Abstract
Background
Ticagrelor and prasugrel are two third-generation oral P2Y12 inhibitors which are more commonly used in clinical practice. However, dyspnea has been consecutively reported in patients using third-generation oral P2Y12 inhibitors. This study aims to compare the risk of dyspnea in patients treated with third-generation P2Y12 inhibitors compared with clopidogrel.
Methods
We systematically searched the PubMed, Cochrane Central Register of Controlled Trials databases, ClinicalTrials.gov and Web of Science for randomized control trials (RCTs) comparing ticagrelor or prasugrel with clopidogrel until July 2019. The primary outcome was the incidence of dyspnea. The risk ratios (RR) and 95% confidence intervals (CI) were estimated using meta-analysis.
Results
We included 25 RCTs involving 63,484 patients in this meta-analysis, including 21 studies on ticagrelor and 4 studies on prasugrel. Compared to the clopidogrel group, third-generation oral P2Y12 inhibitors were associated with an increased risk of dyspnea compared with clopidogrel (RR 2.15, 95% CI 1.59–2.92), which was consistent in the analysis of ticagrelor (RR 2.65, 95% CI 1.87–3.76). However, the adverse effect was not found among patients receiving prasugrel therapy (RR 1.03, 95% CI 0.86–1.22). The increased dyspnea risk of ticagrelor was consistent in subgroups with different follow-up durations (≤ 1 month RR 1.87, 95% CI 1.56–2.24; 1–6 months RR 4.19, 95% CI 1.99–8.86; > 6 months 2.45, 95% CI 1.13–5.34).
Conclusions
Ticagrelor has a higher risk of dyspnea than clopidogrel, which was not observed in patients using prasugrel.
Similar content being viewed by others
Introduction
Antiplatelet agents are the common primary therapy for patients with acute coronary syndrome (ACS), especially in patients undergoing percutaneous coronary intervention (PCI) [1]. Clopidogrel is the most commonly prescribed drug of antiplatelet agents. However, clopidogrel has a delayed onset and a modest antiplatelet effect. New antiplatelet agents, ticagrelor and prasugrel, were developed as third-generation oral P2Y12 inhibitors, which inhibit platelets more rapidly and persistently than clopidogrel. Several large randomized controlled trials (RCTs) have confirmed the superiority of third-generation P2Y12 inhibitors over clopidogrel in preventing ischemic vascular events [2,3,4].
Bleeding, as the most common side effects of third-generation oral P2Y12 inhibitors, has been well evaluated in previous studies [5,6,7]. Dyspnea was another important side effect, which was commonly reported in third-generation oral P2Y12 inhibitors users. The PLATO (Platelet Inhibition and Patient Outcomes) study showed that dyspnea was more common in the ticagrelor group than in the clopidogrel group (13.8% vs. 7.8%) [2]. More cases with dyspnea were also reported among patients taking prasugrel in the study of TRITON-TIMI-38 (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis in Myocardial Infarction) [4]. However, the risk of dyspnea was not well established in previous studies on third-generation oral P2Y12 inhibitors, which mainly focused on their efficacy or the risk of bleeding. Therefore, we performed this meta-analysis of RCTs to compare the risk of dyspnea in patients taking third-generation oral P2Y12 inhibitors with clopidogrel.
Methods
This meta-analysis was carried out according to the methods recommended in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [8].
Search strategy
RCTs comparing the safety of ticagrelor or prasugrel with clopidogrel published before July 2019 were identified from PubMed, Cochrane Central Register of Controlled Trials databases, ClinicalTrials.gov and Web of Science. The following terms, dyspnea or dyspnoea, and prasugrel or CS-747 or 640,315 or ticagrelor or AZD6140, and randomized controlled trial or random* were used.
Inclusion and exclusion criteria
Two independent reviewers reviewed the eligible studies. Disagreements were resolved by discussion with a third reviewer. The inclusion criteria were as follows: 1) full-text RCTs; 2) comparing ticagrelor or prasugrel with clopidogrel; 3) dyspnea was reported as one of the safety endpoints; 4) in English. The exclusion criteria were as follows: 1) incomplete data; 2) reanalysis or subgroup analysis of previous RCTs; 3) including healthy subjects only or involving animals.
Endpoints of evaluation
The primary outcome was the risk of dyspnea in patients taking third-generation oral P2Y12 inhibitors compared with clopidogrel. Dyspnea was reported by the participants and judged by the investigators. However, most studies did not specify the definition of dyspnea.
Data extraction and risk of bias analysis
Characteristics and data of included studies were extracted by two independent reviewers, and disagreements were settled by discussion with a third reviewer. The extracted data included the year of publication, the study country or area, sample size, indications for treatment, dose of drugs, the duration of treatment, the duration of follow-up, and frequency of dyspnea in each study arm. Results of included studies were also checked in ClinicalTrials.gov. All randomized patients were included in this meta-analysis. Specifically, if the number of patients who received at least one dose of study drugs was specified in the included studies, this number would be used alternatively. If the data were incomplete, authors would be contacted for more information. The Cochrane Collaboration’s tool was used to assess the risk of bias of the studies [9]. Random sequence generation, allocation concealment, blinding, incomplete outcome data, and selective outcome reporting were assessed.
Statistical methods
Pooled risk ratios (RRs) and corresponding 95% confidence intervals (CIs) were calculated in order to evaluate the risk of dyspnea for the third-generation oral P2Y12 inhibitors, in which random effects models were applied. For sensitivity analysis, we also computed the estimates using fixed effects model. I2 were taken as the determinant of heterogeneity and P value (< 0.1) indicated statistically significant. We regarded I2 values of < 25%, 25–50%, and > 50% as evidence of low, moderate, and high levels of heterogeneity, respectively [10]. Publication bias was assessed by using funnel plots. Begg’s rank correlation test and the Egger’s linear regression test were performed to test the symmetry of funnel plot [11, 12].
Furthermore, we also performed subgroup analyses on individual drug (ticagrelor or prasugrel), studies with standard dosage of drugs (maintenance dose of ticagrelor 90 mg twice per day, prasugrel 10 mg once per day and clopidogrel 75 mg once per day), studies involving Asian subjects, and studies according to study follow-up (≤ 1 month, 1–6 months, > 6 months). In addition, sensitivity analysis was also performed after excluding studies with high risk of bias or excluding the study with the largest sample size. R software, version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria, 2018) was used to perform this meta-analysis.
Results
Study characteristics and study quality
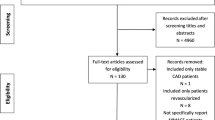
The study selection process is outlined in Fig. 1. After removing the duplicates, 216 relevant citations were identified, which yielded 25 studies fulfilling the inclusion criteria, including 21 studies comparing ticagrelor with clopidogrel [2, 3, 13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31] and 4 studies comparing prasugrel with clopidogrel [4, 32,33,34]. For study of Ge 2010 [32], the data was from ClinicalTrials.gov. A total of 64,049 patients were involved in the randomization, and 63,484 patients who received at least one dose of study drugs were included in the final analysis. The characteristics of included studies were summarized in Table 1. There were 10 ticagrelor studies [17,18,19,20,21,22,23, 25, 27, 31] and 1 prasugrel study [32] carried out in Asian population. Considering the dosage of study drugs, standard maintenance dose was used in 12 ticagrelor studies [2, 3, 15,16,17, 20, 21, 24,25,26, 28, 29] and 2 prasugrel studies [4, 34].
The quality assessment of the included studies is displayed in Table S1 and Figure S1. High risk bias was observed in some trials. As several studies were open-label trials [16, 23,24,25, 28, 30], performance bias and detection bias would be high. Though studies of Dehghani 2017 [26] and TREAT 2018 [29] were also open-label, the clinical endpoint assessment was blinded. In most studies, however, generation of random sequence and allocation concealment were not reported. Other biases were low in most studies.
Dyspnea risk of third-generation P2Y12 inhibitors
All of the 25 studies were included in the analysis on dyspnea, involving a total of 63,484 patients (ticagrelor 20,152 vs clopidogrel 19,523; prasugrel 12,037 vs clopidogrel 11,772). In the included studies, 2512 (7.8%) cases of dyspnea were reported in the third-generation P2Y12 inhibitors group, and 1420 (4.5%) in clopidogrel group. Overall, third-generation P2Y12 inhibitors was associated with a higher risk of dyspnea compared with clopidogrel (RR 2.15, 95% CI 1.59–2.92, See Fig. 2). However, high heterogeneity was observed in this analysis with the I2 of 85% (P < 0.01). The result was consistent in subgroup analysis of ticagrelor (RR 2.65, 95% CI 1.87–3.76), but not in analysis of prasugrel (RR 1.03, 95% CI 0.86–1.22).
In addition, in subgroup analysis of studies with standard dose of drugs, the result was consistent (overall, RR 2.25, 95% CI 1.56–3.24; ticagrelor, RR 2.51, 95% CI 1.66–3.79; prasugrel, RR 1.58, 95% CI 0.43–5.80). Similar result was also observed in analysis of Asian studies (overall, RR 2.25, 95% CI 1.56–3.24; ticagrelor, RR 2.51, 95% CI 1.66–3.79; prasugrel, RR 1.58, 95% CI 0.43–5.80).
In the sensitivity analysis excluding studies with high risk of bias, 10 ticagrelor studies and all 4 prasugrel studies were included. The results remained consistent that third-generation P2Y12 inhibitors increased the risk of dyspnea (RR 2.22 95% CI 1.49–3.32), and it was also only observed among ticagrelor studies (RR 3.27, 95% CI 1.75–6.14). After excluding the largest study, the PLATO study, ticagrelor was still associated with increased risk of dyspnea (RR 2.90, 95% CI 1.85–4.55).
As the increased risk was only found among patients taking ticagrelor, subgroup analysis according to study follow-up duration was only performed in the ticagrelor studies. We found that ticagrelor increased the risk of dyspnea compared with clopidogrel in all follow-up durations, in which the RR was 1.87 (95 CI 1.56–2.24), 4.19 (95% CI 1.99–8.86) and 2.45 (95% CI 1.13–5.34) for follow-up duration less than 1 month, 1–6 months and more than 6 months, respectively. The results are presented in Fig. 3.
Severity of dyspnea
In the study of Husted 2006 [13], 29 instances of dyspnea were reported by the 23 ticagrelor treated patients, in which 21 were mild and 8 were moderate. But none of them was associated with congestive heart failure or bronchospasm. Three out of fourteen (21.4%) of patients reported dyspnea in the ticagrelor group stopped the study drug owing to dyspnea in the Onset/offset study [15], however, it was only 0.9% in the ticagrelor group and 0.1% in the clopidogrel group in the study of PLATO [2]. It was similar in study of TREAT that few patients discontinued the study drug because of dyspnea (19 of 1913 [1.0%] patients in the ticagrelor group and none in the clopidogrel group). In contrast, dyspnea was one of the most common causes of study drug discontinuation in the study of EUCLID (Examining Use of Ticagrelor in Peripheral Artery Disease) [3].
For patients reported dyspnea in the study of DISPERSE-22007 [14], 27% of the patients had resolution of this symptom within 24 h, 25% after 24 h, and 48% experienced persistent symptoms during treatment (> 15 days). In study of Zhang 2016, there was only 1 patient in whom dyspnea lasted for 1 month, for others dyspnea was tolerable and mild or moderate dyspnoea disappeared within 1 week [23].
Publication bias
Publication bias were not detected in the overall analysis of dyspnea involving 25 studies (P value for Begg’s test: 0.350; P value for Egger’s test: 0.246). The funnel plot is presented in Figure S2.
Discussion
In this study, we performed a meta-analysis using 25 RCTs and found that third-generation oral P2Y12 inhibitors have a higher risk of dyspnea than clopidogrel. Compared to clopidogrel, patients taking ticagrelor had twice-fold increase in the risk of dyspnea. In addition, the increased risk of dyspnea for ticagrelor was consistent in subgroup analyses by follow-up duration. However, the adverse effect was not found among patients receiving prasugrel therapy. In addition, ticagrelor induced dyspnea reported in most studies was likely to be mild to moderate or transient, and few patients discontinued ticagrelor because of dyspnea.
The result was similar with the meta-analysis of Caldeira et al., in which they found that ticagrelor, cangrelor, and elinogrel have an increased incidence of dyspnea compared with clopidogrel or prasugrel [35]. But only 5 ticagrelor studies and 1 prasugrel study were included in this study. Tan et al. also assessed the dyspnea risk of ticagrelor and prasugrel as a secondary analysis in a meta-analysis, however, many non-RCTs were included in this meta-analysis [36]. In our meta-analysis, we included 25 studies that reported dyspnea when comparing efficacy or safety of third-generation oral P2Y12 inhibitors with clopidogrel, which are all RCTs and decreases the heterogeneity.
A review by Cattaneo and Faioni discussed the dyspnea of new antiplatelet drugs and hypothesized that dyspnea could be related to the reversibility of drug [37]. The study of Caldeira et al. also support this hypothesis that reversible P2Y12 antagonists ticagrelor, cangrelor, and elinogrel have an higher risk of dyspnea in increasing order when compared with irreversible P2Y12 inhibitors such as clopidogrel or prasugrel [35]. This is consistent with the results of our analysis that ticagrelor had a higher risk of dyspnea than clopidogrel, which was not observed in prasugrel.
The mechanism of P2Y12 inhibitors, especially ticagrelor, related dyspnea is till be proven. Current hypothesis is inhibition of P2Y12 inhibitors, particularly reversible inhibitors, on sensory neurons increasing the sensation of dyspnea [37]. It could be related to the reversibility of drug. Previous also found that dyspnea was mainly found in reversible P2Y12 inhibitors, including cangrelor, elinogrel and ticagrelor [35, 37], which was consistent with our analysis. Another hypothesis is ticagrelor stimulates pulmonary vagal C fibers by inhibiting adenosine reuptake and increasing of extracellular adenosine levels [38]. But there are also evidences against the hypothesis of increased extracellular adenosine by ticagrelor [38].
The DISPERSE-2 trial reported that the increased rate of dyspnea was dose-dependent [14]. But we found that ticagrelor had a higher risk of dyspnea in subgroup studies with standard drug dose, which is commonly used in clinical practice. Though dyspnea was more common in patients using ticagrelor, most of the cases were mild or moderate. A part of patients with dyspnea after taking ticagrelor discontinued the study drug, but the rate varied in different studies. It was only 0.9% in the ticagrelor group in the study of PLATO [2], while it reached 21.4% in the onset/offset study among patients who reported dyspnea [15]. It is consistent in previous studies that this symptom did not last long. Therefore, dyspnea may not be the major concern when using third-generation oral P2Y12 inhibitors.
There are several limitations in this study. Firstly, the definition was not specified in the included studies, which may affect the generalization of this study. Second, the follow-up duration ranged among included trials. But we performed a subgroup analysis on ticagrelor stratified by the follow-up duration and we found the result was consistent in studies with different follow-up durations. Third, only four studies on prasugrel were included as most studies did not reported the results of dyspnea. But the current finding on prasugrel was consistent with previous meta-analyses [35, 36].
Conclusions
In conclusion, a higher risk of dyspnea was found in patients treated with ticagrelor compared with clopidogrel, while it was not observed in patients using prasugrel. Most of the cases, however, were mild or moderate, in spite of a higher risk of dyspnea.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Abbreviations
- ACS:
-
Acute coronary syndrome
- CI:
-
Confidence interval
- EUCLID:
-
Examining use of Ticagrelor in peripheral artery disease
- PCI:
-
Percutaneous coronary intervention
- PLATO:
-
Platelet inhibition and patient outcomes
- RCT:
-
Randomized controlled trials
- RR:
-
Risk ratio
- TRITON-TIMI-38:
-
Trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with Prasugrel-thrombolysis in myocardial infarction
References
Jneid H, Anderson JL, Wright RS, et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2012;60(7):645–81.
Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045–57.
Hiatt WR, Fowkes FG, Heizer G, et al. Ticagrelor versus Clopidogrel in symptomatic peripheral artery disease. N Engl J Med. 2017;376(1):32–40.
Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001–15.
Bundhun PK, Huang F. Post percutaneous coronary interventional adverse cardiovascular outcomes and bleeding events observed with prasugrel versus clopidogrel: direct comparison through a meta-analysis. BMC Cardiovasc Disord. 2018;18(1):78.
Kheiri B, Osman M, Abdalla A, et al. Ticagrelor versus clopidogrel after fibrinolytic therapy in patients with ST-elevation myocardial infarction: a systematic review and meta-analysis of randomized clinical trials. J Thromb Thrombolysis. 2018;46(3):299–303.
Guo CG, Chen L, Chan EW, et al. Systematic review with meta-analysis: the risk of gastrointestinal bleeding in patients taking third-generation P2Y12 inhibitors compared with clopidogrel. Aliment Pharmacol Ther. 2019;49(1):7–19.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Husted S, Emanuelsson H, Heptinstall S, Sandset PM, Wickens M, Peters G. Pharmacodynamics, pharmacokinetics, and safety of the oral reversible P2Y12 antagonist AZD6140 with aspirin in patients with atherosclerosis: a double-blind comparison to clopidogrel with aspirin. Eur Heart J. 2006;27(9):1038–47.
Cannon CP, Husted S, Harrington RA, et al. Safety, tolerability, and initial efficacy of AZD6140, the first reversible oral adenosine diphosphate receptor antagonist, compared with clopidogrel, in patients with non-ST-segment elevation acute coronary syndrome: primary results of the DISPERSE-2 trial. J Am Coll Cardiol. 2007;50(19):1844–51.
Gurbel PA, Bliden KP, Butler K, et al. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation. 2009;120(25):2577–85.
Bonello L, Laine M, Kipson N, et al. Ticagrelor increases adenosine plasma concentration in patients with an acute coronary syndrome [randomized controlled trial; research support, non-U.S. Gov't]. J Am Coll Cardiol. 2014;63(9):872–7. https://doi.org/10.1016/j.jacc.2013.09.067.
Goto S, Huang CH, Park SJ, Emanuelsson H, Kimura T. Ticagrelor vs. clopidogrel in Japanese, Korean and Taiwanese patients with acute coronary syndrome -- randomized, double-blind, phase III PHILO study. Circ J. 2015;79(11):2452–60.
Li P, Yang Y, Chen T, et al. Ticagrelor overcomes high platelet reactivity in patients with acute myocardial infarction or coronary artery in-stent restenosis: a randomized controlled trial. Sci Rep. 2015;5:13789.
He MJ, Liu B, Sun DH, et al. One-quarter standard-dose ticagrelor better than standard-dose clopidogrel in Chinese patients with stable coronary artery disease: a randomized, single-blind, crossover clinical study [comparative study; randomized controlled trial]. Int J Cardiol. 2016;215:209–13. https://doi.org/10.1016/j.ijcard.2016.04.087.
Lu Y, Yao R, Li Y, Li L, Zhao L, Zhang Y. Clinical effect of ticagrelor administered in acute coronary syndrome patients following percutaneous coronary intervention [journal: article]. Exp Ther Med. 2016;11(6):2177–84. https://doi.org/10.3892/etm.2016.3224.
Wang S, Yang X, Li Z, Zhang B, Cheng Y. Safety and efficacy of ticagrelor with emergency percutaneous coronary intervention in senile patients with ST-segment elevation myocardial infarction and dementia [journal: article]. Int J Clin Exp Med. 2016;9(6):11831–7.
Xue HJ, Shi J, Liu B, et al. Comparison of half- and standard-dose ticagrelor in Chinese patients with NSTE-ACS [journal: article]. Platelets. 2016;27(5):440–5. https://doi.org/10.3109/09537104.2015.1135890.
Zhang Y, Zhao Y, Pang M, et al. High-dose clopidogrel versus ticagrelor for treatment of acute coronary syndromes after percutaneous coronary intervention in CYP2C19 intermediate or poor metabolizers: a prospective, randomized, open-label, single-Centre trial. Acta Cardiol. 2016;71(3):309–16.
Campo G. Vieceli Dalla Sega F, Pavasini R, et al. biological effects of ticagrelor over clopidogrel in patients with stable coronary artery disease and chronic obstructive pulmonary disease. Thromb Haemost. 2017;117(6):1208–16.
Choi KN, Jin HY, Shin HC, et al. Comparison of the antiplatelet effects of once and twice daily low-dose Ticagrelor and Clopidogrel after percutaneous coronary intervention. Am J Cardiol. 2017;120(2):201–6.
Dehghani P, Lavoie A, Lavi S, et al. Effects of ticagrelor versus clopidogrel on platelet function in fibrinolytic-treated STEMI patients undergoing early PCI. Am Heart J. 2017;192:105–12.
Gu X, Fu X, Wang Y, et al. Comparison of ticagrelor and high-dose clopidogrel on the platelet functions in patients with inadequate response to clopidogrel [journal: article]. Am J Cardiovasc Dis. 2017;7(1):1–8.
Zafar MU, Baber U, Smith DA, et al. Antithrombotic potency of ticagrelor versus clopidogrel in type-2 diabetic patients with cardiovascular disease. Thromb Haemost. 2017;117(10):1981–8.
Berwanger O, Nicolau JC, Carvalho AC, et al. Ticagrelor vs Clopidogrel after Fibrinolytic therapy in patients with ST-elevation myocardial infarction: a randomized clinical trial. JAMA Cardiol. 2018;3(5):391–9.
Orme RC, Parker WAE, Thomas MR, et al. Study of two dose regimens of Ticagrelor compared with Clopidogrel in patients undergoing percutaneous coronary intervention for stable coronary artery disease (STEEL-PCI). Circulation. 2018;138(13):1290-300.
Wu HB, Tian HP, Wang XC, et al. Clinical efficacy of ticagrelor in patients undergoing emergency intervention for acute myocardial infarction and its impact on platelet aggregation rate. Am J Transl Res. 2018;10(7):2175–83.
Ge JB, Zhu JR, Hong BK, et al. Prasugrel versus clopidogrel in Asian patients with acute coronary syndromes: design and rationale of a multi-dose, pharmacodynamic, phase 3 clinical trial. Curr Med Res Opin. 2010;26(9):2077–85.
Roe MT, Armstrong PW, Fox KA, et al. Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. N Engl J Med. 2012;367(14):1297–309.
Trenk D, Stone GW, Gawaz M, et al. A randomized trial of prasugrel versus clopidogrel in patients with high platelet reactivity on clopidogrel after elective percutaneous coronary intervention with implantation of drug-eluting stents: results of the TRIGGER-PCI (testing platelet reactivity in patients undergoing elective stent placement on Clopidogrel to guide alternative therapy with Prasugrel) study. J Am Coll Cardiol. 2012;59(24):2159–64.
Caldeira D, Pinto FJ, Ferreira JJ. Dyspnea and reversibility profile of P2Y12 antagonists: systematic review of new antiplatelet drugs. Am J Cardiovasc Drugs. 2014;14:303–11.
Tan QT, Jiang X, Huang SC, et al. The clinical efficacy and safety evaluation of ticagrelor for acute coronary syndrome in general ACS patients and diabetic patients: A systematic review and meta-analysis. PLoS One. 2017;12(5):e0177872.
Cattaneo M, Faioni EM. Why does ticagrelor induce dyspnea? Thromb Haemost. 2012;108(6):1031–6.
Parodi G, Storey RF. Dyspnoea management in acute coronary syndrome patients treated with ticagrelor. Eur Heart J Acute Cardiovasc Care. 2015;4(6):555–60.
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
NZ was responsible for the conception and design of this study. NZ and WSX performed the analysis, drafted the paper and interpreted the results. WSX, OL and BZ involved in data collection and the revision of the paper. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Table S1.
List of potential bias of included studies. Figure S1. Potential bias of included studies using Cochrane Risk Bias. Figure S2. Funnel plot of the overall analysis.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, N., Xu, W., Li, O. et al. The risk of dyspnea in patients treated with third-generation P2Y12 inhibitors compared with clopidogrel: a meta-analysis of randomized controlled trials. BMC Cardiovasc Disord 20, 140 (2020). https://doi.org/10.1186/s12872-020-01419-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-020-01419-y