Abstract
Background
Isolated splenic artery dissection (SAD) is extremely rare, life-threatening, and particularly difficult to diagnose. Moreover, SAD presenting as digestive hemorrhage has not been reported.
Case presentation
A 44-year-old man presented with recurrent life-threatening hematochezia. Magnetic resonance and computed tomographic angiography showed isolated SAD with an intrapancreatic hematoma. Selective angiography confirmed the diagnosis of rupture of SAD. Hemosuccus pancreaticus was considered the potential mechanism of digestive hemorrhage. It was successfully managed by endovascular coil embolization.
Conclusions
Isolated SAD is especially rare but fatal. Rupture of SAD should be considered in the differential diagnosis as a rare cause of digestive hemorrhage. Endovascular coil embolization is effective in treating ruptured SAD.
Similar content being viewed by others
Background
Splenic artery dissection (SAD) usually originates from dissection of the celiac trunk or the abdominal aorta [1]. Isolated SAD is extremely rare but life-threatening [2]. To date, only eight cases with isolated SAD have been reported in literature and six (75%) had dissection rupture. Of these six cases, five died and only one was successfully treated by open surgery [2]. Ruptured SAD usually presents with abdominal pain and results in massive retroperitoneal or intraperitoneal hemorrhage [2]. However, to our knowledge, it has not been reported that ruptured SAD presents with painless hematochezia. Herein, we present a case of spontaneous rupture of isolated SAD presenting as recurrent, life-threatening digestive hemorrhage.
Case presentation
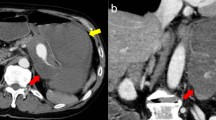
A 44-year-old man was referred from a local hospital with a 30-day history of intermittent hematochezia. No abdominal pain was noted, and his physical examination was unremarkable. His medical history included a 6-month history of hypertension, no histories of abdominal trauma, surgery, or pancreatitis, and no use of nonsteroidal anti-inflammatory drugs. Laboratory examinations, including tumor markers, were within normal limits, except for anemia (hemoglobin: 77 g/L [normal: 120–160]). Esophagogastroduodenoscopy, colonoscopy, and capsule endoscopy were performed, but these procedures failed to find the source of the bleeding. Contrast-enhanced computed tomography (CT) showed a 3.6 × 3.4 cm2 hypodense mass (Fig. 1a, white arrow) which was suspected to be a neoplasm and a dilated splenic artery (Fig. 1b, white arrow). Magnetic resonance (MR) angiography demonstrated that the mass was an intrapancreatic hematoma (Fig. 1c, white arrow) close to the protruding splenic artery, which was separated into two lumens at the proximal segment (Fig. 1d, white arrow). CT angiography confirmed isolated dissection of the splenic artery with a typical “double-lumen” sign (Fig. 1e, f, white arrows).
Preoperative images: a Contrast-enhanced computed tomography (CT) reveals a 3.6 × 3.4 cm2 intrapancreatic hypodense mass (white arrow) close to the splenic artery (SA). b CT shows dilation of the calcified SA (white arrow). c Magnetic resonance angiography shows a hematoma (white arrow) close to the protruding SA. d The proximal SA is divided into two lumens. e, f CT Angiography confirms isolated splenic artery dissection (SAD) with a typical “double-lumen” sign (white arrows). The SA was partially thrombosed in the proximal segment
Hematochezia recurred eight hours after the patient was admitted to our hospital. The patient suffered from hemorrhagic shock and received fluid resuscitation and blood transfusions. A diagnosis of ruptured SAD was considered. Digital subtraction angiography was performed emergently. The right femoral artery was percutaneously punctured. A 6 F, 50-cm-long sheath (Cook Inc, Bloomington, IN) was introduced over a 0.035-in., 150-cm guidewire. Selective superior and inferior mesenteric artery angiography procedures were unremarkable. Celiac artery angiography via a 5 F Cobra catheter confirmed the true (Fig. 2a, white arrow) and false (black arrows) lumens of SAD. The distal false lumen (large black arrow) was obviously dilated and deviated from the true lumen, which was consistent with the site of the hematoma on CT and MR. Spontaneous rupture of isolated SAD was diagnosed. Subsequently, endovascular embolization was performed. A 2.6-F, 125-cm-long microcatheter (Asahi Intecc, Nagoya, Japan) with a 0.018-in., 180-cm-long microguidewire (Asahi Intecc) was introduced through the Cobra catheter into the distal splenic artery. Both proximal and distal ends of the lesion were embolized via the microcatheter with ten Tornado microcoils (Cook Inc, Fig. 2b).
The patient recovered uneventfully after endovascular treatment. Contrast-enhanced MR at the two-month follow-up showed the occluded main trunk of the splenic artery with a chronic hematoma (Fig. 3, white arrow). The patient has been followed up for one year, and there was no recurrence of digestive hemorrhage. Written informed consent was obtained from the patient for publication of this case report.
Discussion and conclusion
To the best of our knowledge, this is the first case of isolated SAD which was successfully treated by endovascular therapy. Although the etiology of SAD remains unclear, hypertension is considered to be a common risk factor [2]. This is consistent with the history of hypertension in the current case. Ruptured SAD usually presents with upper abdominal or left flank pain and causes intraperitoneal or retroperitoneal hemorrhage [2]. However, in the current case, it presented with intermittent hematochezia and resulted in digestive hemorrhage. To our knowledge, SAD resulting in digestive hemorrhage has not been reported in literature. Herein, we present the first case of SAD presenting as recurrent digestive hemorrhage. Ruptured SAD should be considered in the differential diagnosis as a rare cause of digestive hemorrhage.
Splenic artery diseases rarely result in digestive hemorrhage. Most of them are false splenic artery aneurysms (SAAs) caused by pancreatitis or pancreatic tumors [3,4,5,6,7]. SAAs can rupture into the stomach or the pancreatic duct and lead to gastric or duodenal hemorrhage [3,4,5,6,7]. The latter hemorrhage is also termed “hemosuccus pancreaticus” [4, 6, 7]. The diagnosis of hemosuccus pancreaticus remains challenging. It can be difficult to identify the bleeding site via endoscopy or digital subtraction angiography because of its rarity, anatomical location, and intermittent characteristics [4, 7]. Endoscopy was able to detect active bleeding via the papilla in only 30% of cases [7].
In this case, endoscopies failed to identify the bleeding site, and histopathological examination was not performed because of endovascular treatment. However, the authors believe that digestive hemorrhage was caused by rupture of SAD. The potential mechanism is considered to involve hemosuccus pancreaticus: SAD ruptured into the pancreatic tail, and the blood from the hematoma entered into the duodenum via the pancreatic duct. This is supported by several observations. First, the hematoma was completely within the pancreas. The site is similar to Yoshikazu’s cases [6]. The SAAs observed in their cases were treated by open surgery, and the communication between the aneurysm and the pancreatic duct was proven by histological examination. Second, hemosuccus pancreaticus is usually intermittent and repetitive [4, 7]. These characteristics were consistent with the present case. Third, primary gastrointestinal or pancreatic lesions were excluded via endoscopies (gastroduodenoscopy, colonoscopy, and capsule endoscopy) and radiological examination (digital subtraction angiography, repeated CT, and MR). SAD rupturing into the stomach was also excluded based on the symptoms (hematochezia rather than hematemesis), endoscopies, and the range of the lesion, which did not exceed the superior edge of the pancreas on CT and MR. Finally, digestive hemorrhage was cured after the use of endovascular coil embolization for SAD. The efficacy has been demonstrated by 1-year follow-up.
Several methods have been reported in the treatment of SAAs, including open resection, laparoscopic resection, and endovascular treatment. For hemosuccus pancreaticus associated with SAAs, if the source of the bleeding is identified on angiography, endovascular treatment should be considered as the first choice of treatment [4, 6, 7]. Surgical treatment remains an important supplement if angiography fails to identify the source of the bleeding or if endovascular treatment is not successful. For SAD, only one patient was successfully managed with open surgery [2]. In the current case, it was successfully treated by endovascular coil embolization. Endovascular treatment is a safe and effective option to ruptured SAD.
In conclusion, isolated SAD is extremely rare but life-threatening. Ruptured SAD should be considered in the differential diagnosis as a rare cause of digestive hemorrhage. Selective angiography is recommended for diagnosis, and endovascular embolization is a safe and effective treatment for ruptured SAD.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- SAD:
-
Splenic artery dissection
- CT:
-
Computed tomography
- MR:
-
Magnetic resonance
- SAAs:
-
Splenic artery aneurysms
- SA:
-
Splenic artery
References
Kang SH, Park HS, Yoon CJ, Shin CS, Yoo KC, Lee T. Mid- to long-term outcomes in management of spontaneous isolated coeliac artery dissection (SICAD). Eur J Vasc Endovasc Surg. 2020;59:247–53.
Desinan L, Scott CA, Piai I, Mazzolo GM. Sudden death due to spontaneous rupture in splenic artery atypical dissection with features of vasculitis: case report and review of the literature. Forensic Sci Int. 2010;200:e1–5.
Patel R, Girgis M. Splenic artery pseudoaneurysm with hemosuccus pancreaticus requiring multimodal treatment. J Vasc Surg. 2019;69:592–5.
Ru N, Zou WB, Qian YY, Tang XY, Zhu JH, Hu LH, et al. A systematic review of the etiology, diagnosis, and treatment of hemosuccus pancreaticus. Pancreas. 2019;48:e47-9.
Panzera F, Inchingolo R, Rizzi M, Biscaglia A, Schievenin MG, Tallarico E, et al. Giant splenic artery aneurysm presenting with massive upper gastrointestinal bleeding: a case report and review of literature. World J Gastroenterol. 2020;26:3110–7.
Toyoki Y, Hakamada K, Narumi S, Nara M, Ishido K, Sasaki M. Hemosuccus pancreaticus: problems and pitfalls in diagnosis and treatment. World J Gastroenterol. 2008;14:2776–9.
Yu P, Gong J. Hemosuccus pancreaticus: a mini-review. Ann Med Surg (Lond). 2018;28:45–8.
Acknowledgements
Not applicable.
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
JJ, LY and DX were participants in the treatment and management of the patient. JJ, LY and DX reviewed and analyzed medical records and imaging data. JJ, LY and DX wrote the article. DX was the major contributor in critical revision of the article. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent to publish
Written informed consent was obtained from the patient for publication of this case report. A copy of the written consent form is available for review by the Editor -in -chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jiang, J., Liu, Y. & Ding, X. Endovascular embolization of spontaneous rupture of isolated splenic artery dissection associated with hemosuccus pancreaticus: a case report. BMC Cardiovasc Disord 21, 335 (2021). https://doi.org/10.1186/s12872-021-02148-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-021-02148-6