Abstract
Background
The combination of compost and biochar (CB) plays an important role in soil restoration and mitigation strategies against drought stress in plants. In the current study, the impact of CB was determined on the characteristics of saline calcareous soil and the productivity of fenugreek (Trigonella foenum-graecum L.) plants. The field trials examined CB rates (CB0, CB10 and CB20 corresponding to 0, 10, and 20 t ha‒1, respectively) under deficit irrigation [DI0%, DI20%, and DI40% receiving 100, 80, and 60% crop evapotranspiration (ETc), respectively] conditions on growth, seed yield (SY), quality, and water productivity (WP) of fenugreek grown in saline calcareous soils.
Results
In general, DI negatively affected the morpho-physio-biochemical responses in plants cultivated in saline calcareous soils. However, amendments of CB10 or CB20 improved soil structure under DI conditions. This was evidenced by the decreased pH, electrical conductivity of soil extract (ECe), and bulk density but increased organic matter, macronutrient (N, P, and K) availability, water retention, and total porosity; thus, maintaining better water and nutritional status. These soil modifications improved chlorophyll, tissue water contents, cell membrane stability, photosystem II photochemical efficiency, photosynthetic performance, and nutritional homeostasis of drought-stressed plants. This was also supported by increased osmolytes, non-enzymatic, and enzymatic activities under DI conditions. Regardless of DI regimes, SY was significantly (P ≤ 0.05) improved by 40.0 and 102.5% when plants were treated with CB10 and CB20, respectively, as similarly observed for seed alkaloids (87.0, and 39.1%), trigonelline content (43.8, and 16.7%) and WP (40.9, and 104.5%) over unamended control plants.
Conclusions
Overall, the application of organic amendments of CB can be a promising sustainable solution for improving saline calcareous soil properties, mitigating the negative effects of DI stress, and enhancing crop productivity in arid and semi-arid agro-climates.
Similar content being viewed by others
Background
Fenugreek (Trigonella foenum-graecum L.; family Fabaceae) is an annual herb indigenous to North Africa and Asia, renowned for its medicinal properties [1]. During the winter season, farmers commonly sow fenugreek seeds alongside fodder crops like clover and barley to enhance the nutritional value of animal feed. Moreover, its tender green leaves and pods are esteemed as edible vegetables for human consumption [2]. Fenugreek seeds contain bioactive compounds such as alkaloids, flavonoids, steroids and saponins along with various secondary metabolites known for their therapeutic benefits, including alkaloidal trigonelline [3,4,5]. Trigonelline is recognized not only for its potential role as an osmoregulator and osmoprotectant under conditions of salt, drought, and oxidative stresses but also as an inducer of Nod genes during the interaction between Rhizobium and leguminous plants [6, 7].
Fenugreek encounters significant challenges throughout its life cycle when confronted with environmental stressors. Escalating temperatures and heat stress, attributed to climate change, pose a threat to fenugreek crops, resulting in reduced yield and compromised seed quality [8,9,10]. As water resources become scarce, fenugreek faces difficulties in sustaining optimal growth and productivity. Although fenugreek prefers slightly acidic to neutral soils, alkaline soils with high carbonate content can impede nutrient availability and hinder plant productivity [11,12,13]. Moreover, excessive salt levels in soils can restrict water uptake and nutrient transport within fenugreek plants [12].
The anticipated rise in demand for freshwater resources, driven by both shifting global climate patterns and rapid population growth underscores the urgency of addressing significant challenges surrounding freshwater scarcity [14]. Currently, inadequate management of irrigation water exacerbates these shortages, posing substantial obstacles to global food security [15]. To mitigate this issue and ensure food security, the development of water-saving techniques on a global scale is imperative [10, 16]. Deficit irrigation (DI) emerges as a promising strategy to enhance water productivity (WP) without incurring substantial yield loss [17]. As such, DI represents a pivotal cultivation approach for delivering water below full crop-water requirements (evapotranspiration), offering a crucial means of conserving irrigation water, whether applied during specific growth stages or throughout the entire growing season [18, 19].
Studies have consistently demonstrated that any restriction in irrigation water availability is likely to lead to diminished growth and yield of annual crops, including fenugreek [5, 20]. This decline can be attributed to inadequate leaching and high risk of soil salinity, both of which can detrimentally impact crop health and sustainability of irrigation practices [21]. Soils exhibiting an electrical conductivity of soil extract (ECe) exceeding 4 dS m−1 are typically indicative of elevated levels of dissolved salts, often termed saline soils [22]. Such soils tend to possess low organic matter (OM) content and elevated pH levels, resulting in compromised nutrient solubility and availability, particularly for phosphorus (P) [23,24,25]. Generally, soils in arid and semi-arid regions with low OM also exhibit reduced water-holding capacity (WHC) and crop productivity [26]. Saline calcareous soils, prevalent in arid regions, further exacerbate challenges related to soil fertility and nutrient uptake by plants [27].
Among the paramount strategies in conservation agriculture to alleviate the adverse impacts of abiotic stresses, such as heavy metals, drought, and salinity, soil organic amendments stand out [12, 28]. The seasonal incorporation of OM emerges as a prevalent method to mitigate its depletion in arid and semi-arid soils [29], thereby enhancing soil permeability and water retention capacity [28, 30]. Notably, OM amendment not only enhances the physiochemical and biological properties of saline calcareous soils but also furnishes a substantial portion of nutrients essential for improved growth and increased crop yields [10, 31].
Numerous endeavors have been undertaken to explore economical methods for water conservation [32]. For example, various studies have scrutinized the efficacy of OM inputs, such as compost and biochar individually [33,34,35,36]. Diacono and Montemurro [31] emphasize that compost represents the final stage of microbial decomposition of organic compounds, characterized by its rich OM and nutritional composition [36].
Compost is recognized for its ability to enrich soil properties, improve crop yields, and enhance WP [37]. Upon incorporation into the soil, microorganisms promptly initiate the decomposition of compost. In contrast, biochar, produced through pyrolysis in oxygen-deprived conditions, exhibits greater durability than compost [38]. The dense carbon (C) structure and aromatic composition of biochar render it more resistant to microbial degradation, thereby augmenting soil OM content [39]. Despite its relatively low nutrient concentration, biochar’s exceptional sorption capacity allows it to retain soil nutrients, mitigating leaching and enhancing water retention [40]. Moreover, the porous nature of biochar not only provides habitats for microorganisms but also fosters microbial activity, thereby bolstering nutrient cycling capabilities [40].
While compost and/or biochar have been investigated independently or in combination for their ability to mitigate various stressors, their combined efficacy as a mixture (CB) in alleviating water stress effects on fenugreek crop productivity in saline calcareous environments remains understudied. In the current study, we proposed that the application of a compost and biochar (CB) mixture would yield synergistic advantages, enhancing soil fertility and improving the growth and yield characteristics of fenugreek plants under DI conditions.
Accordingly, the objectives of this study were: (i) evaluate the impact of using CB as an organic amendment on the physio-chemical properties of soil; (ii) investigate how CB influences morpho-physiological traits, osmoprotectants, photosynthetic efficiency, and enzymatic and non-enzymatic antioxidants in fenugreek plants subjected to drought stress; and (iii) assess seed yield (SY), seed alkaloid and trigonelline content and WP of fenugreek under varying application rates of CB in saline calcareous soil, both with full and deficit irrigation. Overall, the findings of this study demonstrate that incorporating CB as a soil amendment can enhance soil quality and improve the yield of fenugreek plants, particularly under conditions of drought stress in arid and semi-arid regions.
Methods
Site description
Two trials were performed in the open field during the growing seasons of 2021–2022 and 2022–2023 in the experimental farm of the Faculty of Agriculture, Fayoum, Egypt (29°17’38” N 30°54’55” E). The climate in the local area is considered arid [41], and the soil is a sandy loam that is saline, calcareous, siliceous, and hyperthermic. It lies between (0.5 and 0.8 m deep) [42].
Basic soil characteristics
Soil pH was assessed in saturated soil-water paste using LI-120, Digital PH Meter (Elico, Sanathnagar, Hyderabad, Telangana, India), and ECe (dS m−1) was measured in saturated soil-water paste extract using CM25 conductivity meter (model 3200, YSI, Inc., Yellow Springs, Ohio, USA) according to Page et al. [43]. Total CaCO3 content was determined volumetrically using the Collin’s Calcimeter method, whereas OM content was measured with the wet combustion method [43].
Available N in the soil was measured using the technique of Stanford et al. [44]. Available P was extracted by 0.5 N of NaHCO3 solution at pH 8.5 as shown by Olsen et al. [45]. The ratio of soil: extract was 1:20, and the extraction time was 30 min of continuous shaking. After the extract had been filtered, an atomic absorption spectrophotometer was used to calculate the extracted P (Perkin-Elmer Model 3300, Glenbrook, Stamford, CT, USA) [46]. After shaking the soil sample with a 1 N C2H7NO2 solution for 30 min, the amount of available K was calculated using flame photometry Model 52-A (Perkin-Elmer) [43]. Bulk density (BD) was determined using the cylinder method [47].
Total porosity (TP) was calculated using particle density (γS) and the dry BD (γd) values by the following equation:
Water holding pores (WHP; 8.62 μm–0.19 μm) and useful pores (UP; < 0.19 μm) were determined by measuring both volumetric water content (θ) and matric potential or suction (ψm).
They were determined in the laboratory using a tension table and pressure plate. A flat porous surface was prepared at one end of each core sample to ensure hydraulic contact with the tension table. The samples were then placed on the saturated surface of the tension table, after which they were subjected to different suctions. The samples were weighed after the equilibrium at each successive suction [47]. Field capacity (θFc) was calculated using the tension table at a tension of 0.33 bar. Available water (AW) was estimated by the difference in water content between θFc and permanent wilting point (PWP) as follows:
The physiochemical characteristics of the tested soil were: ECe = 8.51 dS m−1, pH = 7.96, total N = 1.3 g kg−1, extractable P = 3.37 mg kg−1, extractable K = 39.52 mg kg−1, OM = 0.92%, CaCO3 = 16.52%, BD = 1.56 kg m−3, and soil moisture content at θFc and wilting point = 18.49% and 8.11%, respectively [48]. The meteorological data of the experimental site are shown in Table (S1).
Treatments and agronomic management
Randomized complete block design (RCBD) with the split plot arrangement was used in this experiment. Treatments were divided into three water applications and three compost-biochar mixture (CBM) rates. Irrigated water was applied as a percentage of crop evapotranspiration (ETc), representing three treatments: full irrigated FI (DI0%) = 100%, DI20% = 80% and DI40% = 60% of ETc, while CB mixtures were CB0 = 0 t ha−1 (control), CB10 = 10 t ha−1 (5 t ha−1 compost + 5 t ha−1 biochar) and CB20 = 20 t ha−1 (10 t ha−1 compost + 10 t ha−1 biochar).
Biochar was obtained by slow pyrolysis of wood of Mangifera indica in a biochar kiln at a temperature range of 350–450 °C. The used compost was prepared from 25 kg of Pelargonium graveolens waste material (25%), 0.5 kg of rice straw (0.5%) to provide some free air pores and maintain the aerobic conditions, 0.5 kg of K-humate (0.5%), and 12 kg of each cattle manure (24%) and green Egyptian clover plants (24%) as a N element source [49]. All ingredients were well blended and then composted in a pile measuring 25 × 2 × 1.6 m (length x width x height). The pile was turned over four times a month during the bio-oxidation stage and regularly sprinkled with water to maintain a 60% (v/w) wet level. The composting process continued from April 20 to July 20, up to the intermix maturation of all composted materials.
Irrigation treatments were set as the main plots, while CB treatments were randomly distributed in the sub-plots. CB was applied to the soil three weeks before planting fenugreek seeds. Table (S2) lists the characteristics of CB employed in the current experiments. Nine treatments were replicated three times, and the entire experimental plots were 27. The fenugreek seeds were acquired from the Department of Medicinal and Aromatic Plants of the Egyptian Ministry of Agriculture in Giza, Egypt. Seeds (120 kg ha−1) of fenugreek (cv. Giza 2) were manually planted on October 14, 2021 (Growing season 2021–2022) and October 17, 2022 (Growing season 2022–2023) in beds (15 m in length × one-meter width). Each of the 15 m2 bed areas contained four planting rows (20 cm apart) and 5–7 cm spacing between plants within rows.
In this experiment, irrigation with two dripper lines per row, one on each side, were placed about 0.5 m apart. The drippers along the lines were spaced at 1.7 m accordingly. During soil preparation, the recommended rate of nitrogen (N), P, and K was 50, 75, and 120 kg ha−1, respectively. N was added in the form of ammonium nitrate (33.5% N), P in the form of calcium super phosphate (15.5% P2O5), and K in the form of potassium sulfate (48% K2O). Appropriate agronomic management and pest control for the fenugreek crop was carried out following the recommendation of the Egyptian Agricultural Research Center, Giza, Egypt.
Irrigation water applied (IWA) and WP
The daily reference evapotranspiration (ETo) was calculated according to the technique of FAO Penman-Monteith equation [48]. As shown in the following equation, ETc was calculated using the ETo and the crop coefficient (Kc). The flow rate of the drip irrigation system was 3 L ha−1. The ETc (mm d−1) was estimated [48], as the following:
Where, Kc = crop coefficient. According to [50, 51], the stage-specific Kc values of fenugreek crop at the initial stage, mid-stage, and late-season stage were 0.69, 1.02, and 0.87, respectively. Epan = evaporation from the Class A pan (mm d−1) and Kpan = the pan evaporation coefficient.
The WP was computed using the formula [19] given below:
Measurements of plant growth and key physiological indices
The measured traits of the fenugreek plants were taken at the full blooming stage (90 days after sowing). These traits were plant height (PH; cm), root length (RL; cm), number of branches and leaves plant−1, and dry weight (DW; g plant−1).
The chlorophyll a fluorescence parameters of fenugreek plants were measured with a portable chlorophyll fluorometer (Handy-PEA, Hansatech, UK). For each treatment, the measurements were performed on fully expanded leaves of five plants in the morning (10:00-1100 AM) after dark adaptation for 20 min. Chlorophyll fluorescence was induced by applying a pulse of saturating red light (650 nm). This measurement yielded the values of the minimum fluorescence (F0), maximum fluorescence (Fm), while the maximal efficiency of PSIΙ photochemistry (Fv/Fm) and the potential activity of PSII (Fv/F0) were calculated according to Maxwell and Johnson [52]. The photosynthetic performance index (PI) was also determined as reported previously [53].
The relative water content (RWC%) and membrane stability index (MSI%) values were calculated following the methods given by [54, 55], respectively. A chlorophyll meter (SPAD-502, Minolta, Osaka, Japan) was used to determine the relative chlorophyll content (SPADchlorophyll).
Measurements of SY and yield-related attributes
Five plants were randomly harvested from each experimental plot on April 17 and 19 of the 2022 and 2023 seasons to determine the number of pods plant−1, SY plant−1 (g), and seed index (1000-seed weight; SI; g). All fenugreek plants in each sub-plot were manually harvested, sun-dried for two days, and then weighed along with the five fenugreek plants sampled before to estimate biological yield (BY; t ha−1), SY (t ha−1) based on 12% moisture, as well as seed harvest index (SHI) by dividing SY by BY in t ha−1.
Assays of oxidative stress indicators, osmoprotectants, and non-enzymatic antioxidants
To assay oxidative stress, hydrogen peroxide (H2O2; µmol mg−1 FW) was determined as previously described [56]. Malondialdehyde (MDA) was also tested in plant tissues [57] to determine the extent of lipid peroxidation. An attenuation coefficient of 155 mM−1 cm−1 was used to compute MDA concentration in µmol mg−1 FW. Total soluble sugars (TSS; mg g−1 DW) were extracted [58] and measured using a UV-160 A UV Visible Recording Spectrometer (Bausch and Lomb analytical systems divisions, Rochester, USA) at 625 nm. Free proline concentration (FProC; mg g−1 DW) was rapidly estimated at 520 nm using the colorimetric approach [59].
By using the methanolic solvent [60], total phenolics (TPhs; mg g−1 DW) were extracted from dried tissues, and the Folin–Ciocalteau phenol method [61] was used for phenolic determination. Soluble proteins were extracted using Moore’s method [62] and extraction yield (%) was determined [63]. The reduced glutathione (GSH) and ascorbic acid (AsA) contents in fresh leaf tissues of fenugreek were determined using the techniques previously outlined [64, 65].
Enzymatic antioxidants and 2,2-diphenyl-1- picrylhydrazyl (DPPH)-scavenging activity
Fresh leaf tissue (0.5 g) was used for superoxide dismutase (SOD), catalase (CAT), glutathione reductase (GR), and ascorbate peroxidase (APX) extraction. Samples were homogenized in 0.1 M ice-cold phosphate buffer (pH = 7.5) containing 0.5 mM EDTA with pre-chilled pestle and mortar. Each homogenate was transferred to centrifuge tubes and centrifuged at 4ºC in a Beckman refrigerated centrifuge for 15 min at 15,000 × g and the supernatant was used for the enzyme activity assay. The concentration of the extracted protein was determined [63]. The activity of SOD (EC 1.15.1.1) was assessed by recording the inhibition of cytochrome reduction in nitroblue tetrazolium at 540 nm [66]. CAT (EC 1.11.1.6) was determined by measuring the decomposition rate of H2O2 at 240 nm [67]. GR (EC 1.6.4.1) was determined by measuring the oxidation of NADPH at 340 nm; whereas ascorbate peroxidase (APX; EC 1.11.1.11) was assessed by monitoring the rate of ascorbate oxidation at 290 nm (E = 2.8 mM−1 cm−1) [68]. DPPH radical-scavenging activity (DPPH RSA) of all the extracts was investigated using DPPH free radical method [69].
Measurements of total alkaloid and trigonelline content
For trigonelline determination, one gram of powdered dried seeds of fenugreek was weighed and mixed with one gram of magnesium oxide (MgO) and 20 ml of distilled water. The mixture was incubated in a water bath at 100 °C for 20 min. After cooling, the mixture was filtered through Whatman paper number 1 (Cytiva, Buckinghamshire, United Kingdom), and its volume was brought to 25 ml with distilled water. The absorbance of the solutions was measured in UV-vis spectrophotometer apparatus at 268 nm. A standard curve was used to calculate the sample’s trigonelline content, which was represented as mg g−1 DW [70, 71].
The preparation of solution and extraction procedures were as recommended by [72]. Extracts were collected in a 10-ml volumetric flask and diluted with chloroform. The absorbance of the complex in chloroform was measured at 470 nm.
Determination of leaf mineral contents
The macro-elements (N, P, K+, Ca2+, and Na+) content of fenugreek leaves was determined by drying and grinding the leaves into a powder. The dried samples were subjected to a digestion process using a solution of HClO4 and H2SO4 (at 1:3 v/v, respectively). N content was assessed using micro-Kjeldahl equipment (Ningbo Medical Instruments Co., Ningbo, China) [73]. Molybdenum blue, diluted H2MoO7S, and 8% (w/v) NaHSO3-H2SO4 were used as standard reagents for quantifying P [74]. K+, Ca2+, and Na+ contents were measured using a Perkin-Elmer Model 52-A Flame Photometer [75].
2.11 Statistical analysis
Before the analysis of variance (ANOVA), Shapiro-Wilk normality and Bartlett homogeneity tests were used to explore if the dataset of each variable was normal and whether the error variances of both seasons were homogeneous. The outputs of these two tests, as pre-ANOVA assumptions, showed that all variables are statistically acceptable to perform ANOVA and Duncan multiple comparison tests (with a 5% confidence interval). A split-plot RCBD was used to base the combined analysis for the two experimental seasons ANOVA [76] and with three replicates using INFOSTAT computer software (v.2019 statistical package, Córdoba University, Córdoba, Argentina) [77].
Results
Soil hydro-physico-chemical properties in response to CB mixture
The main hydro-physico-chemical characteristics of soil were markedly (P ≤ 0.05) affected by the amendments of CB10 or CB20 (Table 1). Soil pH, ECe, and BD for each of the CB10- and CB20-amended soils were 3.0 and 4.8%, 20.4 and 28.2%, and 1.9 and 5.1%, respectively, significantly (P ≤ 0.05) lower than without CB amendment. However, CB10- and CB20-amended soils exhibited a progressive improvement in OM by 26.1 and 53.3%, soil N by 100.0 and 450.0%, P by 62.6 and 92.0%, and K by 12.7 and 44.7%, WHP by 35.8 and 59.3%, UP by 53.7 and 85.9%, TP by 15.0 and 22.1%, and soil water content at FC by 30.3 and 31.4% and available water (AW) by 43.3 and 48.8%, respectively, as compared to an unamended saline calcareous soil (Table 1).
Growth attributes and dry matter of fenugreek plants
Characteristics of shoot-root formation in fenugreek plants cultivated in salty calcareous soil were negatively affected by the reduction of soil moisture conditions. Drought stress at DI20% and DI40% levels significantly (P ≤ 0.05) decreased PH by 15.8 and 27.4%, number of branches plant−1 by 24.0 and 45.2% and number of leaves plant−1 by 37.2 and 56.0%, RL by 15.5 and 27.1%, and dry matter plant−1 by 32.5 and 54.5%, respectively, when compared to FI level (Table 2). Adding CB to saline calcareous soil at a rate of 10 or 20 t ha−1 pronouncedly (P ≤ 0.05) improved the PH by 34.9 or 77.8%, number of branches plant−1 by 46.2 or 88.0%, number of leaves plant−1 by 90.2 or 154.3%, RL by 11.9 or 23.3%, and dry matter plant−1 by 97.8 or 237.8%, respectively, compared to unamended (CB0) control fenugreek plants (Table 2).
The interactive effect of DI levels and CB rates showed considerable improvements in the number of branches plant−1, number of leaves plant−1, and dry matter plant−1 of fenugreek plants under saline calcareous soil conditions. The FI × CB20-treated fenugreek plants showed the maximum increases in number of branches plant−1 by 245.2%, number of leaves plant−1 by 393.5%, and dry matter plant−1 by 798.0%, compared to DI40% × CB0-treated plants displaying the minimum mean values of these characteristics (Table 2).
Cell integrity and leaf photosynthetic efficiency
DI strategies and CB application rates individually or in combinations (DI × CB) significantly (P ≤ 0.05) affected cell integrity and leaf photosynthetic efficiency of fenugreek plants in terms of RWC, MSI, SPADchlorophyll, PSIΙ photochemical efficiency (Fv/Fm) and PSII potential photochemical activity (Fv/F0) and PI (Table 3). Compared to FI fenugreek plants, drought stress at DI20% or DI40% markedly (P ≤ 0.05) decreased RWC by 16.8 or 31.2%, MSI by 6.5 or 20.4%, SPAD by 9.5 or 41.9%, Fv/Fm by 3.6 or 14.3%, Fv/F0 by 11.2 or 30.4%, and PI by 28.2 or 60.0%, respectively (Table 3).
Under saline calcareous soil conditions, the application rate of 10 or 20 t CB ha−1 significantly (P ≤ 0.05) improved all the traits mentioned above by 11.1 or 27.7%, 22.1 or 43.5%, 61.6 or 91.2%, 6.8 or 12.2%, 29.7 or 44.0%, and 58.3 or 123.3%, respectively, compared to CB0-treated plants (control) (Table 3). When the interaction of DI × CB was applied, the best results for cell integrity and leaf photosynthetic efficiency were obtained at FI × CB20 and DI20% × CB20 interactions, which significantly (P ≤ 0.05) improved MSI by 87.8 and 70.4%, SPAD by 248.1 and 218.0%, and Fv/F0 by 154.0 and 136.4%, respectively, compared to DI40% × CB0 interaction over the two growing seasons (Table 3).
Yield and yield-related attributes and WP
DI stress induced by DI20% and DI40% levels also negatively affected fenugreek yield and yield-related attributes but positively affected WP (Table 4). There were significant (P ≤ 0.05) decreases in the number of pods plant−1, SY plant−1, SI, SHI, BY, and SY by 26.4 or 50.5%, 36.8 or 58.8%, 20.7 or 33.0%, 10.3 or 18.7%, 1.9 or 13.7%, and 12.6 or 30.1%, respectively, in plants supplied with DI20% or DI40% compared to FI plants (Table 4). The WP; however, significantly (P ≤ 0.05) increased by 10.0 or 16.7%, respectively by the same DI treatments (Table 4).
Saline calcareous soil amended with CB at the rate of 10 t ha−1 significantly (P ≤ 0.05) increased number of pods plant−1 (117.5%), SY plant−1 (47.0%), SI (19.5%), SHI (14.9%), BY (20.9%), and SY (40.0%), and WP (40.9%) that was further enhanced by 185.3% of number of pods plant−1, 126.5% of SY plant−1, 53.4% of SI, 29.2% of SHI, 56.4% of BY, 102.5% of SY, and 104.5% of WP in plants treated with 20 t CB ha−1, compared to unamended control planted in the same soil (Table 4).
There was a significant (P ≤ 0.05) effect of the DI × CB interaction on fenugreek yield and yield-related attributes and WP under saline calcareous soil. For example, the highest number of pods plant−1, SY plant−1, SI, SHI, BY and SY were obtained under FI × CB20 interaction with 465.3%, 532.1%, 133.0%, 53.4%, 95.2%, and 200.0%, respectively, higher than in plants of DI40% × CB0 interaction (Table 4). Thus, this resulted in the lowest values for all these attributes across the two growing seasons when DI40% × CB0 interaction was applied. The greatest WP values, representing 0.97 and 0.89 kg seed m−3, were obtained under DI40% × CB20 and DI20% × CB20 interactions, respectively, with 142.5% and 122.5% higher than FI × CB0 interaction, which recorded the lowest WP value (0.40 kg seed m−3) across the two growing seasons (Table 4).
Oxidative stress indicators, osmoprotectants, and non-enzymatic antioxidants activity
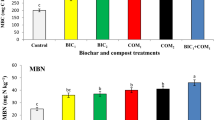
The current results elucidated that DI20% or DI40% treatments significantly (P ≤ 0.05) increased H2O2 by 5.1 or 9.9%, MDA by 19.1 or 46.8%, TSS by 43.8 or 71.9%, TPC by 17.2 or 36%, FProC by 15.8 or 24.2%, AsA by 101.2 or 30.9%, GSH by 234.8 or 147.8%, and TPhs by16.6 or 41.9%, respectively, compared to FI treatment (Table 5; Fig. 1). Compared to CB0 treatment, saline calcareous soil amended with 10 or 20 t CB ha−1 significantly (P ≤ 0.05) lowered H2O2 by 3.1% or 8.8% and MDA by 25.2 or 54.3%. However, it increased TSS by 25.8 or 103.2%, TPC by 10.6 or 22.6%, FProC by 7.2 or 15.3%, AsA by 31.9 or 52.7%, GSH by 64.7 or 97.1% and TPhs by 14.4 or 25.3%, respectively, compared to CB0 treatment (Table 5; Fig. 1).
Effect of the application of CB mixture along with different DI levels on leaf (A) AsA; (B) GSH; and (C) TPhs of fenugreek (Trigonella foenum-graecum L.) plants grown under saline calcareous soil conditions. Vertical bar indicates mean ± standard error based on three replicates and different letters for each DI, CB, or DI × CB levels indicate significant differences according to the Duncan test (P ≤ 0.05). CB, compost and biochar; DI, deficit irrigation; AsA, ascorbic acid; glutathione; GSH; TPhs, total phenols. FI, full irrigation (DI0%) control received 100% of crop evapotranspiration (ETc), and DI20% and DI40%, received 80% and 60% ETc, respectively; CB0, CB10 and CB20, CB mixture (1:1; w/w) at 0, 10 and 20 t ha−1, respectively
The DI × CB interaction also impacted oxidative stress indicators, osmoprotectants, and antioxidant activity of fenugreek plants raised in calcareous, saline soil over the two seasons. On average of the two seasons, the highest H2O2 (34.2 nmol g−1 FW) and MDA (2.89 µmol g−1 FW) levels in fenugreek leaves were recorded in the DI40% × CB0 treatment, where the lowest H2O2 (28.4 nmol g−1 FW) and MDA (0.82 µmol g−1 FW) levels were observed in FI × CB20 treatment (Table 5). Likewise, the highest levels of TSS, TPC, FProC, and TPhs were also noticeable when DI40% × CB20 treatment was applied (Table 5). However, leaves of fenugreek plants treated with DI20% × CB20 accumulated higher AsA and GSH levels than DI20% × CB0 or DI20% × CB10 treatment (Fig. 1).
Enzymatic antioxidants and DPPH RSA
In response to DI20% and DI40%, plants significantly (P ≤ 0.05) increased the activity of SOD by 23.8 and 42.9% (Fig. 2A), CAT by 20.2 and 34.0% (Fig. 2B), APX by 23.5 and 43.2% (Fig. 2C), GR by 44.8 and 81.0% (Fig. 2D), respectively, compared to FI treatment. Similarly, DPPH RSA was significantly (P ≤ 0.05) increased by 9.8% in DI20% and 12.1% in DI40% when compared to FI (Fig. 3A). Moreover, an increment was noticed in the activation of the aforementioned antioxidative enzymes and DPPH RSA when the saline calcareous soil was amended with 10 or 20 t CB ha−1 (Figs. 2 and 3A). In fenugreek plants treated with CB10, the activities of SOD, CAT, APX and GR were 11.3, 18.6, 14.0, and GR 13.3% greater than in CB0, respectively. The same enzymes were also higher by 14.1, 24.7, 24.5, and 14.7%, respectively, than plants treated with CB20. The antioxidant activity of DPPH RSA was increased by 8.7 and 13.3% in fenugreek plants treated with CB10 or CB20, respectively, compared to that in CB0.
Effect of the application of CB mixture along with different DI levels on enzymatic activity of (A) SOD; (B) CAT; (C) APX; and (D) GR of fenugreek (Trigonella foenum-graecum L.) plants grown under saline calcareous soil conditions. Vertical bar indicates mean ± standard error based on three replicates and different letters for each DI, CB, or DI × CB levels indicate significant differences according to the Duncan test (P ≤ 0.05). CB, compost and biochar; DI, deficit irrigation; SOD, superoxide dismutase; CAT, catalase; APX, ascorbate peroxidase; GR, glutathione reductase. FI, full irrigation (DI0%) control received 100% of crop evapotranspiration (ETc), and DI20% and DI40%, received 80% and 60% ETc, respectively; CB0, CB10 and CB20, CB mixture (1:1; w/w) at 0, 10 and 20 t ha−1, respectively
Effect of the application of CB mixture along with different DI levels on leaf (A) DPPH RSA; and seed (B) alkaloids and (C) trigonelline contents of fenugreek (Trigonella foenum-graecum L.) plants grown in saline calcareous soil. Vertical bar indicates mean ± standard error based on three replicates and different letters for each DI, CB, or DI × CB levels indicate significant differences according to the Duncan test (P ≤ 0.05). CB, compost and biochar; DPPH, 2,2-diphenyl-1- picrylhydrazyl; DPPH RSA, DPPH radical-scavenging activity; DI, deficit irrigation; DW, dry weight; FI, full irrigation (DI0%) control received 100% of crop evapotranspiration (ETc), and DI20% and DI40%, received 80% and 60% ETc, respectively; CB0, CB10 and CB20, CB mixture (1:1; w/w) at 0, 10 and 20 t ha−1, respectively
The DI × CB interaction significantly (P ≤ 0.05) increased the activity of antioxidant indicators of the fenugreek plant under saline calcareous soil conditions in both seasons. This was evidenced by the higher activities of SOD (66.1%; Fig. 2A), CAT (102.9%; Fig. 2B), APX (108.4%; Fig. 2C), GR (107.5%; Fig. 2D), and DPPH RSA (28.8%; Fig. 3A) in plants under DI40% × CB20 interaction than those obtained under FI × CB0 interaction.
Seed alkaloids and trigonelline content
Pooled data from the two years showed that the effects of DI strategy, CB rate, and their interaction on the total seed alkaloids and trigonelline contents were significant (P ≤ 0.05; Fig. 3B & C). Once increasing drought stress severity to DI20% and DI40% levels, the total seed alkaloids, and trigonelline content significantly (P ≤ 0.05) increased by 27.9 and 19.9%, and 12.5 and 50.0%, respectively, compared to those that do not suffer from drought stress. The total seed alkaloids and trigonelline contents in dry fenugreek seeds under CB20 treatment were 5.74 and 0.69 mg g DW−1, (approximately 87.0 and 43.8%, respectively) greater than that under unamended (CB0) treatment.
The DI × CB interaction significantly (P ≤ 0.05) affected the total seed alkaloids and trigonelline contents (Fig. 3B & C). Compared to FI (DI0%)-treated plants, stressed plants cultivated in saline calcareous soil at the rate of 20 t ha−1 CB elevated the total seed alkaloids content by 134.9% and 103.2% and trigonelline contents by 66.7% and 117.9% in response to moderate to severe drought stress conditions, respectively (Fig. 3B & C).
Leaf mineral content
Throughout the two growing seasons of fenugreek, the DI level, CB rate, and their interaction had substantial (P ≤ 0.05) impacts on the leaf mineral contents of fenugreek grown in the tested saline calcareous soil (Table 6). In general, plants subjected to DI20% or DI40% levels showed reduced nutrient uptake of N, P, K+ and Ca2+ as well as K+/Na+ ratio by 8.6 or 18.5%, 17.6 or 39.5%, 2.9 or 5.0%, 23.5 or 42.6% and 1.7 or 6.6%, respectively, compared to fully irrigated plants (Table 6). In contrast, more increment was noted by 2.2 to 5.9% in Na+ uptake in response to drought stress and the intensity increased from DI20% to DI40% compared to the non-stressed treatment (Table 6).
Compared to the unamended treatment, nutrients uptake of fenugreek plants grown in saline calcareous soil amended with CB10 or CB20 treatments were significantly (P ≤ 0.05) increased by 9.8 or 17.0% for N, 17.6 or 46.6% for P, 4.5 or 9.4% for K+, 24.4 or 63.4% for Ca2+, and 37.4 or 69.5% for K+/Na+ ratio; however, leaf Na+ content was reduced by 23.0 or 36.2% respectively (Table 6). Compared with DI40% × CB0 interaction, Na+ content was significantly (P ≤ 0.05) lower by 42.4%, but 95.7% higher in the K+/Na+ ratio in leaf tissues of fenugreek plants under FI × CB20 interaction, which was similar to that in plants treated with the combinations of DI20% × CB20 and DI40% × CB20 (Table 6).
Discussion
This study illustrated the positive impact of CB mixture as a soil amendment to improve soil physiochemical characteristics, soil-water interactions, and nutrient retention. This aligns with previous research indicating enhanced physical, chemical, and biological functions in soils following CB incorporation [35, 37, 78]. Consequently, properties, such as TP, WHC and BD, OM, and NPK content show improvement across various soil types with CB application. Our results underscore the potential of CB as a crucial component in water management aimed at boosting the growth, development, and productivity of fenugreek plants cultivated in saline calcareous soil under conditions of water scarcity.
The diminished productivity observed in non-CB-amended soil in this study could be due to the adverse effects induced by DI on various aspects of fenugreek growth and physiology. These effects include compromised root-shoot growth parameters such as PH, leaf number plant−1, branch number plant−1, root length, and plant dry matter, as well as reductions in leaf tissue water content (measured by MSI and RWC) and leaf photosynthetic efficiency (assessed by Fv/Fm, Fv/F0 and PI) under conditions of saline calcareous soil (Tables 2 and 3). Due to DI stress in saline calcareous soil, similar reductions in growth, yield, and related components of fenugreek (e.g., increased flower or pod abortion, reduced seed sets, fewer seeds pod−1, and diminished seed size) have been previously reported [8, 79,80,81,82]. These detrimental effects are likely attributable to the elevated soil ECe and pH levels, coupled low WHP, TP, and water retention capacity (Table 1). These soil conditions impede root proliferation, limit aeration, and hinder water and nutrient uptake by plant roots, ultimately leading to reduced productivity.
In addition, the decreased SHI of fenugreek under DI stress, indicating limited allocation of photo-assimilates to seeds, likely contributes to the reduction in SY [83]. However, the application of CB10 and CB20 to saline calcareous soil resulted in substantial increases in yield and its associated attributes of fenugreek plants compared to those in CB0-amended soil under full- or DI regimes.
The combined application of CB20 and DI20% effectively mitigated the adverse effects of water deficit on fenugreek growth, resulting in notable enhancement in PH, leaf count plant−1, branch count plant−1, pod count plant−1, SY plant−1, and BY with values closely resembling those observed in plants treated with FI × CB20. Compared to untreated soils (CB0), the incorporation of 10 or 20 t CB ha−1 improved fenugreek growth, SY, and related components. The observed growth enhancement may be linked to increased CB decomposition and soil nutrient mineralization [84]. In addition, CB application could enhance soil structure, nutrients supply, and humic acid provision, thereby enhancing soil’s capacity to retain both nutrients and water [12, 85], a phenomenon supported by our findings.
The enhanced SY observed following the application of CB10 and CB20 can be attributed to several factors. Firstly, the increase in soil OM and fertility facilitates greater availability of water and nutrients for plant uptake [12, 85]. Secondly, the application of CB induces significant modifications in soil physico-chemical properties, including enhanced soil structure, reduced soil Na+ content, increased root proliferation [10], and improved water and nutrient-uptake efficiency (Table 1), all contributing to enhanced seed production. Lastly, the decrease in soil ECe and pH resulting from CB application promotes the uptake of certain micronutrients and aids in the regulation of the soil solution ionic balance, further supporting SY enhancement [26, 86].
RWC and MSI have emerged as significant indicators of drought tolerance and cellular membrane integrity, reflecting the extent of oxidative stress. Similar to other reports [10, 87], MSI and RWC exhibited a decline in fenugreek plants subjected to DI stress (Table 3), a change likely stemming from reduced levels of endogenous abscisic acid, a key regulator of stomatal closure [88]. The preservation of water transport due to the turgidity of mesophyll cells and leaf tissue thickness might be another reason of decline [81]. However, CB application can ameliorate plant water status, including RWC and MSI, even in the presence of limited soil water moisture. This beneficial effect can be attributed to the capacity of compost and/or biochar to enhance soil water retention, thereby increasing AW content in plants [89, 90]. This observation aligns with findings by Abd El-Mageed et al. [12], who noted that the combined CB application augmented water content in plant tissues grown in salt-affected soil under soil water deficit.
CB serves as an effective carrier and source of essential nutrients, including N, P, K+ and Ca2+, enriching the soil solution and reducing rhizospheric leaching [91]. Its porous organic nature enables CB to enhance the RWC and MSI of fenugreek [92], thereby enhancing water retention capacity, overall aeration porosity, and nutrient bioavailability within the soil. Consequently, the incorporation of CB can lead to reduced irrigation water demands while simultaneously improving soil conditions for plant growth [10].
Plants employ different adaptations to cope with decreased photosynthetic activity. One strategy involves adjusting pigment composition, wherein plants may alter the ratio of chlorophyll a and b to optimize light absorption [93]. In response to environmental stress, plants often close stomata to minimize water loss, thereby limiting the availability of CO2 available for photosynthesis and subsequent dry matter accumulation [79]. Furthermore, plants activate their antioxidant defense system, producing antioxidants such as AsA and GSH, to alleviate oxidative damage induced by abiotic stress [94, 95].
Similar to lupine plants thriving in saline calcareous soil [10], drought imposition resulted in a significant decrease in the relative chlorophyll content (SPADchlorophyll) and photosynthetic efficiency (Fv/Fm, Fv/F0 and PI) in stressed fenugreek plants compared to their non-stressed counterparts under well-watered conditions. The diminished enzyme activity under drought stress leads to a reduction in chlorophyll production [79]. In addition, drought stress can instigate the disruption of chloroplast membrane integrity, consequently promoting the degradation or breakdown of chlorophyll molecules. This degradation contributes to the overall chlorophyll content in plant cells [27]. Therefore, the decline in chlorophyll content may be attributed to the generation of reactive oxygen species (ROS) and the increased activities of the chlorophyll-degrading enzymes [25, 29].
The decrease in the number of leaves plant−1 induced by drought stress significantly contributes to the reduction in crop yield by impeding the process of photosynthesis [20]. Drought-induced reduction in leaf area commonly occurs as a mechanism to mitigate water loss through canopy transpiration [96]. Our observations align with previous findings [10, 83], indicating lower plant water status, decreased photosynthetic pigments, reduced performance in photosynthetic parameters such as PI and Fv/Fm, and diminished leaf area under drought stress conditions. These factors collectively contribute to reductions in RL, shoot biomass (e.g., OM) and overall SY and its associated attributes.
Enhancing WP stands as a critical important strategy in addressing the global water scarcity challenge, focusing on maximizing crop yields per unit of water consumed. Particularly in irrigated agricultural settings, the emphasis on improving WP outweighs the priority of increasing yield potential per unit area for growers [97]. Our field experiments revealed that fenugreek plants subjected to severe drought stress (DI40%) increased WP (Table 3). These plants demonstrated resilience to water deficits by achieving significantly higher yields while utilizing less irrigation water, highlighting their potential in water-saving cultivation practices [79].
Fenugreek plants cultivated in saline calcareous soil, amended with either 10 or 20 t CB ha−1, exhibited significant increases in WP by 40.9%, and 104.5%, respectively, compared to non-CB-amended plants. Notably, the application of DI40% × CB20 demonstrated the highest WP, reaching 142.5%. Furthermore, DI20% × CB20 treatment conserved an additional 20% of water while enhancing WP to 122.5% compared to FI without CB, in agreement with findings from previous studies [89]. Obadi et al. [98] also observed enhanced WP in drought-stressed pepper plants supplemented with a CB mixture (2:2).
Drought stress typically leads to oxidative damage, evidenced by increased levels of MDA and accumulation of ROS, such as H2O2, in fenugreek leaves. These phenomena potentially contribute to membrane damage and lipid peroxidation in plant cells [80, 99], ultimately affecting fenugreek leaf water relations.
The application of CB resulted in an increase in the accumulation of osmolytes, such as TSS, TPC and FProC (Table 5), along with non-enzymatic antioxidants including AsA, GSH and TPhs (Fig. 1). This suggests that these physio-metabolic adaptive mechanisms could enhance tolerance to salinity and drought stresses [100, 101]. Previous studies have indicated that organic osmoprotectants present in CB-treated fenugreek plants are associated with the osmotic regulation, safeguarding cellular membrane integrity under severe DI conditions [12]. Essential ROS-scavenging mechanisms in plants involve enzymatic activities of SOD, CAT, APX and GR, and DPPH radical-scavenging activity (DPPH RSA). Hence, the regulation of enzymatic components within the antioxidant machinery is crucial for maintaining the “delicate” balance between the production and elimination of ROS and MDA levels in stressed fenugreek plants [102]. The observed enhanced growth and yield, associated with elevated GSH levels, could be attributed to the critical role of GSH in mitigating ROS-induced damage and enhancing tolerance in fenugreek and other plant species [79, 100, 103].
Trigonelline, a pyridine alkaloid compound present in fenugreek and other plant species [104], serves as an important osmoregulatory metabolite, playing a key role in regulating osmotic pressure induced by drought [96]. Studies have shown an elevation in trigonelline concentration in fenugreek and lupin seeds under salinity and drought conditions [10, 83, 105]. In our investigation, DI increased trigonelline levels, yet the application of CB not only reduced trigonelline and total alkaloid contents but also alleviated the adverse effects of DI stress on fenugreek plants. The high accumulation of secondary metabolites, such as trigonelline, in environmentally stressed fenugreek seeds likely serves to counteract excessive production of ROS and the resulting photoinhibition damage [106, 107]. Moreover, plants exposed to abiotic stresses accelerate nitrate accumulation and hinder protein biosynthesis in plant tissues [108], facilitating their incorporation into secondary metabolites, such as alkaloids [108, 109].
The reduction in nutritional status of N, P, K+, and Ca2+ in fenugreek plants exposed to DI in saline calcareous soil may be attributed to the constrained kinetics of nutrient uptake, closely linked to diminished soil moisture levels [110]. As documented previously [111], the accumulation of excessive Na+ ions in the cells of fenugreek plants grown in saline calcareous soil disrupts ionic balance and restricts the uptake of other essential nutrients such as N, P, K+, and Ca2+. However, the application of CB positively influences ionic equilibrium and enhances nutrient uptake under saline calcareous soil conditions. CB serves as an additional element source of OM, N, P, and K+, directly augmenting nutrient levels in the soil. In leaf tissues of DI-stressed fenugreek, the incorporation of 10 or 20 t, CB ha−1 increased the concentrations of N, P, K+, and Ca2+ as well as the K+/Na+ ratio while decreasing Na+ ions concentration (Table 6). Notably, N availability was significantly improved with the provision of irrigation and CB [112]. The decrease in Na+ ion concentration in fenugreek leaves can be attributed to the application of CB, acting as a biochar-containing soil amendment with a high affinity for adsorbing Na+ ions on its surface, thereby facilitating Na+ leaching from the plant rhizosphere and promoting the restoration of saline soil conditions [113].
Singh et al. [114] suggest that maintaining a high K+/Na+ ratio during drought and/or salinity stress may represent a plant’s adaptive response to uphold cytosolic cation balance, thereby preserving cellular osmotic pressure and turgor. In our investigation, the application of CB resulted in elevated levels of Ca2+ and K+ compared to Na+. Consequently, the K+/Na+ ratio substantially increased in fenugreek plant tissues under DI stress conditions. During environmental stresses, it is imperative to sustain the structural and functional integrity of plant membranes with adequate levels of K+ and Ca2+ [100, 115]. Similar observations regarding the restoration of ionic homeostasis and enhancement of nutrient profiles under drought stress through CB application have been reported in eggplant [12], fenugreek [34], and sainfoin [99].
It is worth mentioning that microbial processes play a crucial role in enhancing the availability and accessibility of essential nutrients crucial for sustaining plant health [116]. CB, as a C-rich amendment, has the potential to serve as a source of nutrients and habitat for soil microorganisms. This, in turn, can contribute to the stabilization of soil structure and the promotion of beneficial rhizospheric microorganisms, including N-fixing bacteria, thereby bolstering plant resilience to environmental stress [117, 118]. Future research avenues could explore the impact of CB application on the diversity of soil microbial communities, their ecological functions, soil enzyme activities, and the functional genes associated with improved crop yield and quality, all through the lens of soil microbial dynamics.
Conclusions
The present study indicated that the application of CB mixture to saline calcareous soils could have many benefits as a soil ameliorant, even under DI stress. Soil application of 10 or 20 t h−1 of CB led to improvements in soil physical (BD, WHP, UP, AW content and θFc), chemical (acidity, ECe, OM, N, and P contents) properties, and potentially beneficial to the rhizosphere microorganisms. These contributed to improvements in succulence (RWC and MSI), quantum efficiency of PSII (leaf greenness, chlorophyll a and PI) and nutritional homeostasis (high N, P, K+, Ca+2, and K+/Na+ ratio and lower Na+) in fenugreek leaves. This was also supported by the increase of osmolytes (TSS, TPC, and FProC), non-enzymatic (AsA, GSH, and TPhs), and enzymatic (SOD, CAT, APX, and GR) antioxidant activities, and DPPH RSA to scavenge ROS (H2O2 and MDA) under drought stress conditions. This suggests that these physio-metabolic adaptative mechanisms can improve stress tolerance in fenugreek. The addition of 20 t ha−1 CB mixture to saline calcareous soil under moderate water deficit (DI20%) could save up to 20% of the water applied yielding higher quality (trigonelline and total alkaloid contents) and WP. Thus, this could be commercially marketed for producing fenugreek crops in saline calcareous soil when irrigation water is limited. It is recommended to water at 80% ETc, combined with 20 t ha‒1 CB, in arid agricultural areas to optimize water use and maintain crop health.
Tables.
Data availability
All datasets generated for this study are included in the article/Supplementary Materials.
References
Yadav SR, Biyani DM, Umekar MJ. Trigonella foenum–graecum: a herbal plant review. World J Pharm Res. 2019;8:402–19.
Altuntas E, Ozgoz E, Taser OF. Some physical properties of fenugreek (Trigonella Foenum-Graceum L.) seeds. J Food Eng. 2005;71:37–43. https://doi.org/10.1016/j.jfoodeng.2004.10.015.
Snehlata HS, Payal DR. Fenugreek (Trigonella foenum-graecum L.): an overview. Int J Curr Pharm. 2012;2:169–87.
Zhou J, Chan L, Zhou S. Trigonelline: a plant alkaloid with therapeutic potential for diabetes and central nervous system disease. Curr Med Chem. 2012;19:3523–31. https://doi.org/10.2174/092986712801323171.
Abou-Sreea AI, Kamal M, El Sowfy DM, Rady MM, Mohamed GF, Al-Dhumri SA, Al-Harbi MS, Abdou NM. Small-sized nanophosphorus has a positive impact on the performance of fenugreek plants under soil-water deficit stress: a case study under field conditions. Biology. 2022;11:115. https://doi.org/10.3390/biology11010115.
Tramontano WA, Jouve D. Trigonelline accumulation in salt-stressed legumes and the role of other osmoregulators as cell cycle control agents. Phytochemistry. 1997;44:1037–40. https://doi.org/10.1016/S0031-9422(96)00715-7.
Shimizu M, Mazzafera P. A role for trigonelline during imbibition and germination of coffee seeds. Plant Biol. 2000;2:605–11. https://doi.org/10.1055/s-2000-16645.
Choudhary S, Bhat TM, Alwutayd KM, Abd El-Moneim D, Naaz N. Salicylic acid enhances thermotolerance and antioxidant defense in Trigonella Foenum Graecum L. under heat stress. Heliyon. 2024;10:e27227. https://doi.org/10.1016/j.heliyon.2024.e27227.
Al-Elwany OA, Hemida KA, Abdel-Razek MA, Abd El-Mageed TA, El-Saadony MT, AbuQamar SF, El-Tarabily KA, Taha RS. Impact of folic acid in modulating antioxidant activity, osmoprotectants, anatomical responses, and photosynthetic efficiency of Plectranthus amboinicus under salinity conditions. Front Plant Sci. 2022;13:887091. https://doi.org/10.3389/fpls.2022.887091.
Shaaban A, Al-Elwany OA, Abdou NM, Hemida KA, El-Sherif A, Abdel-Razek MA, Semida WM, Mohamed GF, Abd El-Mageed TA. Filter mud enhanced yield and soil properties of water-stressed Lupinus termis L. in saline calcareous soil. J Soil Sci Plant Nutr. 2023;22. https://doi.org/10.1007/s42729-021-00755-y.
Akula R, Ravishankar GA. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav. 2011;6:1720–31. https://doi.org/10.4161/psb.6.11.17613.
Abd El-Mageed TA, Shaaban A, Abd El-Mageed SA, Semida WM, Rady MO. Silicon defensive role in maize (Zea mays L.) against drought stress and metals-contaminated irrigation water. Silicon. 2020;13:2165–76. https://doi.org/10.1007/s12633-020-00690-0.
Abdelfattah MA, Rady MM, Belal HE, Belal EE, Al-Qthanin R, Al-Yasi HM, Ali EF. Revitalizing fertility of nutrient-deficient virgin sandy soil using leguminous biocompost boosts Phaseolus vulgaris performance. Plants. 2021;10:1637. https://doi.org/10.3390/plants10081637.
Manners R, Varela-Ortega C, Van Etten J. Protein-rich legume and pseudo-cereal crop suitability under present and future European climates. Eur J Agron. 2020;113:125974. https://doi.org/10.1016/j.eja.2019.125974.
Abdelhafez AA, Metwalley SM, Abbas HH. Irrigation: water resources, types and common problems in Egypt. In: Omran ES, Negm A, editors. Technological and modern irrigation environment in Egypt. Springer Water. Cham: Springer; 2020. p. 15–34. https://doi.org/10.1007/978-3-030-30375-4_2.
Kadiresan K, Khanal PR. Rethinking irrigation for global food security. Irrig Drain. 2018;67:8–11. https://doi.org/10.1002/ird.2219.
Al-Qthanin RN, AbdAlghafar I, Sabry D, Fikry AM, AlEnezi NA, Elesawi I, AbuQamar SF, Gad MM, El-Tarabily KA. Impact of rice straw mulching on water Consumption and productivity of orange trees [Citrus sinensis (L.) Osbeck]. Agric Water Manag. 2024;298:108862. https://doi.org/10.1016/j.agwat.2024.108862.
Fereres E, Soriano MA. Deficit irrigation for reducing agricultural water use. J Exp Bot. 2007;58:147–59. https://doi.org/10.1093/jxb/erl165.
Fernández J, Alcon F, Diaz-Espejo A, Hernandez-Santana V, Cuevas M. Water use indicators and economic analysis for on-farm irrigation decision: a case study of a super high density olive tree orchard. Agric Water Manag. 2020;237:106074. https://doi.org/10.1016/j.agwat.2020.106074.
Kaplan M, Kaymaz E, Varol IS, Ciftci B, Gokalp Z. Herbage yield and biochemical characterization of fenugreek (Trigonella foenum-graecum L.) under different irrigation levels. Ind Crops Prod. 2024;210:118071. https://doi.org/10.1016/j.indcrop.2024.118071.
Cucci G, Lacolla G, Boari F, Mastro MA, Cantore V. Effect of water salinity and irrigation regime on maize (Zea mays L.) cultivated on clay loam soil and irrigated by furrow in Southern Italy. Agric Water Manag. 2019;222:118–24. https://doi.org/10.1016/j.agwat.2019.05.033.
Richards LA. Diagnosis and improvement of saline and alkaline soils. Soil Sci. 1947;64:432.
Leytem A, Mikkelsen R. The nature of phosphorus in calcareous soils. Better Crops. 2005;89:11–3.
Semida WM, Abd El-Mageed TA, Hemida K, Rady MM. Natural bee-honey based biostimulants confer salt tolerance in onion via modulation of the antioxidant defense system. J Hortic Sci Biotechnol. 2019;94:632–42. https://doi.org/10.1080/14620316.2019.1592711.
Iqbal B, Kong F, Ullah I, Ali S, Li H, Wang J, Khattak W, Zhou A. Phosphorus application improves the cotton yield by enhancing reproductive organ biomass and nutrient accumulation in two cotton cultivars with different phosphorus sensitivity. Agronomy. 2020;10:153. https://doi.org/10.3390/agronomy10020153.
Yu OY, Harper M, Hoepfl M, Domermuth D. Characterization of biochar and its effects on the water holding capacity of loamy sand soil: comparison of hemlock biochar and switchblade grass biochar characteristics. Environ Prog Sustain. 2017;36:1474–9. https://doi.org/10.1002/ep.12592.
Taalab AS, Ageeb GW, Siam HS, Mahmoud SA. Some characteristics of calcareous soils. A review. Middle East J Agric Res. 2019;8:96–105.
El-Samnoudi IM, Ibrahim AE-AM, Abd El Tawwab AR, Abd El-Mageed TA. Combined effect of poultry manure and soil mulching on soil properties, physiological responses, yields and water-use efficiencies of sorghum plants under water stress. Commun Soil Sci Plant Anal. 2019;50:2626–39. https://doi.org/10.1080/00103624.2019.1671445.
Hu S, Ding Y, Zhu C. Sensitivity and responses of chloroplasts to heat stress in plants. Front Plant Sci. 2020;11:375. https://doi.org/10.3389/fpls.2020.00375.
Gangwar K, Singh K, Sharma S, Tomar O. Alternative tillage and crop residue management in wheat after rice in sandy loam soils of Indo-Gangetic plains. Soil Tillage Res. 2006;88:242–52. https://doi.org/10.1016/j.still.2005.06.015.
Diacono M, Montemurro F. Long-term effects of organic amendments on soil fertility. In: Lichtfouse E, Hamelin M, Navarrete M, Debaeke P, editors. Sustainable Agriculture Volume 2. Dordrecht: Springer; https://doi.org/10.1007/978-94-007-0394-0_34.
Badal E, Abd El-Mageed T, Buesa I, Guerra D, Bonet L, Intrigliolo DS. Moderate plant water stress reduces fruit drop of Rojo Brillante persimmon (Diospyros kaki) in a Mediterranean climate. Agric Water Manag. 2013;2013(119):154–60. https://doi.org/10.1016/j.agwat.2012.12.020.
Abd El-Mageed TA, El-Sherif AM, Abd El-Mageed SA, Abdou NM. A novel compost alleviate drought stress for sugar beet production grown in Cd-contaminated saline soil. Agric Water Manag. 2019;226:105831. https://doi.org/10.1016/j.agwat.2019.105831.
Bitarafan Z, Asghari HR, Hasanloo T, Gholami A, Moradi F, Khakimov B, Liu F, Andreasen C. The effect of charcoal on medicinal compounds of seeds of fenugreek (Trigonella foenum-graecum L.) exposed to drought stress. Ind Crops Prod. 2019;131:323–9. https://doi.org/10.1016/j.indcrop.2019.02.003.
Khan I, Iqbal B, Khan AA, Inamullah, Rehman A, Fayyaz A, Shakoor A, Farooq TH, Wang Lx. The interactive impact of straw mulch and biochar application positively enhanced the growth indexes of maize (Zea mays L.) Crop. Agronomy. 2022;12:2584. https://doi.org/10.3390/agronomy12102584.
Siedt M, Schäffer A, Smith KE, Nabel M, Roß-Nickoll M, Van Dongen JT. Comparing straw, compost, and biochar regarding their suitability as agricultural soil amendments to affect soil structure, nutrient leaching, microbial communities, and the fate of pesticides. Sci Total Environ. 2021;751:141607. https://doi.org/10.1016/j.scitotenv.2020.141607.
Abd El-Mageed TA, El-Samnoudi IM, Ibrahim AE-AM, Abd El Tawwab AR. Compost and mulching modulates morphological, physiological responses and water use efficiency in Sorghum bicolor L. Moench under low moisture regime. Agric Water Manag. 2018;208:431–9. https://doi.org/10.1016/j.agwat.2018.06.042.
Das M, Roychoudhury A. ROS and responses of antioxidant as ROS-scavengers during environmental stress in plants. Front Environ Sci. 2014;2:53. https://doi.org/10.3389/fenvs.2014.00053.
Ayaz M, Feizienė D, Tilvikienė V, Akhtar K, Stulpinaitė U, Iqbal R. Biochar role in the sustainability of agriculture and environment. Sustainability. 2021;13:1330. https://doi.org/10.3390/su13031330.
Rasse DP, Weldon S, Joner EJ, Joseph S, Kammann CI, Liu X, O’toole A, Pan G, Kocatürk-Schumacher NP. Enhancing plant N uptake with biochar-based fertilizers: limitation of sorption and prospects. Plant Soil. 2022;475:213–36. https://doi.org/10.1007/s11104-022-05365-w.
Ponce VM, Pandey RP, Ercan S. Characterization of drought across climatic spectrum. J Hydrol Eng. 2000;5:222–4.https://doi.org/10.1061/(ASCE)1084-0699(2000)5:2(222).
Soil Survey Staff. Soil Taxonomy: A basic system of soil classification for making and interpreting soil surveys. United States Department of Agriculture (USDA), Natural Resources Conservation Service. Second Edition, No. 436. Washington, DC: Agriculture Handbook, USDA; 1999.
Page AI, Miller RH, Keeny DR. Methods of soil analysis, Part 2: Chemical and microbiological properties. Madison: American Society of Agronomy, Madison Inc; 1982. p. 1159.
Stanford G, Carter JN, Westermann DT, Meisinger JJ. Residual nitrate and mineralizable soil nitrogen in relation to nitrogen uptake by irrigated sugar beets 1. Agron J. 1977;69:303–8. https://doi.org/10.2134/agronj1977.00021962006900020025x.
Olsen SR, Cole CV, Watanabe FS. Estimation of available phosphorus in soils by extraction with sodium bicarbonate. USDA Circular No. 939. Washington DC: US Government Printing Office; US Department of Agriculture; 1945.
Parnell JJ, Terry RE, Nelson Z. Soil chemical analysis applied as an interpretive tool for ancient human activities in Piedras Negras, Guatemala. J Archaeol Sci. 2002;29:379–404. https://doi.org/10.1006/jasc.2002.0735.
Klute A, Dirksen C. Hydraulic conductivity and diffusivity: laboratory methods. Methods of soil analysis: Part 1 physical and mineralogical. Methods. 1986;5:687–734. https://doi.org/10.2136/sssabookser5.1.
Allen RG, Pereira LS, Raes D, Smith M. Crop evapotranspiration - guidelines for computing crop water requirements - FAO Irrigation and drainage paper 56. Volume 300. Rome, Italy: FAO - Food and Agriculture Organization of the United Nations; 1998. p. D05109.
Idrovo-Novillo J, Gavilanes-Terán I, Angeles Bustamante M, Paredes C. Composting as a method to recycle renewable plant resources back to the ornamental plant industry: agronomic and economic assessment of composts. Process Saf Environ Prot. 2018;116:388–95. https://doi.org/10.1016/j.psep.2018.03.012.
Abd-Elghany G, El-Shazly MM, Hashem HAEA. Water management for fenugreek plant and its response to bio fertilization in North Sinai. Egypt J Appl Sci. 2017;32:494–515.
Kumar D, Rank HD. Comparison of fenugreek crop evapotranspiration measured by a micro-lysimeter, field water balance method and automatic closed canopy chamber. IJAEB. 2021;14:29–49. https://doi.org/10.30954/0974-1712.01.2021.5.
Maxwell K, Johnson G. N. Chlorophyll fluorescence—a practical guide. J Exp Bot. 2000;51:659–68. https://doi.org/10.1093/jxb/51.345.659.
Clark AJ, Landolt W, Bucher JB, Strasser RJ. Beech (Fagus sylvatica) response to ozone exposure assessed with a chlorophyll a fluorescence performance index. Environ Pollut. 2000;109:501–7. https://doi.org/10.1016/S0269-7491(00)00053-1.
Premachandra GS, SaneokaB H, Ogata S. Cell membrane stability, an indicator of drought tolerance, as affected by applied nitrogen in soybean. J Agric Sci. 1990;115:63–6. https://doi.org/10.1017/S0021859600073925.
Hayat S, Hayat Q, Alyemeni M, Wani A, Pichtel J, Ahmad A. Role of proline under changing environments: a review. Plant Signal Behav. 2012;7:1456–66. https://doi.org/10.4161/psb.21949.
Patterson BD, Macrae EA, Ferguson IB. Estimation of hydrogen peroxide in plant extracts using titanium (IV). Anal Biochem. 1984;139:487–92. https://doi.org/10.1016/0003-2697(84)90039-3.
Heath RL, Packer L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189–98. https://doi.org/10.1016/0003-9861(68)90654-1.
Irigoyen J, Einerich D, Sánchez-Díaz M. Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol Plant. 1992;84:55–60. https://doi.org/10.1111/j.1399-3054.1992.tb08764.x.
Bates LS, Waldren RP, Teare I. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–7. https://doi.org/10.1007/BF00018060.
Sauvesty A, Page F, Huot J. A simple method for extracting plant phenolic compounds. Can J Res. 1992;22:654–9. https://doi.org/10.1139/x92-087.
Meyers KJ, Watkins CB, Pritts MP, Liu RH. Antioxidant and antiproliferative activities of strawberries. J Agric Food Chem. 2003;51:6887–92. https://doi.org/10.1021/jf034506n.
Moore TC. Effects of certain synthetic plant growth regulators on the development of selected species. In: Research experiences in plant physiology. Berlin, Heidelberg: Springer; 1974. p. 307–23. https://doi.org/10.1007/978-3-642-96168-7_20.
Bradford N. A rapid and sensitive method for the quantitation microgram quantities of a protein isolated from red cell membranes. Anal Biochem. 1976;72:e254. https://doi.org/10.1006/abio.1976.9999.
Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980;106:207–12. https://doi.org/10.1016/0003-2697(80)90139-6.
Mukherjee S, Choudhuri M. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol Plant. 1983;58:166–70. https://doi.org/10.1111/j.1399-3054.1983.tb04162.x.
Zhao X, Xie H, Zhao X, Zhang J, Li Z, Yin W, Yuan A, Zhou H, Manan S, Nazar M, Iqbal B, Li G, Du D. Combined inhibitory effect of Canada goldenrod invasion and soil microplastics on rice growth. Int J Environ Res Public Health. 2022;19:11947. https://doi.org/10.3390/ijerph191911947.
Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–6. https://doi.org/10.1016/s0076-6879(84)05016-3.
Rao MV, Paliyath G, Ormrod DP. Ultraviolet-B-and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol. 1996;110:125–36. https://doi.org/10.1104/pp.110.1.125.
Brand-Williams W, Cuvelier M, Berset C. Antioxidative activity of phenolic composition of commercial extracts of sage and rosemary. LWT Food Sci Technol. 1995;28:25–30. https://doi.org/10.1016/S0023-6438(95)80008-5.
Martín MJ, Pablos F, Bello MA, González AG. Determination of trigonelline in green and roasted coffee from single column ionic chromatography. Z Anal Chem. 1997;357:357–8. https://doi.org/10.1007/s002160050169.
Mohamadi N, Pournamdari M, Sharififar F, Ansari M. Simultaneous spectrophotometric determination of trigonelline, diosgenin and nicotinic acid in dosage forms prepared from fenugreek seed extract. Iran J Pharm Res. 2020;19:153–9. https://doi.org/10.22037/ijpr.2019.1100790.
Shamsa F, Monsef H, Ghamooshi R, Verdian-Rizi M. Spectrophotometric determination of total alkaloids in some Iranian medicinal plants. Thai J Pharm Sci. 2008;32:17–20.
Jacobs MB. Micro-Kjeldahl method for biologicals. J Am Pharm Assoc. 1951;40:151-3. https://doi.org/10.1002/jps.3030400309.
Jackson M. Soil chemical analysis prentice. New Delhi: Hall of India Private Limited; 1967. p. 498.
Chapman HD, Pratt PF. Methods of analysis for soils, plants and waters. Soil Sci. 1962;93:68. https://doi.org/10.1097/00010694-196201000-00015.
Goos P, Vandebroek M. Outperforming completely randomized designs. J Qual Technol. 2004;36:12–26. https://doi.org/10.1080/00224065.2004.11980249.
Di Rienzo J, Casanoves F, Balzarini M, Gonzalez L, Tablada M, Robledo C. InfoStat Group. Facultad de Ciencias Agropecuarias: Universidad Nacional de Córdoba, Argentina; 2016.
Leogrande R, Vitti C, Castellini M, Garofalo P, Samarelli I, Lacolla G, Montesano FF, Spagnuolo M, Mastrangelo M, Stellacci AM. Residual effect of compost and biochar amendment on soil chemical, biological, and physical properties and durum wheat response. Agronomy. 2024;14:749. https://doi.org/10.3390/agronomy14040749.
Nyaupane S, Poudel MR, Panthi B, Dhakal A, Paudel H, Bhandari R. Drought stress effect, tolerance, and management in wheat – a review. Cogent Food Agric. 2024;10:2296094. https://doi.org/10.1080/23311932.2023.2296094.
Maleki M, Shojaeiyan A, Mokhtassi-Bidgoli A. Differential responses of two fenugreek (Trigonella foenum-graecum L.) landraces pretreated with melatonin to prolonged drought stress and subsequent recovery. BMC Plant Biol. 2024;24:161. https://doi.org/10.1186/s12870-024-04835-w.
Dehkordi RA, Roghani SR, Mafakheri S, Asghari B. Effect of biostimulants on morpho-physiological traits of various ecotypes of fenugreek (Trigonella foenum-graecum L.) under water deficit stress. Sci Hortic. 2021;283:110077. https://doi.org/10.1016/j.scienta.2021.110077.
Bhutia PH, Sharangi AB. Effect of dates of sowing and soil moisture level in different growth stages and yield dynamics of fenugreek (Trigonella foenum-graecum L). Natl Acad Sci Lett. 2016;39:77–80. https://doi.org/10.1007/s40009-016-0428-2.
Maleki M, Shojaeiyan A, Mokhtassi-Bidgoli A. Genotypic variation in biochemical and physiological responses of fenugreek (Trigonella foenum-graecum L.) landraces to prolonged drought stress and subsequent rewatering. Sci Hortic. 2021;287:110224. https://doi.org/10.1016/j.scienta.2021.110224.
Antonangelo JA, Sun X, Zhang H. The roles of co-composted biochar (COMBI) in improving soil quality, crop productivity, and toxic metal amelioration. J Environ Manage. 2021;277:111443. https://doi.org/10.1016/j.jenvman.2020.111443.
Teodoro M, Trakal L, Gallagher BN, Šimek P, Soudek P, Pohořelý M, Beesley L, Jačka L, Kovář M, Seyedsadr S, Mohan D. Application of co-composted biochar significantly improved plant-growth relevant physical/chemical properties of a metal contaminated soil. Chemosphere. 2020;242:125255. https://doi.org/10.1016/j.chemosphere.2019.125255.
Garau M, Pinna MV, Nieddu M, Castaldi P, Garau G. Mixing compost and biochar can enhance the chemical and biological recovery of soils contaminated by potentially toxic elements. Plants. 2024;13:284. https://doi.org/10.3390/plants13020284.
Ashok K, Sharma K. Leaf water content-a simple indicator of drought tolerance in crop plants. Indian J Agric Sci. 2010;80:1095–7.
Rajasheker G, Jawahar G, Jalaja N, Kumar SA, Kumari PH, Punita DL, Karumanchi AR, Reddy PS, Rathnagiri P, Sreenivasulu N, Bilhan P, Kishor K. Role and regulation of osmolytes and ABA interaction in salt and drought stress tolerance. In: Khan MIR, Reddy PS, Ferrante A, Khan NA, editors. Plant Signaling molecules. Editors: Woodhead Publishing, Duxford, UK. pp; 2019. p. 417–36. https://doi.org/10.1016/B978-0-12-816451-8.00026-5.
Zahra MB, Aftab ZEH, Haider MS. Water productivity, yield and agronomic attributes of maize crop in response to varied irrigation levels and biochar–compost application. J Sci Food Agric. 2021;101:4591–604. https://doi.org/10.1002/jsfa.11102.
Werdin J, Fletcher TD, Rayner JP, Williams NS, Farrell C. Biochar made from low density wood has greater plant available water than biochar made from high density wood. Sci Total Environ. 2020;705:135856. https://doi.org/10.1016/j.scitotenv.2019.135856.
Sg L, Jjo O, Adeleke R, St M. The potential of biochar to enhance concentration and utilization of selected macro and micronutrients for chickpea (Cicer arietinum) grown in three contrasting soils. Rhizosphere. 2021;17:100289. https://doi.org/10.1016/j.rhisph.2020.100289.
Figueiredo C, Lopes H, Coser T, Vale A, Busato J, Aguiar N, Novotny E, Canellas L. Influence of pyrolysis temperature on chemical and physical properties of biochar from sewage sludge. Arch Agron Soil Sci. 2018;64:881–9. https://doi.org/10.1080/03650340.2017.1407870.
Liu C, Wang Y, Pan K, Wang Q, Liang J, Jin Y, Tariq A. The synergistic responses of different photoprotective pathways in dwarf bamboo (Fargesia Rufa) to drought and subsequent rewatering. Front Plant Sci. 2017;8:489. https://doi.org/10.3389/fpls.2017.00489.
Tikhonov AN. Induction events and short-term regulation of electron transport in chloroplasts: an overview. Photosynth Res. 2015;125:65–94. https://doi.org/10.1007/s11120-015-0094-0.
Banakar MH, Amiri H, Ardakani MRS, Ranjbar GH. Susceptibility and tolerance of fenugreek (Trigonella Foenum-Graceum L.) to salt stress: physiological and biochemical inspections. Environ Exp Bot. 2022;194:104748. https://doi.org/10.1016/j.envexpbot.2021.104748.
Wang S, Hoch G, Grun G, Kahmen A. Water loss after stomatal closure: quantifying leaf minimum conductance and minimal water use in nine temperate European tree species during a severe drought. Tree Physiol. 2024;44:tpae027. https://doi.org/10.1093/treephys/tpae027.
Jahromi MN, Razzaghi F, Zand-Parsa S. Strategies to increase barley production and water use efficiency by combining deficit irrigation and nitrogen fertilizer. Irrig Sci. 2022;1–15. https://doi.org/10.1007/s00271-022-00811-0
Obadi A, Alharbi A, Abdel-Razzak H, Al-Omran A. Biochar and compost as soil amendments: effect on sweet pepper (Capsicum annuum L.) growth under partial root zone drying irrigation. Arab J Geosci. 2020;13:508. https://doi.org/10.1007/s12517-020-05529-x.
Roy R, Núñez-Delgado A, Wang J, Kader MA, Sarker T, Hasan AK, Dindaroglu T. Cattle manure compost and biochar supplementation improve growth of Onobrychis viciifolia in coal-mined spoils under water stress conditions. Environ Res. 2022;205:112440. https://doi.org/10.1016/j.envres.2021.112440.
Rehman H, Alharby HF, Bamagoos AA, Abdelhamid MT, Rady MM. Sequenced application of glutathione as an antioxidant with an organic biostimulant improves physiological and metabolic adaptation to salinity in wheat. Plant Physiol Biochem. 2021;158:43–52. https://doi.org/10.1016/j.plaphy.2020.11.041.
Roy R, Núñez-Delgado A, Sultana S, Wang J, Battaglia ML, Sarker T, Seleiman MF, Barmon M, Zhang R. Additions of optimum water, spent mushroom compost and wood biochar to improve the growth performance of Althaea rosea in drought-prone coal-mined spoils. J Environ Manage. 2021;295:113076. https://doi.org/10.1016/j.jenvman.2021.113076.
Chen Y-E, Cui J-M, Su Y-Q, Zhang C-M, Ma J, Zhang Z-W, Yuan M, Liu W-J, Zhang H-Y, Yuan S. Comparison of phosphorylation and assembly of photosystem complexes and redox homeostasis in two wheat cultivars with different drought resistance. Sci Rep. 2017;7:12718. https://doi.org/10.1038/s41598-017-13145-1.
Nguyen KH, Mostofa MG, Watanabe Y, Tran CD, Rahman MM, Tran L-SP. Overexpression of GmNAC085 enhances drought tolerance in Arabidopsis by regulating glutathione biosynthesis, redox balance and glutathione-dependent detoxification of reactive oxygen species and methylglyoxal. Environ Exp Bot. 2019;161:242–54. https://doi.org/10.1016/j.envexpbot.2018.12.021.
Ashihara H. Trigonelline (N-methylnicotinic acid) biosynthesis and its biological role in plants. Nat Prod Commun. 2008;3:1423–8. https://doi.org/10.1177/1934578X0800300906.
Tavangar A, Karami L, Hedayat M, Gholamreza A. Effect of salinity and drought stress on morphological and biochemical properties of two Iranian fenugreek (Trigonella foenum-graecum) populations. Not Bot Horti Agrobot. 2021;49:12038–12038. https://doi.org/10.15835/nbha49212038.
Afshar RK, Chaichi MR, Jovini MA, Jahanzad E, Hashemi M. Accumulation of silymarin in milk thistle seeds under drought stress. Planta. 2015;242:2265–9. https://doi.org/10.1007/s00425-015-2265-9.
Beygi Z, Nezamzadeh Z, Rabiei M, Mirakhorli N. Enhanced accumulation of trigonelline by elicitation and osmotic stresses in fenugreek callus culture. Plant Cell Tissue Organ Cult. 2021;147:169–74. https://doi.org/10.1007/s11240-021-02055-w.
Yahyazadeh M, Meinen R, Hänsch R, Abouzeid S, Selmar D. Impact of drought and salt stress on the biosynthesis of alkaloids in Chelidonium majus L. Phytochemistry. 2018;152:204–12. https://doi.org/10.1016/j.phytochem.2018.05.007.
Baričevič D, Zupančič A. The impact of drought stress and/or nitrogen fertilization in some medicinal plants. J Herbs Spices Med Plants. 2002;9:53–64. https://doi.org/10.1300/J044v09n02_08.
Bista DR, Heckathorn SA, Jayawardena DM, Mishra S, Boldt JK. Effects of drought on nutrient uptake and the levels of nutrient-uptake proteins in roots of drought-sensitive and-tolerant grasses. Plants. 2018;7:28. https://doi.org/10.3390/plants7020028.
Belmecheri-Cherifi H, Albacete A, Martínez-Andújar C, Pérez-Alfocea F, Abrous-Belbachir O. The growth impairment of salinized fenugreek (Trigonella foenum-graecum L.) plants is associated to changes in the hormonal balance. J Plant Physiol. 2019;232:311–9. https://doi.org/10.1016/j.jplph.2018.11.016.
Marshall J, Muhlack R, Morton BJ, Dunnigan L, Chittleborough D, Kwong CW. Pyrolysis temperature effects on biochar–water interactions and application for improved water holding capacity in vineyard soils. Soil Syst. 2019;3:27.
Yan S, Gao Y, Tian M, Tian Y, Li J. Comprehensive evaluation of effects of various carbon-rich amendments on tomato production under continuous saline water irrigation: overall soil quality, plant nutrient uptake, crop yields and fruit quality. Agric Water Manag. 2021;255: 106995. https://doi.org/10.1016/j.agwat.2021.106995.
Singh D, Yadav NS, Tiwari V, Agarwal PK, Jha B. A SNARE-like superfamily protein SbSLSP from the halophyte Salicornia brachiata confers salt and drought tolerance by maintaining membrane stability, K+/Na+ ratio, and antioxidant machinery. Front Plant Sci. 2016;7:737.
Zhu JK. Abiotic stress signaling and responses in plants. Cell. 2016;167:313–24. https://doi.org/10.1016/j.cell.2016.08.029.
Siebert J, Sünnemann M, Auge H, Berger S, Cesarz S, Ciobanu M, Guerrero-Ramírez NR, Eisenhauer N. The effects of drought and nutrient addition on soil organisms vary across taxonomic groups, but are constant across seasons. Sci Rep. 2019;9:1–12. https://doi.org/10.1038/s41598-018-36777-3.
Nadeem SM, Imran M, Naveed M, Khan MY, Ahmad M, Zahir ZA, Crowley DE. Synergistic use of biochar, compost and plant growth-promoting rhizobacteria for enhancing cucumber growth under water deficit conditions. J Sci Food Agric. 2017;97:5139–45. https://doi.org/10.1002/jsfa.8393.
Mithu MMH, Mia S, Suhi AA, Tahura S, Biswas P, Kader MA, Kassim S, Makino T. Biochar enriched compost elevates mungbean (Vigna radiata L.) yield under different salt stresses. Crop Pasture Sci. 2022;74:79–89. https://doi.org/10.1071/CP21653.
Acknowledgements
This research was funded by Khalifa Center for Biotechnology and Genetic Engineering-UAEU, grant number 12R028; Abu Dhabi Award for Research Excellence-Department of Education and Knowledge (Grant number: 21S105), and UAEU Program of Advanced Research (Grant number: 21S169).
Not applicable.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization: A.S., T.A.E.-M., S.AQ., K.E.-T., M.E.-S. and O.A.-E.; methodology, A.S., K.H., and T.A.E.-M.; validation: W.S., S.AQ., and K.E.-T.; formal analysis: A.S., K.H., T.A.E.-M., S.AQ., and K.E.-T.; investigation: A.S., W.S., and O.A.-E.; Resources: A.S., T.A.E.-M., S.AQ., K.E.-T., M.E.-S., and O.A.-E.; data curation: A.S., S.AQ., and K.E.-T.; preparation and writing original draft: A.S., K.H., S.AQ., and K.E.-T.; writing review and editing: A.S., K.H., T.A.E.-M., W.S., S.AQ., K.E.-T., M.E.-S., and O.A.-E.; supervision: A.S., S.AQ., K.E.-T., and O.A.-E.; project administration: S.AQ. and K.E.-T.; funding acquisition: S.AQ. and K.E.-T. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study does not involve any human or animal tissue materials. It does not require ethical approval. All methods were carried out in accordance with local and national regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shaaban, A., Hemida, K.A., Abd El-Mageed, T.A. et al. Incorporation of compost and biochar enhances yield and medicinal compounds in seeds of water-stressed Trigonella foenum-graecum L. plants cultivated in saline calcareous soils. BMC Plant Biol 24, 538 (2024). https://doi.org/10.1186/s12870-024-05182-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-024-05182-6