Abstract
Background
Alfalfa (Medicago sativa L.) is an essential leguminous forage with high nutrition and strong adaptability. The TIFY family is a plant-specific transcription factor identified in many plants. However, few reports have been reported on the phylogenetic analysis and gene expression profiling of TIFY family genes in alfalfa.
Result
A total of 84 TIFY genes belonging to 4 categories were identified in alfalfa, including 58 MsJAZs, 18 MsZMLs, 4 MsTIFYs and 4 MsPPDs, respectively. qRT-PCR data from 8 genes in different tissues revealed that most MsTIFY genes were highly expressed in roots. The expression of MsTIFY14 was up-regulated after different times in both thrips-resistant and susceptible alfalfa after thrips feeding, and the expression of the remaining MsTIFYs had a strong correlation with the time of thrips feeding. Different abiotic stresses, including drought, salt, and cold, could induce or inhibit the expression of MsTIFY genes to varying degrees. In addition, the eight genes were all significantly up-regulated by JA and/or SA. Interestingly, MsTIFY77 was induced considerably by all the biotic, abiotic, or plant hormones (JA or SA) except ABA.
Conclusion
Our study identified members of the TIFY gene family in alfalfa and analyzed their structures and possible functions. It laid the foundation for further research on the molecular functions of TIFYs in alfalfa.
Similar content being viewed by others
Introduction
Alfalfa (Medicago sativa L.) is one of the most essential plants for the development of animal husbandry and dairy industry [1]. With the expansion of planting area and the improvement of intensive planting, alfalfa planting also faces uncertain factors such as low yield, diseases and insect pests [2, 3]. To resist these problems, researchers have identified multiple defense mechanisms at the molecular level of plants by mobilizing a wide range of stress-response genes [4]. Therefore, for the development of the alfalfa industry, more attention should be paid to identifying critical functional genes of alfalfa and breeding resistant varieties [5].
Transcription factors (TFs) have a unique structure and regulate the transcription process of genes by binding to the regulatory region’s DNA sequence [6,7,8]. TFs participate in regulating plant growth and development by activating or inhibiting gene expression and play a vital role in the stress response of plants [9, 10]. Initially, this protein was referred to as ZIM (Zinc-finger protein expressed in Inflorescence Meristem) proteins due to their C2C2-GATA zinc-finger structure. Subsequently, they were renamed the TIFY family, drawing from the conserved core motif TIF[F/Y]XG [11]. According to the conserved domain types, the TIFY family can be divided into four categories: TIFY, ZML, PPD, and JAZ [12]. The TIFY transcription factors play a significant role in the growth and development of plants, stress response, and hormone signal transduction [13]. To date, studies on the TIFY gene family have been reported in many plants. A total of 18, 20, 21, 38 and 47 TIFY genes were identified and analyzed in Arabidopsis thaliana [11], Oryza sativa [14], Byachypodium distachyon [15], Soybean [16] and Maize [17]. These reports are favorable resources for the study of the TIFY gene family. In rice, overexpression of OsTIFYs genes can not only increase grain size through enhanced accumulation of carbohydrates in the stem, but also significantly increase salt and dehydration tolerance [18, 19]. Similarly, overexpression of TdTIFY11a can promote wheat germination under salt stress [20]. In addition, many studies have proposed that TIFY family members, especially the JAZ subfamily proteins, are critical regulators of the JA signaling pathway [21,22,23,24]. Thus, TIFY TFs have major significance in enhancing plant tolerance to various stresses.
However, there have been no reports in terms of the TIFY gene family in alfalfa. In this study, 84 genes were screened from the alfalfa TIFY gene family, and the conserved motif, phylogenetic analysis, and protein interaction network diagram prediction of the family member were systematically analyzed. Quantitative Real-Time PCR was used to analyze the gene expression of TIFY in different tissues (roots, stems, old leaves, tender leaves, flowers, pods, and seeds), biotic stress (thrips), abiotic stresses (drought, salt, and cold) and hormones stresses (JA, SA, and ABA). These findings will provide valuable insights for the functional characterization of TIFY genes in alfalfa.
Result
Identification and classification of TIFY proteins
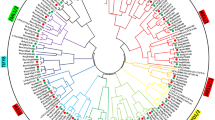
According to the results of a BLAST alignment and the HMM, there were 16, 19, 20, 18, 21, and 84 genes were screened from the whole genome of Physcomitrella patens, Selaginella moellendorffii, Oryza sativa, Arabidopsis thaliana, Medicago truncatula and Medicago sativa (Table 1), respectively. The nomenclature of the TIFY family genes in Arabidopsis and Oryza sativa is based on the UniProt database (www.uniprot.org). Physcomitrella patens, Selaginella moellendorffii, Medicago truncatula, and Medicago sativa TIFY family genes were based on the order in which the sequences are arranged on the chromosome (Tab.S1). Furthermore, according to the characteristics of the conserved domain, they were classified into four subfamilies, namely TIFY, JAZ, PPD, and ZML (Table 1). In addition, the phylogenetic tree analysis of the above 178 amino acid sequences was carried out, and then we further classified them according to the phylogenetic tree of the JAZ subfamily sequence and the characteristics of the motif sequence, and then divided them into five sublevels of JAZ.I, II, III, IV, and V branch (Fig. 1).
The total number of genes and the number of genes in different subfamilies obtained after analysis and separated by our method are basically consistent with those reported in Bai [12]. Since Medicago sativa was tetraploid, while Medicago truncatula and other plants were diploid, the number of genes in alfalfa was significantly expanded. 84 TIFY genes were identified in total, including 4 MsTIFYs, 58 MsJAZs (25 class I, 13 class II, 4 class III, 9 class IV, and 7 Class V), 4 MsPPDs, and 18 MsZMLs. P. patens and O. sativa did not possess PPD subfamily genes.
The results showed that TIFY family genes had low sequence similarity among different species, with an average homology of 46.53%. The TIFY subfamily was found in Bryophyta, Lycophyta, monocotyledonous, and dicotyledonous plants. However, in each subfamily, the TIFY evolutionary relationships of plants from different species were more similar. For example, the TIFY evolutionary relationships of dicotyledonous plants were more clustered in one branch. This suggests that TIFY evolved later than the differentiation of bryophyta, Lycophyta, monocotyledon and dicotyledon.
Structure analysis of the TIFY gene family in alfalfa
Basic information about the members of the alfalfa TIFY gene family was listed in Table S2. The results showed that the full-length coding sequences (CDS), protein length, isoelectric points (pI), molecular weights (Mw), chromosomes and initiation sites are quite different. According to the analysis of chromosome gene sequence length, the most extended TIFY family in alfalfa was MsTIFY30, with a length of 16,712 bp, and the shortest gene was MsTIFY20, with a length of 342 bp. According to the analysis of protein sequence length, it was found that the sequence length of the TIFY family in alfalfa was 87 ~ 429aa. The molecular weight is 9.162 KDa~46.23 KDa.
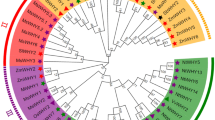
The structural characteristics of the TIFY family evolutionary tree, motif and domain genes in alfalfa were analyzed (Fig. 2). A total of 10 conserved motifs were detected in the MsTIFY genes, which were designated Motifs 1–10, and the members in the same subfamily shared similar conserved motifs. The TIFY subfamily only has the TIFY conserved domain, and the corresponding motif is composed of motif 1 and motif 5 in series. PPD consists of the PPD motif (motif 7), TIFY conserved domain, and JAS/CCT-2, including a sequence of motif 9 at the C-terminal. ZML consists of TIFY, CCT, and GATA domains, and the motif composition is different from other subfamilies, consisting of motif 1, motif 10, motif 2, motif 4, and motif 3. The JAZ subfamily is the largest, with 58 members, and is made up of TIFY conserved domains and JAS/CCT-2. Based on motifs, conserved domain, sequence alignment and phylogenetic tree analysis, the JAZ subfamily was divided into four groups (25 in JAZ I, 13 in JAZ II, 4 in JAZ III, 9 in JAZ IV, and 7 in JAZ V). The presence of subfamily-specific conversed motifs in the subfamily may play a critical role in functional specificity.
Analysis of genetic structure characteristics of the TIFY family phylogenetic tree, motif, and domain genes in alfalfa. The innermost circle is the TIFY family evolutionary tree of alfalfa, the middle is motif sequence composition characteristics, and the outer circle is domain gene structure characteristics
TIFY protein-protein interaction (PPI) network in alfalfa
Using protein-protein interactions to connect unknown functional proteins into protein interaction networks will help understand the biological functions of proteins [25, 26]. In this study, Arabidopsis was used as a background to predict the potential interacting proteins associated with the protein function of MsTIFYs (Tab.S3). A total of 76 TIFY family genes found their positions in the interaction network. It was divided into three clusters according to the degree of their interaction with other family genes (Fig. 3). The results showed that the MsTIFY proteins interacted with proteins such as nuclear-localized protein, Jasmonate-zim-domain protein 8, MYC-related transcriptional activator, Coronatine-insensitive protein 1, Jasmonate-zim-domain protein 3, DNA-binding family protein MYC and GATA transcription factor 24. It is speculated that MsTIFY proteins may work synergistically with other proteins in jasmonic acid-mediated plant resistance defense.
MsTIFY proteins interaction network diagram. Red mark cluster I, green mark cluster 2, and blue mark cluster 3. The right table shows the genes that may be involved in the interaction of the three clusters. The red letters represent the genes identified in the left figure, and the genes below the genes are other homologous genes of this gene in alfalfa
Expression of MsTIFY genes in different tissues
In order to understand the expression profile of MsTIFY genes in different tissues, the expression of 8 MsTIFY genes in roots, stems, old leaves, tender leaves, flowers, pods, and seeds were analyzed by qRT-PCR (Fig. 4; Tab.S4). The results showed that MsTIFY11, MsTIFY14, MsTIFY18, MsTIFY58 and MsTIFY71 were highly expressed in roots. MsTIFY77 was preferentially expressed in stems, and MsTIFY28 was highest expressed in tender leaves. MsTIFY41 was preferentially expressed in older leaves and highly expressed in roots and stems. The expression level of the flower was relatively high in MsTIFY28, MsTIFY58 and MsTIFY77. The results showed that the MsTIFY genes had overlapping but spatially varying tissue expression, indicating that they may play different roles in specific tissues.
qRT-PCR analysis of the expression patterns of eight MsTIFY genes in seven tissues in alfalfa. R: roots; St: stems; OL: old leaves; TL: tender leaves; F: flowers; P: pods; S: seeds. The error bars indicate the standard errors of three biological replicates. Columns with different letters are significantly different(P < 0.05)
Expression of MsTIFY genes in response to thrips infection
To clarify the expression patterns of the alfalfa TIFY gene family under biotic stresses, we analyzed the expression levels of 8 genes (MsTIFY11, MsTIFY14, MsTIFY18, MsTIFY28, MsTIFY41, MsTIFY58, MsTIFY71 and MsTIFY77) in two alfalfa varieties, ‘Caoyuan No.2’ (a thrips-sensitive variety) and ‘Caoyuan No.4’ (a thrips-resistant variety), at different times (0d, 3d, 7d, 10d and 14d) with thrips infection (Fig. 5; Tab.S5).
The results showed that all 8 MsTIFY genes were induced by the thrips infection in 3d in both ‘Caoyuan No.2’ and ‘Caoyuan No.4’, suggesting that these genes may play essential roles in the early response to biotic stress in alfalfa (Fig. 5A, B). In detail, MsTIFY11 was significantly up-regulated only in the 3d thrips infection but downregulated in 7d, 10d, and 14d thrips infection in both varieties. In addition, MsTIFY14 and MsTIFY77 were highly induced by 14d thrips infection in both varieties. These results suggested that different MsTIFY genes play roles in various stages of plant-insect interactions.
In Caoyuan No.2, MsTIFY28 reached the highest level after 3d of thrips infection, and then it gradually decreased. MsTIFY18 and MsTIFY58 showed significant up-regulation after 10d of thrips infection, while the expression of MsTIFY77 showed an upward trend. Interestingly, MsTIFY11, MsTIFY14, and MsTIFY71 showed the highest expression levels on the 3d of thrips infection, which then rapidly decreased and gradually increased again (Fig. 5A). The expression patterns of MsTIFYs in ‘Caoyuan No.4’ were similar to those of ‘Caoyuan No.2’ (Fig. 5B).
qRT-PCR analysis of expression patterns of eight MsTIFY genes under thrips stress of ‘Caoyuan No.2’ and ‘Caoyuan No.4’. A: The expression patterns of eight MsTIFY genes under thrips stress of Caoyuan No.2. B: The expression patterns of eight MsTIFY genes under the stress of Caoyuan No.4. The error bars indicate the standard errors of three biological replicates. Columns with different letters are significantly difference(P < 0.05)
Expression of MsTIFY genes in response to abiotic stress
To explore the potential functions of the MsTIFY genes in response to abiotic stress, the expression of 8 screened MsTIFY genes in response to drought, salt, and cold in ‘Caoyuan No.4’ were analyzed (Fig. 6; Tab.S6).
The results showed that all the MsTIFYs genes were up-regulated under different degrees of drought stress (Fig. 6A). In detail, a significant up-regulation of the MsTIFY11 expression was observed at different status of drought stress by up to 3.87- to 7.69- fold of the control. For MsTIFY14, MsTIFY28, MsTIFY41, MsTIFY58, and MsTIFY71, the expression level significantly increased at 2 h and then declined and maintained the control level. In addition, transcripts of MsTIFY18 and MsTIFY77 increased in the later stage of drought stress by up to 81.89- and 36-fold of the control, respectively.
Under salt stress, the expression level of the majority of MsTIFYs genes was fluctuation rising, including MsTIFY11, MsTIFT14, MsTIFY18, MsTIFY41 and MsTIFY77 (Fig. 6B). For MsTIFY28, the gene expression was down to 0.12 to 0.68 folds of the control. The expression of MsTIFY71 clearly declined except at 12 h, and MsTIFY58 expression increased before 4 h and then gradually decreased.
The results showed that MsTIFY genes presented different expression patterns under cold treatment at 4℃ (Fig. 6C). In detail, the expression of MsTIFY77 was significantly up-regulated of the control. The expression of MsTIFY11, MsTIFY14, MsTIFY18, and MsTIFY28 gradually increased in the earlier stage of cold treatment but progressively declined with the extension of treatment time. The expression of MsTIFY58 and MsTIFY71 were significantly lower than those of control except at 6 h. Interestingly, the expression level of MsTIFY41 clearly declined to about half of the control except at 12 h.
Expression of MsTIFY genes in response to plant hormones
Hormones are essential factors affecting plant growth and development [27, 28]. The expression levels of 8 genes in response to JA, SA, and ABA treatments in ‘Caoyuan No.4’ were analyzed by qRT-PCR (Fig. 7; Tab.S7).
The results showed that all MsTIFY genes were up-regulated at different times after spraying JA (Fig. 7a). In detail, the expression levels of MsTIFY11 and MsTIFY41 were gradually increased. MsTIFY14, MsTIFY18, MsTIFY58, and MsTIFY71 had similar expression patterns, which gradually increased or maintained in the earlier stage while declining at 12 h. In addition, the MsTIFY28 and MsTIFY77 expression levels fluctuated and rose.
For SA treatment, all MsTIFY genes showed a similar expression pattern (Fig. 7b), which gradually increased at 3 h and decreased sharply at 6–8 h and then up to the control level except MsTIFY18.
The results showed that all the MsTIFYs genes decreased at different times under ABA treatment except MsTIFY11 (Fig. 7C). In detail, the expression of MsTIFY14, MsTIFY18, MsTIFY28, MsTIFY41, MsTIFY58, and MsTIFY77 were clearly declined to about 0.2- to 0.4- fold of the control. In contrast to these genes, a significant upregulation of MsTIFY11 was observed at any treatment time and reached 47.48- fold of the control at 3 h.
To further clarify the response of the 8 MsTIFYs genes to different treatments, correspondence analysis was performed (Fig. 8). The results showed that MsTIFY41 was more closely related to JA, MsTIFY11 was more closely related to ABA, and MsTIFY28 and MsTIFY71 were more closely related to SA. Furthermore, MsTIFY14 was closer to thrips stress and cold stress, while MsTIFY58 was mainly closer to thrips stress. In addition, MsTIFY18 and MsTIFY77 were closer to the late stage of drought, salt and cold stress MsTIFY14, MsTIFY18, MsTIFY58 and MsTIFY77 had closer correspondences to thrips, drought, salt, and cold stress.
Discussion
The TIFY gene family is unique in plants [29]. In the study on the TIFY gene family, the number of TIFY genes in most plants was not more than 30, such as Arabidopsis with 18 [11], rice with 20 [18], Terrestris with 21 [30], cotton with 21 [31], Moso bamboo with 24 [32], tomato with 26 [33], kiwifruit with 27 [34] and peanut with 29 [35]. We identified 84 TIFY genes in alfalfa, the largest number of TIFY family members studied so far, which might be caused by the doubling of the alfalfa genome. In addition, different research methods may also lead to differences in the numbers identified. For instance, 30 TIFY genes were identified before [36], but 47 were identified in the latest study [17]. Wheat had 49 [20] and then 63 [37]. As a result, newer methods can identify more gene family members. The analysis of family members showed that among the TIFY gene family, the JAZ subfamily had the most family members, while the TIFY and PPD families had fewer family members. For example, among the 15 TIFY gene family members in watermelon, there were 8 CIJAZs, 4 CIZMLs, 2 CITIFYs, and 1 ClPPDs [38]. Among the 12 TIFY gene families in birch, there were 7 BpJAZs, 3 BpZMLs, 1 BpTIFYs, and 1 BpPPDs [39]. We identified 84 TIFY genes in alfalfa (Table 1), including 58 MsJAZs, 18 MsZMLs, 4 MsTIFYs, and 4 MsPPDs, which were similar to those found in other species. In addition, TIFY family genes were found in bryophytes, stonecrops, monocotyledons, and dicotyledons (Table 1; Fig. 1)., but within each subfamily, plants originating from the same phylum have closer TIFY evolutionary relationships. For example, dicotyledons are more clustered on one branch of the TIFY evolutionary relationship. This suggests that the genes of the dicotyledonous TIFY family evolved later than those of the bryophyte phylum, the lithophyte phylum, and the monocotyledonous phylum.
TIFY family genes had diverse structural domains. In this study, TIFY, JAS/CCT-2, CCT and GATA were found in the TIFY proteins (Tab.S8). The motif structure in each TIFY subfamily had mostly conservative domains. The MsTIFY gene family was classified into four subfamilies (TIFY, JAZ, ZML, and PPD) according to the characteristics of its conserved domain, and the JAZ subfamily could be further divided into five subgroups. This is consistent with previous studies [39]. AtTIFY8 interacts with the transcription factor REVOLUTA of HD-ZIP III and regulates leaf senescence [40]. In this study, MsTIFY50, MsTIFY55, MsTIFY56, and MsTIFY57 cluster into the same clade, suggesting that they may have similar functions (Fig. 2). OsTIFY11b can increase carbohydrate accumulation in stem and leaf sheath [19], regulate the growth and development of stem and leaf [14], and improve salt tolerance [20]. In our study, OsTIFY11b was clustered into the same branch with 38 MsTIFYs, among which it was close to MsTIFY3, MsTIFY4, MsTIFY11, MsTIFY12, and MsTIFY21, was predicted to have similar functions (Fig. 1). In addition, qRT-PCR also found that MsTIFY11 was highly expressed in roots, stems and leaves (Fig. 4), and was significantly up-regulated after salt stress (Fig. 5), so it is speculated that MSTIFY11 plays a vital role in regulating stem and leaf growth and salt stress tolerance.
MsTIFY family genes have tissue specificity. The expression profiles of 8 MsTIFYs were identified in 7 different tissues (roots, stems, old leaves, tender leaves, flowers, pods and seeds) (Fig. 4). The results showed that most MsTIFYs were highly expressed in roots, especially MsTIFY14, MsTIFY71, MsTIFY18, MsTIFY11 and MsTIFY58, suggesting that TIFY gene family may participate root growth and development. MsTIFY77 was expressed preferentially in stems, and MsTIFY28 was expressed highest in young leaves. MsTIFY41 was expressed preferentially in old leaves and was highly expressed in roots and stems. Interestingly, OsTIFY1a and OsTIFY1b are highly expressed in leaves [14], which are in the same clade as MsTIFY41 in the evolutionary tree (Fig. 2). In rice [19], most OsTIFY genes are mainly expressed in leaves. CmJAZ delays the flowering of chrysanthemums. In Astragalus [41], JAZ proteins interact with haemoglobin via TIFY domains and are involved in nodal development and nitrogen fixation.
MsTIFY family genes play an essential role in biotic stresses. The expression of the same gene was different in different alfalfa varieties. In sorghum [42], after JA treatment and aphid infection, the expression of SbJAZ1, SbJAZ5, SbJAZ13, and SbJAZ16 in resistant varieties was up-regulated. In apples [43], the expression level of MsTIFY10B-a and MsTIFY9-c in resistant strains increased 34 times and 5.2 times after insect infestation. In maize [44], ZmJAZ1 and ZmCOI1a responded to the feeding of autumn armyworms (Spodoptera frugiperda). In the studies on the response of the TIFY gene family to biological stress, there are only a few studies on infectious pathogens and almost no studies on butt worms. In cucumber [45], CsJAZ1 and CsJAZ2 showed significant changes in infection with four diseases (powdery mildew, downy mildew, stem blight, and grey mould). In tea plants [46], Colletotrichum camelliae mainly up-regulated the expression levels of CsJAZ1 and CsJAZ10. In this study, thrips susceptible and thrips resistant alfalfa varieties with different various degrees of thrips damage were selected to observe the expression changes of 8 MsTIFYs genes (Fig. 5). The results showed that 8 MsTIFYs were significantly up-regulated in both varieties at 3d of thrips feeding, indicating that they could positively regulate the early defense of alfalfa against thrips. MsTIFY14 and MsTIFY77 were also significantly up-regulated in the expression of thrips on the 7d and 14d of thrips feeding, suggesting that they may have an important role in alfalfa defense against thrips at later stages.
A growing number of studies have shown that TIFY transcription factors play an important regulatory role in abiotic stresses [47,48,49,50]. In wheat [20], salt treatment induced the expression of TdTIFY11. In bamboo [32], 50% of PeTIFY genes could be up-regulated by dehydration stress. In soybean [16], salt stress induced GmTIFY10e and GmTIFY10g. In rice [14], overexpression of OsTIFY11a increased tolerance to salt and dehydration stress. In our study, most MsTIFYs were up-regulated by drought, salt, and cold stress (Fig. 6). Drought significantly induced MsTIFY11, MsTIFY18, MsTIFY28, MsTIFY71, and MsTIFY77. Salt stress significantly induced MsTIFY18, MsTIFY41, and MsTIFY77, and cold significantly induced MsTIFY28 and MsTIFY77. In watermelon [38], JA activated 8 genes and inhibited 1 gene, among which CIJAZ1 and CIJAZ7 were most significantly induced. In tomato [33], SlJAZ1, SlJAZ3, SlJAZ6, SlJAZ7 and SlJAZ11 were significantly induced by JA. Although ABA could induce some genes, their expression levels were not as high as those of JA. However, in grapes [51], many of the TIFY genes were responsive to JA and ABA but not SA or ET. In our study, it was also found that almost all MsTIFYs were induced by JA and SA, and ABA significantly inhibited the expression of most MsTIFYs (Fig. 7). Therefore, we speculated that JA and SA could induce the expression of MsTIFY family genes. In contrast, ABA could inhibit most of them. Therefore, the results indicate that MsTIFY genes actively respond to biotic and abiotic stresses.
Further, the correspondence analysis of 8 genes with the expression of genes after different stresses revealed that MsTIFY58 was mainly related to insect stress, and MsTIFY18 and MsTIFY77 were mainly involved in the late stage of drought, salt and cold stresses. MsTIFY58 may be important for responding to insect stress. MsTIFY14 was in response to both thrips stress and cold stress. In addition, presumably based on correspondence analysis MsTIFY41 and MsTIFY11 may play more dominant roles mainly in regulating JA and ABA, respectively. MsTIFY28 and MsTIFY71 genes may primarily play an important role in the response to SA and early cold stress.
Materials and methods
Identification of the TIFY gene family in alfalfa
To identify more comprehensive TIFY family genes, the sequence of Physcomitrella patens of Bryophyta, Selaginella moellendorffii of Lycopodiophyta, Oryza sativa of Monocots, Arabidopsis thaliana and Medicago truncatula in Eudicots was downloaded from the Ensemblplants database (http://plants.ensembl.org/). However, we didn’t find a genome database of alfalfa in the Ensemblplants database. Thus, the protein sequence and corresponding CDS sequence of alfalfa were obtained from Alfalfa Breeder’s Toolbox database (https://alfalfatoolbox.org/) by homologous sequence alignments.
The sequence characteristics of the TIFY domain (PF06200) were searched in the Pfam database (http://pfam.xfam.org/), and the HMM file was formed as a reference file for conservative domain comparison [52]. Then, according to Bai [12], the HMMER program was used to compare the protein sequence data in the above genomic data respectively and screen E-value > 1e-6, the amino acid sequence with a score higher than 20 [53, 54]. The BLASTP program was then used to compare these sequences to Arabidana seed sequences. Proteins containing the TIFY domain were separated into different subfamilies based on the presence or absence of TIFY, PPD, CCT-2, CCT, or ZML domains by removing duplications, incorrect sequencing, incomplete read frames, or incomplete domain sequences.
Conserved motif and phylogenetic analysis of MsTIFY proteins
To explore the phylogenetic relationship of MsTIFY transcription factors, the molecular evolutionary relationship of the MsTIFY gene family was elucidated by constructing an evolutionary tree. The sequences were aligned with MAFFT using the ‘auto’ strategy and normal alignment mode [55]. Gap sites were removed with trimAl using “noallgaps” command [56]. The best-fit model for 178 amino acid sequences of 6 different plants was JTT + R5. The best-fit model for the MsTIFY family in Medicago sativa was JTT + G4. Maximum likelihood phylogenies were inferred using IQ-TREE under the JTT + R5 or JTT + G4 model for 20,000 ultrafast bootstraps, as well as the Shimodaira–Hasegawa–like approximate likelihood-ratio test [57,58,59]. The conserved motif of MsTIFY was determined using MEME online analytical tools (https://meme-suite.org/meme/tools/meme). In addition, NCBI Conserved Domains were used to analyze the Conserved domain of alfalfa TIFY family amino acid sequence [60].
Protein interaction network diagram prediction for MsTIFYs
Cystoscope version 3.7.0 was used to search for the interaction factors of these proteins to construct PPI networks for the MsTIFY gene family and related families. The search was conducted on the STRING database (https://string-db.org/) and the organism selected was Arabidopsis thaliana. These proteins were then mapped to the MsTIFY family in Medicago sativa with the following cutoff values: identity ≥ 30 and E-value ≤ 1E-10. If the value exceeds this value, it will not be displayed. Then, the single gene with the highest hit ratio (lowest E value) was selected to represent each protein in the network, and the related protein regulatory network was established.
Plant materials and stress treatments
Alfalfa seeds (Caoyuan No.4 and Caoyuan No.2) were bred at Inner Mongolia Agricultural University, China. All plants were cultivated in pots (H10 cm × D12 cm, one plant per pot) containing field-collected soil in a greenhouse with a relative humidity of 60 ± 5% and 70 ± 5% at 30 ± 5 °C and 20 ± 5 °C during day and night, respectively. Plants were watered every other day.
Thrips infections were treated as described by Tu et al. [61]. with some modifications. When the seedlings were about 40 days old, they were randomly and equally divided into two groups: (1) 100 thrips per plant were placed onto the leaves and covered by a cage with 300-mesh nylon cloth, and (2) In order to avoid the interference of growth conditions on the test results, this test was sampled at the same time (at the same time at 54 days of growth), and thrips injection was carried out 14, 10, 7, 3 and 1 days before sampling, respectively, and alfalfa that was not inoculated with thrips on the day of sampling was used as a control. For salt and drought stresses, the seedlings (Caoyuan No.4) were treated with 250mmol/L NaCl and air drought [62] and harvested at 0 h, 2 h, 4 h, 8 h, 12 h, and 24 h. For cold stress, the seedlings were placed in a low-temperature incubator at four °C, and samples were collected at 0 h, 2 h, 6 h, 12 h, 24 h, and 48 h. In addition, for hormone treatments, including salicylic acid (SA), abscisic acid (ABA), and jasmonic acid (JA) treatments, 0.5mmol/L SA, 10umol/LABA, and 100umol/L MeJA were sprayed on the plants, to minimize errors, spray each plant five times, then seal it with a transparent plastic bag to prevent hormone volatilization, and harvest at the same time at 0 h, one h, 3 h, 6 h and 12 h. Samples of roots, stems, mature leaves, young leaves, flowers, seeds, and pods of alfalfa were collected for tissue-specific expression analysis. All samples were set in triplicate. Immediately after sampling, the samples were snap-frozen in liquid nitrogen and stored at -80 °C for subsequent analysis.
Quantitative real-time PCR analysis
Eight MsTIFYs were screened for qRT-PCR experiments. The total RNA of different samples was extracted using OminiPlant RNA Kit (Cwbio, Beijing, China) according to the manufacturer’s instructions. RNA concentration and purity were measured by a NanoDrop ND1000 spectrophotometer. Total RNA was used for first-strand cDNA synthesis using EasyQuick RT MasterMix (Cwbio, Beijing, China). The synthesized cDNA was used as a template for gene expression analysis. The qRT-PCR experiments were performed with 2×MagicSYBR Misture on a 7500 Real-time PCR system (Applied Biosystems, Foster City, CA, USA), using the alfalfa β-actin gene as a reference gene. NCBI Primer-BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi) was used to design primers (Tab.S8). Three independent biological replicates and two technical replicates for each sample were used for the qRT-PCR. The gene expression was quantified by the 2−ΔΔCT method [63].
Statistical analysis
Excel 2010 was used for the statistics calculation of relevant data, SPSS Statistics 18.0 software was used for the analysis of variance, and a student t-test was used to compare the mean values at a 5% significance level. Use the Origin 2019 software to make bar charts. SAS was used to analyze 8 genes in correspondence with their expression after different stresses.
Conclusion
In this study, a total of 84 TIFY genes were identified in the alfalfa, including 58 MsJAZs, 18 MsZMLs, 4 MsTIFYs, and 4 MsPPDs. qRT-PCR data of 8 random selected genes in different tissues, biotic and abiotic stress revealed that most of MsTIFY genes were highly expressed in roots and leaves. All 8 MsTIFY genes were significantly induced by early thrips feeding in the resistant and susceptible alfalfa varieties, MsTIFY14 and MsTIFY77 may be concurrently involved in alfalfa defense against thrips in the late stages. Different abiotic stresses, including drought, salt, and cold, could induce or inhibit the expression of 8 MsTIFY genes to varying degrees. In addition, MsTIFY genes were significantly up-regulated by JA and SA. Interestingly, MsTIFY77 was induced considerably by all the biotic, abiotic, or plant hormones (JA or SA) except ABA. Our results laid the foundation for further study for further study on molecular functions of TIFYs in alfalfa.
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files]. The datasets supporting our conclusions regarding the current study are included in the manuscript and the additional file. We did not generate any sequencing data in this study. We have used already published genomic data. The sequence of Physcomitrella patens of Bryophyta, Selaginella moellendorffii of Lycopodiophyta, Oryza sativa of Monocots, Arabidopsis thaliana, and Medicago truncatula in Eudicots was downloaded from the Ensembl plants database (http://plants.ensembl.org/). The protein sequence and corresponding CDS sequence of alfalfa were obtained from Alfalfa Breeder’s Toolbox database (https://alfalfatoolbox.org/).
References
Shen C, Du H, Chen Z, Lu H, Zhu F, Chen H, Meng X, Liu Q, Liu P, Zheng L, et al. The chromosome-level genome sequence of the Autotetraploid Alfalfa and Resequencing of Core Germplasms provide genomic resources for Alfalfa Research. Mol Plant. 2020;13(9):1250–61.
Wang X, Miao J, Kang W, Shi S. Exogenous application of salicylic acid improves freezing stress tolerance in alfalfa. Front Plant Sci. 2023;14:1091077.
Zhang C, Shi S, Wu F. Effects of Drought stress on Root and physiological responses of different Drought-Tolerant Alfalfa varieties. Scientia Agricultura Sinica. 2018;51(5):868–82.
Jun L. Transform alfalfa research. Nat Plants. 2020;6(6):596.
Wang Y, Ruan Q, Zhu X, Wang B, Wei B, Wei X. Identification of Alfalfa SPL gene family and expression analysis under biotic and abiotic stresses. Sci Rep. 2023;13(1):84.
Song L, Li W, Chen X. Transcription factor is not just a transcription factor. Trends Plant Sci. 2022;27(11):1087–9.
Leben K, Strmsek Z, Lebar T, Verbic A, Dragovan M, Omersa N, Anderluh G, Jerala R. Binding of the transcription activator-like effector augments transcriptional regulation by another transcription factor. Nucleic Acids Res. 2022;50(11):6562–74.
Fan S, Wang J, Qin B, Wang L. Analytic methods and application of plant transcription fact. Mol Plant Breed. 2019;17(15):5003–9.
Chen N, Shao Q, Lu Q, Li X, Gao Y, Xiao Q. Research progress on function of NAC transcription factors in tomato (Solanum lycopersicum L). Euphytica 2023, 219(1).
Zou X, Sun H. DOF transcription factors: specific regulators of plant biological processes. Front Plant Sci. 2023;14:1044918.
Vanholme B, Grunewald W, Bateman A, Kohchi T, Gheysen G. The TIFY family previously known as ZIM. Trends Plant Sci. 2007;12(6):239–44.
Bai Y, Meng Y, Huang D, Qi Y, Chen M. Origin and evolutionary analysis of the plant-specific TIFY transcription factor family. Genomics. 2011;98(2):128–36.
Yang R-j, Zhang Z-b, Wu Z-y. Progress of the Structural and Functional Analysis of Plant Transcription Factor TIFY Protein Family. Biotechnol Bull. 2020;36(12):121–8.
Ye H, Du H, Tang N, Li X, Xiong L. Identification and expression profiling analysis of TIFY family genes involved in stress and phytohormone responses in rice. Plant Mol Biol. 2009;71(3):291–305.
Zhang L, You J, Chan Z. Identification and characterization of TIFY family genes in Brachypodium distachyon. J Plant Res. 2015;128(6):995–1005.
Liu Y, Zheng L, Jin L, Liu Y, Kong Y, Wang Y, Yu T, Chen J, Zhou Y, Chen M, et al. Genome-wide analysis of the soybean TIFY Family and Identification of GmTIFY10e and GmTIFY10g response to salt stress. Front Plant Sci. 2022;13:845314.
Sun P, Shi Y, Valerio AGO, Borrego EJ, Luo Q, Qin J, Liu K, Yan Y. An updated census of the maize TIFY family. PLoS ONE. 2021;16(2):e0247271.
Hakata M, Muramatsu M, Nakamura H, Hara N, Kishimoto M, Iida-Okada K, Kajikawa M, Imai-Toki N, Toki S, Nagamura Y, et al. Overexpression of TIFY genes promotes plant growth in rice through jasmonate signaling. Biosci Biotechnol Biochem. 2017;81(5):906–13.
Hakata M, Kuroda M, Ohsumi A, Hirose T, Nakamura H, Muramatsu M, Ichikawa H, Yamakawa H. Overexpression of a rice TIFY gene increases grain size through enhanced accumulation of carbohydrates in the stem. Biosci Biotechnol Biochem. 2012;76(11):2129–34.
Ebel C, BenFeki A, Hanin M, Solano R, Chini A. Characterization of wheat (Triticum aestivum) TIFY family and role of Triticum Durum TdTIFY11a in salt stress tolerance. PLoS ONE. 2018;13(7):e0200566.
Li Z, Luo X, Ou Y, Jiao H, Peng L, Fu X, Macho A, Liu R, He Y. JASMONATE-ZIM DOMAIN proteins engage polycomb chromatin modifiers to modulate Jasmonate signaling in Arabidopsis. Mol Plant. 2021;14(5):732–47.
An JP, Wang XF, Zhang XW, You CX, Hao YJ. Apple B-box protein BBX37 regulates jasmonic acid mediated cold tolerance through the JAZ-BBX37-ICE1-CBF pathway and undergoes MIEL1-mediated ubiquitination and degradation. New Phytol. 2021;229(5):2707–29.
Zhang F, Yao J, Ke J, Zhang L, Lam V, Xin X, Zhou X, Chen J, Brunzelle J, Griffin P, et al. Structural basis of JAZ repression of MYC transcription factors in jasmonate signalling. Nature. 2015;525(7568):269–73.
Guo Q, Yoshida Y, Major I, Wang K, Sugimoto K, Kapali G, Havko N, Benning C, Howe G. JAZ repressors of metabolic defense promote growth and reproductive fitness in Arabidopsis. Proc Natl Acad Sci USA. 2018;115(45):E10768–77.
Kang Y, Xu Y, Wang X, Pu B, Yang X, Rao Y, Chen J. HN-PPISP: a hybrid network based on MLP-Mixer for protein-protein interaction site prediction. Brief Bioinform 2023, 24(1).
Yang X, Yang S, Qi H, Wang T, Li H, Zhang Z. PlaPPISite: a comprehensive resource for plant protein-protein interaction sites. BMC Plant Biol. 2020;20(1):61.
Yuan H, Liu W, Lu Y. CATALASE2 coordinates SA-Mediated repression of both Auxin Accumulation and JA Biosynthesis in Plant defenses. Cell host Microbe. 2017;21(2):143–55.
Xiong X, Sun S, Zhu Q, Zhang X, Liu F, Li Y, Xue F, Sun J. Transcriptome analysis and RNA interference reveal GhGDH2 regulating Cotton Resistance to Verticillium Wilt by JA and SA Signaling pathways. Front Plant Sci. 2021;12:654676.
Jia K, Yan C, Zhang J, Cheng Y, Li W, Yan H, Gao J. Genome-wide identification and expression analysis of the JAZ gene family in turnip. Sci Rep. 2021;11(1):21330.
Pecrix Y, Staton S, Sallet E, Lelandais-Brière C, Moreau S, Carrère S, Blein T, Jardinaud M, Latrasse D, Zouine M, et al. Whole-genome landscape of Medicago truncatula symbiotic genes. Nat Plants. 2018;4(12):1017–25.
Zhao G, Song Y, Wang C, Butt H, Wang Q, Zhang C, Yang Z, Liu Z, Chen E, Zhang X, et al. Genome-wide identification and functional analysis of the TIFY gene family in response to drought in cotton. Mol Genet Genomics: MGG. 2016;291(6):2173–87.
Huang Z, Jin S, Guo H, Zhong X, He J, Li X, Jiang M, Yu X, Long H, Ma M, et al. Genome-wide identification and characterization of TIFY family genes in Moso Bamboo (Phyllostachys edulis) and expression profiling analysis under dehydration and cold stresses. PeerJ. 2016;4:e2620.
Chini A, Ben-Romdhane W, Hassairi A, Aboul-Soud M. Identification of TIFY/JAZ family genes in Solanum lycopersicum and their regulation in response to abiotic stresses. PLoS ONE. 2017;12(6):e0177381.
Tao J, Jia H, Wu M, Zhong W, Jia D, Wang Z, Huang C. Genome-wide identification and characterization of the TIFY gene family in kiwifruit. BMC Genomics. 2022;23(1):179.
Sen S, Dasgupta M. Involvement of Arachis hypogaea Jasmonate ZIM domain/TIFY proteins in root nodule symbiosis. J Plant Res. 2021;134(2):307–26.
Zhang Z, Li X, R Y, Han M, Wu Z. Isolation, structural analysis, and expression characteristics of the maize TIFY gene family. Mol Genet Genomics: MGG. 2015;290(5):1849–58.
Xie S, Cui L, Lei X, Yang G, Li J, Nie X, Ji W. The TIFY Gene Family in Wheat and its progenitors: genome-wide identification, evolution and expression analysis. Curr Genom. 2019;20(5):371–88.
Yang Y, Ahammed G, Wan C, Liu H, Chen R, Zhou Y. Comprehensive Analysis of TIFY Transcription Factors and Their Expression Profiles under Jasmonic Acid and Abiotic Stresses in Watermelon. International journal of genomics 2019, 2019:6813086.
Lv G, Han R, Shi J, Chen K, Liu G, Yu Q, Yang C, Jiang J. Genome-wide identification of the TIFY family reveals JAZ subfamily function in response to hormone treatment in Betula platyphylla. BMC Plant Biol. 2023;23(1):143.
Galan AGA, Doll J, Saile S, Wünsch M, Roepenack-Lahaye E, Pauwels L, Goossens A, Bresson J, Zentgraf U. The Non-JAZ TIFY protein TIFY8 of Arabidopsis thaliana interacts with the HD-ZIP III Transcription Factor REVOLUTA and regulates Leaf Senescence. Int J Mol Sci 2023, 24(4).
Li Y, Xu M, Wang N, Li Y. A JAZ protein in Astragalus sinicus interacts with a Leghemoglobin through the TIFY Domain and is involved in Nodule Development and Nitrogen fixation. PLoS ONE. 2015;10(10):e0139964.
Shrestha K, Huang Y. Genome-wide characterization of the sorghum JAZ gene family and their responses to phytohormone treatments and aphid infestation. Sci Rep. 2022;12(1):3238.
Mei C, Zhang X, Yan P. Identification of TIFY Family in Apple and their expression analysis under insect stress. Acta Horticulturae Sinica. 2021;48(2):233–42.
Han Y, Luthe D. Key genes in the JAZ Signaling Pathway are Up-Regulated faster and more abundantly in Caterpillar-Resistant Maize. J Chem Ecol. 2022;48(2):179–95.
Dai Z, Dong S, Miao H, Liu X, Han J, Li C, Gu X, Zhang S. Genome-wide identification of TIFY genes and their response to various Pathogen infections in Cucumber (Cucumis sativus L). Sci Hort. 2022;295:110814.
Zhang X, Ran W, Zhang J, Ye M, Sun X. Genome-wide identification of the Tify Gene Family and their expression profiles in response to biotic and abiotic stresses in tea plants (Camellia sinensis). Int J Mol Sci. 2020;21(21):115.
He X, Kang Y, Li W, Liu W, Xie P, Liao L, Huang L, Yao M, Qian L, Liu Z, et al. Genome-wide identification and functional analysis of the TIFY gene family in the response to multiple stresses in Brassica napus L. BMC Genomics. 2020;21(1):736.
Saha G, Park J, Kayum M, Nou I. A genome-wide analysis reveals stress and hormone responsive patterns of TIFY Family genes in Brassica rapa. Front Plant Sci. 2016;7:936.
Li L, Liu Y, Huang Y, Li B, Ma W, Wang D, Cao X, Wang Z. Genome-wide identification of the TIFY Family in Salvia miltiorrhiza reveals that SmJAZ3 interacts with SmWD40-170, a relevant protein that modulates secondary metabolism and development. Front Plant Sci 2021, 12.
Wu H, Ye H, Yao R, Zhang T, Xiong L. OsJAZ9 acts as a transcriptional regulator in jasmonate signaling and modulates salt stress tolerance in rice. Plant Science: Int J Experimental Plant Biology. 2015;232:1–12.
Zhang Y, Gao M, Singer S, Fei Z, Wang H, Wang X. Genome-wide identification and analysis of the TIFY gene family in grape. PLoS ONE. 2012;7(9):e44465.
Mistry J, Chuguransky S, Williams L, Qureshi M, Salazar G, Sonnhammer E, Tosatto S, Paladin L, Raj S, Richardson L, et al. Pfam: the protein families database in 2021. Nucleic Acids Res. 2021;49(D1):D412–9.
Eddy S. Profile hidden Markov models. Bioinf (Oxford England). 1998;14(9):755–63.
Grewal J, Krzywinski M, Altman N. Markov models - hidden Markov models, vol. 16; 2019.
Katoh K, Standley D. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30(4):772–80.
Capella-Gutierrez S, Silla-Martínez J, Gabaldón T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinf (Oxford England). 2009;25(15):1972–3.
Nguyen L, Schmidt H, Haeseler Av, Minh BQ. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 2015;32(1):268–74.
Guindon S, Dufayard J, Lefort V, Anisimova M, Hordijk W, Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 2010;59(3):307–21.
Minh B, Nguyen M, Haesele A. Ultrafast approximation for phylogenetic bootstrap. Mol Biol Evol. 2013;30(5):1188–95.
Marchler-Bauer A, Bo Y, Han L, He J, Lanczycki C, Lu S, Chitsaz F, Derbyshire M, Geer R, Gonzales N, et al. CDD/SPARCLE: functional classification of proteins via subfamily domain architectures. Nucleic Acids Res. 2017;45(D1):D200–3.
Tu X, Liu Z, Zhang Z. Comparative transcriptomic analysis of resistant and susceptible alfalfa cultivars (Medicago sativa L.) after thrips infestation. BMC Genomics. 2018;19(1):116.
Yang P, Zhang P, Li B, Hu T. Effect of nodules on dehydration response in alfalfa (Medicago sativa L). Environ Experimental Bot. 2013;86(none):29–34.
Adnan M, Morton G, Hadi S. Analysis of rpoS and bolA gene expression under various stress-induced environments in planktonic and biofilm phase using 2(-∆∆CT) method. Mol Cell Biochem. 2011;357(1–2):275–82.
Funding
This work was supported by Inner Mongolia Autonomous Region Colleges and Universities ' Youth Science and Technology Talent Support Project ‘, China (NJYT23009), projects of the National Natural Science Foundation of China (32160333), the National Natural Science Foundation of Inner Mongolia, China (2021MS03011), Construction of alfalfa molecular breeding system and germplasm creation(BR22-11-12), Inner Mongolia Autonomous Region Postgraduate Research Innovation Program(S20231109Z).
Author information
Authors and Affiliations
Contributions
Zhiqiang Zhang and Fengling Shi designed and performed the experiments; Qi Chen, Shuang Shuang and Xiaowei Huo performed the main experiments; Rui Dai and Yan Zhang analyzed all the data; Qi Chen and Rui Dai wrote the manuscript. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The ‘Caoyuan No.2’ and ‘Caoyuan No.4’ alfalfa were grown and collected from Inner Mongolia Agricultural University, China, and all samples from these cultivar was adopted for all experiment. No specific permits are required for sample collection in this study. For experimental research and field studies on plants, including the collection of plant material, we comply with relevant institutional, national, and international guidelines and legislation. We declared that wild plants have not been collected/used in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, Q., Dai, R., Shuang, S. et al. Genome-wide investigation of the TIFY transcription factors in alfalfa (Medicago sativa L.): identification, analysis, and expression. BMC Plant Biol 24, 840 (2024). https://doi.org/10.1186/s12870-024-05378-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-024-05378-w