Abstract
Background
High-sensitivity C-reactive protein (hs-CRP) plays an important role in hypoalbuminemia as a representative of inflammation, which is closely associated with poor prognosis among patients with coronary artery disease (CAD). The present study aimed to evaluate the independent and joint effects of high hs-CRP levels and hypoalbuminemia on long-term mortality among CAD patients.
Methods
A total of 1449 CAD patients were included from a prospective, multicenter, observational cohort study (REICIN, NCT01402232) of patients referred for coronary angiography (CAG). The primary endpoint was long-term all-cause death.
Results
During a median follow-up of 2.9 (2.0–3.0) years, a total of 107 (7.4%) patients died. The long-term mortality was higher among CAD patients with high hs-CRP levels (> 3 mg/L) than those with the low hs-CRP levels (≤ 3 mg/L; 10.7% versus 4.1%; hazard ratio [HR] 2.49; 95% confidence interval [CI] 1.48–4.17). Similarly, CAD patients with hypoalbuminemia had higher mortality than those without hypoalbuminemia (12.2% versus 4.9%; HR 1.93; 95% CI 1.20–3.08). When hs-CRP and albumin were combined, CAD patients with high hs-CRP levels (> 3 mg/L) and with hypoalbuminemia were at the highest risk of death compared with their reference group (hs-CRP ≤ 3 mg/L and albumin > 35 g/L; HR 3.79; 95% CI 1.91–7.52).
Conclusions
High hs-CRP levels and hypoalbuminemia were independently and jointly associated with long-term mortality among CAD patients. Patients with high hs-CRP levels and hypoalbuminemia had the highest risk of long-term mortality compared with other groups.
Similar content being viewed by others
Background
The Global Burden of Disease study indicated that coronary artery disease (CAD) remains the leading cause of morbidity and mortality [1]. Current studies emphasize the clinical importance of evaluating the prognosis of patients with CAD by using simple indicators.
Increasing evidence has shown that chronic inflammatory reaction, which is clinically presented as high-sensitivity C-Reactive Protein (hs-CRP), plays an important role in the development of CAD. Therefore, it is feasible for hs-CRP to predict adverse clinical events among CAD patients. Accordingly, both Momiyama et al. and Kim et al. have indicated that higher hs-CRP levels were found to be associated with a significantly increased risk for further cardiovascular events [2,3,4]. However, data concerning the association between hs-CRP and the risk of long-term mortality are limited.
Serum albumin, a major protein found in the extracellular fluid compartment, contributes to maintaining diverse physiological functions, such as anti-inflammatory, antioxidant, anticoagulant, and antiplatelet aggregation activity, as well as a colloid osmotic effect [5]. Further, studies have demonstrated that hypoalbuminemia is associated with poor survival in CAD patients [6, 7]. Interestingly, there is also a strong association between hypoalbuminemia and inflammation, which may be driven by the acute-phase response [8,9,10]. For example, inflammation is a driving force of reduced albumin levels, while hypoalbuminemia reflects the physiological stress from inflammation [11,12,13]. Notably, the Glasgow Prognostic Score, which calculated via serum albumin and inflammation indicators, and has been widely used in the field of oncology, has been shown to accurately predict the poor prognosis of patients with tumors in many studies [14,15,16].
However, the joint effect of hs-CRP and hypoalbuminemia among CAD patients remains unclear. Therefore, the current study aimed to test whether high hs-CRP levels can, independently and jointly with hypoalbuminemia, predict an increased risk of all-cause mortality among CAD patients in a multicenter prospective Reduction of Risk for Contrast-Induced Nephropathy (REICIN) study.
Population, materials, and methods
Data sources and study population
Based on REICIN study data, the current study is a prospective, multicenter, observational cohort study to assess a method of reducing risk for contrast-induced nephropathy among patients given coronary angiography (CAG) who were admitted to one of 12 teaching hospitals in the Guangdong, Fujian, and Xinjiang Provinces of China from January 2013 to February 2016. Details of the site investigators and hospitals are presented in Additional file 1: Table S1. Patients were recruited according to the inclusion and exclusion criteria (Additional file 1: Table S2). Details of the cohort procedure are provided in Additional file 1: Fig. S1 and the inclusion/exclusion criteria are described in Additional file 1: Table S2. Based on the analysis of 4271 patients given CAG, the exclusion criteria were: patients who did not have a diagnosis of CAD (n = 746); patients who were missing follow-up information (n = 266); and patients whose data for serum levels of hs-CRP and albumin were missing (n = 1810). After exclusions, a total of 1449 CAD patients were enrolled (Fig. 1).
Measurements of albumin and hs-CRP
Blood samples, including serum albumin, creatinine, glucose, hs-CRP, and other hematologic parameters, were obtained from all patients the morning after admission. Serum albumin levels were measured by the photometric method in the hospital laboratory. We used a high-sensitivity CRP enzyme-linked immunosorbent assay (ELISA) kit to determined hs-CRP levels. Both hs-CRP and serum albumin were detected in the same laboratory and the same measurement methods were used in all centers.
Clinical definition
CAD was defined as a chronic coronary syndrome or acute coronary syndrome [17,18,19]. Chronic kidney disease (CKD) was defined as an estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2. eGFR was calculated by the Modification of Diet in Renal Disease (MDRD) equation [20]. Anemia was defined according to the World Health Organization criteria, i.e. baseline hematocrit value < 39% for men and < 36% for women. Hypoalbuminemia was defined as albumin < 35 g/L.
Study endpoints and clinical follow-up
The primary endpoint was long-term all-cause mortality, which was defined as any death after the date of enrollment. This information was monitored and recorded by research assistants and trained nurses through outpatient interviews and via telephone. The proportion of subjects that were unable to complete follow-up did not exceed 10%.
Statistical analysis
Descriptive statistics are reported as the mean (SD), median (interquartile range [IQR]) for continuous variables, and proportions for categorical variables. Differences between groups were analyzed with Student’s t-tests and one-way analyses of variance (ANOVAs) as appropriate. Categorical data were analyzed by Pearson chi-squared tests. We used log-transformation (log10) for describing hs-CRP concentration and used a restricted cubic splines (RCS) model to examine the associations between albumin/log10 hs-CRP and outcomes. The cut-offs for hs-CRP (≤ 3, or > 3 mg/L) and albumin (< 35, or ≥ 35 g/L) were based on previous studies [21,22,23,24,25]. We divided patients into four groups: Group 1 (non-hypoalbuminemia and hs-CRP ≤ 3 mg/L; Group 2 (hypoalbuminemia and hs-CRP ≤ 3 mg/L), Group 3 (non-hypoalbuminemia and hs-CRP > 3 mg/L), and Group 4 (hypoalbuminemia and hs-CRP > 3 mg/L). To estimate the effect of different groups on long-term all-cause mortality, Kaplan–Meier curves were constructed. Additionally, receiver-operator characteristic (ROC) curves were used to identify the optimal sensitivity for the observed range of albumin and hs-CRP among the four groups (Additional file 1: Fig. S2). Differences in survival rates were tested with the log-rank test. We also used density curves to assess the distribution of hs-CRP among acute coronary syndrome (ACS) and choric CAD (Additional file 1: Fig. S3).
Cox proportional hazard models were used to investigate the independent and joint effects of hs-CRP and albumin on long-term all-cause death among CAD patients. Hazard ratios and 95% CIs are reported. In the Cox hazard model, albumin and hs-CRP/log10 hs-CRP were presented as continuous and their tertiles (Table 2 and Additional file 1: Fig. S4). According to clinical experience and current studies, we adjusted the covariates in multivariate model, including demographic characteristics [age > 65, gender, and smoking], diagnosis [hypertension, chronic kidney diseases, acute myocardial infarction, stroke, diabetes mellitus, percutaneous coronary intervention, ejection fraction reduced heart failure, hyperlipidemia, and anemia], and medical information [angiotensin-converting enzyme inhibitor/angiotensin receptor blockers, β-blockers, and statins] [26,27,28]. Multivariate and interaction test was used to analyze the joint effect of hs-CRP and albumin on mortality (Table 3). We also performed a subgroup analysis among four prespecified subgroups (ACS or Choric CAD, older [age > 65] or younger [age ≤ 65], male or female, anemia or non-anemia) to assess the independent and joint effects of hs-CRP and albumin on long-term all-cause mortality and calculated the P interaction to assess the relationship between the endpoints and subgroups. Because of relatively small sample size, adjustment for multivariate not performed (Additional file 1: Table S4).
Sensitivity analysis
We proceed a sensitivity analysis that those with severe hypoalbuminemia (≤ 25 g/L) (n = 7) are excluded from our included subjects to see whether the results were consistent. In addition, we also divided patients into four groups: group 1 (non-hypoalbuminemia and age ≤ 65; group 2 (hypoalbuminemia and age ≤ 65), group 3 (non-hypoalbuminemia and age > 65), and group 4 (hypoalbuminemia and age > 65). All data analyses were performed using R (version 4.0.3; R Core Team, Vienna, Austria). Two-tailed P values < 0.05 were considered statistically significant.
Results
Clinical characteristics
A total of 1449 patients were included in the study. Based on albumin levels (< 35 or ≥ 35 g/L), patients were divided into two groups; 501 (34.6%) had hypoalbuminemia and 948 (65.4%) did not have hypoalbuminemia. Under different albumin level groups, patients were further divided into two groups according to hs-CRP level (≤ 3 or > 3 mg/L). The mean overall age was 63.5 ± 10.8 years, and 329 (22.7%) were female. A total of 1041 (71.8%) patients underwent percutaneous coronary intervention (PCI) treatment, 317 (21.9%) patients were identified as having CKD, 426 (29.4%) patients had diabetes mellitus (DM), and 98 (7.6%) had ejection fraction reduced heart failure (EFrHF). In the hypoalbuminemia group, the mean age overall was 66.7 ± 9.5 years, and 127 (25.3%) were female. A total of 378 (75.4%) patients underwent PCI treatment, 159 (31.7%) patients were identified as having CKD, 157 (31.3%) patients had DM, and 61 (13.1%) had EFrHF. More data on the baseline characteristics of the study population are detailed in Table 1 and Additional file 1: Table S3.
Primary outcomes
During a median follow-up of 2.9 years (interquartile range: 2.0 to 3.0 years), a total of 107 (7.4%) patients died. As can be seen in Fig. 2, there was a significant linear association between both hs-CRP and albumin with the risk of all-cause death. As you can see in Table 2, when albumin was a continuous variable, it was significantly associated with long-term mortality (HR 0.90, 95% CI 0.85–0.95, P < 0.001), whereas the result of log10 (hs-CRP) was opposite (HR 1.51, 95% CI 1.09–2.09, P = 0.014). Subsequently, in the multivariate model, with full adjustment, the lowest albumin level (T1) was correlated with a greater risk of mortality with albumin at the highest level (T3; HR 2.55, 95% CI 1.29–5.02, P = 0.007, P for trend = 0.005); highest hs-CRP level (T3) was associated with the greater risk of mortality with the lowest hs-CRP level (T1; HR 2.10, 95% CI 1.17–3.78, P = 0.013, P for trend = 0.013).
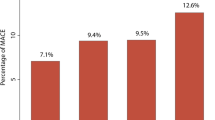
As can be seen in Fig. 3 and Table 3, when hs-CRP levels (≤ 3 mg/L vs. > 3 mg/L) and hypoalbuminemia (yes or no) were taken together, the Kaplan–Meyer curves showed that the cumulative hazard of death risk significantly differed among the four groups and that Group 4 (hs-CRP > 3 mg/L and hypoalbuminemia) had the highest risk of death when compared to the other groups (Group1 vs Group 2 vs Group 3 vs Group 4: 2.9% vs 7.9% vs 8.7% vs 13.5%). Cox proportional hazard regression analyses estimated that the highest risk of death was in Group 4 (hypoalbuminemia and hs-CRP > 3 mg/L) in multivariate model, (HR 3.79, 95% CI 1.91–7.52, P < 0.001). The analyses also revealed an additive effect of hypoalbuminemia and increased hs-CRP in predicting the risk of all-cause mortality. Further, results were consistent across all subgroups, even among the ACS (yes vs. no) and age groups (older [age > 65] vs. younger [age ≤ 65]; P-interaction > 0.05; Additional file 1: Table S4).
Sensitivity analysis
Our overall findings were consistent after performing the sensitivity analyses described in the methods (Additional file 1: Table S5). We note that the patients with hypoalbuminemia had lower HDLC level and more complications, such as anemia, AMI, CKD and more. However, in the group of age ≤ 65, there was no difference in some complications, and the incidence of hypoalbuminemia was significantly lower than that in the group of age > 65 (Additional file 1: Table S6).
Discussion
We systematically assessed the significance of concomitant high hs-CRP levels and hypoalbuminemia on long-term all-cause mortality among CAD patients. We found that concomitant high hs-CRP levels (> 3 mg/L) and hypoalbuminemia (< 35 g/L) increased the risk of long-term all-cause mortality among CAD patients near four-fold.
Previous studies have shown that hs-CRP level, which is a biomarker of systemic inflammation, has been reported to predict cardiovascular events among a wide variety of population such as CAD patients [29,30,31]. For example, Momiyama et al. indicated that an hs-CRP level > 1.0 mg/L was an independent predictor for cardiovascular events in Japanese patients with stable CAD [3]. Kim et al. showed that elevated levels of hs-CRP (≥ 3 mg/L) was an independent predictor of long-term cardiovascular outcomes only among ST-elevated myocardial infarction (STEMI) patients with a long ischemic time[2]. While both of these studies are excellent proof-of-concepts, the absence of long-term all-cause death is a limitation. It should also be mentioned that studies have suggested inflammation may play a different role in ACS and stable CAD on various elements, such as cytokine levels and proinflammatory status [32]. Although the distribution of hs-CRP was different among ACS and stable CAD patients, the increased risk relation of combined hs-CRP to albumin was consistent, which may rely on the mechanism of inflammation in hypoalbuminemia.
Conversely, epidemiologic studies have reported that hypoalbuminemia concentration predicted adverse outcomes in several populations, such as individuals with ACS [33,34,35]. Touma et al. indicated patients with hypoalbuminemia were older, and with increased comorbidity, than patients with normal albumin levels [36]. The pathological process in these conditions can be inflammatory, degenerative, or malignant, and has been confirmed in various medical conditions, as shown in recent studies [37, 38].
The prognostic relevance of serum albumin in cardiovascular disorders primarily refers to hypoalbuminemia and inflammation. Wiedermann indicated that preexisting hypoalbuminemia contributes to the risk of acute infectious diseases, and that acute loss of albumin in systemic inflammatory reactions further complicates the clinical course of all trauma, medical, and surgical conditions [12]. The high-sensitivity Modified Glasgow Prognostic Score are measures that utilize hs-CRP and albumin levels, and have been widely used in the field of oncology. Previous studies [39, 40] have demonstrated their ability in predicting poor prognosis in patients with tumors. CAD has a similar pathophysiological process to the tumor, which is related to inflammation and hypoalbuminemia. Our study indicated that a high hs-CRP level increases the risk of long-term all-cause mortality, especially in CAD patients with hypoalbuminemia. What is exciting is that our conclusion coincides with Hideki Wada, et al.’s study [27]. We included an interaction test in additive scale for the joint effect of hs-CRP and hypoalbuminemia on mortality. We found that the P for interaction between hs-CRP and albumin was 0.04. Our study indicated that, especially for CAD patients with high hs-CRP levels, hypoalbuminemia increased the risk of long-term all-cause mortality. Serum albumin concentration is defined by a complex interaction of several factors, including inflammation, nutrition, and dialysis efficacy. Therefore, the prognostic value of serum albumin is mainly explained by inflammation [11,12,13].
Furthermore, we noted that the population with hypoalbuminemia was likely to be older and had more complications. Our study showed among middle-aged subjects, risks of outcomes in individuals who had high CRP levels alone or in individuals who had low serum albumin levels alone attenuate or even lose statistical power. It indicated that exploring hypoalbuminemia and high CRP levels in the elderly has greater significance and more attention should be paid to the treatment of hypoalbuminemia and high CRP levels in the elderly in subsequent studies.
Our study showed that it was very meaningful to assess the degree of serum albumin and inflammation in patients with CAD. Better prognosis hinges on timely recognition of, and action toward, clinical clues. CAD patients with hypoalbuminemia must receive medical tests including computed tomography, urine analysis and more to determine the reason for albumin decrease, and subsequently adopt effective therapy. For example, when nutritional intake is lacking, a multidisciplinary nutrition team may give them nutritional support nutritional supplementation. Interdisciplinary approaches should be considered in these patients. Furthermore, some treatments that reduce CAD-related risks factors (such as smoking, hyperlipemia, hypertension, DM,, and overweight) can also reduce inflammation [41]. In addition, lipid-lowering therapy can reduce inflammation in atherosclerosis by severely controlling low-density lipoprotein cholesterol [42].
Limitations
This study systematically examined the significance of concomitant hypoalbuminemia and high hs-CRP levels on long-term all-cause mortality among CAD patients. The abundant data extracted from multiple centers allowed us to control a variety of confounders during analyses. Nevertheless, there are several limitations to the current study. First, information about cause-specific mortality was not available in this study, which restricted our ability to examine the significance of concomitantly high hs-CRP levels and hypoalbuminemia with cause-specific mortality. Second, although hs-CRP and CRP are usually tested together in the clinical practice, we strictly did not include the patients without the data of hs-CRP, even if these patients had CRP. As a result, selection bias should be existed. However, there was no special tendency for clinicians to choose these two tests in general and the difference of mortality rates between excluded subjects and including subjects were no statistic significance. However, our study was based on a multicenter prospective cohort study including all CAD patients. Thus, future testing should be conducted on a larger population of CAD patients. Third, only inpatient information about baseline hs-CRP and albumin were contained in our study. Therefore, follow-up information of hs-CRP and albumin should be collected to better understand their status and the effects of changes after discharge. Fourth, we did not have access to several variables that may have influenced the final results, including body mass index and diet structure. Finally, the current study lacked corresponding information on malignant diseases that may cause protein-energy malnutrition and inflammation. Further studies that assess the role of malignant diseases are warranted to corroborate these results.
Conclusions
High hs-CRP levels, as well as hypoalbuminemia, are effective prognostic factors of long-term all-cause mortality in CAD patients, especially in elderly patients. Importantly, CAD patients with high hs-CRP levels (> 3 mg/L) and hypoalbuminemia (< 35 g/L) exhibited a three-fold increased risk of long-term all-cause mortality. Clinically, prospective large scale population studies are necessary to more deeply explore high hs-CRP levels and hypoalbuminemia in CAD patients with a high risk of mortality.
Availability of data and materials
The datasets used and analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- AMI:
-
Acute myocardial infarction
- CAD:
-
Coronary artery disease
- CKD:
-
Chronic kidney disease
- hs-CRP:
-
High-sensitivity C-reactive protein
- PCI:
-
Percutaneous coronary intervention
References
Dai H, Much AA, Maor E, Asher E, Younis A, Xu Y, Lu Y, Liu X, Shu J, Bragazzi NL. Global, regional, and national burden of ischemic heart disease and its attributable risk factors, 1990–2017: results from the global Burden of Disease Study 2017. Eur Heart J Qual Care Clin Outcomes. 2020.
Kim KH, Kim W, Kang WY, Hwang SH, Cho SC, Kim W, Jeong MH. The impact of ischemic time on the predictive value of high-sensitivity C-reactive protein in ST-segment elevation myocardial infarction patients treated by primary percutaneous coronary intervention. Korean Circ J. 2013;43(10):664–73.
Momiyama Y, Kawaguchi A, Kajiwara I, Ohmori R, Okada K, Saito I, Konishi M, Nakamura M, Sato S, Kokubo Y, et al. Prognostic value of plasma high-sensitivity C-reactive protein levels in Japanese patients with stable coronary artery disease: the Japan NCVC-Collaborative Inflammation Cohort (JNIC) Study. Atherosclerosis. 2009;207(1):272–6.
Koenig W. High-sensitivity C-reactive protein and atherosclerotic disease: from improved risk prediction to risk-guided therapy. Int J Cardiol. 2013;168(6):5126–34.
Arques S. Human serum albumin in cardiovascular diseases. Eur J Intern Med. 2018;52:8–12.
Suzuki S, Hashizume N, Kanzaki Y, Maruyama T, Kozuka A, Yahikozawa K. Prognostic significance of serum albumin in patients with stable coronary artery disease treated by percutaneous coronary intervention. PLoS ONE. 2019;14(7):e0219044.
Shiyovich A, Bental T, Assali A, Vaknin-Assa H, Kornowski R, Perl L. Changes over time in serum albumin levels predict outcomes following percutaneous coronary intervention. J Cardiol. 2020;75(4):381–6.
Yeun JY, Levine RA, Mantadilok V, Kaysen GA. C-reactive protein predicts all-cause and cardiovascular mortality in hemodialysis patients. Am J Kidney Dis. 2000;35(3):469–76.
Mitch WE. Malnutrition: a frequent misdiagnosis for hemodialysis patients. J Clin Investig. 2002;110(4):437–9.
Mitch WE. Proteolytic mechanisms, not malnutrition, cause loss of muscle mass in kidney failure. J Renal Nutr. 2006;16(3):208–11.
de Mutsert R, Grootendorst DC, Indemans F, Boeschoten EW, Krediet RT, Dekker FW. Association between serum albumin and mortality in dialysis patients is partly explained by inflammation, and not by malnutrition. J Renal Nutr. 2009;19(2):127–35.
Wiedermann CJ. Hypoalbuminemia as surrogate and culprit of infections. Int J Mol Sci. 2021;22(9):4496.
Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. 2003;63(3):793–808.
Lu X, Guo W, Xu W, Zhang X, Shi Z, Zheng L, Zhao W. Prognostic value of the Glasgow prognostic score in colorectal cancer: a meta-analysis of 9839 patients. Cancer Manag Res. 2019;11:229–49.
Hao X, Wei Y, Wei X, Zhou L, Wei Q, Zhang Y, Huang W, Feng R. Glasgow prognostic score is superior to other inflammation-based scores in predicting survival of diffuse large B-cell lymphoma. Oncotarget. 2017;8(44):76740–8.
Zhu L, Chen S, Ma S, Zhang S. Glasgow prognostic score predicts prognosis of non-small cell lung cancer: a meta-analysis. Springerplus. 2016;5:439.
Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, et al. ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407–77.
Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64(24):e139–228.
Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87–165.
Aguiar-Souto P, Ferrante G, Del Furia F, Barlis P, Khurana R, Di Mario C. Frequency and predictors of contrast-induced nephropathy after angioplasty for chronic total occlusions. Int J Cardiol. 2010;139(1):68–74.
Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511.
Kurtul A, Ocek AH, Murat SN, Yarlioglues M, Demircelik MB, Duran M, Ergun G, Cay S. Serum albumin levels on admission are associated with angiographic no-reflow after primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction. Angiology. 2015;66(3):278–85.
Oduncu V, Erkol A, Karabay CY, Kurt M, Akgün T, Bulut M, Pala S, Kirma C. The prognostic value of serum albumin levels on admission in patients with acute ST-segment elevation myocardial infarction undergoing a primary percutaneous coronary intervention. Coron Artery Dis. 2013;24(2):88–94.
Wei XB, Jiang L, Liu YH, Feng D, He PC, Chen JY, Yu DQ, Tan N. Prognostic value of hypoalbuminemia for adverse outcomes in patients with rheumatic heart disease undergoing valve replacement surgery. Sci Rep. 2017;7(1):1958.
Chi G, Gibson CM, Liu Y, Hernandez AF, Hull RD, Cohen AT, Harrington RA, Goldhaber SZ. Inverse relationship of serum albumin to the risk of venous thromboembolism among acutely ill hospitalized patients: analysis from the APEX trial. Am J Hematol. 2019;94(1):21–8.
Blomberg J, Lagergren P, Martin L, Mattsson F, Lagergren J. Albumin and C-reactive protein levels predict short-term mortality after percutaneous endoscopic gastrostomy in a prospective cohort study. Gastrointest Endosc. 2011;73(1):29–36.
Wada H, Dohi T, Miyauchi K, Doi S, Naito R, Konishi H, Tsuboi S, Ogita M, Kasai T, Okazaki S, et al. Independent and combined effects of serum albumin and C-reactive protein on long-term outcomes of patients undergoing percutaneous coronary intervention. Circ J. 2017;81(9):1293–300.
Liu ZY, Tang JN, Cheng MD, Jiang LZ, Guo QQ, Zhang JC, Zhang ZL, Song FH, Wang K, Fan L, et al. C-reactive protein-to-serum albumin ratio as a novel predictor of long-term outcomes in coronary artery disease patients who have undergone percutaneous coronary intervention: analysis of a real-world retrospective cohort study. Coronary Artery Dis. 2021.
Ridker PM. High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation. 2001;103(13):1813–8.
Pu LJ, Lu L, Xu XW, Zhang RY, Zhang Q, Zhang JS, Hu J, Yang ZK, Ding FH, Chen QJ, et al. Value of serum glycated albumin and high-sensitivity C-reactive protein levels in the prediction of presence of coronary artery disease in patients with type 2 diabetes. Cardiovasc Diabetol. 2006;5:27.
Matsubara T, Naruse K, Arakawa T, Nakao M, Yokoi K, Oguri M, Marui N, Amano T, Ichimiya S, Ohashi T, et al. Impact of pitavastatin on high-sensitivity C-reactive protein and adiponectin in hypercholesterolemic patients with the metabolic syndrome: the PREMIUM Study. J Cardiol. 2012;60(5):389–94.
Parisi V, Petraglia L, Cabaro S, D’Esposito V, Bruzzese D, Ferraro G, Urbani A, Grieco FV, Conte M, Caruso A, et al. Imbalance between interleukin-1β and interleukin-1 receptor antagonist in epicardial adipose tissue is associated with non ST-segment elevation acute coronary syndrome. Front Physiol. 2020;11:42.
Phillips A, Shaper AG, Whincup PH. Association between serum albumin and mortality from cardiovascular disease, cancer, and other causes. Lancet (London, England). 1989;2(8677):1434–6.
Plakht Y, Gilutz H, Shiyovich A. Decreased admission serum albumin level is an independent predictor of long-term mortality in hospital survivors of acute myocardial infarction. Soroka Acute Myocardial Infarction II (SAMI-II) project. Int J Cardiol. 2016;219:20–4.
Klonoff-Cohen H, Barrett-Connor EL, Edelstein SL. Albumin levels as a predictor of mortality in the healthy elderly. J Clin Epidemiol. 1992;45(3):207–12.
Touma E, Bisharat N. Trends in admission serum albumin and mortality in patients with hospital readmission. Int J Clin Pract. 2019;73(6):e13314.
Yeun JY, Kaysen GA. Acute phase proteins and peritoneal dialysate albumin loss are the main determinants of serum albumin in peritoneal dialysis patients. Am J Kidney Dis. 1997;30(6):923–7.
Levitt DG, Levitt MD. Human serum albumin homeostasis: a new look at the roles of synthesis, catabolism, renal and gastrointestinal excretion, and the clinical value of serum albumin measurements. Int J Gen Med. 2016;9:229–55.
Takeno S, Hashimoto T, Shibata R, Maki K, Shiwaku H, Yamana I, Yamashita R, Yamashita Y. The high-sensitivity modified Glasgow prognostic score is superior to the modified Glasgow prognostic score as a prognostic predictor in patients with resectable gastric cancer. Oncology. 2014;87(4):205–14.
Osugi J, Muto S, Matsumura Y, Higuchi M, Suzuki H, Gotoh M. Prognostic impact of the high-sensitivity modified Glasgow prognostic score in patients with resectable non-small cell lung cancer. J Cancer Res Ther. 2016;12(2):945–51.
Hansson GK, Robertson AK, Söderberg-Nauclér C. Inflammation and atherosclerosis. Annu Rev Pathol. 2006;1:297–329.
Aikawa M, Rabkin E, Sugiyama S, Voglic SJ, Fukumoto Y, Furukawa Y, Shiomi M, Schoen FJ, Libby P. An HMG-CoA reductase inhibitor, cerivastatin, suppresses growth of macrophages expressing matrix metalloproteinases and tissue factor in vivo and in vitro. Circulation. 2001;103(2):276–83.
Acknowledgements
Not applicable.
Funding
Beijing Lisheng Cardiovascular Health Foundation Pilot Fund (No. LHJJ20141751). The National Science Foundation of China (No. 81970311 and No. 82070360). Study on the function and mechanism of the potential target for early warning of cardiorenal syndrome after acute myocardial infarction based on transformism (DFJH201919). Clinical Medicine Research Fund of Guangdong Province (2019ZX01).
Author information
Authors and Affiliations
Contributions
Research idea and study design: YL and JL; Data acquisition: RHT, QL, KBM, WW, YRY, ZDH, WGL, and BW; Data analysis/interpretation: YRY and HZH; Statistical analysis: HZH and SQC, Supervision and mentorship: YL, JYC, NT and LLC; Writing guidance: YL and LLC. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study and all of its protocols were approved by the institutional Ethics Research Committee of Guangdong Provincial People’s Hospital (No. GDREC2012141H). The study protocol conformed to the principles outlined in the Declaration of Helsinki and was approved by the Guangdong Provincial People’s Hospital ethics committee. Written informed consent to participate in the study was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary instructions 1–10:
1. Investigators or sub-investigators; 2. The inclusion criteria and exclusion criteria of REICIN cohort; 3. Baseline characteristics of the patients; 4. Crude mortality and Cox proportional hazard ratios of different subgroups; 5. Sensitivity analysis; 6. Characteristics between subjects with and without hypoalbuminemia; 7. The flow of participants through REICIN cohort; 8. receiver-operator characteristic (ROC) curves; 9. Density curve of hs-CRP among ACS and chronic CAD; 10. Dose-response relationship between hs-CRP tertiles and unadjusted hazard ratios and 95% confidence intervals of long-term all-cause mortality, stratified by status of albumin.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Huang, H., Yu, Y., Chen, L. et al. Independent and joint effects of high-sensitivity c-reactive protein and hypoalbuminemia on long-term all-cause mortality among coronary artery disease: a prospective and multicenter cohort study. BMC Cardiovasc Disord 21, 613 (2021). https://doi.org/10.1186/s12872-021-02431-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-021-02431-6