the diabetic threshold [1]. Prediabetes is characterized by impaired fasting glucose (IFG), impaired glucose tolerance (IGT) and glycated haemoglobin (Hb1Ac) [2]. These parameters of prediabetes are risks for progressing to type 2 diabetes mellitus and its complications(T2DM) [3, 4]. Approximately 25% of individuals with prediabetes will progress to T2DM and 70% of those individuals will develop overt diabetes complications within their lifetime [5, 6]. Cardiovascular disease (CVD) is the leading cause of mortality and morbidity in T2DM worldwide and myocardial infarction (MI) contributes significantly to CVD mortality [7,8,9]. In T2DM reactive oxygen species (ROS) generation within the cardiomyocytes exceeds antioxidant defence leading to oxidative damage [10, 11]. The polyol pathway depletes nicotinamide adenine dinucleotide phosphate (NADPH), which is essential for generating glutathione while producing NADH, a substrate for NADH oxidase [12]. The expression of aldose reductase (AR) and sorbitol dehydrogenase (SDH) is increased in the diabetic heart [13]. Studies have reported that AR expression accelerates atherosclerosis in diabetic mice [14]. Atherosclerosis reduces myocardial blood flow and subsequently leads to myocardial infarction (MI) [15]. Furthermore, overexpression of AR in cardiomyocytes leads to a greater infarct area [16, 17].
Alternatively, hyperglycaemia in T2DM increases NADH oxidase1 [18]. NADH oxidase1 is one enzyme that produces excessive ROS within a diabetic heart [18]. Studies show that overexpression of NADH oxidase 1 impairs endothelial vaso-relaxation in animal models of T2DM and may lead to MI [19]. Cardiac troponins (cTnI) and (cTnT) are released as an indication of myocardial damage due to myocardial ischemic injury [20]. Loss of viable myocardium is histological evidence of MI [21]. Elevation of NADH oxidase 1 is reported to play a role in endotoxin-induced cardiomyocyte apoptosis [22].
Studies have demonstrated that T2DM related complications begin during prediabetes [23, 24]. Studies in our laboratory using the high-fat-high-carbohydrate (HFHC) diet-induced model of prediabetes have demonstrated that kidney and brain injury occurs during prediabetes [25, 26]. However, any injury to the myocardium during prediabetes have not been investigated. Hence this study aims to assess changes in myocardial tissue in an HFHC diet-induced model of prediabetes.
Materials and methods
Aim
This study aimed to assess myocardial tissue injury in diet-induced prediabetes.
Animals
Male Sprague–Dawley (150–180 g) rats were obtained from the Biomedical Research Unit (BRU), University of KwaZulu Natal (UKZN). The animals were kept under standard experimental conditions at room temperature (225 ± 2 °C), humidity (55 ± 5%), and 12 h day:12 h night cycle. The animals consumed a standard rat chow (Meadow Feeds, South Africa) and water ad libitum for two weeks to acclimatize before being exposed to an experimental diet (high-fat high carbohydrate). The high-fat high carbohydrate (HFHC) is composed of carbohydrates (55%kcal/g), fats (30%kcal/g), and proteins (15%kcal/g) as previously described [27]. All experimental procedures were conducted in line with ARRIVE guidelines and according to the ethics and animal care guidelines of the Animal Research Ethics Committee (AREC) of UKZN, Durban, South Africa. The study was approved by the UKZN AREC (Ethics No: AREC024/018D).
Experimental design
After two weeks of acclimatization, the animals were grouped into a non-prediabetic (n = 6) control group and prediabetic group (n = 6). The non-prediabetic group was fed standard rat chow and water ad libitum. The prediabetes group was fed an HFHC diet and water supplemented with fructose (15%) for 20 weeks to induce prediabetes. After 20 weeks, prediabetes was confirmed using the American Diabetes Association (ADA) criteria for diagnosis of prediabetes. Animals with fasting blood glucose (FBG) concentrations of 5.6–7.1 mmol/L, oral glucose tolerance test (OGTT) 2-h glucose concentration of 7.1–11.1 mmol/L and glycated haemoglobin (Hb1Ac) concentration of 5.7–6.4% were considered prediabetic. FBG was determined using the tail-prick method and measured using a One-Touch select glucometer (Lifescan, Malta, United Kingdom). OGTT was conducted as per laboratory established protocol [27,28,29,30,31]. Briefly, after 12 h fasting period, FBG was measured (time, 0 min) in all the animals. Glucose (0.86 g/kg, p.o.) was loaded into the animals via oral gavage (18-gauge gavage needle, 38 mm long curved with 21/4 mm ball end). Glucose concentrations were measured at 30-, 60-, and 120-min following glucose loading.
Tissue harvesting
The animals were placed in a gas chamber (BRU, UKZN, South Africa) and anesthetized with 100 mg/kg of Isoform (Safeline Pharmaceuticals Ltd, Roodeport, South Africa) for 3 min to collect blood samples. Blood samples were collected by cardiac puncture into precooled heparinized containers in an unconscious state. The blood samples were centrifuged (Eppendorf centrifuge 5403, Germany) at 4 °C , 503 g for 15 min to collect plasma. The plasma was stored at – 80 °C in a Bio Ultra freezer (Snijders Scientific, Tilburg, Holland). The hearts of all the animals were excised, cut in half, rinsed with cold standard saline solution, weighed, and snapped frozen in liquid nitrogen before storage in Bio Ultra freezer at – 80 \(^\circ{\rm C}\) for biochemical analysis and in formalin for histological analysis [30, 32].
Biochemical analysis
Oxidative stress
Aldose reductase, NADH oxidase 1 and Malondialdehyde (MDA)
Aldose reductase and NADH oxidase 1 activity was measured in the heart tissue homogenate using their respective sandwich ELISA according to the manufacturer’s protocol (Fine Biotech Co., Ltd., Wuhan, China). Lipid peroxidation was estimated by determining the concentration of MDA in the heart tissue homogenate according to a previously described protocol [30, 33].
SOD and GPx
The antioxidant status of the heart homogenates was determined by using a specific ELISA kit to analyse the concentration of superoxide dismutase (SOD) and glutathione peroxidase (GPx) according to the instructions of the manufacturer (Elabscience Biotechnology Co., Ltd., Houston, TX, USA).
Cardiac injury
cTnT and cTnI
Cardiac troponins (cTnT and cTnI) were measured from heart tissue homogenate using specific rat sandwich ELISA according to the protocol from the manufacturer (Elabscience Biotechnology Co., Ltd., Houston, TX, USA).
Heart tissue histology
Heart tissues were fixed in formalin overnight, paraffin-embedded and processed for sectioning. 0.5 μM sections (Robert-Bosch-Straße, Walldorf, Baden-Württemberg, Germany) were made and stained with haematoxylin and eosin (H&E) to analyse the cardiomyocyte size and the arrangement of cardiomyocyte myofibers and apoptotic cells using Leica microsystems for analysis Leica Scanner, SCN400 and Slide Path Gateway LAN software for analysis (Leica Microsystems CMS, Wetzlar, Germany).
Statistical analysis
All data were expressed as means ± SEM. Statistical comparisons were performed with Graph Pad instat Software (version 5.00, GraphPad Software, Inc., San Diego, California, USA) using the Student's unpaired two-sided t-test. Pearson correlation coefficient was calculated to find the association between HbA1c and cardiac troponins. A value of p < 0.05 was considered statistically significant.
Results
Body weight and heart weight
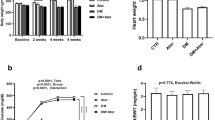
Body weight and heart weight was measured at the end of prediabetes induction period between the NPD and PD groups. Figure 1B shows a significant increase (p < 0.0001) in body weight in the PD (681.7 ± 6.22 g) compared to the NPD (382.5 ± 2.54 g) group. Figure 2B shows a significant increase (p < 0.0001) in heart weight in the PD group (1.76 ± 0.02 g) compared to the NPD group (1.52 ± 0.01 g).
FBG, OGTT and AUC and HbA1c between the NPD and PD group. Values are presented as mean ± SEM (n = 6 in each group). A * Indicates p = 0.0020. B * Indicates p = 0.0215, p = 0.0386. ** Indicates p = 0.0020, 0.0095. C* Indicates p = 0.043. NPD, non-prediabetes; PD, prediabetes; FBG, fasting blood glucose; OGTT, oral glucose tolerance; AUC, area under the curve; HbA1c, glycated haemoglobin
ADA prediabetes diagnosis parameters
FBG, OGTT and HbA1c concentration was measured at the end of prediabetes induction between the NPD and PD groups. Figure 2A shows that there was a significant increase (p = 0.0020) in FBG in the PD (4.93 ± 0.20 mmol/L) group compared to the NPD group (4.00 ± 0.103 mmol/L). Figure 2B shows that glucose concentration started significantly high in the PD group (p = 0.0020) compared to the NPD group at time 0. Glucose concentration remained significantly higher in the PD compared to the NPD as depicted at times 30 min and 120 min with p = 0.0215 and p = 0.0386, respectively. The AUC graph in Fig. 2B shows a significant increase (p = 0.0095) in glucose concentration in the PD group compared to NPD. Figure 2C shows that there was a significant increase (p = 0.043) in HbA1c concentration in the PD group (6.49 ± 0.32 mmol/mol) compared to the NPD group (4.32 ± 0.89 mmol/mol).
Oxidative stress
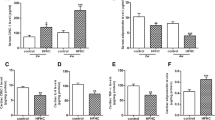
The concentration of AKR1B1, NOX1, MDA, SOD and GPx was measured between the NDP and PD group at the end of the prediabetes induction period. Figure 3 depicts an increase in oxidative stress as indicated by an increase in oxidative stress biomarkers and a decrease in antioxidant enzymes. Figure 3A shows an insignificant increase in the concentration of AKR1B1 (p = 0.4419) and a significant increase in NOX1 (p = 0.0156) and MDA (p = 0.0007) concentration in the PD group compared to the NPD group. Figure 3B shows a significant decrease in the concentration of SOD (SODNPD = 71.44 ± 0.59 ng/mL and SODPD = 63.61 ± 0.83 ng/mL) and GPx (GPxNPD = 1791 ± 47.04 ng/mL and GPxPD = 1547 ± 37.89 ng/mL) in the PD group compared to the NPD group with p < 0.0001 and p = 0.0033 respectively.
The concentration of AKR1B1, NOX1, MDA, SOD and GPx between the NPD and PD group. Values are presented as mean ± SEM. (n = 6 in each group). A NOX1: * Indicates p = 0.0156. MDA *** Indicates p = 0.0007. GPx ** p = 0.0033. B SOD **** indicates p < 0.0001. NPD, non-prediabetes; PD, prediabetes; AKR1B1, aldose reductase; NOX1(NADH oxidase 1), nicotinamide adenine dinucleotide phosphate oxidase 1; MDA, malondialdehyde; SOD, superoxide dismutase; GPx, glutathione peroxidase
Cardiac injury
Cardiac troponins (cTnT and cTnI)
The concentration of cTnT and cTnI was measured between the NPD and PD groups at the end of the prediabetes induction period. Figure 4 shows that there was an increase in mean cTnT concentration (989.5 ± 48.60 pg/mL) in the PD group compared to NPD group (456.50 ± 280.10 pg/mL); however, the increase was insignificant (p = 0.0814). The concentration of cTnI was increased (522.20 ± 6.30 pg/ml) in the PD group compared to the NDP group (496.20 ± 11.34 pg/mL). The increase insignificant (p = 0.0799).
Myocardial morphology
Heart tissue was processed for histological analysis at the end of the prediabetes induction period. Figure 5 shows H & E photomicrographs from the NPD (A) and PD (B) myocardium at a 20X250µm magnification. In Fig. 5A, the myocardium has a regular shape of myofibers and a nucleus that is located centrally. In Fig. 5B, myofibers are disarrayed and consist of fibrous fibres.
Correlation
The association between HbA1c and the cardiac troponins (cTnT and cTnI) was calculated between the NPD and PD group. There was an insignificant negative association between HbA1c and cTnT as well as cTnI in the NPD group (r = − 0.74; p = 0.2552) and (r = − 0.84; p = 0.0722) respectively. The association between HbA1c and cTnT including cTnI was positive and insignificant in the PD group (r = 0.37; p = 0.6273) and (r = 0.06; p = 0.9188) respectively.
Discussion
T2DM is a significant risk factor for CVD [34, 35]. However, T2DM is preceded by prediabetes which is an intermediate state of hyperglycaemia [3]. Complications associated with T2DM are reported to begin during the prediabetes stage [36, 37]. Studies in our laboratory have demonstrated that hepatic and renal dysfunction and injury seen in T2DM are present during prediabetes [38, 39]. However, the risk factors of myocardial tissue injury have not been investigated in diet-induced prediabetes. Hence this study was conducted to assess myocardial tissue injury in diet-induced prediabetes.
Hyperglycaemia is due to impaired insulin secretion or impaired insulin because of pancreatic beta-cell dysfunction or insulin resistance [40]. In states of insulin resistance, beta-cells compensate by increasing insulin secretion to maintain glucose homeostasis. However, insulin levels increase and glucose remains elevated [41]. Insulin resistance and hyperinsulinemia result in impaired glucose tolerance [42]. IFG is obtained from fasting plasma glucose and occurs because of poor glucose regulation, resulting in raised plasma glucose even after an overnight fast. [42]. IGT is associated with peripheral insulin resistance, and IFG is associated with hepatic insulin resistance and endogenous glucose production [4]. According to a study by Bacha and colleagues, obese adolescents who show signs of glucose dysregulation, including IFG, IGT, or both, are more likely to have impaired insulin secretion rather than reduced insulin sensitivity [3]. HbA1c is a product of a non-enzymatic reaction between glucose and haemoglobin. HbA1c increases as the average plasma glucose level increase and reflects average plasma glucose over a long period [43, 44]. In the present study, the PD group depicted a significantly impaired glucose tolerance, FBG and HbA1c concentration compared to the NPD group. These results coincide with the results published by Siboto et al., [45]. The PD group also had a significantly higher body weight and heart weight compared to the NPD. High body weight and heart weight can be attributed to the high calorie consumed for 20 weeks.
Aldose reductase (AR) reduces glucose to fructose in the polyol pathway, thus shifting excess glucose from metabolism [46]. This reaction reduces NADPH which is essential for glutathione formation while producing NADH, a substrate for NADH oxidase [12]. Activation of AR puts a strain on the NADPH system and subsequently on the glutathione reductase/glutathione peroxidase system, which protects against oxidative stress [47, 48]. In this study, there was an insignificant increase in AKR1B1 concentration in PD compared to the NDP group. These results contradict the finding by Daniels et al., who recently reported a significant increase in cardiac fructose and sorbitol in T2DM subjects. Sorbitol positively correlated with diastolic dysfunction. In Zucker Diabetes Fatty rats, fructose metabolism enzymes were markedly increased [49]. This study analysed intermediates of the polyol and then inferred that the enzymes of the polyol pathway are increased since intermediates of the pathway are elevated. AR reductase is active in chronic hyperglycaemia. The present study analysed AR in intermediate hyperglycaemia. Intermediate hyperglycaemia could be the reason that we did not find evidence of statistical significance.
NOX 1 is one of the cellular sources of ROS [50]. NOX1 catalyses the transfer of electrons to O2 generation O2− (superoxide) and H2O2 (hydrogen peroxide) [51]. Hyperglycaemia activates NOXI through PKC-dependent Rac1 activation [52]. NOX1 is also activated by the activation of the polyol pathway [12, 53]. In this study, there was a significant increase in NOX1 concentration in PD compared to the NPD group. These results coincide with the findings by Xu et al., in which wild type mice fed high-fat-high sucrose (HFHS) diet demonstrated an increase in Mac-2, IL-1β and nitrosative stress in cardiac tissue by comparison to NOX-1 knockout mice, indicating that NOX1 contributes to oxidative stress, endothelial activation and myocardial inflammation [54]. According to literature, ROS generation results in cardiac oxidative stress, associated with increased cardiac fibrosis, reduced cardiac contractility and ultimately cardiac dysfunction [55]. NOX1-induced ROS plays a role in endotoxin-induced cardiomyocyte apoptosis [22]. NOX1 elevation risks myocardial injury and myocardial infarction by inducing oxidative stress and inflammation.
Lipids present in plasma and cell membranes are subjected to ROS attack and peroxidation. Lipid peroxidation products are toxic to a cell and require removal by GSH [53]. MDA is a biomarker of oxidative stress formed as a lipid peroxidation product [56]. The level of MDA is increased in T2DM [57]. In the present study, there was a significant increase in MDA concentration in PD compared to the NPD group. These results are in accordance with the results reported by Su et al., in which subjects with diabetes and those with prediabetes had a significantly increased MDA concentration and a decreased SOD activity compared to subjects with standard glucose tolerance [58]. In the HFHC diet-induced prediabetes model, Akinnuga et al., reported a significant increase in MDA concentration in the prediabetes group compared to non-prediabetes [30].
Reactive oxygen species (ROS) are highly reactive molecules that regulate vascular tone and cell proliferation [59]. Antioxidant enzymes prevent abnormal ROS production and lipid peroxidation. Enzymes such as SOD converts superoxide to hydrogen peroxide, which is then transformed into water by catalase or glutathione peroxidase (GPx) [60]. Glutathione reductase is a hydrogen donor to GPx; therefore, it is vital for the activity of GPx [61]. In this study, there was a significant decrease in SOD and GPx. These results concur with the results published by Mabuza et al., in which the prediabetes group showed a significant decrease in SOD and GPx in the cardiac tissue [33]. An increase in AR activity is one of the many factors that cause a decrease in GPx. AR depletes NADH, a substrate for GSH. AR metabolizes GSH-lipid-derived aldehyde adducts, which decreases GSH and subsequently increases oxidative stress [61].
The myocardium releases cardiac troponins (cTn) in proportion to the degree of myocardial tissue injury and disruption of the myocyte membrane [62,63,64]. Though these cardiac injury markers are usually tested in the event of cardiac ischemia, several studies have reported an elevation of these markers in subclinical myocardial damage in diabetes [37, 65]. A study by Selvin et al., demonstrated an incidence of elevated cTn in diabetic people [66]. Elevated cTn has a strong correlation with the adverse cardiovascular outcome whether a coronary disease is present or not [67]. In Table 1, there was an insignificant increase in cTnT and cTnI concentration between the PD and NPD groups in the current study. Interestingly, the concentration of cTnT in PD (989.5 ± 48.6 pg/mL) was double the concentration in NPD (456.5 ± 250.1 pg/mL) whereas the concentration of cTnI in PD (522.2 ± 6.3 pg/mL) is 1 × factor higher than the concentration in NPD (496.2 ± 11.3 pg/mL). These results suggest cTnT is markedly elevated and is a sensitive marker of cardiac injury in prediabetes. In Table 1, we reported an insignificant negative association between HbA1c and the cardiac troponin (cTnT and cTnI) in the NPD group, whereas the association was positive in the PD group. This study is per a recently published study by Witkowski et al., in which prediabetes is associated with major adverse cardiac events (MACE) and increasing hs-cTnT levels associated with an increased risk for 3-year MACE and 5-year all-cause mortality in the entire cohort [68].Interestingly, there was no correlation between the levels of hs-cTnT and either FBG or HbA1c [69]. Matsumoto observed a significant positive correlation between HbA1c and hs-cTnT in participants with T2DM. The concentration of hs-cTnT was further positively associated with oxidative stress markers [70]. These findings contradict the findings of this study. We suggest that this could be the difference in the study groups, methodology, and hyperglycaemia duration.
In a cross-sectional study conducted by Kerr and colleagues, there was a significant increase in cTnI levels in T2DM compared to non-diabetes subjects [71]. These results are different from the findings of this study reported in Table 1. We did not find evidence of a statistical difference in the concentration of cTnI between the NPD and the PD group. We speculate this to be because the study consisted of 6 rats per group and had intermediate hyperglycaemia whereas Kerr reported a cross-sectional study with T2DM participants. Odum and Young investigated the level of cTnI, CK-MB and myoglobin and their relation to CVD risk in T2DM. None of the participants had an elevation of all three biomarkers, and only 4.3% had an elevation of two biomarkers [72]. In the present study, we analysed cTnT and cTnI. Though there was an insignificant increase, the concentration of cTnT in the PD group doubled the concentration in the NPD group, whereas the concentration of cTnI increased by 1. This difference in cTn concentration suggests that testing one biomarker may not detect the risk or detect the injury.
In a study associating subclinical myocardial injury with arterial stiffness in T2DM patients, hs-cTnI had an insignificant positive correlation with HbA1c and arterial stiffness [73]. These studies follow the present study as we did not find a statistically significant association between HbA1c and cTnI. Detection of cardiac biomarkers in prediabetes indicates a subclinical myocardial injury during prediabetes.
Elevated ROS in the cardiac tissue results in cardiac oxidative stress, cardiac fibrosis, cardiac dysfunction and potentially cardiac events such as MI [55]. This study observed increased AR, NOX1, MDA, and decreased antioxidant enzymes in the PD group compared to the NPD group myocardium. Oxidative stress through the polyol pathway is reported to decrease SERCA activity, thereby decreasing Ca2+and subsequently resulting in contractile dysfunction [74]. Contractile dysfunction is also caused by myocardial apoptosis and fibrosis [75]. Studies have demonstrated that myocardial fibrosis and deranged myofibers are hyperglycaemia induced myocardial structural changes [76]. In the present study, in Fig. 5 we observed disarrayed myofibers and the deposition of fibrous networks in the PD myocardium. In contrast, the NPD group's nucleus is centrally located, and the myofibers are regularly shaped. This observation aligns with Kusaka et al., who reported that the cardiac tissue of the SHrcp rat model of metabolic syndrome with prediabetes displayed oxidative stress, inflammation, hypertrophy, and fibrosis compared to the control [77].
Conclusion
The findings of this study demonstrate that prediabetes is associated with myocardial injury through oxidative stress. Future studies are to use immunohistochemistry to investigate the cardiac contractile function and include more cardiac biomarkers.
Availability of data and materials
The datasets generated during and analysed during the current study are not publicly available due to concerns of misuse and the more aspects of the study are to be added. However, datasets are available from the corresponding author on reasonable request.
Abbreviations
- %:
-
Percentage
- ADA:
-
American Diabetes Association
- AGE:
-
Advanced glycation end-product
- AKR1B1:
-
Aldo-keto reductase family 1, member 1
- ANGΙΙ:
-
Angiotensin 2
- AR:
-
Aldose reductase
- AREC:
-
Animal Research Ethics Committee
- AUC:
-
Area under the curve
- BRU:
-
Biomedical Research Unit
- Ca2+ :
-
Calcium
- CAT:
-
Catalase
- CK-MB:
-
Creatine kinase-myocardial band
- Co.:
-
Company
- cTn:
-
Cardiac troponin
- cTnI:
-
Cardiac troponin I
- cTnT:
-
Cardiac troponin T
- CVD:
-
Cardiovascular disease
- ELISA:
-
Enzyme-linked immunosorbent assay
- eNOS:
-
Endothelial nitric oxide synthase
- FBG:
-
Fasting blood glucose
- GPx:
-
Glutathione reductase
- GSH:
-
Glutathione
- H&E:
-
Haematoxylin and eosin
- H2O2 :
-
Hydrogen peroxide
- HbA1c:
-
Glycated haemoglobin
- HFD:
-
High-fat diet
- HFHC:
-
High-fat-high-carbohydrate
- HFHS:
-
High-fat-high-sucrose
- HNE:
-
4-Hydroxynonenal
- Hr:
-
Hour
- hs-cTnT:
-
High sensitivity cardiac troponin T
- IFG:
-
Impaired fasting glucose
- IGT:
-
Impaired glucose tolerance
- IL-1β:
-
Interleukin 1-beta
- Kg:
-
Kilogram
- Ltd:
-
Limited
- MACE:
-
Major adverse cardiac event
- MDA:
-
Malondialdehyde
- MI:
-
Myocardial infarction
- min:
-
Minute
- mmol/L:
-
Millimoles per litre
- mRNA:
-
Messenger ribonucleic acid
- NADH:
-
Reduced nicotinamide adenine dinucleotide
- NOX1:
-
NADPH oxidase 1 encoding gene
- NPD:
-
Non-prediabetes
- O2 :
-
Oxygen
- O2 − :
-
Superoxide ion
- OGTT:
-
Oral glucose tolerance test
- pg:
-
Picogram
- PKC:
-
Protein kinase C
- r:
-
Pearson’s correlation coefficient
- Rac1:
-
Ras-related C3 botulinum toxin substrate 1
- ROS:
-
Reactive oxygen species
- SDH:
-
Sorbitol dehydrogenase
- SEM:
-
Standard error of the mean
- SERCA:
-
Sarco/endoplasmic reticulum Ca2+-ATPase
- SOD:
-
Superoxide dismutase
- T2DM:
-
Type 2 diabetes mellitus
- TX:
-
Texas
- UKZN:
-
University of KwaZulu Natal
- USA:
-
United States of America
References
Bansal N. Prediabetes diagnosis and treatment: a review. World J Diabetes. 2015;6(2):296–303.
Diabetes Canada Clinical Practice Guidelines Expert C, Punthakee Z, Goldenberg R, Katz P. Definition, Classification and Diagnosis of Diabetes, Prediabetes and Metabolic Syndrome. Can J Diabetes. 2018;42 Suppl 1:S10-S5.
Bacha F, Lee S, Gungor N, Arslanian SA. From pre-diabetes to type 2 diabetes in obese youth: pathophysiological characteristics along the spectrum of glucose dysregulation. Diabetes Care. 2010;33(10):2225–31.
Buysschaert M, Medina JL, Bergman M, Shah A, Lonier J. Prediabetes and associated disorders. Endocrine. 2015;48(2):371–93.
Hostalek U. Global epidemiology of prediabetes-present and future perspectives. Clin Diabetes Endocrinol. 2019;5(1):1–5.
Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379(9833):2279–90.
Susan van D, Beulens JW, Yvonne T van der S, Grobbee DE, Nealb B. The global burden of diabetes and its complications: an emerging pandemic. European Journal of Cardiovascular Prevention & Rehabilitation. 2010;17(1_suppl):s3-s8.
Gamede M, Mabuza L, Ngubane P, Khathi A. Plant-derived oleanolic acid (OA) ameliorates risk factors of cardiovascular diseases in a diet-induced pre-diabetic rat model: effects on selected cardiovascular risk factors. Molecules. 2019;24(2):340.
Nielsen SH, Mouton AJ, DeLeon-Pennell KY, Genovese F, Karsdal M, Lindsey ML. Understanding cardiac extracellular matrix remodeling to develop biomarkers of myocardial infarction outcomes. Matrix Biol. 2019;75–76:43–57.
Anderson EJ, Kypson AP, Rodriguez E, Anderson CA, Lehr EJ, Neufer PD. Substrate-specific derangements in mitochondrial metabolism and redox balance in the atrium of the type 2 diabetic human heart. J Am Coll Cardiol. 2009;54(20):1891–8.
Heather LC, Clarke K. Metabolism, hypoxia and the diabetic heart. J Mol Cell Cardiol. 2011;50(4):598–605.
Tang W, Martin KA, Hwa J. Aldose reductase, oxidative stress, and diabetic mellitus. Front Pharmacol. 2012;3:87.
Ananthakrishnan R, Li Q, Gomes T, Schmidt AM, Ramasamy R. Aldose reductase pathway contributes to vulnerability of aging myocardium to ischemic injury. Exp Gerontol. 2011;46(9):762–7.
Vikramadithyan RK, Hu Y, Noh H-L, Liang C-P, Hallam K, Tall AR, et al. Human aldose reductase expression accelerates diabetic atherosclerosis in transgenic mice. J Clin Investig. 2005;115(9):2434–43.
Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93(1):137–88.
Son N-H, Ananthakrishnan R, Yu S, Khan RS, Jiang H, Ji R, et al. Cardiomyocyte aldose reductase causes heart failure and impairs recovery from ischemia. PLoS ONE. 2012;7:e46549.
Jannapureddy S, Sharma M, Yepuri G, Schmidt AM, Ramasamy R. Aldose reductase: an emerging target for development of interventions for diabetic cardiovascular complications. Front Endocrinol. 2021;12:78.
Kayama Y, Raaz U, Jagger A, Adam M, Schellinger IN, Sakamoto M, et al. Diabetic cardiovascular disease induced by oxidative stress. Int J Mol Sci. 2015;16(10):25234–63.
Dikalova AE, Góngora MC, Harrison DG, Lambeth JD, Dikalov S, Griendling KK. Upregulation of Nox1 in vascular smooth muscle leads to impaired endothelium-dependent relaxation via eNOS uncoupling. Ame J Physiol Heart Circ Physiol. 2010;299(3):H673–9.
de Lemos JA. Increasingly sensitive assays for cardiac troponins: a review. JAMA. 2013;309(21):2262–9.
Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth Universal Definition of Myocardial Infarction (2018). Circulation. 2018;138(20):e618–51.
Matsuno K, Iwata K, Matsumoto M, Katsuyama M, Cui W, Murata A, et al. NOX1/NADPH oxidase is involved in endotoxin-induced cardiomyocyte apoptosis. Free Radical Biol Med. 2012;53(9):1718–28.
Vistisen D, Witte DR, Brunner EJ, Kivimäki M, Tabak A, Jørgensen ME, et al. Risk of cardiovascular disease and death in individuals with prediabetes defined by different criteria: the Whitehall II study. Diabetes Care. 2018;41(4):899–906.
Axelsen LN, Calloe K, Braunstein TH, Riemann M, Hofgaard JP, Liang B, et al. Diet-induced pre-diabetes slows cardiac conductance and promotes arrhythmogenesis. Cardiovasc Diabetol. 2015;14(1):87.
Mkhize B, Mosili P, Ngubane P, Sibiya N, Khathi A. Diet-induced prediabetes: Effects on the systemic and renal renin-angiotensin-aldosterone system. 2020.
Mosili P, Mkhize BC, Ngubane P, Sibiya N, Khathi A. The dysregulation of the hypothalamic-pituitary-adrenal axis in diet-induced prediabetic male Sprague Dawley rats. Nutr Metab (Lond). 2020;17(1):104.
Luvuno M, Mbongwa H, Khathi A. Development of a novel prediabetes animal model using a high fat high carbohydrate diet: Implications for type 2 diabetes. PLoS ONE. 2017;13:8–14.
Khathi A, Serumula MR, Myburg RB, Van Heerden FR, Musabayane CT. Effects of Syzygium aromaticum-derived triterpenes on postprandial blood glucose in streptozotocin-induced diabetic rats following carbohydrate challenge. PLoS ONE. 2013;8(11):e81632.
Gamede M, Mabuza L, Ngubane P, Khathi A. The effects of plant-derived oleanolic acid on selected parameters of glucose homeostasis in a diet-induced pre-diabetic rat model. Molecules. 2018;23(4):794.
Akinnuga AM, Siboto A, Khumalo B, Sibiya NH, Ngubane P, Khathi A. Bredemolic acid improves cardiovascular function and attenuates endothelial dysfunction in diet-induced prediabetes: effects on selected markers. Cardiovasc Ther. 2020;2020:1–9.
Khathi A, Luvuno M, Mabandla M. Voluntary ingestion of a high-fat high-carbohydrate diet: a model for prediabetes. PONTE Int Sci Res J. 2018. https://doi.org/10.21506/j.ponte.2018.5.11.
Luvuno M, Mabandla M, Khathi A. Voluntary ingestion of a high-fat high-carbohydrate diet: a model for prediabetes. Ponte Int Sci Res J. 2018. https://doi.org/10.21506/j.ponte.2018.5.11.
Mabuza LP, Gamede MW, Maikoo S, Booysen IN, Ngubane PS, Khathi A. Cardioprotective effects of a ruthenium (II) Schiff base complex in diet-induced prediabetic rats. Diabetes Metab Syndr Obes. 2019;12:217–23.
Mazzone T, Chait A, Plutzky J. Cardiovascular disease risk in type 2 diabetes mellitus: insights from mechanistic studies. Lancet. 2008;371(9626):1800–9.
King RJ, Grant PJ. Diabetes and cardiovascular disease: pathophysiology of a life-threatening epidemic. Herz. 2016;41(3):184–92.
Di Pino A, Scicali R, Calanna S, Urbano F, Mantegna C, Rabuazzo AM, et al. Cardiovascular risk profile in subjects with prediabetes and new-onset type 2 diabetes identified by HbA(1c) according to American Diabetes Association criteria. Diabetes Care. 2014;37(5):1447–53.
Selvin E, Lazo M, Shen L, Rubin J, McEvoy JW, Hoogeveen RC, et al. Diabetes, pre-diabetes, and progression of subclinical myocardial damage. Circulation. 2014;129(suppl_1):A16-A.
Gamede M, Mabuza L, Ngubane P, Khathi A. Preventing the onset of diabetes-induced chronic kidney disease during prediabetes: The effects of oleanolic acid on selected markers of chronic kidney disease in a diet-induced prediabetic rat model. Biomed Pharmacother. 2021;139: 111570.
Akinnuga AM, Siboto A, Khumalo B, Sibiya NH, Ngubane P, Khathi A. Bredemolic acid ameliorates selected liver function biomarkers in a diet-induced prediabetic rat model. Can J Gastroenterol Hepatol. 2020. https://doi.org/10.1155/2020/2475301.
Baynest HW. Classification, pathophysiology, diagnosis and management of diabetes mellitus. J Diabetes Metabol. 2015. https://doi.org/10.4172/2155-6156.1000541.
Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88.
Association AD. 2. Classification and diagnosis of diabetes. Diabetes care. 2015;38(Supplement 1):S8-S16.
Sherwani SI, Khan HA, Ekhzaimy A, Masood A, Sakharkar MK. Significance of HbA1c test in diagnosis and prognosis of diabetic patients. Biomarker Insights. 2016;11:BMI.S38440.
Organization WH. Use of glycated haemoglobin (HbA1c) in diagnosis of diabetes mellitus: abbreviated report of a WHO consultation. World Health Organization; 2011.
Siboto A, Akinnuga AM, Khumalo BN, Ismail MB, Booysen IN, Sibiya NH, et al. The effects of a [3+ 1] oxo-free rhenium (V) compound with uracil-derived ligands on selected parameters of glucose homeostasis in diet-induced pre-diabetic rats. Obes Med. 2020;19: 100258.
Lj Y. Redox imbalance stress in diabetes mellitus: Role of the polyol pathway. Anim Models Exp Med. 2018;1(1):7–13.
González RG, Barnett P, Aguayo J, Cheng H-M, Chylack L. Direct measurement of polyol pathway activity in the ocular lens. Diabetes. 1984;33(2):196–9.
Srivastava SK, Ramana KV, Bhatnagar A. Role of aldose reductase and oxidative damage in diabetes and the consequent potential for therapeutic options. Endocr Rev. 2005;26(3):380–92.
Daniels LJ, Annandale M, Koutsifeli P, Li X, Bussey CT, van Hout I, et al. Elevated myocardial fructose and sorbitol levels are associated with diastolic dysfunction in diabetic patients, and cardiomyocyte lipid inclusions in vitro. Nutr Diabetes. 2021;11(1):1–7.
Zhang Y, Tocchetti CG, Krieg T, Moens AL. Oxidative and nitrosative stress in the maintenance of myocardial function. Free Radical Biol Med. 2012;53(8):1531–40.
Liu J, Yin Y, Song Z, Li Y, Jiang S, Shao C, et al. NADH: flavin oxidoreductase/NADH oxidase and ROS regulate microsclerotium development in Nomuraea rileyi. World J Microbiol Biotechnol. 2014;30(7):1927–35.
Inoguchi T, Sonta T, Tsubouchi H, Etoh T, Kakimoto M, Sonoda N, et al. Protein kinase C–dependent increase in reactive oxygen species (ROS) production in vascular tissues of diabetes: role of vascular NAD (P) H oxidase. J Am Soc Nephrol. 2003;14(suppl 3):S227–32.
Mahjoub S, Masrour-Roudsari J. Role of oxidative stress in pathogenesis of metabolic syndrome. Caspian J Intern Med. 2012;3(1):386.
Xu L, Balzarolo M, Robinson EL, Lorenz V, Verde GD, Joray L, et al. NOX1 mediates metabolic heart disease in mice and is upregulated in monocytes of humans with diastolic dysfunction. Cardiovasc Res. 2021. https://doi.org/10.1093/cvr/cvab349.
Faria A, Persaud SJ. Cardiac oxidative stress in diabetes: Mechanisms and therapeutic potential. Pharmacol Ther. 2017;172:50–62.
Liu Y, Zhao J, Lu M, Wang H, Tang F. Retinoic acid attenuates cardiac injury induced by hyperglycemia in pre-and post-delivery mice. Can J Physiol Pharmacol. 2020;98(1):6–14.
Rehman K, Akash MSH. Mechanism of generation of oxidative stress and pathophysiology of type 2 diabetes mellitus: how are they interlinked? J Cell Biochem. 2017;118(11):3577–85.
Su Y, Liu XM, Sun YM, Jin HB, Fu R, Wang YY, et al. The relationship between endothelial dysfunction and oxidative stress in diabetes and prediabetes. Int J Clin Pract. 2008;62(6):877–82.
Barton M, Meyer MR, Prossnitz ER. Nox1 downregulators: a new class of therapeutics. Steroids. 2019;152: 108494.
Johansen JS, Harris AK, Rychly DJ, Ergul A. Oxidative stress and the use of antioxidants in diabetes: linking basic science to clinical practice. Cardiovasc Diabetol. 2005;4(1):1–11.
Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell longev. 2014;2014:360438.
Metzger JM, Westfall MV. Covalent and noncovalent modification of thin filament action: the essential role of troponin in cardiac muscle regulation. Circ Res. 2004;94(2):146–58.
O’brien P, Smith D, Knechtel T, Marchak M, Pruimboom-Brees I, Brees D, et al. Cardiac troponin I is a sensitive, specific biomarker of cardiac injury in laboratory animals. Lab Anim. 2006;40(2):153–71.
Chan D, Ng LL. Biomarkers in acute myocardial infarction. BMC Med. 2010;8(1):34.
Raposeiras-Roubín S, Barreiro-Pardal C, Álvarez E, González Juanatey JR. Is glycated hemoglobin an accurate enough predictor of subclinical myocardial injury or a simple precursor of advanced glycation end products? J Am Coll Cardiol. 2012;60(2):166–7.
Selvin E, Lazo M, Chen Y, Shen L, Rubin J, McEvoy JW, et al. Diabetes mellitus, prediabetes, and incidence of subclinical myocardial damage. Circulation. 2014;130(16):1374–82.
Al-Otaiby MA, Al-Amri HS, Al-Moghairi AM. The clinical significance of cardiac troponins in medical practice. J Saudi Heart Assoc. 2011;23(1):3–11.
Witkowski M, Wu Y, Hazen SL, Tang WHW. Prognostic value of subclinical myocardial necrosis using high-sensitivity cardiac troponin T in patients with prediabetes. Cardiovasc Diabetol. 2021;20(1):171.
Witkowski M, Wu Y, Hazen SL, Tang W. Prognostic value of subclinical myocardial necrosis using high-sensitivity cardiac troponin T in patients with prediabetes. Cardiovasc Diabetol. 2021;20(1):1–11.
Hitsumoto T. Factors associated with high-sensitivity cardiac troponin T in patients with type 2 diabetes mellitus. J Nippon Med Sch. 2015;82(6):274–80.
Karar T, Elfaki EM, Qureshi S. Determination of the serum levels of troponin I and creatinine among Sudanese type 2 diabetes mellitus patients. J Nat Sci Biol Med. 2015;6(Suppl 1):S80.
Odum EP, Young EE. Elevated cardiac troponin I, creatine kinase and myoglobin and their relationship with cardiovascular risk factors in patients with type 2 diabetes. Diabetes Metab Syndr. 2018;12(2):141–5.
Yiu K-H, Zhao C-T, Chen Y, Siu C-W, Chan Y-H, Lau K-K, et al. Association of subclinical myocardial injury with arterial stiffness in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2013;12(1):94.
Tang WH, Cheng WT, Kravtsov GM, Tong XY, Hou XY, Chung SK, et al. Cardiac contractile dysfunction during acute hyperglycemia due to impairment of SERCA by polyol pathway-mediated oxidative stress. Am J Physiol Cell Physiol. 2010;299(3):C643–53.
Danilova I, Sarapultsev P, Medvedeva S, Gette I, Bulavintceva T, Sarapultsev A. Morphological restructuring of myocardium during the early phase of experimental diabetes mellitus. Anat Rec. 2015;298(2):396–407.
Adeghate E, Singh J. Structural changes in the myocardium during diabetes-induced cardiomyopathy. Heart Fail Rev. 2014;19(1):15–23.
Kusaka H, Koibuchi N, Hasegawa Y, Ogawa H, Kim-Mitsuyama S. Empagliflozin lessened cardiac injury and reduced visceral adipocyte hypertrophy in prediabetic rats with metabolic syndrome. Cardiovasc Diabetol. 2016;15(1):1–14.
Acknowledgements
The authors would like to acknowledge Dr Kogi Moodley and Mr Denis Makhubela for their technical assistance, the Biomedical Research Unit of the University of KwaZulu Natal for the provision of animals and assistance and the National Research Fund Scholarship, South Africa for financial support.
Funding
This study was funded by the University of KwaZulu Natal College of Health Science (CHS).
Author information
Authors and Affiliations
Contributions
NG was involved in the study design, conducted experiments, analysed and interpreted data and was also involved in manuscript writing. AK was involved in study concept, study design, analysis and interpretation of data, manuscript editing and writing and provided funding. PSN was involved in the study design, analysis and interpretation of data, and was involved in writing the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All experimental procedures were conducted in line with ARRIVE guidelines and according to the ethics and animal care guidelines of the Animal Research Ethics Committee (AREC) of UKZN, Durban, South Africa. The study was approved by the UKZN AREC (Ethics No: AREC024/018D).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. The association between antioxidant enzymes (SOD and GPx) and oxidative stress biomarkers (NOX1 and MDA) was calculated between the NPD and PD group. In the NPD group there was an insignificant positive correlation (SOD and NOX1: r = 0.47, p = 0.5268), (SOD and MDA: r = 0.72, p = 0.1741), (GPx and NOX1: r = 0.70, p = 0.3033), (GPx and MDA: 0.23, p = 0.6908) whereas in the PD group there was an insignificant negative association (SOD and NOX1: r = −0.72, p = 0.2846), (SOD and MDA: r = −0.66, p = 0.2218), (GPx and NOX1: r = −0.91, p = 0.0950), (GPx and MDA: r = −0.22, p = 0.7240) between the antioxidant enzymes and oxidative stress biomarkers.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gumede, N., Ngubane, P. & Khathi, A. Assessing the risk factors for myocardial infarction in diet-induced prediabetes: myocardial tissue changes. BMC Cardiovasc Disord 22, 350 (2022). https://doi.org/10.1186/s12872-022-02758-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-022-02758-8