Abstract
Background
The homodomain-leucine zipper (HD-Zip) is a conserved transcription factor family unique to plants that regulate multiple developmental processes including lignificaion. Stone cell content is a key determinant negatively affecting pear fruit quality, which causes a grainy texture of fruit flesh, because of the lignified cell walls.
Results
In this study, a comprehensive bioinformatics analysis of HD-Zip genes in Chinese white pear (Pyrus bretschneideri) (PbHBs) was performed. Genome-wide identification of the PbHB gene family revealed 67 genes encoding PbHB proteins, which could be divided into four subgroups (I, II, III, and IV). For some members, similar intron/exon structural patterns support close evolutionary relationships within the same subgroup. The functions of each subgroup of the PbHB family were predicted through comparative analysis with the HB genes in Arabidopsis and other plants. Cis-element analysis indicated that PbHB genes might be involved in plant hormone signalling and external environmental responses, such as light, stress, and temperature. Furthermore, RNA-sequencing data and quantitative real-time PCR (RT-qPCR) verification revealed the regulatory roles of PbHB genes in pear stone cell formation. Further, co-expression network analysis revealed that the eight PbHB genes could be classified into different clusters of co-expression with lignin-related genes. Besides, the biological function of PbHB24 in promoting stone cell formation has been demonstrated by overexpression in fruitlets.
Conclusions
This study provided the comprehensive analysis of PbHBs and highlighted the importance of PbHB24 during stone cell development in pear fruits.
Similar content being viewed by others
Background
Transcription factors (TFs) are important regulators of gene transcription that bind to a specific nucleotide sequence upstream, which usually contains a DNA-binding domain, transactivation domain, oligomerization site, and nuclear localisation signal [1]. Genes encoding TFs are generally part of large multigene families, play an important role in controlling biological processes [2, 3]. Homeobox genes encode a homeodomain (HD) protein domain, which participates in a wide variety of developmental processes, and were first identified in Drosophila [4]. The first plant homeobox-containing gene KNOTTED1 was identified in maize (Zea mays) by transposon tagging [5]. Henceforth, several Homeobox-containing genes have been extensively investigated in a wide variety of plants.
In plants, homeobox family genes are classified into 14 subclasses based on the HD sequences and the characteristic codomains: homeodomain associated to a leucine zipper (HD-Zip) I to IV, BEL, Knotted related homeobox (KNOX), zinc finger associated to a homeodomain (ZF-HD), Wuschel related homeobox (WOX), plant homeodomain associated to a finger domain (PHD finger), DDT, NDX (Nodulin homeobox genes), Luminidependens (LD), AWADEE and PINTOX [6]. Members of the HD-Zip family have a leucine zipper motif (LZ) immediately following the HD protein domain [7]. The HD and LZ motifs are present in the TFs of species in other eukaryotic kingdoms; however, their combination in a single protein is unique to plants [8]. HD-Zips have been extensively investigated and functionally characterised in many species. In Arabidopsis thaliana, HD-Zip is involved in several biological processes such as organ and vascular development [9], secondary cell wall (SCW) development [10], hormone action mediation [11], meristem regulation [12], and responses to environmental conditions [13].
Forty-seven HD-Zips in Arabidopsis thaliana [7], 63 in Populus trichocarpa [14], 33 in grapes (Vitis vinifera) [15], 43 in potato [16] have been identified. Recently, HD-Zips in Rosaceae, including apple (Malus domestica) [17], peach (Prunus persica) [18], strawberry (Fragaria vesca) [19], and Prunus mume [20] have been identified and functionally characterised. However, the distribution of HD-Zips in Chinese white pear has not been fully identified and characterised.
Pears (Pyrus spp.), belonging to the rose family (Rosaceae), are one of the most important fruit trees worldwide, and are cultivated in all temperate-zone countries [21]. Pear fruit is commonly eaten fresh or canned as a nutritious food, even in dietary medicine for the prevention of PM2.5 and Covid-19 among people with multiple underlying diseases [22]. Stone cells are the key determinants reducing pear fruit quality, and have been identified as thick-walled tissue cells; their formation was attributed to the thickening of the SCWs with lignin and cellulose deposition and programmed cell death [23, 24]. HD-Zip family proteins have been reported to be involved in various growth and developmental processes, such as lignification and SCW formation [25, 26], flowering [27], fruit development [28], and reponse to phytohormone and environmental signals [29, 30]. Therefore, the well-assembled genome sequence for Chinese white pear allowed us to identify and analyse HD-Zip genes at the whole-genome level to predict their function in pear stone cell formation. In our previous study, bioinformatics was used to identify HD-Zip genes in Chinese white pear, and analyse the gene structure, chromosomal location, collinearity, conserved motifs, phylogenetic relationships, and cis-acting elements of promoters. Functional prediction through comparative analysis of the HD-Zip gene in Arabidopsis helped characterise genes from other species. This study lays a solid foundation for further investigation of the biological functions of HD-Zip TFs in pears.
Materials and methods
Plant materials
The pear fruit samples used in this study were harvested from an orchard in Gaoyou City, Jiangsu Province, China, at different fruit developmental stages in 2021. Pear trees under uniform cultivation without disease or insect infections were randomly selected. After harvesting, the pears were transported to the laboratory at Nanjing Agricultural University (Nanjing, China). The collected samples were cut by using a sharp blade, frozen in liquid nitrogen, and then stored at -80 °C for further analysis.
Identification of HD-Zip family members
The HD-Zip conserved domain homeodomain (PF00046) and homeobox associated leucine zipper domain (PF02183) model files were downloaded from the PFAM website (https://www.pfam.org) to obtain the Hidden Markov Model (HMM) seed file. HMMER v.3.2 software was used to search the pear protein database (http://peargenome.njau.edu.cn/) (E-value = e− 10) [31]. BLAST searches was performed using Arabidopsis HD-Zip genes as queries against the Chinese white pear genome databases. The redundant sequences were removed based on their identification numbers and chromosomal locations. The InterProScan program was used to confirm the presence of conserved HD-Zip domains and analysed the completeness of the HD-Zip gene domains using Pfam (http://pfam.janelia.org/) and SMART (http://smart.embl-heidelberg.de/).
Physical and chemical properties analysis of pear HD-Zip proteins
The physicochemical properties of pear HD-Zip proteins were predicted using the ExPASy website (https://www.expasy.org/). The transmembrane structures were predicted using the TMHMM Server software (version 2.0; http://www.cbs.dtu.dk/services/TMHMM/). The subcellular locations were predicted using WOLF PSORT (https://wolfpsort.hgc.jp/) and CELLO (http://cello.life.nctu.edu.tw/).
Multiple sequence alignments and phylogenetic relationship analysis
The amino acid sequences of HD-Zips of Chinese white pear and Arabidopsis were extracted and aligned with MAFFT v.7.4 software using default parameters [32, 33]. The HD-Zip proteins in pears were aligned and divided into different domains using the Jalview software v.2.10 [34]. ClustalX software was used to verify these results [35, 36]. The phylogenetic trees were constructed using IQ-TREE v.1.6 using the Maximum Likelihood model with 1000 bootstrap replicates. The phylogenetic treeS were visualised using the Figtree 1.4.3 software and iTOL website (https://itol.embl.de/).
Motif composition and gene structure analysis
The conserved motifs of the PbHB genes were analysed using Motif EM for motif elicitation (MEME) v.5.0 software based on the protein sequences [37]. The chromosome location of the PbHB genes was extracted from the gene annotation files. The coding and non-coding regions and pattern of exon-introns were characterised using the Gene Structure and Display Server (GSDS) software (version 2.0; http://gsds.cbi.pku.edu.cn/index.php). Motif composition and gene structure analyses were performed using TBtools software [38].
Gene collinearity relationships
The MCScanX (multiple collinearity scan) toolkit was used for collinearity analysis between multiple genomes and the pear genome, and homologous regions between pears and Arabidopsis were anchored. The relationships of gene collinearity were visualised using the TBtools software and the Python package circos (https://github.com/Tanghaibao/circos) [38].
Cis-element analysis
The 2,000 bp upstream region of each PbHB gene was extracted using Tbtools software and submitted to the PlantCARE database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) to predict cis elements [39, 40]. Sequence features of PbHBs were analysed as discribed [41, 42]. The obtained results are visualized using TBtools software [38].
Co-expression network analysis
The expression of genes related to lignin biosynthesis in pear fruits was used to explore the expression patterns of lignin biosynthetic genes and differentially expressed HD-Zips. The expression similarity between gene pairs was characterised using Pearson’s correlation coefficient (PCC) values. The values were then filtered using Excel software (the parameter was set to > 0.6). Visualisation of the data was performed using the Cytoscape software.
RT-qPCR analysis
RT-qPCR was performed to determine gene expression levels. The TRIzol kit provided by Tiangen Biotech (Beijing, China) Co., LTD was used to extract total RNAs from the collected plant materials, as instructed by the manufacturer. Single-stranded cDNA was synthesised using a cDNA Reverse Transcription Kit (Takara Bio, Shiga, Japan) according to the manufacturer’s instructions. RT-qPCR was conducted with the SYBR® Green Master (Roche, Basel, Switzerland) in a sequence detection system LightCycler 480 (Roche). The 2−ΔΔCT method was used to calculate the relative expression levels [43, 44].
Gene cloning, vector construction, and transient overexpression in pear fruit
The full-length of PbHB24 was cloned with primer pairs containing Xba I and BamH I restriction sites using high performance DNA polymerase KOD FX Neo (TOYOBO, Japan). The fusion construct 35 S:: PbHB24 was generated by inserting the gene fragment into the pCAMBIA1300 vector using the One-step Rapid Cloning Kit. Then, The plasmids of empty vector and 35S:: PbHB24 fusion constructs were transferred to GV3101 according to its illustration. Agrobacterium cells were injected into the flesh of ‘Dangshansuli’ fruits at 35 Days after full bloom (DAFB) using needleless syringes as discribed previously [45]. Twenty fruits were injected with each construct. Samples were collected 7 days after injection. Stone cell contents and acetyl bromide lignin contents of pear fruit were measured as described before [24, 45]. Stone cell and lignin measurements were replicated for at least six biological replicates.
Results
Identification, genome distribution, and collinearity of HD-Zip members in Chinese white pear
We searched the entire Chinese white pear genome for genes that encode proteins with the HD-Zip conserved domains. Ultimately, 56 PbHB genes were identified using Pfam and interProScan, after manually checked. Among these 56 PbHB genes, 48 genes were unevenly distributed on the chromosomes, and the other eight genes could be mapped to the unanchored scaffolds (Fig. 1, Table S1). The molecular weights (MW) of these PbHB genes ranged from 19505.22 to 114045.3 Da. The predicted isoelectric points range from 4.61 to 9.46. Gene subcellular localization prediction indicated that all the PbHB proteins were possibly located in the nucleus (Table S1).
Based on their position on the chromosomes, the HD-Zip genes in pears were denominated as PbHB1-PbHB56 (Table S1). As shown in Fig. 1, no PbHB gene was located on chromosome 4 or 16. Chromosomes 1 and 13 are short but contain three and five PbHB genes, respectively. A few PbHB genes were well-proportioned and mapped to one chromosome but were mostly distributed on certain fragments of chromosomes (Fig. 1). This may be attributed to uneven duplication events in pear chromosome fragments. The evolution of the PbHB family has been promoted by different gene duplication modes, including whole-genome duplication (WGD), tandem duplication (TD), transpose duplication (TRD), and dispersed duplication (DSD). All PbHB genes were assigned as WGD, TD, TRD, or DSD. Among the duplication events, DSD played a significant role in the evolution of PbHB (Table S2).
Multiple alignment, classification, and sequence features of PbHBs
Multiple alignment analysis was performed to investigate the homologous domain sequence features of the 56 HD-Zip proteins in pears (Fig. S1). The frequencies of the most widespread amino acids in the HD and LZ-domain were obtained (Fig. 2). The predicted HD and LZ-domain are highly conserved across plant lineages. The HD domain is responsible for DNA binding, and LZ is located immediately at the C-terminus of HD and is involved in protein-protein interactions. The results showed the basic regions of the HD and LZ domains in Chinese white pear.
HD- and LZ domains are highly conserved across all HD-Zip proteins in the pear genome. Sequence logos of the HD- and LZ-domain are based on the sequence alignments of 56 PbHB proteins. Bit score indicates the information content for each position in the sequence. Multiple alignment analysis of 56 PbHB proteins was performed with ClustalW (see Supplementary Fig. S1)
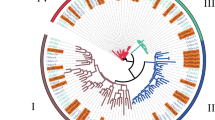
To further investigate the phylogenetic relationships between PbHB proteins, a phylogenetic tree was constructed, which revealed that the 56 PbHB proteins of Chinese white pear could be divided into four subfamilies:18, 18, 5, and 15 members, which were identified separately in subfamilies I, II, III, and IV, respectively (Fig. 3A). Overall, 10 motifs were detected in the PbHB proteins. In the same subfamily, the number and types of conserved motifs were similar, whereas those of PbHBs were diverse in different subfamilies. Similar intron/exon structural patterns were observed in the same type of genomic structures of the 56 PbHB genes, strongly supporting their close evolutionary relationship (Fig. 3B). The exon numbers of subfamilies III and IV were much higher than those of subfamilies I and II. All the PbHBs contained at least one exon, with a maximum of 21 exons (Table S3).
Phylogenetic relationships and gene structures of PbHBs. (A) Phylogenetic tree of 56 PbHB proteins. Phylogenetic tree was created in IQ-TREE v.1.6 using the Maximum Likelihood model with 1,000 bootstrap replicates. (B) Gene structure of PbHB family TFs in Chinese white pear. Abscissa represents the length of the nucleic acid sequence (bp)
Phylogenetic relationships of HD-Zip proteins in Rosaceae and Arabidopsis
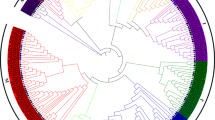
To determine the evolutionary relationships of the HD-Zip family in Rosaceae, a phylogenetic tree was constructed using the protein sequences of six Rosaceae species: 56 HD-Zip proteins from P. bretschneideri, 61 from Pyrus communis, 80 from Malus domestica, 33 from Prunus persica, 28 from Fragaria vesca, 42 from Prunus mume and 47 from Arabidopsis (Fig. S2, Table S4). They were divided into four subfamilies (I, II, III, and IV). Among the six Rosaceae species, P. persica and P. mume belong to Prunoideae, F. vesca belongs to Rosoideae, and P. bretschneideri, P. communis, and M. domestica belong to the Maloideae. A recent duplication event in Maloideae, but not in Prunoideae or Rosoideae, probably contributed to the expansion of the HD-Zip gene family in Maloideae (Fig. 3; Table 1). Notably, the HD-Zip genes of all subfamilies were duplicated in Maloideae except for the pear subfamily III. It is currently unknown whether this is the cause of stone cell occurrence in pear fruits.
Comparison analysis reveals the putative functions of pear HD-Zip TFs
Orthologous and paralogous analyses are indispensable in comparative genomics. Through evolutionary tree analysis, homologous genes clustered in the same branches and sub-branches often have similar functions [46]. To date, the most extensive quantitative evaluation of plant HD-Zip genes has been conducted in Arabidopsis. Therefore, a phylogenetic tree of P. bretschneideri and Arabidopsis was constructed to classify the functions of PbHBs based on well-characterised HD genes in Arabidopsis. The clusters of orthologous and paralogous genes was identified, which helped characterise each subfamily of the PbHB gene family (Fig. 4A). Proteins within a subfamily sharing the same motif are likely to exhibit similar biological functions. Motif composition analysis showed that HD-Zip genes were remarkably conserved between pears and Arabidopsis, indicating their similar functions (Fig. 4B). Subfamilies I and II were similar, containing only the HD and LZ-domain, whereas subgroups III and IV were significantly different, containing multiple other motifs (Fig. 4B). Subgroups with specific motifs may also have specific functions.
Phylogenetic analysis and motif compositions of HD-Zips from pear and Arabidopsis. (A) Phylogenetic tree of HD-Zip proteins from pear and Arabidopsis was constructed with IQ-TREE v.1.6 software. PbHBs were clustered into 4 distinct clades, marked by curves of different colours. (B) Schematic representation of the conserved motifs of the PbHBs identified by MEME. Each motif is indicated by a coloured box numbered at the bottom. Abscissa represents the amino acid length (C) Schematic representation of the distinctive domains exhibited by each subfamily of HD-Zip proteins. Abbreviations: MEKHLA domain, named after the highly conserved amino acids Met, Glu, Lys, His, Leu, Ala; N-term, N-terminus consensus; SAD, START adjacent domain; START, steroidogenic acute regulatory protein-related lipid transfer domain
There are few reports on the biological functions of HD-Zip proteins in other model plants, such as poplar, rice, and tomato. In this study, the biological functions of HD-Zip genes were characterised by homology comparison (Table 2). As shown in Table 2, HD-Zip I proteins are involved ABA response, fruit ripening, lignification, thermotolerance, blue-light signalling, salinity tolerance, and source-to-sink partitioning; HD-Zip II proteins are involved in shade avoidance, auxin response, illumination, ABA signalling, and gynoecium development; HD-Zip III proteins are involved in cambium and meristem formation, vascular development, SCW development, auxin biosynthesis, signalling, and transport, abscission, JA-isoleucine biosynthesis, brassinosteroid synthesis, and lignin biosynthesis; HD-Zip IV proteins are involved in lignification, specification of the epidermis, cell elongation, gibberellic acid signalling, floral organ identity, flowering phenotype, flowering time and fertility, drought tolerance, glandular trichome initiation and cuticle development, flag leaf development, and trichome elongation. The particular orthologous and paralogous proteins in pears were identified andtheir functions were characterised (Table 2). Results revealed that PbHB17, PbHB7, PbHB22, PbHB13, PbHB39 might be involved in SCW thickening and lignin formation because their homolog genes in Arabidopsis and other plants are known to play crucial roles in the regulation of SCW thickening and lignin formation; PbHB1, PbHB2, PbHB3, PbHB15, PbHB56, PbHB52, PbHB31, PbHB27, PbHB11, PbHB54, PbHB25, and PbHB39 might be involved in plant hormone signalling; and PbHB1, PbHB2, PbHB13, PbHB56, PbHB52, PbHB31, PbHB27 might be involved in stress response (Table 2). This result enhances our understanding of the putative roles of PbHB proteins.
Cis-regulatory element assessment analysis of PbHBs
To reveal the biological processes in which PbHB genes might be involved, the 2,000 bp upstream region of all PbHB genes was extracted to identify the cis-acting elements. After prediction using the PlantCARE website, some elements with unknown functions were eliminated, and 15 important elements were selected for further analysis. The distribution of the promoter elements in the four subfamilies is shown in Fig. 5. Overall, the number and distribution of the light-responsive components were the highest (Fig. 5A). All the PbHB genes contain light-responsive elements (Fig. 5B). Second, some hormone signals are also widely distributed in the PbHB promoters, such as MeJA responsiveness, abscisic acid responsiveness, and salicylic acid responsiveness, indicating that PbHB genes play a critical role in the response to plant hormones. Moreover, several stress-related elements were detected, including low-temperature responsiveness, defence, and stress responsiveness (Fig. 5A, B). These results indicate that PbHB genes may be involved in plant hormone signalling and external environmental responses such as light, temperature, and stress.
Cis-acting elements analysis of the promoter of PbHB genes. (A) Distribution of 15 cis-acting elements in the promoters of 56 PbHB genes. Abscissa represents the promoter sequence length. Scale bar indicates 300 bp. Different coloured boxes indicate different types of cis-acting elements. (B) Number of 15 cis-acting elements in promoters of 56 PbHB genes. The colour of the box indicates the number of cis-acting elements
Expression profiling of HD-Zips in pear fruits
Stone cell content increases rapidly during the early stages of pear fruit development and is discontinued after the middle stage of development (~ 55 days after flowering) [83]. Based on RNA-sequencing data, 40 HD-Zip genes were expressed during fruit development (Fig. 6, Table S5). Fourteen of these genes were highly expressed during the early stages of fruit development, indicating their putative roles in stone cell formation. It is worth noting that all HD-Zip genes of subfamily III were specifically highly expressed in the early stages, indicating their crucial role in stone cell development.
Heatmap indicates the expression pattern of HD-Zip genes in pear fruits. Gene expression of 40 PbHB genes was scaled according to the bar in colour that measures Z-scores of mean FPKM values. Five genes indicated in red means HD-Zip genes of subfamily III. Detailed information has listed in Table S5. Red represents up-regulated and green represents down-regulated. DAF, days after flowering
Co-expression network analysis of PbHB genes and lignin biosynthetic genes
Co-expression network analysis was performed to elucidate the relationship between PbHB genes and lignin biosynthetic genes. Eight PbHB genes were classified into different co-expression clusters with lignin-related genes, including PbHB27, PbHB49, PbHB43, PbHB22, PbHB10, PbHB39, PbHB7, and PbHB24 (Fig. 7; Table S6). These PbHBs were highly correlated with genes involved in lignin biosynthesis, which are highly expressed in the early stages of fruit development. Of these PbHB genes, PbHB24 showed the highest correlation, indicating that it is a putative regulator of stone cell development.
Co-expression network of PbHBs with lignin-related genes. RNA-seq data were used to measure the expression similarity between gene pairs as PCC values. Detailed information is listed in Table S6. Size and colour depth of each object represent degree values measured by CytoNCA [84]. The values were then filtered with Excel software (with the parameter set as > 0.6). Data were visualised using Cytoscape software
RT-qPCR analysis
The high conservation of the HD-Zip III genes throughout evolution indicates that they likely play a significant role in lignin biosynthesis and SCWs thickening. To reveal the potential role of HD-Zip III genes in pears, we monitored the expression of five HD-Zip III genes (PbHB7, PbHB10, PbHB22, PbHB24, and PbHB39) in different pear tissues and fruits of D. angshansuli at different developmental stages (Fig. 8). They were all highly expressed in the lignified tissues of pear trees, including the roots, stems, and early flesh. In the later stages of fruit development, their expression levels were extremely low, except for PbHB22. Stone cell formation occurs in the early stages of fruit development. A total of 188 genes involved in lignin biosynthesis were identified in the transcriptome data, which showed that they were highly expressed at two early periods of pear fruit development compared to other developmental periods (Table S6). Here, the expression trends of these HD-Zip genes in fruits correlated with stone cell development, indicating their regulatory role in stone cell formation.
Tissue-specific expression of pear HD-Zip III genes. Primer sequences are listed in Table S7. Bar values represent the mean ± SD of three biological replicates. Different coloured bars represent different genes
Overexpression PbHB24 promotes stone cell formation in pear fruits
To verify the function of PbHB24 in stone cell formation, the transient overexpression of PbHB24 was conducted in ‘Dangshansuli’ at 35DAFB. Increases in lignin staining were observed at the injection sites under the overexpression of PbHB24 7 days after injection (Fig. 9A). RT-qPCR showed that PbHB24 was successfully overexpressed at the 35 S::PbHB24 injection sites (Fig. 9B). The stone cell and lignin contents of overexpression sites were also increased compared with fruits injected with the control vector (Fig. 9C, D). Furthermore, the expression of multiple lignin biosynthetic genes were upregulated after PbHB24 overexpression (Fig. 9E). These data indicate the positive regulatory role of PbHB24 in stone cell formation in pear fruits.
Transient overexpression of PbHB24 in pear fruitlets. (A) Phloroglucinol staining of the pulp of ‘Dangshansuli’ fruitlets after Agrobacterium-mediated overexpression. 35 S::EV, fruitlets injected by empty vector as a control; 35 S::PbHB24, PbHB24 overexpression vector mediated by 35 S strong promoter. (B) Relative expression of PbHB24 7 days after injection. The error bars are the means ± SD (n = 3). (C) Stone cell content of pear fruits 7 days after injection. The error bars are the means ± SD (n = 12). (D) Acetyl bromide lignin content of pear fruits 7 days after injection. The error bars are the means ± SD (n = 6). The vertical bars are the means ± SD of biological replicates and asterisks indicate significant differences by two-tailed Student’s t-test (*P < 0.05, **P < 0.01)
Discussion
Plants produce lignin and thickened SCWs to equip plants with mechanical strength for erect growth and the capacity for long-distance conduction. The stone cells of pear fruits negatively affect fruit quality because of their lignified cell walls [24, 85]. Thus far, knowledge of the molecular mechanisms involved in stone cell formation is limited. The development of stone cells begins with differentiation of the lateral meristem and vascular cambium into secondary xylem mother cells, followed by cell expansion, secondary wall deposition, programmed cell death, and stone cell formation. Emerging evidence suggests that HD-Zip genes are involved in the vascular cambium, secondary wall deposition, and lignin biosynthesis (Table 2). This study was conducted through the identification, gene structure, and expression analysis of the HD-Zip family members to reveal their potential role in stone cell formation.
HD-Zip protein plays a wide range of roles in plant development, including vascular development, organ formation, meristem maintenance, stress response, and environmental and hormone signalling responses. Analysis of the promoter elements of the 56 PbHB genes revealed that environmental and plant hormone-responsive elements were most widely distributed in the promoters of pear HD-Zip genes. HD-Zip II TF HAHB10 participates in the induction of flowering and control of phytohormone-mediated responses to biotic stress in sunflowers [86]. The genes in HD-Zip II are mostly known for their functions in shade avoidance and light environment responses [87, 88]. PIF4 and the miR166-HB15 module modulate vascular development, SCW thickening, and consequently, stem-lodging susceptibility at elevated temperatures [26]. In loquat fruit, the EjbHLH14-EjHB1-EjPRX12 module is involved in the methyl jasmonate alleviation of chilling-induced lignin deposition [49]. These studies confirmed that HD-Zip genes might be crucial molecular hubs for connecting plant environmental/plant hormone signals with plant developmental signals.
In many plants, gene co-expression networks are increasingly being used to predict gene function and search for speculative targets of TFs [89]. Candidate genes involved in the regulation of anthocyanin and organic biosynthesis were prioritised based on co-expression analysis [90]. In grapes, the gene co-expression database VTCdb (http://vtcdb.adelaide.edu.au/Homethroat) offers an online platform for transcriptional regulatory inference [91]. Here, we predicted that HD-Zip III genes are critical regulators of lignification in stone cell formation, based on co-expression analysis. A previous study in Arabidopsis and the high conservation of HD-Zip III genes indicated that it likely plays a significant role in lignin biosynthesis and SCWs thickening. In Arabidopsis, miR165/166 and its downstream targets, five HD-Zip III genes, play conserved roles in vascular development and SCW formation in vascular plants [92]. HD-Zip III genes are characterised by an HD-Zip domain for DNA binding and protein dimerisation, and a highly conserved lipid or steroid-binding steroidogenic acute regulatory protein-related lipid transfer (START) domain. The two NAC master switches, SND1 and NST2, are upregulated in athb15 mutants, confirming that AtHB15 functions as a negative regulator of secondary wall-related regulatory pathways [10]. REV promotes lignin biosynthesis by binding to the promoter of the lignin biosynthesis gene PHENYLALANINE AMMONIA LYASE4 (PAL4) [69]. These studies demonstrate that HD-Zip III genes play a crucial role in lignification; however, knowledge of their specific molecular network is still limited.
During secondary growth, xylem and phloem are further expanded via the differentiation of cells derived from division in the cambium. Almost all developmental fate decisions, including vascular specification, patterning, and differentiation, are regulated by TFs belonging to the HD-Zip III family. TFs are important regulators of gene transcription that bind to specific upstream DNA sequences [1]. HD-Zip I genes are generally encoded by 35 kDa proteins and exhibit a highly conserved HD domain, a less conserved LZ domain, and no other similarities. PCR-assisted binding-site selection and footprinting assays revealed the ability of HD-Zip I proteins to recognise and bind to the pseudopalindromic sequence [CAAT(A/T)ATTG] [93]. The HD-Zip II and III binding sites share the same core sequence [AAT(G/C)ATT] [88, 94]. HD-Zip class IV TFs bind to the L1BX-like sequence, [TAAATG(C/T)A] [95]. These reports establish a solid foundation for studying HD gene regulation during lignification and SCWs thickening. Future studies should focus on identifying the molecular pathways through which the PbHB genes regulate lignin biosynthesis in stone cells.
Data availability
The datasets analyzed during the current study are included within the article and its supplementary information fIles. The RNA sequencing data is available in the NCBI BioProject database (https://www.ncbi.nlm.nih.gov/bioproject.) under the accession numbers PRJNA825067 and The Pear Expression Database (http://www.peardb.org.cn/).
Abbreviations
- HD-Zip:
-
Homodomain-leucine zipper
- RT-qPCR:
-
Quantitative real-time PCR
- TFs:
-
Transcription factors
- KNOX:
-
Knotted related homeobox
- ZF-HD:
-
Zinc finger associated to a homeodomain
- PHD:
-
Plant homeodomain associated to a finger domain
- LD:
-
Luminidependens
- LZ:
-
Leucine zipper motif
- SCW:
-
secondary cell wall
- MEME:
-
Motif EM for motif elicitation
- WGD:
-
Whole-genome duplication
- TD:
-
Tandem duplication
- TRD:
-
Transpose duplication
- DSD:
-
Dispersed duplication
References
Liu L, White MJ, MacRae TH. Transcription factors and their genes in higher plants functional domains, evolution and regulation. Eur J Biochem. 1999;262(2):247–57.
Yufei W, Ahmad N, Jiaxin C, Lili Y, Yuying H, Nan W, Min Z, Libo J, Na Y, Xiuming L. CtDREB52 transcription factor regulates UV-B-induced flavonoid biosynthesis by transactivating CtMYB and CtF3′H in Safflower (Carthamus tinctorius L). Plant Stress. 2024;11:100384.
Wang Y, Ge H, Ahmad N, Li J, Wang Y, Liu X, Liu W, Li X, Wang N, Wang F et al. Genome-wide identification of MADS-Box Family genes in Safflower (Carthamus tinctorius L.) and functional analysis of CtMADS24 during flowering. Int J Mol Sci 2023, 24(2).
Garber RL, Kuroiwa A, Gehring WJ. Genomic and cDNA clones of the homeotic locus Antennapedia in Drosophila. Embo j. 1983;2(11):2027–36.
Vollbrecht E, Veit B, Sinha N, Hake S. The developmental gene Knotted-1 is a member of a maize homeobox gene family. Nature. 1991;350(6315):241–3.
Mukherjee K, Brocchieri L, Bürglin TR. A comprehensive classification and evolutionary analysis of plant homeobox genes. Mol Biol Evol. 2009;26(12):2775–94.
Ariel FD, Manavella PA, Dezar CA, Chan RL. The true story of the HD-Zip family. Trends Plant Sci. 2007;12(9):419–26.
Schena M, Davis RW. HD-Zip proteins: members of an Arabidopsis homeodomain protein superfamily. Proc Natl Acad Sci USA. 1992;89(9):3894–8.
Prigge MJ, Clark SE. Evolution of the class III HD-Zip gene family in land plants. Evol Dev. 2006;8(4):350–61.
Du Q, Avci U, Li S, Gallego-Giraldo L, Pattathil S, Qi L, Hahn MG, Wang H. Activation of miR165b represses AtHB15 expression and induces pith secondary wall development in Arabidopsis. Plant J. 2015;83(3):388–400.
Nakamura M, Katsumata H, Abe M, Yabe N, Komeda Y, Yamamoto KT, Takahashi T. Characterization of the class IV homeodomain-leucine Zipper gene family in Arabidopsis. Plant Physiol. 2006;141(4):1363–75.
Ohashi-Ito K, Iwamoto K, Yamagami A, Nakano T, Fukuda H. HD-ZIP III-dependent local promotion of brassinosteroid synthesis suppresses vascular cell division in Arabidopsis root apical meristem. Proc Natl Acad Sci USA. 2023;120(15):e2216632120.
Henriksson E, Olsson AS, Johannesson H, Johansson H, Hanson J, Engström P, Söderman E. Homeodomain leucine zipper class I genes in Arabidopsis. Expression patterns and phylogenetic relationships. Plant Physiol. 2005;139(1):509–18.
Hu R, Chi X, Chai G, Kong Y, He G, Wang X, Shi D, Zhang D, Zhou G. Genome-wide identification, evolutionary expansion, and expression profile of homeodomain-leucine zipper gene family in poplar (Populus trichocarpa). PLoS ONE. 2012;7(2):e31149.
Li Z, Zhang C, Guo Y, Niu W, Wang Y, Xu Y. Evolution and expression analysis reveal the potential role of the HD-Zip gene family in regulation of embryo abortion in grapes (Vitis vinifera L). BMC Genom. 2017;18(1):744.
Li W, Dong J, Cao M, Gao X, Wang D, Liu B, Chen Q. Genome-wide identification and characterization of HD-ZIP genes in potato. Gene. 2019;697:103–17.
Liu K, Han X, Liang Z, Yan J, Cong P, Zhang C. Genome-wide identification, classification, and expression analysis of the HD-Zip transcription factor family in apple (Malus domestica Borkh). Int J Mol Sci 2022, 23(5).
Wang Z, Wu X, Zhang B, Xiao Y, Guo J, Liu J, Chen Q, Peng F. Genome-wide identification, bioinformatics and expression analysis of HD-Zip gene family in peach. BMC Plant Biol. 2023;23(1):122.
Wang Y, Fan J, Wu X, Guan L, Li C, Gu T, Li Y, Ding J. Genome-wide characterization and expression profiling of HD-Zip genes in ABA-mediated processes in Fragaria vesca. Plants 2022, 11(23).
Li L, Zheng T, Zhuo X, Li S, Qiu L, Wang J, Cheng T, Zhang Q. Genome-wide identification, characterization and expression analysis of the HD-Zip gene family in the stem development of the woody plant Prunus mume. PeerJ. 2019;7:e7499.
Hummer KE, Janick J. Rosaceae: Taxonomy, Economic Importance, Genomics. In: Genetics and Genomics of Rosaceae Edited by Folta KM, Gardiner SE. New York, NY: Springer New York; 2009: 1–17.
Hong SY, Lansky E, Kang SS, Yang M. A review of pears (Pyrus spp.), ancient functional food for modern times. BMC Complement Med Ther. 2021;21(1):219.
Smith WW, THE COURSE OF STONE CELL FORMATION IN PEAR FRUITS. Plant Physiol. 1935;10(4):587–611.
Tao S, Khanizadeh S, Zhang H, Zhang S. Anatomy, ultrastructure and lignin distribution of stone cells in two Pyrus species. Plant Sci. 2009;176(3):413–9.
Prigge MJ, Otsuga D, Alonso JM, Ecker JR, Drews GN, Clark SE. Class III homeodomain-leucine zipper gene family members have overlapping, antagonistic, and distinct roles in Arabidopsis development. Plant Cell. 2005;17(1):61–76.
Wei H, Song Z, Xie Y, Cheng H, Yan H, Sun F, Liu H, Shen J, Li L, He X et al. High temperature inhibits vascular development via the PIF4-miR166-HB15 module in Arabidopsis. Curr Biol 2023.
Cai Y, Bartholomew ES, Dong M, Zhai X, Yin S, Zhang Y, Feng Z, Wu L, Liu W, Shan N, et al. The HD-ZIP IV transcription factor GL2-LIKE regulates male flowering time and fertility in cucumber. J Exp Bot. 2020;71(18):5425–37.
Li F, Fu M, Zhou S, Xie Q, Chen G, Chen X, Hu Z. A tomato HD-zip I transcription factor, VAHOX1, acts as a negative regulator of fruit ripening. Hortic Res. 2023;10(1):uhac236.
Ahmad S, Chen Y, Shah AZ, Wang H, Xi C, Zhu H, Ge L. The homeodomain-leucine Zipper genes Family regulates the Jinggangmycin Mediated Immune Response of Oryza sativa to Nilaparvata lugens, and Laodelphax striatellus. Bioeng (Basel Switzerland) 2022, 9(8).
Sharif R, Raza A, Chen P, Li Y, El-Ballat EM, Rauf A, Hano C, El-Esawi MA. HD-ZIP Gene Family: potential roles in improving Plant Growth and regulating stress-responsive mechanisms in plants. Genes 2021, 12(8).
He W, Liu H, Li Y, Wu Z, Xie Y, Yan X, Wang X, Miao Q, Chen T, Rahman S-, et al. Genome-wide characterization of B-box gene family in Artemisia annua L. and its potential role in the regulation of artemisinin biosynthesis. Ind Crops Prod. 2023;199:116736.
Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30(4):772–80.
Hou Y, Wang Y, Liu X, Ahmad N, Wang N, Jin L, Yao N, Liu X. A cinnamate 4-HYDROXYLASE1 from Safflower promotes flavonoids Accumulation and stimulates antioxidant Defense System in Arabidopsis. Int J Mol Sci 2023, 24(6).
Clamp M, Cuff J, Searle SM, Barton GJ. The Jalview Java alignment editor. Bioinf (Oxford England). 2004;20(3):426–7.
Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22(22):4673–80.
Sun J, Zhang X, Fu C, Ahmad N, Zhao C, Hou L, Naeem M, Pan J, Wang X, Zhao S. Genome-wide identification and expression analysis of GA20ox and GA3ox genes during pod development in peanut. PeerJ. 2023;11:e16279.
Bailey TL, Williams N, Misleh C, Li WW. MEME: discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res 2006, 34(Web Server issue):W369–373.
Chen C, Chen H, Zhang Y, Thomas HR, Frank MH, He Y, Xia R. TBtools: an integrative Toolkit developed for interactive analyses of big Biological Data. Mol Plant. 2020;13(8):1194–202.
Rombauts S, Déhais P, Van Montagu M, Rouzé P. PlantCARE, a plant cis-acting regulatory element database. Nucleic Acids Res. 1999;27(1):295–6.
Yingqi H, Ahmad N, Yuanyuan T, Jianyu L, Liyan W, Gang W, Xiuming L, Yuanyuan D, Fawei W, Weican L et al. Genome-wide identification, expression analysis, and subcellular localization of Carthamus tinctorius bHLH transcription factors. Int J Mol Sci 2019, 20(12).
Li H, Li L, ShangGuan G, Jia C, Deng S, Noman M, Liu Y, Guo Y, Han L, Zhang X, et al. Genome-wide identification and expression analysis of bZIP gene family in Carthamus tinctorius L. Sci Rep. 2020;10(1):15521.
Hong Y, Ahmad N, Zhang J, Lv Y, Zhang X, Ma X, Xiuming L, Na Y. Genome-wide analysis and transcriptional reprogrammings of MYB superfamily revealed positive insights into abiotic stress responses and anthocyanin accumulation in Carthamus tinctorius L. Mol Genet Genomics. 2022;297(1):125–45.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 – ∆∆CT method. Methods. 2001;25(4):402–8.
Wang Y, Li Z, Ahmad N, Sheng X, Iqbal B, Naeem M, Wang N, Li F, Yao N, Liu X. Unraveling the functional characterization of a jasmonate-induced flavonoid biosynthetic CYP45082G24 gene in Carthamus tinctorius. Funct Integr Genom. 2023;23(2):172.
Wang Q, Gong X, Xie Z, Qi K, Yuan K, Jiao Y, Pan Q, Zhang S, Shiratake K, Tao S. Cryptochrome-mediated blue-light signal contributes to lignin biosynthesis in stone cells in pear fruit. Plant Science: Int J Experimental Plant Biology. 2022;318:111211.
Li L, Stoeckert CJ Jr., Roos DS. OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Res. 2003;13(9):2178–89.
Olsson A, Engström P, Söderman E. The homeobox genes ATHB12 and ATHB7encode potential regulators of growth in response to water deficit in Arabidopsis. Plant Mol Biol. 2004;55(5):663–77.
Himmelbach A, Hoffmann T, Leube M, Höhener B, Grill E. Homeodomain protein ATHB6 is a target of the protein phosphatase ABI1 and regulates hormone responses in Arabidopsis. Embo j. 2002;21(12):3029–38.
Zhang M, Shi Y, Liu Z, Zhang Y, Yin X, Liang Z, Huang Y, Grierson D, Chen K. An EjbHLH14-EjHB1-EjPRX12 module is involved in methyl jasmonate alleviation of chilling-induced lignin deposition in loquat fruit. J Exp Bot. 2022;73(5):1668–82.
Wu Z, Li T, Zhang D, Teng N. Lily HD-Zip I transcription factor LlHB16 promotes thermotolerance by activating LlHSFA2 and LlMBF1c. Plant Cell Physiol. 2022;63(11):1729–44.
Wang Y, Henriksson E, Söderman E, Henriksson KN, Sundberg E, Engström P. The Arabidopsis homeobox gene, ATHB16, regulates leaf development and the sensitivity to photoperiod in Arabidopsis. Dev Biol. 2003;264(1):228–39.
Zhao S, Wang H, Jia X, Gao H, Mao K, Ma F. The HD-Zip I transcription factor MdHB7-like confers tolerance to salinity in transgenic apple (Malus domestica). Physiol Plant. 2021;172(3):1452–64.
Raminger BL, Miguel VN, Zapata C, Chan RL, Cabello JV. Source-to-sink partitioning is altered by changes in the expression of the transcription factor AtHB5 in Arabidopsis. J Exp Bot. 2023;74(6):1873–89.
Carabelli M, Morelli G, Whitelam G, Ruberti I. Twilight-Zone and canopy shade induction of the Athb-2 homeobox gene in green plants. Proc Natl Acad Sci USA. 1996;93(8):3530–5.
Sawa S, Ohgishi M, Goda H, Higuchi K, Shimada Y, Yoshida S, Koshiba T. The HAT2 gene, a member of the HD-Zip gene family, isolated as an auxin inducible gene by DNA microarray screening, affects auxin response in Arabidopsis. Plant J. 2002;32(6):1011–22.
Ciarbelli AR, Ciolfi A, Salvucci S, Ruzza V, Possenti M, Carabelli M, Fruscalzo A, Sessa G, Morelli G, Ruberti I. The Arabidopsis homeodomain-leucine zipper II gene family: diversity and redundancy. Plant Mol Biol. 2008;68(4–5):465–78.
Baek W, Bae Y, Lim CW, Lee SC. Pepper homeobox abscisic acid signalling-related transcription factor 1, CaHAT1, plays a positive role in drought response. Plant Cell Environ. 2023;46(7):2061–77.
Carabelli M, Turchi L, Ruzza V, Morelli G, Ruberti I. Homeodomain-Leucine Zipper II family of transcription factors to the limelight: central regulators of plant development. Plant Signal Behav 2013, 8(9).
Zúñiga-Mayo VM, Marsch-Martínez N, de Folter S. JAIBA, a class-II HD-ZIP transcription factor involved in the regulation of meristematic activity, and important for correct gynoecium and fruit development in Arabidopsis. Plant J. 2012;71(2):314–26.
Zhu Y, Song D, Xu P, Sun J, Li L. A HD-ZIP III gene, PtrHB4, is required for interfascicular cambium development in Populus. Plant Biotechnol J. 2018;16(3):808–17.
Zhu Y, Song D, Sun J, Wang X, Li L. PtrHB7, a class III HD-Zip gene, plays a critical role in regulation of vascular cambium differentiation in Populus. Mol Plant. 2013;6(4):1331–43.
Xu C, Shen Y, He F, Fu X, Yu H, Lu W, Li Y, Li C, Fan D, Wang HC, et al. Auxin-mediated Aux/IAA-ARF-HB signaling cascade regulates secondary xylem development in Populus. New Phytol. 2019;222(2):752–67.
Müller CJ, Valdés AE, Wang G, Ramachandran P, Beste L, Uddenberg D, Carlsbecker A. PHABULOSA mediates an auxin signaling loop to regulate vascular patterning in Arabidopsis. Plant Physiol. 2016;170(2):956–70.
Huang T, Harrar Y, Lin C, Reinhart B, Newell NR, Talavera-Rauh F, Hokin SA, Barton MK, Kerstetter RA. Arabidopsis KANADI1 acts as a transcriptional repressor by interacting with a specific cis-element and regulates auxin biosynthesis, transport, and signaling in opposition to HD-ZIPIII factors. Plant Cell. 2014;26(1):246–62.
Liu X, Cheng L, Li R, Cai Y, Wang X, Fu X, Dong X, Qi M, Jiang CZ, Xu T, et al. The HD-Zip transcription factor SlHB15A regulates abscission by modulating jasmonoyl-isoleucine biosynthesis. Plant Physiol. 2022;189(4):2396–412.
Bang SW, Lee DK, Jung H, Chung PJ, Kim YS, Choi YD, Suh JW, Kim JK. Overexpression of OsTF1L, a rice HD-Zip transcription factor, promotes lignin biosynthesis and stomatal closure that improves drought tolerance. Plant Biotechnol J. 2019;17(1):118–31.
Baima S, Possenti M, Matteucci A, Wisman E, Altamura MM, Ruberti I, Morelli G. The arabidopsis ATHB-8 HD-zip protein acts as a differentiation-promoting transcription factor of the vascular meristems. Plant Physiol. 2001;126(2):643–55.
Kim J, Jung JH, Reyes JL, Kim YS, Kim SY, Chung KS, Kim JA, Lee M, Lee Y, Narry Kim V, et al. microRNA-directed cleavage of ATHB15 mRNA regulates vascular development in Arabidopsis inflorescence stems. Plant J. 2005;42(1):84–94.
Taylor-Teeples M, Lin L, de Lucas M, Turco G, Toal TW, Gaudinier A, Young NF, Trabucco GM, Veling MT, Lamothe R, et al. An Arabidopsis gene regulatory network for secondary cell wall synthesis. Nature. 2015;517(7536):571–5.
Otsuga D, DeGuzman B, Prigge MJ, Drews GN, Clark SE. REVOLUTA regulates meristem initiation at lateral positions. Plant J. 2001;25(2):223–36.
Robischon M, Du J, Miura E, Groover A. The Populus class III HD ZIP, popREVOLUTA, influences cambium initiation and patterning of woody stems. Plant Physiol. 2011;155(3):1214–25.
Carlsbecker A, Lee JY, Roberts CJ, Dettmer J, Lehesranta S, Zhou J, Lindgren O, Moreno-Risueno MA, Vatén A, Thitamadee S, et al. Cell signalling by microRNA165/6 directs gene dose-dependent root cell fate. Nature. 2010;465(7296):316–21.
Sun J, Cui X, Teng S, Kunnong Z, Wang Y, Chen Z, Sun X, Wu J, Ai P, Quick WP, et al. HD-ZIP IV gene Roc8 regulates the size of bulliform cells and lignin content in rice. Plant Biotechnol J. 2020;18(12):2559–72.
Rombolá-Caldentey B, Rueda-Romero P, Iglesias-Fernández R, Carbonero P, Oñate-Sánchez L. Arabidopsis DELLA and two HD-ZIP transcription factors regulate GA signaling in the epidermis through the L1 box cis-element. Plant Cell. 2014;26(7):2905–19.
Meyer HM, Teles J, Formosa-Jordan P, Refahi Y, San-Bento R, Ingram G, Jönsson H, Locke JC, Roeder AH. Fluctuations of the transcription factor ATML1 generate the pattern of giant cells in the Arabidopsis sepal. eLife 2017, 6.
Kamata N, Okada H, Komeda Y, Takahashi T. Mutations in epidermis-specific HD-ZIP IV genes affect floral organ identity in Arabidopsis thaliana. Plant J. 2013;75(3):430–40.
Abe M, Katsumata H, Komeda Y, Takahashi T. Regulation of shoot epidermal cell differentiation by a pair of homeodomain proteins in Arabidopsis. Development. 2003;130(4):635–43.
Koornneef M, Hanhart CJ, van der Veen JH. A genetic and physiological analysis of late flowering mutants in Arabidopsis thaliana. Mol Gen Genet. 1991;229(1):57–66.
Yu H, Chen X, Hong YY, Wang Y, Xu P, Ke SD, Liu HY, Zhu JK, Oliver DJ, Xiang CB. Activated expression of an Arabidopsis HD-START protein confers drought tolerance with improved root system and reduced stomatal density. Plant Cell. 2008;20(4):1134–51.
Ueda M, Aichinger E, Gong W, Groot E, Verstraeten I, Vu LD, De Smet I, Higashiyama T, Umeda M, Laux T. Transcriptional integration of paternal and maternal factors in the Arabidopsis zygote. Gene Dev. 2017;31(6):617–27.
Zhang X, Wang Y, Zhu X, Wang X, Zhu Z, Li Y, Xie J, Xiong Y, Yang Z, He G, et al. Curled flag leaf 2, encoding a cytochrome P450 protein, regulated by the transcription factor Roc5,influences flag leaf development in rice. Front Plant Sci. 2020;11:616977.
Hua B, Chang J, Xu Z, Han X, Xu M, Yang M, Yang C, Ye Z, Wu S. HOMEODOMAIN PROTEIN8 mediates jasmonate-triggered trichome elongation in tomato. New Phytol. 2021;230(3):1063–77.
Cai Y, Li G, Nie J, Lin Y, Nie F, Zhang J, Xu Y. Study of the structure and biosynthetic pathway of lignin in stone cells of pear. Sci Hortic. 2010;125(3):374–9.
Tang Y, Li M, Wang J, Pan Y, Wu FX. CytoNCA: a cytoscape plugin for centrality analysis and evaluation of protein interaction networks. Bio Syst. 2015;127:67–72.
Wang L, Jing M, Ahmad N, Wang Y, Wang Y, Li J, Li X, Liu W, Wang N, Wang F et al. Tracing key molecular regulators of lipid biosynthesis in Tuber Development of Cyperus esculentus using transcriptomics and Lipidomics Profiling. Genes 2021, 12(10).
Dezar CA, Giacomelli JI, Manavella PA, Ré DA, Alves-Ferreira M, Baldwin IT, Bonaventure G, Chan RL. HAHB10, a sunflower HD-Zip II transcription factor, participates in the induction of flowering and in the control of phytohormone-mediated responses to biotic stress. J Exp Bot. 2011;62(3):1061–76.
Reymond MC, Brunoud G, Chauvet A, Martínez-Garcia JF, Martin-Magniette ML, Monéger F, Scutt CP. A light-regulated genetic module was recruited to carpel development in Arabidopsis following a structural change to SPATULA. Plant Cell. 2012;24(7):2812–25.
Turchi L, Baima S, Morelli G, Ruberti I. Interplay of HD-Zip II and III transcription factors in auxin-regulated plant development. J Exp Bot. 2015;66(16):5043–53.
Lee T, Kim H, Lee I. Network-assisted crop systems genetics: network inference and integrative analysis. Curr Opin Plant Biol. 2015;24:61–70.
Sweetman C, Sadras VO, Hancock RD, Soole KL, Ford CM. Metabolic effects of elevated temperature on organic acid degradation in ripening Vitis vinifera fruit. J Exp Bot. 2014;65(20):5975–88.
Wong DC, Sweetman C, Drew DP, Ford CM. VTCdb: a gene co-expression database for the crop species Vitis vinifera (grapevine). BMC Genom. 2013;14:882.
Du Q, Wang H. The role of HD-ZIP III transcription factors and miR165/166 in vascular development and secondary cell wall formation. Plant Signal Behav. 2015;10(10):e1078955.
Chan RL, Gago GM, Palena CM, Gonzalez DH. Homeoboxes in plant development. Biochim Biophys Acta. 1998;1442(1):1–19.
Sessa G, Morelli G, Ruberti I. The Athb-1 and – 2 HD-Zip domains homodimerize forming complexes of different DNA binding specificities. Embo j. 1993;12(9):3507–17.
Abe M, Takahashi T, Komeda Y. Identification of a cis-regulatory element for L1 layer-specific gene expression, which is targeted by an L1-specific homeodomain protein. Plant J. 2001;26(5):487–94.
Funding
This work was supported by National Natural Science Foundation of China (31972361 and U2003121), also partially by the Grant-in-Aids for Scientific Research (KAKENHI: 21H02184, 21K19111, 20K20372) from the Japan Society for the Promotion of Science (JSPS), and Achievement Transformation Fund Project of Sanya Institute of Nanjing Agricultural University (NAUSY-CG-YB06).
Author information
Authors and Affiliations
Contributions
QW and KS conceived and designed the study. QW and YYW performed the experiments. KQ and ZX prepared stuffs for the research. FZ and CH conducted the co-expression analysis. LYW and MR performed gene expression analysis. SZ provided the methodology and resources. QW wrote the manuscript. KS and ST revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
We declare that we have provided consent to publish this article and have provided ethical approval for this submission. The manuscript constitutes an original research work and has never been submitted elsewhere, either completely, in part, or in another form for publication. Our research did not involve any human or animal subjects, material, or data. The plant materials used in this study were collected in an orchard in a gardening field of Baoying County, Gaoyou City, Jiangsu Province and conserved by Nanjing agricultural university.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, Q., Wang, Y., Zhang, F. et al. Genome-wide characterisation of HD-Zip transcription factors and functional analysis of PbHB24 during stone cell formation in Chinese white pear (Pyrus bretschneideri). BMC Plant Biol 24, 444 (2024). https://doi.org/10.1186/s12870-024-05138-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-024-05138-w