Abstract
Background
Patients with coronary artery disease (CAD) combined with diabetes have a higher risk of cardiovascular events, and high-sensitivity C-reactive protein (hs-CRP)-to-albumin ratio (CAR) is a novel inflammatory biomarker. However, whether the CAR can identify high-risk patients with CAD and type 2 diabetes (T2DM) remains unclear.
Methods
The present study was based on a prospective and observational cohort with 10,724 individuals who undergo percutaneous coronary intervention (PCI) in Fu Wai Hospital throughout the year 2013 consecutively enrolled. The primary endpoint was all-cause mortality. The secondary endpoint was cardiac mortality. CAR was calculated with the formula: hs-CRP (mg/L)/albumin (g/L). According to the optimal cut-off value of CAR for all-cause mortality, patients were divided into higher CAR (CAR-H) and lower CAR (CAR-L) groups.
Results
A total of 2755 patients with T2DM who underwent PCI and received dual antiplatelet therapy were finally enrolled. During a follow-up of 5 years (interquartile range: 5.0–5.1 years), 126 (4.6%) all-cause mortalities and 74 (2.7%) cardiac mortalities were recorded. In the multivariable Cox model, CAR-H was associated with a higher risk of all-cause mortality (hazard ratio [HR]: 1.634, 95% confidence interval [CI] 1.121–2.380, p = 0.011) and cardiac mortality (HR: 1.733, 95% CI 1.059–2.835, p = 0.029) compared with CAR-L. When comparing the predictive value, CAR was superior to hs-CRP for all-cause mortality (area under the curve [AUC] 0.588 vs. 0.580, p = 0.002) and cardiac mortality (AUC 0.602 vs. 0.593, p = 0.004).
Conclusion
In this real-world cohort study, a higher level of CAR was associated with worse 5-year outcomes among diabetic patients with PCI.
Similar content being viewed by others
Introduction
Globally, approximately 32.2% of patients with type 2 diabetes mellitus (T2DM) simultaneously suffer coronary artery disease (CAD) [1]. Patients with CAD are at significantly higher risk of adverse events once combined with diabetes mellitus (DM). The accurate identification and subsequent treatment of high-risk patients with CAD and DM to reduce adverse prognosis has been a hotspot of current research. Inflammation plays a crucial role in the progression of coronary atherosclerotic disease and DM. There is growing evidence that the higher levels of inflammatory biomarkers are significantly associated with an increased risk of adverse cardiovascular events in patients with CAD or DM [2,3,4].
As acute phase proteins of inflammation, both high-sensitivity C-reactive protein (hs-CRP) and albumin are vital indicators that reflect the inflammation grade. However, their acute phase responses to inflammation are distinct, with hs-CRP levels rising and albumin levels falling [5]. Previous studies have explored hs-CRP and albumin and prognosis of CAD, respectively. On the one hand, in patients with CAD, it was shown that hs-CRP was strongly associated with disease severity [6] and adverse cardiovascular events [2, 3]. On the other hand, in patients with T2DM, hs-CRP was associated with an increased risk of adverse cardiovascular events [7] and mortality [8]. Albumin is an important nutritional indicator that is associated with inflammatory and hemostatic processes. Hypoalbuminemia is an independent risk factor for in-hospital and long-term prognosis in patients with acute coronary syndromes (ACS) and myocardial infarction (MI) [9,10,11]. Therefore, early prediction of the effect of inflammation on the risk of death and cardiovascular events in PCI patients combined with T2DM (individuals at high risk among patients with CAD [12]) is an essential clinical issue. The hs-CRP-to-albumin ratio (CAR) is a very novel indicator of inflammation, but to date, the association between CAR levels and long-term prognosis in a diabetic population with PCI has not been reported in the literature.
Recent studies have shown that CAR is a more accurate indicator than albumin and hs-CRP alone in terms of the evaluation of systemic inflammatory status as well as the determination of prognosis of patients with cancer and critical illness. CAR is a prognostic factor in esophageal [13], hepatocellular [14], and ovarian cancers [15]. In patients with CAD, it is strongly associated with the disease severity [16, 17], coronary thrombotic load [18] and prognosis [19,20,21]. It is an important clinical issue to assess whether CAR can predict the risk of all-cause mortality and cardiac mortality in PCI patients with T2DM. Therefore, we aimed to use a large, real-world and long-term follow-up dataset to evaluate the effect of CAR on the risk of long-term adverse cardiovascular events in patients with T2DM treated with PCI.
Methods
Study design and patients
The present study was based on a real-world, prospective, single-centre and observational cohort. From January 2013 to December 2013, 10,724 patients who were treated with PCI were consecutively and prospectively enrolled in Fu Wai Hospital, National Center for Cardiovascular Diseases, National Clinical Research Center for Cardiovascular Diseases and State Key Laboratory of Cardiovascular Diseases (Beijing, China). Among them, 3238 patients with T2DM. After excluding 36 patients not treated with dual antiplatelet therapy (DAPT) at baseline, 3 patients treated with oral anticoagulants and 205 who received only balloon dilatation without stent, 2994 eligible diabetic patients who undergo PCI with DAPT were enrolled. Furthermore, we excluded 239 patients lost to follow-up, with a final sample size of 2755 (92.0%) for statistical analysis (Fig. 1).
After PCI, DAPT consisting of aspirin 100 mg daily and clopidogrel 75 mg daily or ticagrelor 90 mg twice daily were administered for at least 12 months in all participants according to the guideline at that time in China [22]. This study complied with the Helsinki Declaration. The Review Board of Fu Wai Hospital approved the study protocol (Approval Number: 2013-449). All participants provided written informed consent.
Parameters measurements
Within 24 h after admission, venous blood was collected from all patients after fasting and was sent to the laboratory within two hours. The serum albumin concentration was measured by an AU5400 automatic biochemical analyzer (Beckman Coulter, Brea, CA, USA), and the serum hs-CRP concentration was measured by an automated biochemical analyzer (Labospect 008, Hitachi 7150, Tokyo, Japan) in the Clinical Chemistry Department of Fu Wai Hospital.
Definition and study outcomes
CAR refers to the hs-CRP to albumin ratio. According to the optimal cut-off value of CAR for all-cause mortality, patients with higher CAR were divided into a higher level of CAR (CAR-H) group, while those with lower CAR were divided into a lower level of CAR (CAR-L) group.
The primary endpoint was all-cause mortality. The secondary endpoint was cardiac mortality. Follow-ups were regularly conducted through clinic visits or by telephone interviews at 30 days, 6 months, 1 year, 2 years and 5 years, with a 92.0% follow-up rate at 5 years. The time to events was calculated as the number of days from the date of performing PCI to the date of the death, the last visit or the last recorded clinical event of participants still alive, whichever came first. All endpoint events were adjudicated centrally by two independent cardiologists, and possible disagreement was resolved by consensus.
Statistical analysis
Continuous variables with a normal distribution were expressed as mean ± standard deviation, while categorical variables were expressed as frequency (percentage). Continuous variables were compared by the Student’s t-test, and the Pearson chi-square test or Fisher’s exact test was used to compare categorical variables.
Receiver operating characteristic (ROC) curve analysis was performed to obtain the area under the curve (AUC) and the Youden’s index. The Youden’s index with the greatest sensitivity and specificity was used to determine the optimal cut-off value for CAR to predict long-term all-cause mortality. Survival curves were constructed by Kaplan–Meier method and compared by the log-rank test. The univariate and multivariate Cox regression analyses were conducted to calculate the Hazard ratios (HRs) and 95% confidence interval (CI) and assess the associations among CAR and 5-year outcomes. The multivariate Cox model was adjusted for the following covariates: sex, body mass index, hypertension, ACS, haemoglobin, white blood cell count, estimated glomerular filtration rate and glucose. Furthermore, to reduce confounding, propensity score matching was performed to adjust for baseline differences between CAR-L and CAR-H groups. The exploratory analyses were performed to assess the effect of CAR in different subgroups on the primary endpoint. Pearson correlation and linear regression analysis were constructed to evaluate the correlation between hs-CRP and albumin. The prediction of CAR and hs-CRP for 5-year outcomes were assessed by using AUC and compared by the Delong test. All statistical analyses were performed at a significance level of two-sided 0.05. Statistical analyses were performed via SPSS 23.0 (IBM Corp., Armonk, New York, USA), R Programming Language version 4.0.3 (R Core Team, 2014) and GraphPad Prism version 8.0.0 for windows (GraphPad Software, San Diego, California USA).
Results
A total of 2755 eligible diabetic patients who undergo PCI with DAPT were included for final analysis (Fig. 1), among whom 126 (4.6%) experienced all-cause mortality and 74 (2.7%) experienced cardiac mortality during a follow-up of 5 years.
Baseline characteristics
The mean age of the 2755 participants was 59.2 ± 9.7 years, 2060 (74.8%) were men and 1552 (56.3%) clinically presented with ACS. Based on the Youden’s index, the optimal cut-off point of CAR is 0.0594. Table 1 shows the comparison of baseline characteristics of CAR-H versus CAR-L. Individuals with higher CAR had less men, a significantly higher body mass index, a higher prevalence of hypertension and ACS, a lower baseline haemoglobin, a higher baseline white blood cell count, a lower baseline estimated glomerular filtration rate and a higher baseline glucose concentration than those with lower CAR (all p < 0.05).
Incidences of 5-year outcomes in CAR-L and CAR-H
The incidences of all-cause mortality (62/964 [6.4%] versus 64/1791 [3.6%], p = 0.001) and cardiac mortality (39/964 [4.0%] versus 35/1791 [2.0%], p = 0.001) were significantly higher in patients with CAR-H than in those with CAR-L.
Survival analysis
The Kaplan–Meier estimates of all-cause mortality and cardiac mortality were shown in Fig. 2. In the univariate Cox model, CAR-H was associated with all-cause mortality (HR: 1.815, 95% CI 1.280–2.574, p < 0.001) and cardiac mortality (HR: 2.085, 95% CI 1.321–3.291, p = 0.002) compared with CAR-L (Table 2). In the multivariable Cox model, patients with higher CAR had a higher risk of all-cause mortality (adjusted HR: 1.634, 95% CI 1.121–2.380, p = 0.011) and a higher risk of cardiac mortality (adjusted HR: 1.733, 95% CI 1.059–2.835, p = 0.029) compared with those with lower CAR (Table 3).
ROC curves of CAR to 5-year follow-up
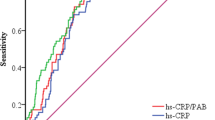
ROC curves were performed to assess the predictive value of CAR for 5-year outcomes as a continuous variable. The CAR showed a predictive value for all-cause mortality with an AUC of 0.588 (95% CI 0.536–0.639), and for cardiac mortality with an AUC of 0.602 (95% CI 0.537–0.667) (Fig. 3).
The comparison between CAR and hs-CRP
The prediction of CAR for all-cause mortality was superior to hs-CRP (AUC 0.588 vs. 0.580, Z=− 3.140, p = 0.002) and the prediction of CAR for cardiac mortality was superior to hs-CRP (AUC 0.602 vs. 0.593, Z=− 2.889, p = 0.004).
Sensitivity analysis
A 1:2 propensity score matching cohort (n = 2122) was obtained from CAR-H (n = 964) versus CAR-L (n = 1791). This analysis showed CAR-H was associated with all-cause mortality (HR:1.484, 95% CI 1.000–2.201) and cardiac mortality (HR: 1.691, 95% CI 1.001–2.856) compared with CAR-L (Additional file 1: Table S1).
Subgroup analyses
Subgroup analyses showed the CAR-H was associated with all-cause mortality relative to CAR-L in certain subsets (age, sex, ACS, hypertension, and SYNTAX score) (Fig. 4).
Correlation analysis between hs-CRP and albumin
The range for albumin was 24.20 to 55.30 g/L. Only 4 (0.1%) patients showed hypoalbuminemia (albumin < 30 g/L). The range for hs-CRP was 0–16.99 mg/L. No patient with hs-CRP > 20 mg/L. Pearson correlation analysis and linear regression analysis showed that hs-CRP was negatively associated with albumin (R2 = 0.043, Standard β=− 0.208, p < 0.001) (Additional file 1: Figure S1).
Discussion
This real-world study of 2755 participants with T2DM who undergo PCI with DAPT with 5 years of follow-up showed that compared with patients with a lower level of CAR, those with a higher level of CAR was associated with an increased risk of long-term mortality including all-cause mortality and cardiac mortality; the prediction of CAR for all-cause mortality and cardiac mortality was superior to hs-CRP; the results remained consistent after propensity score matching.
CAR is associated with the risk of long-term mortality in patients with CAD combined with T2DM
CAR is a novel biomarker of inflammation. The present study is the first to report that although receiving PCI and DAPT, CAR still can reflect long-term residual inflammation risk in patients with CAD combined with T2DM. CAR is composed of two inflammatory factors: hs-CRP and albumin. In recent years, the association of hs-CRP and albumin and poor prognosis in patients with CAD has been a hotspot, yielding relatively consistent findings. Many previous studies have shown that hs-CRP is strongly associated with a higher incidence of long-term ischemic events in patients with CAD treated with PCI [2, 3], and decreased serum albumin is associated with susceptibility and poor prognosis of cardiovascular disease [9,10,11, 23]. Wada et al. showed that the presence of low serum albumin and high CRP levels had a synergistic effect on elevated long-term ischemic risk in patients undergoing PCI [24]. Meanwhile, in studies of diabetic patients, a recent meta-analysis has shown that hs-CRP is associated with a high risk of developing T2DM [4]. It has also been shown that chronic low-grade inflammation, as determined by measurement of hs-CRP, plays an important role in the development of T2DM [25] and may be a therapeutic target for reducing residual cardiovascular risk in patients with T2DM [26]. Albumin is an important biomarker for monitoring the pathophysiology of DM [27]. The albumin levels are reduced by approximately 20% in diabetic patients compared to normal subjects and basic trials have shown that treatment of diabetic rats with insulin restores albumin to normal levels [28].
CAR is a significant inflammatory biomarker for assessing prognosis in patients with certain cancer [15, 29,30,31], and has been shown to have a better predictive value than hs-CRP and albumin alone in terms of ischemic events [21, 32]. Meanwhile, CAR has a better diagnostic value for CAD than other inflammatory indicators (neutrophil to lymphocyte ratio, and monocyte to lymphocyte ratio, etc.) [33] and can be used as a predictor of atherosclerosis and CAD [34]. To date, there have been several studies on the prognostic value of CAR in CAD. However, it is worth noting that firstly, the number of people with CAD included in these studies was small, with the majority of the study population being only a few hundred, except for three which included 1396 [19], 1630 [20] and 2243 [21] patients. Secondly, the duration of follow-up was short. The investigators observed mostly in-hospital or short-term events with the longest mean follow-up being 37.59 months [20]. Finally, none of these studies performed further subgroup analyses in the DM population, so the predictive value of CAR in patients with CAD combined with DM is unclear. In terms of death, Liu et al. [20] showed that CAR was an independent predictor of long-term all-cause mortality and cardiac death in patients with CAD undergoing PCI (n = 1630). Among patients with ST-segment elevated MI (STEMI), Söğüt et al. (n = 116) [35] and Çınar et al. (n = 2,243) [21] showed that CAR was an independent predictor of in-hospital mortality and long-term all-cause mortality, respectively. Cheng et al. [36] found in patients with coronary chronic total occlusion (n = 664) that patients with higher CAR had an increased risk of all-cause mortality. In addition to the ischaemic aspect of death. Acet et al. [32] showed that CAR was an independent predictor of major adverse cardiac events (MACE) at 6 months in patients with STEMI (n = 539). Wang et al. [37] showed that CAR was independently associated with in-hospital and short-term MACE in patients with ACS (n = 652). Aksu et al. [19] found in STEMI patients (n = 1396) that CAR was an independent predictor of stent restenosis.
All of the above studies support the predictive value of CAR for ischaemic adverse events in patients with CAD. It has been suggested that CAR is a predictor of diabetes mellitus [38] and that higher CAR significantly increases the risk of the serious postoperative complication “systemic inflammatory response syndrome”, which is further increased in elderly patients with DM [39]. In summary, it is important to explore the effect of CAR on long-term prognosis in PCI patients with CAD combined with DM. However, there is a lack of literature on this subject. Therefore, in this study, 2755 patients with T2DM were selected from 10,724 patients with CAD treated with PCI for analysis. Our results showed that higher levels of CAR were strongly associated with a higher risk of long-term all-cause mortality and cardiac mortality in diabetic patients with PCI and that CAR was independently associated with poor prognosis, regardless of ACS.
Compared to previous CAR-related studies in patients with CAD, the total population with CAD enrolled in this study (n = 10,724) is the largest in number in this field to date and is more representative. It is worth pointing out that this study focused on patients at higher risk in the population with CAD (T2DM). Besides, this result remained consistent after propensity score matching, indicating reliable stability. Patients with CAD combined with diabetes represent a larger proportion of the population, and assessing CAR in this group of patients may have clinical implications for the management of high-risk individuals. Diabetics account for a large proportion of patients with CAD [12], and the assessment of CAR in these patients may be of clinical implications for the management of high-risk individuals.
Potential mechanisms and clinical implications
Potential mechanisms to the pathophysiology of elevated CAR associated with poor prognosis of death in patients with PCI combined with T2DM are as follows. Our previous studies have shown that higher levels of hs-CRP are associated with high platelet reactivity [40], which is associated with an increased risk of ischaemic events [41]. In addition, CRP promotes smooth muscle cell proliferation, affects human macrophage polarization [42] enhances thromboxane activity [42] and ultimately leads to atherosclerosis and thrombosis. Albumin has a number of important physiological functions, such as antioxidant and anticoagulant functions [43]. With regard to the characteristic of antioxidants, serum albumin contains a free cysteine at the − 34 position, called the Cys34 residue, which has redox properties [44]. With this, serum albumin inhibits lipid peroxidation [45] and increases glutathione levels [46], acting as an anti-atherogenic agent. With regard to the characteristic of anticoagulation, albumin Cys-34 binds nitric oxide, resulting in the formation of nitrosoalbumin, which prolongs the biological activity of nitric oxide, acting as a vasodilator and inhibitor of platelet aggregation agent [47]. In particular, serum albumin in diabetic patients is glycated, impairing its ligand to bind to albumin and further impairing antioxidant and anticoagulant properties. Although we know that inflammatory hypotheses have been proposed in the development of CAD and DM, the exact mechanisms linking high hs-CRP and low serum albumin to increased risk of CAD and DM are not yet clear. Therefore, further studies are needed to elucidate the underlying mechanisms.
In conclusion, this study showed that patients with T2DM treated with PCI who have higher CAR levels were associated with a higher risk of long-term mortality (both all-cause and cardiac mortality) compared to those with lower CAR levels. In the future, it’s necessary to routinely screen this simple and readily available novel inflammatory biomarker, CAR, in patients with DM after PCI. Controlling residual inflammation risk can have further cardiovascular benefits, and anti-inflammatory therapy has yielded some encouraging results in recent years [48], but to our knowledge, anti-inflammatory drugs mostly focus on the levels of hs-CRP and interleukin-1β. Our research points out that in the future, we might pay attention to serum albumin levels at the same time, especially in patients with type 2 diabetes who undergo PCI. In other words, we are looking forward to a therapeutic target aimed at simultaneously lowering hs-CRP levels and raising serum albumin levels. In the future, whether long-term control of CAR levels can reduce the risk of events in patients with CAD combined with DM is worth further study.
Limitations
Our study had several limitations. First, this was a single-centre, observational study. Therefore, this study has the inherent defects of an observational study, and the extrapolation of our conclusions requires further verification. Second, we did not routinely evaluate serum hs-CRP and albumin concentrations which will fluctuate normally during the follow-up. Third, although we attempted to adjust as many important confounding factors as possible, unmeasured confounding factors still cannot be ruled out as related to the risk of outcomes. Fourth, the discrimination of CAR for all-cause mortality and cardiac mortality was poor to fair.
Conclusion
This large-sample, real-world study shows that higher level of CAR was associated with the risk of 5-year all-cause mortality and cardiac mortality in diabetic patients who undergo PCI with DAPT. CAR showed a predictive value for 5-year outcomes, which was superior to hs-CRP. This finding may shed a light on better management of patients with PCI, indicating that more attention should be paid to the inflammatory marker CAR in treatment in the current era of DAPT.
Availability of data and materials
Due to ethical restrictions related to the consent given by subjects at the time of study commencement, our datasets are available from the corresponding author upon reasonable request after permission of the Institutional Review Board of State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Diseases.
Abbreviations
- ACS:
-
Acute coronary syndrome
- DAPT:
-
Dual antiplatelet therapy
- DM:
-
Diabetes mellitus
- T2DM:
-
Type 2 diabetes mellitus
- CAD:
-
Coronary artery disease
- hs-CRP:
-
High-sensitivity C reactive protein
- PCI:
-
Percutaneous coronary intervention
- MI:
-
Myocardial infarction
- STEMI:
-
ST-segment elevated myocardial infarction
- CAR:
-
hs-CRP-to-albumin ratio
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under the curve
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- MACE:
-
Major adverse cardiac events
References
Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol. 2018;17(1):83.
Wada H, Dohi T, Miyauchi K, Shitara J, Endo H, Doi S, Naito R, Konishi H, Tsuboi S, Ogita M, et al. Preprocedural high-sensitivity C-reactive protein predicts long-term outcome of percutaneous coronary intervention. Circ J. 2016;81(1):90–5.
Liu R, Xu F, Ma Q, Zhou Y, Liu T. C-reactive protein level predicts cardiovascular risk in Chinese young female population. Oxid Med Cell Longev. 2021;2021:6538079.
Yang X, Tao S, Peng J, Zhao J, Li S, Wu N, Wen Y, Xue Q, Yang CX, Pan XF. High-sensitivity C-reactive protein and risk of type 2 diabetes: a nationwide cohort study and updated meta-analysis. Diabetes Metab Res Rev. 2021;37(8):e3446.
Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(6):448–54.
Liu Y, Jia SD, Yao Y, Tang XF, Xu N, Jiang L, Gao Z, Chen J, Yang YJ, Gao RL, et al. Impact of high-sensitivity C-reactive protein on coronary artery disease severity and outcomes in patients undergoing percutaneous coronary intervention. J Cardiol. 2020;75(1):60–5.
Hwang YC, Morrow DA, Cannon CP, Liu Y, Bergenstal R, Heller S, Mehta C, Cushman W, Bakris GL, Zannad F, et al. High-sensitivity C-reactive protein, low-density lipoprotein cholesterol and cardiovascular outcomes in patients with type 2 diabetes in the EXAMINE (examination of cardiovascular outcomes with alogliptin versus standard of care) trial. Diabetes Obes Metab. 2018;20(3):654–9.
Yamagishi S. Cardiovascular disease in recent onset diabetes mellitus. J Cardiol. 2011;57(3):257–62.
González-Pacheco H, Amezcua-Guerra LM, Sandoval J, Martínez-Sánchez C, Ortiz-León XA, Peña-Cabral MA, Bojalil R. Prognostic implications of serum albumin levels in patients with acute coronary syndromes. Am J Cardiol. 2017;119(7):951–8.
Bicciré FG, Pastori D, Tanzilli A, Pignatelli P, Viceconte N, Barillà F, Versaci F, Gaudio C, Violi F, Tanzilli G. Low serum albumin levels and in-hospital outcomes in patients with ST segment elevation myocardial infarction. Nutr Metab Cardiovasc Dis. 2021;31(10):2904–11.
Plakht Y, Gilutz H, Shiyovich A. Decreased admission serum albumin level is an independent predictor of long-term mortality in hospital survivors of acute myocardial infarction. soroka acute myocardial infarction II (SAMI-II) project. Int J Cardiol. 2016;219:20–4.
Mosenzon O, Alguwaihes A, Leon JLA, Bayram F, Darmon P, Davis TME, Dieuzeide G, Eriksen KT, Hong T, Kaltoft MS, et al. CAPTURE: a multinational, cross-sectional study of cardiovascular disease prevalence in adults with type 2 diabetes across 13 countries. Cardiovasc Diabetol. 2021;20(1):154.
Liu Z, Shi H, Chen L. Prognostic role of pre-treatment C-reactive protein/albumin ratio in esophageal cancer: a meta-analysis. BMC Cancer. 2019;19(1):1161.
Wu MT, He SY, Chen SL, Li LF, He ZQ, Zhu YY, He X, Chen H. Clinical and prognostic implications of pretreatment albumin to C-reactive protein ratio in patients with hepatocellular carcinoma. BMC Cancer. 2019;19(1):538.
Liu Y, Chen S, Zheng C, Ding M, Zhang L, Wang L, Xie M, Zhou J. The prognostic value of the preoperative c-reactive protein/albumin ratio in ovarian cancer. BMC Cancer. 2017;17(1):285.
Çağdaş M, Rencüzoğullari I, Karakoyun S, Karabağ Y, Yesin M, Artaç I, Iliş D, Çağdaş ÖS, Tezcan AH, Tanboğa HI. Assessment of relationship between C-reactive protein to albumin ratio and coronary artery disease severity in patients With acute coronary syndrome. Angiology. 2019;70(4):361–8.
Karabağ Y, Çağdaş M, Rencuzogullari I, Karakoyun S, Artaç İ, İliş D, Atalay E, Yesin M, Halil Gürsoy MO, Tanboğa I. Relationship between C-reactive protein/albumin ratio and coronary artery disease severity in patients with stable angina pectoris. J Clin Lab Anal. 2018. https://doi.org/10.1002/jcla.22457.
Duman H, Çinier G, Bakırcı EM, Duman H, Şimşek Z, Hamur H, Değirmenci H, Emlek N. Relationship between C-reactive protein to albumin ratio and thrombus burden in patients with acute coronary syndrome. Clin Appl Thromb Hemost. 2019;25:1076029618824418.
Aksu U, Gulcu O, Aksakal E, Kalkan K, Öztürk M, Korkmaz AF, Uslu A, Demirelli S. The association between CRP / albumin ratio and in-stent restenosis development in patients with ST-segment elevation myocardial infarction. J Clin Lab Anal. 2019;33(4):e22848.
Liu ZY, Tang JN, Cheng MD, Jiang LZ, Guo QQ, Zhang JC, Zhang ZL, Song FH, Wang K, Fan L, et al. C-reactive protein-to-serum albumin ratio as a novel predictor of long-term outcomes in coronary artery disease patients who have undergone percutaneous coronary intervention: analysis of a real-world retrospective cohort study. Coron Artery Dis. 2021;32(3):191–6.
Çınar T, Çağdaş M, Rencüzoğulları İ, Karakoyun S, Karabağ Y, Yesin M, Sadioğlu Çağdaş Ö, Tanboğa H. Prognostic efficacy of C-reactive protein/albumin ratio in ST elevation myocardial infarction. Scand Cardiovasc J. 2019;53(2):83–90.
Chinese guideline for percutaneous coronary intervention (pocket guideline). Zhonghua Xin Xue Guan Bing Za Zhi. 2012;40(4):271–7.
Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA. 1998;279(18):1477–82.
Wada H, Dohi T, Miyauchi K, Doi S, Naito R, Konishi H, Tsuboi S, Ogita M, Kasai T, Okazaki S, et al. Independent and combined effects of serum albumin and C-reactive protein on long-term outcomes of patients undergoing percutaneous coronary intervention. Circ J. 2017;81(9):1293–300.
Effoe VS, Correa A, Chen H, Lacy ME, Bertoni AG. High-sensitivity C-reactive protein is associated with incident type 2 diabetes among African Americans: the Jackson heart study. Diabetes Care. 2015;38(9):1694–700.
Sharif S, Van der Graaf Y, Cramer MJ, Kapelle LJ, de Borst GJ, Visseren FLJ, Westerink J. Low-grade inflammation as a risk factor for cardiovascular events and all-cause mortality in patients with type 2 diabetes. Cardiovasc Diabetol. 2021;20(1):220.
Guerin-Dubourg A, Catan A, Bourdon E, Rondeau P. Structural modifications of human albumin in diabetes. Diabetes Metab. 2012;38(2):171–8.
Peavy DE, Taylor JM, Jefferson LS. Correlation of albumin production rates and albumin mRNA levels in livers of normal, diabetic, and insulin-treated diabetic rats. Proc Natl Acad Sci U S A. 1978;75(12):5879–83.
Kuboki A, Kanaya H, Nakayama T, Konno W, Goto K, Nakajima I, Kashiwagi T, Hirabayashi H, Haruna SI. Prognostic value of C-reactive protein/albumin ratio for patients with hypopharyngeal and laryngeal cancer undergoing invasive surgery involving laryngectomy. Head Neck. 2019;41(5):1342–50.
Matsubara T, Takamori S, Haratake N, Fujishita T, Toyozawa R, Ito K, Shimokawa M, Yamaguchi M, Seto T, Okamoto T. Identification of the best prognostic marker among Immunonutritional parameters using serum C-reactive protein and albumin in non-small cell lung cancer. Ann Surg Oncol. 2021;28(6):3046–54.
Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Shibuya N, Kubota K. Clinical significance of the C-reactive protein to albumin ratio for survival after surgery for colorectal cancer. Ann Surg Oncol. 2016;23(3):900–7.
Acet H, Güzel T, Aslan B, Isik MA, Ertas F, Catalkaya S. Predictive value of C-reactive protein to albumin ratio in ST-segment elevation myocardial infarction patients treated with primary percutaneous coronary intervention. Angiology. 2021;72(3):244–51.
Tanriverdi Z, Gungoren F, Tascanov MB, Besli F, Altiparmak IH. Comparing the diagnostic value of the C-reactive protein to albumin ratio with other inflammatory markers in patients with stable angina pectoris. Angiology. 2020;71(4):360–5.
Erdöl MA, Gayretli Yayla K. Relationship between C-reactive protein to albumin ratio and coronary artery calcium score and CAD-RADS scores with coronary computed tomography angiography. Turk J Med Sci. 2021;51(5):2674–82.
Söğüt Ö, Akdemir T, Can MM. Prognostic value of the C-reactive protein to albumin ratio in patients undergoing primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Turk J Med Sci. 2021;51(3):1281–8.
Cheng L, Meng Z, Wang Q, Jian Z, Fan P, Feng X, Qiao X, Yang J, Yuan Z, Li B, et al. The usefulness of C-Reactive protein to albumin ratio in the prediction of adverse cardiovascular events in coronary chronic total occlusion undergoing percutaneous coronary intervention. Front Cardiovasc Med. 2021;8:731261.
Wang W, Ren D, Wang CS, Li T, Yao HC, Ma SJ. Prognostic efficacy of high-sensitivity C-reactive protein to albumin ratio in patients with acute coronary syndrome. Biomark Med. 2019;13(10):811–20.
Cho AR, Lee SB, Hong KW, Jung DH. C-reactive protein-to-albumin ratio and 8-year incidence of type 2 diabetes: the korean genome and epidemiology study. Acta Diabetol. 2021;58(11):1525–32.
Chen C, Chen X, Chen J, Xing J, Hei Z, Zhang Q, Liu Z, Zhou S. Association between preoperative hs-crp/albumin ratio and postoperative sirs in elderly patients: a retrospective observational cohort study. J Nutr Health Aging. 2022;26(4):352–9.
Li J, Yuan D, Jiang L, Tang X, Xu J, Song Y, Chen J, Qiao S, Yang Y, Gao R, et al. Similar inflammatory biomarkers reflect different platelet reactivity in percutaneous coronary intervention patients treated with clopidogrel: a large-sample study from China. Front Cardiovasc Med. 2021;8:736466.
Montalescot G, Rangé G, Silvain J, Bonnet JL, Boueri Z, Barthélémy O, Cayla G, Belle L, Van Belle E, Cuisset T, et al. High on-treatment platelet reactivity as a risk factor for secondary prevention after coronary stent revascularization: a landmark analysis of the ARCTIC study. Circulation. 2014;129(21):2136–43.
Devaraj S, Jialal I. C-reactive protein polarizes human macrophages to an M1 phenotype and inhibits transformation to the M2 phenotype. Arterioscler Thromb Vasc Biol. 2011;31(6):1397–402.
Fanali G, di Masi A, Trezza V, Marino M, Fasano M, Ascenzi P. Human serum albumin: from bench to bedside. Mol Aspects Med. 2012;33(3):209–90.
He XM, Carter DC. Atomic structure and chemistry of human serum albumin. Nature. 1992;358(6383):209–15.
Neuzil J, Stocker R. Free and albumin-bound bilirubin are efficient co-antioxidants for alpha-tocopherol, inhibiting plasma and low density lipoprotein lipid peroxidation. J Biol Chem. 1994;269(24):16712–9.
Cantin AM, Paquette B, Richter M, Larivée P. Albumin-mediated regulation of cellular glutathione and nuclear factor kappa B activation. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1539–46.
Keaney JF Jr, Simon DI, Stamler JS, Jaraki O, Scharfstein J, Vita JA, Loscalzo J. NO forms an adduct with serum albumin that has endothelium-derived relaxing factor-like properties. J Clin Invest. 1993;91(4):1582–9.
Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, et al. Antiinflammatory therapy with Canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–31.
Acknowledgements
Thanks for the data collection, analysis, revision, and monitoring by all contributors in this study.
Funding
This work was supported by the National Key Research and Development Program of China [Nos. 2016YFC1301300 and 2016YFC1301301], the CAMS Innovation Fund for Medical Sciences (CIFMS) [2020-I2M-C&T-B-052] and the Young and middle-aged talents in the XPCC Science and Technology Project [2020CB012].
Author information
Authors and Affiliations
Contributions
LJW and ZXY contributed to the concept and design of the study; LJW wrote the manuscript; LJW conducted the statistical analysis; ZXY and YJQ revised the intellectual content; LJW, ZP, LYL, YKL, TXF, and XJJ contributed to data collection; YWX, QSB, XB, YYJ, and GRL contributed to interpretation of data. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study complied with the principles of the Declaration of Helsinki and was approved by the Review Board of Fuwai hospital. Written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors have no competng interests to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Figure S1.
Correlation analysis between hs-CRP and albumin. Table S1. Propensity score-matched analysis.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, J., Zhu, P., Li, Y. et al. A novel inflammatory biomarker, high-sensitivity C-reactive protein-to-albumin ratio, is associated with 5-year outcomes in patients with type 2 diabetes who undergo percutaneous coronary intervention. Diabetol Metab Syndr 15, 14 (2023). https://doi.org/10.1186/s13098-022-00977-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-022-00977-9