Abstract
Background
Spot blotch is a serious foliar disease of barley (Hordeum vulgare L.) plants caused by Bipolaris sorokiniana, which is a hemibiotrophic ascomycete that has a global impact on productivity. Some Trichoderma spp. is a promising candidate as a biocontrol agent as well as a plant growth stimulant. Also, the application of nanomaterials in agriculture limits the use of harmful agrochemicals and helps improve the yield of different crops. The current study was carried out to evaluate the effectiveness of Trichoderma. cf. asperellum and the biosynthesized titanium dioxide nanoparticles (TiO2 NPs) to manage the spot blotch disease of barley caused by B. sorokiniana and to assess the plant’s innate defense response.
Results
Aloe vera L. aqueous leaf extract was used to biosynthesize TiO2 NPs by reducing TiCl4 salt into TiO2 NPs and the biosynthesized NPs were detected using SEM and TEM. It was confirmed that the NPs are anatase-crystalline phases and exist in sizes ranging from 10 to 25 nm. The T. cf. asperellum fungus was detected using morphological traits and rDNA ITS analysis. This fungus showed strong antagonistic activity against B. sorokiniana (57.07%). Additionally, T. cf. asperellum cultures that were 5 days old demonstrated the best antagonistic activity against the pathogen in cell-free culture filtrate. Also, B. sorokiniana was unable to grow on PDA supplemented with 25 and 50 mg/L of TiO2 NPs, and the diameter of the inhibitory zone increased with increasing TiO2 NPs concentration. In an in vivo assay, barley plants treated with T. cf. asperellum or TiO2 NPs were used to evaluate their biocontrol efficiency against B. sorokiniana, in which T. cf. asperellum and TiO2 NPs enhanced the growth of the plant without displaying disease symptoms. Furthermore, the physiological and biochemical parameters of barley plants treated with T. cf. asperellum or TiO2 NPs in response to B. sorokiniana treatment were quantitively estimated. Hence, T. cf. asperellum and TiO2 NPs improve the plant’s tolerance and reduce the growth inhibitory effect of B. sorokiniana.
Conclusion
Subsequently, T. cf. asperellum and TiO2 NPs were able to protect barley plants against B. sorokiniana via enhancement of chlorophyll content, improvement of plant health, and induction of the barley innate defense system. The present work emphasizes the major contribution of T. cf. asperellum and the biosynthesized TiO2 NPs to the management of spot blotch disease in barley plants, and ultimately to the enhancement of barley plant quality and productivity.
Key points
• T. cf. asperellum showed strong antagonistic activity against B. sorokiniana invitro.
• Plant- based synthesis of TiO2 NPs (10- 25 nm) using Aloe vera L. aqueous leaf extract.
• B. sorokiniana triggers morphological and biochemical changes in barley plants, causing spot blotch disease.
• T. cf. asperellum or green synthesized TiO2 NPs positively increased the host plant's tolerance against this disease by inducing of osmolytes and antioxidant defense-related enzyme production.
Similar content being viewed by others
Background
Barley (Hordeum vulgare L.) is the world's fourth most-produced cereal [1, 2], coming after wheat, rice, and corn. According to estimates by Triticase et al. [3], 21% of barley production was used in the malting and brewing industries, 70% went towards animal feed, and only roughly 6% was consumed by humans.
In agriculture, plant diseases contribute significantly to the depletion of natural resources and are thought to be a significant factor in global food production's yearly decline [4, 5]. In particular, soil-borne pathogens pose a serious threat, with fungi being the most active [6]. Due to changes in agricultural practices over the past few years, the proliferation of various phytopathogenic fungi, including Fusarium spp, Rhizoctonia spp, Alternaria spp, Botrytis spp, and Helminthosporium spp. has been damaging to crops with significant economic losses [7,8,9,10,11,12]. Barley is an excellent example of such crops that are exposed to various fungal pathogens, among which Bipolaris sorokiniana causes leaf blight (also known as spot blotch), black point, and other foliar and root diseases.
Spot blotch disease is one of the most serious diseases of barley and can reduce yield by more than 30% and have an impact on malting quality [13, 14]. It happens in warm, humid areas of the world. B. sorokiniana fungus may infect the plant's coleoptiles, crowns, culms, leaves, and roots [15]. Small brown patches that are first caused by spot blotches grow into dark brown blotches [16]. A zone of yellow leaf tissue with different widths that separates leaf spots may cause the production of shriveled seeds and reduce yield [14, 17]. Management of spot blotch disease has relied on the application of fungicides, cultural practices, disease-resistant cultivars, and biological control agents by beneficial microbes [18,19,20]. However, its management by beneficial microorganisms is a promising biocontrol strategy, as these beneficial microorganisms are essential for increasing nutrient availability, promoting plant development, combating soil-borne pathogens, and stimulating the plant's immune system [20,21,22].
Since they are considered symbiotic, opportunistic, and non-virulent, Trichoderma spp. have been used as biological control agents against plant pathogenic fungi instead of synthetic pesticides [23,24,25]. Their biological control strategies involve activating various processes, either indirectly through the competition for resources and space, the stimulation of plant growth and defense systems, or directly through mycoparasitism and antibiosis [26, 27]. Some Trichoderma spp. is associated with numerous plants through endophytic associations and can colonize the root surface [28]. Because of this symbiotic association, the plant is efficiently protected from pathogens [29, 30], where Trichoderma causes the expression of genes involved in plants' defensive mechanisms when it interacts with them [31, 32] and stimulates root development and plant growth [33,34,35,36]. For example, Morais et al. [27] investigated that Trichoderma spp. was used to assess the antagonistic activity against Colletotrichum truncatum, Lasiodiplodia theobromae, Sclerotium delphinii, and Macrophomina phaseolina. Also, T. viride and T. harzianum evidenced high efficiency against A. alternata and Drechslera halodes [37]. When employed to control R. solani and F. oxysporum f. sp. lycopersici in tomato plants, T. atrobrunneum and T. simmonsii significantly increased stem height and fresh weight in pathogen-treated tomato plants [38].
Another way to enhance the defense machinery of plants is through the use of modern technologies, i.e. nanotechnology, which can be extremely helpful in addressing this problem by restricting the use of damaging agrochemicals and assisting in boosting the yield of different crops [39, 40]. Nanoparticles (NPs) are recognized as a plant growth stimulant that modifies physiological, biochemical, and physicochemical pathways [41, 42]. Recently, titanium dioxide nanoparticles (TiO2 NPs), a type of metal oxide nanomaterial, have gained popularity as an environmentally friendly and clean photocatalyst due to their optical qualities, chemical stability, and non-toxicity [43,44,45]. These NPs have powerful oxidizing properties that produce free radicals like superoxide anion radicals, which inhibit the growth of microorganisms. As a result, they can be used in the agriculture industry to protect plants and inactivate various pathogenic infections [46].
By taking advantage of the reducing qualities of plant secondary metabolites, green synthesis of NPs can advantageously synthesize functional NPs [45, 47]. These benefits include producing biologically active nanomaterials, using inexpensive reactants, and having an environmentally favorable synthesis process [40]. TiO2 NPs made from plants have the potential to decrease the severity of diseases and stimulate plant growth by quickly absorbing on the surface of the plant and pathogens [48]. Currently, it is crucial to recognize the plant's defense mechanism against fungal pathogens. Few scientific papers have discussed the function of TiO2 NPs in reducing disease severity and their effects on plant biochemistry and productivity in response to fungal stress [49]. Hence, in this study, we attempted to go deeply into investigating the impact of T. cf. asperellum and green synthesized TiO2 NPs on the growth of barley plants and the severity of spot blotch disease caused by B. sorokiniana. As well, their effects on biochemical analysis and the defense-related enzymes in barley plants were also evaluated.
Results
Molecular identification of Trichoderma sp. and Bipolaris sp.
The morphological identification (Fig. 1) of Trichoderma sp. and Bipolaris sp. was verified through the use of ITS1/ITS4 primers in a molecular investigation of the ITS rDNA sequence (18S-28S rRNA), flanking ITS1 (5.8S rRNA), and ITS2 as T. cf. asperellum and B. sorokiniana. The retrieved ITS sequence was entered into NCBI GenBank as accession no.: OP108262 and OP714480 using the BLAST program, and in order to compare with other relevant strains, the sequence data was aligned. Phylogenetic analysis was performed using MEGA version 7 software, which stands for molecular evolutionary genetic analysis. Based on the ITS gene sequences, the phylogenetic tree displayed in Fig. 1c was constructed using the neighbor-joining method with 1000 bootstrap repetitions. Multiple sequence alignments were performed using the ClustalW programme in the MEGA7 software using the closest homologous sequences that were chosen. The maximum composite likelihood approach was used to compute the evolutionary distances.
Morphological and molecular characterization of (A) T. cf. asperellum (biocontrol agent): (a) colony on PDA, (b) vegetative mycelium microstructure showing conidiophore and phialides, (c) phylogenetic tree (B) B. sorokiniana (pathogen): (a) colony on PDA, (b) vegetative mycelium microstructure showing hyphae and conidia, (c) phylogenetic tree
In vitro antifungal assay
T. cf. asperellum was isolated from the rhizosphere of plants grown in the Egyptian soil and showed an inhibitory effect against B. sorokiniana. The dual culture test illustrated in Fig. 2A showed the inhibition zone between B. sorokiniana and T. cf. asperellum, where T. cf. asperellum exhibited strong antagonistic activity against B. sorokiniana (57.07%) that proved its potent activity as compared to B. sorokiniana (Fig. 2B). Also, Fig. 2 (C) showed the antagonistic effect of T. cf. asperellum cell-free culture filtrate against B. sorokiniana. Figure 2 (D-F) showed the mycoparasitism of T asperellum against mycelium of B. sorokiniana, intensively vacuolated deformed mycelium of B. sorokiniana and T. cf. asperellum and B. sorokiniana hyphal interaction.
Dual culture for the antagonistic effect evaluation of T. cf. asperellum against B. sorokiniana. A T. cf. asperellum and B. sorokiniana interaction, (B) B. sorokiniana control, (C) In vitro control of B. sorokiniana using cell-free culture filtrate of (150 µL) by well diffusion method after 5 days of incubation. D-F Mycoparasitism of T. cf. asperellum against mycelium of B. sorokiniana (D) intensively vacuolated deformed mycelium of B. sorokiniana, (E) and (F) T.cf. asperellum and B. sorokiniana hyphal interaction
Characterization of TiO2 NPs' morphology and optics
An early indicator of synthesis is the transformation of TiCl4 from a milky off-white to a reddish brown color after 4 h of stirring with A. vera aqueous extract. As shown in Fig. 3A and B, the SEM and TEM analysis of TiO2 NPs indicated that they are tetragonal in shape, with the majority of the nano-forms being found in the 10 to 25 nm size range.
Antifungal activity of TiO2 NPs against B. sorokiniana
The well diffusion method was used to determine the effect of two different concentrations of green-synthesized TiO2 NPs against B. sorokiniana. The results are displayed in Fig. 3 (C). The results showed that on PDA agar plates treated with 25 and 50 mg/L of TiO2 NPs, B. sorokiniana was not able to grow and that the diameter of the inhibition zone increased with TiO2 NPs concentration. Based on these findings, a concentration of 50 mg/L showed a promising antifungal action against B. sorokiniana.
Efficacy of T. cf. asperellum on growth attributes of barley against B. sorokiniana in response to TiO2 NPs
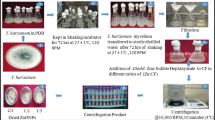
Infected barley plants with B. sorokiniana showed a decline in their morphological attributes (Fig. 4A-C). Figure 4(D) showed typical spot blotch symptoms on barley plant leaves challenged with B. sorokiniana. The barley height, shoot and root fresh weight (fwt), and dry weight (dwt) (Table 1) were significantly reduced compared to the healthy control. However, T. cf. asperellum significantly increased the barley growth parameters compared to the B. sorokiniana- infected plants and also showed a reduction in disease symptoms caused by B. sorokiniana. T. cf. asperellum increased barley height, shoot, and root fwt by 33.3, 20.7, and 34%, respectively. Generally, barley plants diseased with B. sorokiniana exhibited a significant decrease in spike fwt and dwt (Fig. 4C and Table 1) reaching 62.9 and 57.9% respectively, compared to control plants. Moreover, the data in Table 1 also revealed that the biosynthesized TiO2 NPs had a positive impact on the growth parameters of barley and improved both fwt and dwt. The percentage enhancement due to TiO2 NPs application of shoot fwt and dwt of B. sorokiniana- infected plants was 30 and 31.4 as compared to B. sorokiniana-infected ones.
In vivo effect of T. cf. asperellum and TiO2 NPs against B. sorokiniana infection on barley plants (A), barley leaves (B), barley spikes (C) and (D) leaf spot blotch on barley leaves after 4 weeks from B. sorokiniana infection. T1, T2 and T3 refers to control, TiO2 NPs and T. cf. asperellum treated plants respectively
Evaluation of barley physio-biochemical characteristics in response to T. cf. asperellum and TiO2 NPs application to B. sorokiniana stress
Barley leaves were tested for the presence of photosynthetic pigments (Chl a, Chl b, and total Chl), which revealed a severe deficiency in their contents of 26.7, 19.58, and 24.67%, respectively, in B. sorokiniana pathogen-challenged plants compared to controls. Interestingly, treating diseased or healthy plants with T. cf. asperellum or TiO2 NPs led to a noticeable improvement in Chl pigments. When compared to healthy plants, T. cf. asperellum had the highest levels of Chl a, Chl b, and carotenoids (13.9, 8.56, and 42.7%, respectively) in its photosynthetic pigment content (Table 2). Also, barley plants treated with TiO2 NPs also displayed improved levels of Chl a, Chl b, and total Chl. In plants under stress, the total Chl content increased in those exposed to TiO2 NPs from 1.523 to 2.082 mg/g leaf fwt.
In terms of total sugar contents (Fig. 5A), barley plants infected with B. sorokiniana showed a marked reduction in their amount (83.133 ± 2.199 mg/g dwt) as compared to uninfected plants (132.86 ± 3.52 mg/g dwt). Spraying T. cf. asperellum or TiO2 NPs had a notable and beneficial impact on total sugars in both healthy and B. sorokiniana-stressed plants (Fig. 5A, Table 3). Moreover, the data in Table 4 revealed a positive Pearson’s correlation between total sugars and pigment fractions (Chl a ‘0.974’, Chl b ‘0.926’, and total pigments ‘0.983’). The results in Fig. 5B and C also revealed a rise in the total protein and proline content in B. sorokiniana-infected plants compared to the healthy plants (negative control). However, barley plants treated with T. cf. asperellum and infected with B. sorokiniana showed further proliferation in their contents (6.194 ± 0.164 mg/g fwt and 132.09 ± 3.49 µmol/g fwt) as compared with the infected ones (4.854 ± 0.128 mg/g fwt and 77.28 ± 2.04 µmol/g fwt), and the same trend was reported for TiO2 NPs.
Influence of T. cf. asperellum and TiO2 NPs on osmolytes: (A) total sugars, (B) protein and (C) proline of barley plants infected with B. sorokiniana pathogen, and uninfected. Data analysis was done by using Duncan’s multiple range test at p ≤ 0.05. The same letters are not significantly different and error bars reflect the standard error of the mean
Reduction of oxidative burst and lipid peroxidation
The findings presented in Fig. 6 (A and B) demonstrated that, in comparison to healthy plants, the B. sorokiniana infection of barley plants resulted in a notable and elevated accumulation of H2O2 and MDA content (26.33 and 79.56%, respectively). It is important to note that T. cf. asperellum caused the least reduction in lipid peroxidation and H2O2 concentration. The application of T. cf. asperellum or TiO2 NPs retarded the values of H2O2 (27.8 and 12.5%) and lipid peroxidation (27.9 and 13.78%) as compared to healthy plants (Fig. 6). Moreover, the elevated concentration of H2O2 in the cellular system positively and significantly correlates (Table 4, r = 0.949**) with the oxidative changes affecting MDA content.
Effect of T. cf. asperellum and TiO2 NPs on stress markers: (A) H2O2, (B) Malondialdehyde (MDA), (C) POX, (D) CAT, (E) PAL and (F) LOX of barley infected with test B. sorokiniana pathogen, and uninfected. Data analysis was done by using Duncan’s multiple range test at p ≤ 0.05. The same letters are not significantly different and error bars reflect the standard error of the mean
Enhancing antioxidant and defense related enzymes activity
POX, CAT, and PAL activities increased in barley plant leaves challenged with B. sorokiniana (42, 17, and 73%) as compared to untreated negative control plants. Remarkably, based on ANOVA results, barley plants sprayed with T. cf. asperellum or TiO2 NPs showed a significant increase in their activities over pathogen-challenged plants (Fig. 6C-E and Table 3). Furthermore, an increase in LOX activity (Fig. 6F) was observed in barley plants infected with B. sorokiniana. Furthermore, under pathogen-challenged conditions, treatment with T. cf. asperellum or TiO2 NPs slightly and non-significantly increased LOX levels in plant leaves when compared to B. sorokiniana-treated plants and untreated control plants.
Discussion
The hemibiotrophic ascomycete B. sorokiniana poses a major danger to the production of barley and other cereal crops. Numerous chemical fungicides have been applied, but since the disease is so sophisticated, the pathogens have evolved to become resistant to these chemicals [50]. Therefore, biological management may be a good choice for high quality and productivity in crop production and sustainable agriculture. Recently, biocontrol agents that employ bacteria and fungi have drawn a lot of interest as a secure and effective disease prevention strategy. To do this, scientists have looked into a variety of microorganisms, including fungi and bacteria like Chaetomium globosum, T. reesei, T. hamatum, T. harzianum and Bacillus subtilis TE3, to manage the spot blotch pathogen [51,52,53].
It is obvious that T. cf. asperellum showed an inhibitory effect against B. sorokiniana. Our results of the antifungal activity of T. cf. asperellum against B. sorokiniana agree with Yassin et al. [37], Matroudi et al. [54] and Mukherjee et al. [55] who studied the efficacy of T. cf. asperellum against a wide range of soil-borne fungi due to the production of antifungal metabolites. Druzhinina et al. [26] and Morais et al. [27] confirmed that the mycoparasitism and antibiosis attributes of some Trichoderma spp. in addition to competition for resources and space, are used by humans for biological control activities. Furthermore, T. cf. asperellum induces morphological modifications that allow it to penetrate the host and hold high quantities of osmotic solutes such as glycerol [55, 56]. Trichoderma attaches to the pathogen through cell-wall carbohydrates that bind to the lectins on the pathogen [57]. The creation of cell wall-degrading enzymes and peptaibols [58] is the next stage, which makes it easier for Trichoderma hypha to enter the parasitized fungus' lumen and for the cell-wall content to be assimilated.
The one-step reaction, environmentally friendly reactants, and cost-effectiveness of the green NPs synthesis make it superior to the chemical and physical approaches. As a result, biocompatible active NPs with several biological and medicinal uses are created [40]. Plant secondary metabolites are important for reducing and stabilizing bulk materials in redox processes during the creation of NPs [59]. A. vera leaf aqueous extract was qualitatively assessed, as earlier mentioned in our previous study [60] and the results showed the presence of some phytochemicals such as phenolics, flavonoids, alkaloids, and tannins. In this study, TiO2 NPs were prepared by green synthesis at pH 9 using phytochemicals in A. vera extract to reduce the TiCl4 salt into TiO2 NPs. An initial sign of synthesis is the change of the milky off-white color of TiCl4 to a pinkish brown color after 4 h of stirring with A. vera aqueous extract. Visual observation of this color change was in line with results presented in Satti et al. [49]. As previously described in our study, according to XRD results, the synthesized TiO2 NPs were pure and formed of only the anatase crystalline phase [60]. Burda et al.'s study [61] confirmed that the two factors affecting TiO2 NPs' physicochemical properties and consequently their antibacterial properties are their shape and crystal structure. More specifically, anatase has the highest levels of photocatalytic and antibacterial activity. The anatase structure is capable of producing OH˙ radicals during a photocatalytic event, which can destroy the pathogen membranes. Using a leaf extract from Trigonella foenum-graecum, Gomathipriya and Subhapriya [62] biosynthesized TiO2 NPs, producing spherical NPs with diameters ranging from 20 to 90 nm. In this study, we evaluated the potential of synthetic TiO2 NPs as fungal pathogen biocontrol agents.
The use of biosynthesized metal NPs to fight fungi that cause plant diseases has increased recently [63,64,65,66]. The results showed that B. sorokiniana was unable to grow on PDA agar plates that had been supplemented with 25 and 50 mg/L of TiO2 NPs; in addition, the diameter of the inhibitory zone increased with increasing TiO2 NPs concentration. Our findings concur with those of Boxi et al. [67] and Irshad et al. [68] that hazardous plant pathogens such as F. solani, Venturia inaequalis, and Ustilago tritici were resistant to the strongest antifungal effects of TiO2 NPs. TiO2 NPs can induce cell damage in Pichia pastoris by impairing the ROS-associated scavenging system, which results in the accumulation of ROS [69]. Additionally, these NPs have the ability to penetrate cells and destroy the fungal cell wall. However, their entry into the cell, diffusion, and endocytosis might cause the creation of ROS, which can impair the performance of numerous intracellular organs [64, 70, 71]. By causing oxidative stress, ROS appears to play a significant role in the antifungal response. Additionally, they can destroy all cellular macromolecules, such as DNA and proteins [63]. NPs can also cause fungal death by disrupting cellular enzymes and interfering with the electron transfer chain [66].
In a field experiment, the antifungal properties of T. cf. asperellum and TiO2 NPs against the biotic stress of the spot blotch disease were investigated. The morphological traits of B. sorokiniana-infected barley plants decreased. However, adding biosynthesized TiO2 NPs or T. cf. asperellum, a fungus that promotes plant growth, greatly improved the growth parameters for barley plants. Our results are in agreement with Khalil et al. [72] results that the foliar application of T. viride and C. globosum either alone or in combination increased tomato fwt and dwt of shoots and roots as compared to control. Also, Abdelhameed and Metwally [25] reported that T. viride enhanced onion plant growth under normal conditions. T. cf. asperellum colonization of cucumber roots has been demonstrated to improve the availability of P and Fe to plants, resulting in appreciable increases in dwt, shoot length, and leaf area [73]. Additionally, auxins made by Trichoderma spp. can promote plant growth and root formation [74].
Under B. sorokiniana stress, Satti et al. [49] demonstrated that the biosynthesized TiO2 NPs improved the agro-morphological traits (fwt and dwt) and yield parameters of wheat plants. Additionally, it was noted that applying TiO2 NPs improved Zea mays growth characteristics [75]. Mishra et al. [76] state that TiO2 NPs may regulate the activity of N metabolism-related enzymes, which facilitates plant uptake of more nutrients as these NPs convert N2 into organic nitrogen in the form of proteins and chlorophyll pigments [77], which in turn raises plant biomass and dwt. A study by Jaberzadeh et al. [78] stated that the foliar application of TiO2 NPs to wheat plants shows a rise in starch and gluten contents, which in turn boosts yield due to increasing rubisco activity. Also, Rizwan et al. [79] reported an increase in soybean yield after treating with TiO2 NPs because of the increased absorption of water by the plants.
The physio-biochemical properties of the barley plants were examined in order to investigate the antifungal effects of T. cf. asperellum and the biosynthesized TiO2 NPs against spot blotch disease in barley plants. Photosynthesis plays an essential role in plant productivity and takes place in green leaves [80]. A severe deficiency in the photosynthetic pigment contents in B. sorokiniana pathogen-challenged plants was detected. This might be due to the plant's failure to capture sunlight and the breakdown of Chl pigments and thus photosynthesis will be diminished [81, 82]. It is interesting that a noticeable improvement in Chl pigments appeared with T. cf. asperellum or TiO2 NPs applications. Our results are consistent with Khalil et al. [72] whose work showed that T. viride and C. globosum foliar application, either alone or in combination, exhibited a considerable increase in Chl contents but a decrease in H2O2 and MDA. The findings of our study also support those of Aldinary et al. [83], who found that the use of fungal endophytes increases the efficiency of photosynthesis due to numerous changes in the chloroplasts and contents of carotene and Chl, as Trichoderma increases gene expression regulating Chl biosynthesis, light-harvesting complex proteins, or Calvin cycle components. Similarly, Satti et al. [49] found that the spot blotch in wheat plants caused by B. sorokiniana resulted in a significant decline in all pigment fractions. Nevertheless, with the application of 40 mg/L TiO2 NPs, an improvement in these pigment fractions appeared. Morteza et al. [75] and Khodakovskaya and Lahiani [84] showed a significant increase in all pigment fractions with TiO2 NPs application, where TiO2 NPs increase plant growth and photosynthesis rate by producing more carbohydrates. Additionally, Rodrìguez-González et al. [85] noted that the photocatalytic capability of TiO2 NPs, which degrade many pesticide types, might be important for protecting plants from diseases since they do not form poisonous or toxic chemicals, leading to a high pathogen disinfection capacity [86]. We hypothesized that the increase in Chl content would have a significant effect on the rate of photosynthesis, enhance the productivity of carbohydrates, and ultimately result in an increase in fwts and dwts.
In order to overcome the negative effects of spot blotch disease caused by B. sorokiniana on morphological and Chl content, barley plants activate dual defense, which is characterized by the enhanced accumulation of different osmolytes (sugars, proteins, and proline) and increased antioxidant and defense-related enzymes (POX, CAT, PAL, and LOX). Sugars participate in physiological processes related to plant growth and development and are also involved in the response to a number of stresses, acting as nutrient and metabolite signalling molecules [87, 88]. A sharp decrease in their amount was recorded in barley plants infected with B. sorokiniana compared to uninfected, and with the applications of T. cf. asperellum or TiO2 NPs a significant and positive effect on total sugars was detected. These results agree with those of Khodakovskaya and Lahiani [84], in which TiO2 NPs produced more carbohydrates, thus promoting growth and photosynthesis rates in plants. Sugars act as osmotic agents, helping maintain plasma membrane integrity [87]. In addition, a study by Abdelhameed and Metwally [25] supported the idea that T. viride contributes to the rise in sugar levels in onion plants. This might be explained by the enhancing effect of T. cf. asperellum and TiO2 NPs in raising Chl concentration (seen above), which had a favorable influence on photosynthetic rate and led to an increase in the production of soluble sugars, boosting fwt and dwt. Furthermore, Ze et al. [89] suggested that TiO2 NPs may enhance light harvesting complex II gene expression in chloroplasts, which is consistent with our finding that TiO2 NP supplementation results in a rise in the concentration of soluble sugars.
Concerning the results of the total protein and proline content, there was an increase in their contents in B. sorokiniana-infected plants compared to the healthy plants. However, barley plants treated with T. cf. asperellum or TiO2 NPs and infected with B. sorokiniana showed further proliferation in their contents. Our findings are consistent with those of Khodakovskaya and Lahiani [84], who found significant differences in the amounts of amide and carbohydrates in cucumber plants treated with TiO2 NPs, suggesting that TiO2 NPs can affect cucumber at the macromolecular level. Also, TiO2 NPs transform N2 into organic nitrogen in the form of proteins, which eventually results in an increase in protein content. Moreover, T. viride caused a substantial increase in protein content in onion plants [35]. Similar to this, it has been noted that proline content increased in A. solani-infected eggplant and tomato [12, 90]. Our findings are consistent with Satti et al.'s [49] observation that spot blotch stress led to significant increases in proline concentration in wheat plants. Furthermore, it was discovered that applying TiO2 NPs increased the proline content of the infected wheat plants. In the same respect, TiO2 NPs were proven to increase proline content in broad bean plants under both normal and abiotic stress conditions by Abdel Latef et al. [91]. In addition to maintaining cell turgor or osmotic balance, accumulating proline also stabilizes membranes to avoid electrolyte leakage and scavenges ROS to prevent protracted oxidative bursts in plants [92,93,94]. Lipids are crucial for preserving the structural integrity of cells. MDA is thought to be the most thiobarbituric acid-reacting molecule that demonstrates the extent of oxidative stress as a result of lipid peroxidation [93, 95, 96]. Infection of barley plants with B. sorokiniana induced a significant and high accumulation of H2O2 and MDA content. It is worth mentioning that the application of TiO2 NPs or T. cf. asperellum reduced the amount of H2O2 and lipid peroxidation. Similar to this, Khalil et al. [72] reported that the application of T. viride controlled the production of H2O2 and lipid peroxidation, maintaining cell homeostasis in tomato plants that were not infected by the pathogen. In accordance with these results, Abdelrhim et al. [97] also showed an augmentation of H2O2 and lipid peroxidation in wheat plants challenged with R. solani and the role of SiO2 NPs in reducing their contents and inducing pathogen disease resistance. As well, Abdalla et al. [60] showed a great reduction in the amounts of H2O2 and lipid peroxidation in soybean plants treated with TiO2 NPs. Moreover, the elevated concentration of H2O2 in the cellular system positively correlates with the oxidative changes affecting MDA content, as it is formed by the reaction of ROS (H2O2 or/and O−2) with lipid molecules [98]. Soliman et al. [99] have reported that infection with A. alternata increased the amount of lipid peroxidation in the pathogen-inoculated pepper leaf samples.
Antioxidant enzymes have a crucial function in scavenging ROS and preventing the oxidative stress that leads to harmful effects on many sensitive molecules [100,101,102,103]. In general, POX, CAT, and PAL activities increased in barley plant leaves infected with B. sorokiniana and there was a further increase in their activities with T. cf. asperellum or TiO2 NPs application. It is known that B. sorokiniana stimulates cereals to activate a variety of secondary metabolic pathways [104]. Bagy et al. [105] reported that POX, which catalyzes H2O2 breakdown, is also implicated in lignification and suberization processes, which reduce pathogenesis and aid in infection prevention. Also, Kaur et al. [14] and Singla et al. [106] stated that PAL, the primary enzyme that connects primary to secondary metabolism, has been linked to the activation of responses against pathogenic fungi in barley. It is also involved in the synthesis of plant secondary antimicrobial substances that are essential for plant disease resistance and plays an essential role in the biosynthesis of lignin precursors [107]. Furthermore, Khalil et al. [72] revealed a substantial enhancement of CAT enzyme activity with T. viride and C. globosum applications. According to Naz et al. [108], a botanical chemical formulation caused cereals to become resistant to the fungus B. sorokiniana by activating POX and PAL enzymes. Similarly, wheat developed stress tolerance against this fungus by upregulating defense-related enzymes, including CAT, ascorbate peroxidase, PAL, and POX [52]. Moreover, Abdelrhim et al. [97] showed that the activity of PAL was intensified as SiO2 NPs were applied in wheat plants against R. solani. Kaur et al. [107], Ferrer et al. [109], Król et al. [110] and Abdelhameed et al. [111] reported that PAL and POX enzymes might serve as markers of induced resistance to fungal diseases. Related study by Metwally and Abdelhamed [112] revealed that NPs could likely boost POX and CAT activities, which directly contribute to overcoming various stresses.
Also, an increase in LOX activity was observed in barley plants infected with B. sorokiniana. Furthermore, treatment with T. cf. asperellum or TiO2 NPs increased LOX levels non-significantly. In the same trend, Ohta et al. [113] found an increase in LOX activity in rice leaves after infection with rice blast fungus, which is correlated with plant resistance against pathogens. As well, Nandini et al. [114] showed an increase in LOX and POX activities in pearl millet plants infected with downy mildew disease using crude proteins extracted from six different Trichoderma spp. This was attributed to the function of LOX in the establishment of the hypersensitive response, a form of programmed cell death that serves as an active defense mechanism [115, 116]. A necrotic lesion develops as a result of the rapid death of plant cells in the area surrounding the infection site, which stops the pathogen from spreading and causes more damage to the surviving plant organ.
Methods
In vitro experiments
Isolation of fungal pathogen (Bipolaris sp.)
The pathogenic fungus from diseased leaves of wheat and barley plants grown in the Egyptian soil of Minia Al-Qamh, El-Sharkia Governorate (30°31′25.4"N 31°21′13.1"E), showing typical spot blotch disease symptoms, was collected in paper bags. Isolation and purification of fungal pathogens were done according to Kumar et al. [117]. The infected wheat and barley plants' leaves were chopped into small pieces and surface sterilized in a 5% sodium hypochloride solution for 5 min, then rinsed in sterile distilled water. After drying in a sterile filter, the plant tissues were incubated for 6 days at 27°C in a potato dextrose agar (PDA) medium. To obtain pure cultures of the pathogen, colonies of the fungus that appeared were transferred to fresh PDA plates.
Isolation of biocontrol agent (Trichoderma sp.)
In the Egyptian soil of Minia Al-Qamh, El-Sharkia Governorate (30°31′25.4"N 31°21′13.1"E), Trichoderma sp. was isolated from barley plant rhizospheres using a serial dilution plate method. One mL of the suspension from each dilution was added aseptically into sterile Petri dishes filled with Rose Bengal medium. Three days later, Trichoderma colonies were selected and grown on PDA media. Pure isolates were made by inoculating the fungal colonies and letting them grow for seven days at 28°C. Trichoderma sp. stock culture was kept viable on PDA slants.
Identification of fungal inoculants
Morphological identification of isolated fungi
By contrasting macroscopic characteristics on agar plates with those of microscopic aspects like hyphal branching pattern and conidial shape, the morphological characteristics of hyphae of Trichoderma sp. and Bipolaris sp., were confirmed [118, 119] respectively. Figure 1 illustrates the morphological growth of both fungal inoculants of Trichoderma sp. (Fig. 1A [a and b]) and Bipolaris sp. (Fig. 1B [a and b]) on PDA plates.
Molecular identification of isolated fungi
The pure isolates of Trichoderma sp. and Bipolaris sp. were sub-cultured on a PDA medium and grown for 5 days at 27°C, and their genomic DNA was isolated using the CTAB technique [120]. The cell walls of fungal mycelia were broken down in the presence of liquid N2 by grinding. After that, the CTAB extraction buffer was added, and the mixture was incubated at 65°C before being purified using phenol, chloroform, and isoamyl alcohol (25:24:1). Cold isopropanol was used to precipitate the genomic DNA, which was then washed twice with cold 70% ethyl alcohol. Finally, the DNA was dissolved in 50 μL of sterilized distilled water.
PCR Amplification and phylogenetic analysis
The ITS1 and ITS2 as well as the inverted 5.8S coding rDNA were amplified using the ITS1 and ITS4 primers. In a total volume of 50 µL, each PCR reaction mixture comprised 5–10 ng of genomic DNA, 1 µM of each ITS1/ITS4 primer, 5 µL of a 10X reaction buffer (50 mM KCl, 50 mM Tris–HCl; pH 8.3, 0.1 mg/mL bovine serum albumin (BSA), 3 mM MgCl2, 200 µM each of dNTP, and 2.5 U of Taq DNA polymerase (Promega, Mannheim, Germany). The PCR technique includes 35 cycles of denaturation at 95°C for 30 s, annealing at 56°C for 30 s, and elongation at 72°C for 1 min. Before DNA sequencing, the PCR amplicon was resolved using an 8% agarose gel and purified using a specific PCR purification kit (Accu Prep® PCR DNA Purification Kit, K-3034–1, Bioneer Corporation, South Korea). MacrogenInc, (South Korea) sequenced the purified PCR products. All inter-transcribed spacer sequencing work was also performed by MacrogenInc, (South Korea) and was carried out on both strands of the submitted DNA fragments [121]. The purified PCR products were sequenced using an ABI 377 DNA. Auto-sequencer (PerkinElmer, Applied Biosystems Div., Waltham, USA) based on the same primers mentioned before.
In vitro evaluation of the antifungal activity of Trichoderma sp. against fungal pathogen
Trichoderma sp. was employed in an in vitro antagonistic assay to assess its biocontrol effects against the pathogenic fungus of barley by the dual culture technique [122]. Mycelial growth discs of 5 mm diam. removed under aseptic conditions from the growing edge of a 5 day-old pure culture of Trichoderma sp. and Bipolaris sp. were transferred and placed on the opposite of a Petri dish (9 cm) containing 20 mL of PDA and were kept 6 cm apart from each other. The plates were incubated for 7 days at 28°C, and the treatments were replicated in triplicate. Control dishes were cultures of Bipolaris sp. without the presence of Trichoderma sp. The growth of the pathogen and Trichoderma sp. was observed constantly, and radial growth was recorded by measuring the mean colony diameter on the 5th day of inoculation. The percent of inhibition (PI) of the test phytopathogenic fungus (Bipolaris sp.) was calculated using the following formula:
R1 was the radial growth of the pathogen without Trichoderma sp., and R2 represents the radial growth of the pathogen inoculated with Trichoderma sp. [123].
Mycoparasitism
For microscopy examination, dual culture for both pathogenic and Trichoderma sp. was examined after 7 days of incubation, the regions where the hyphae of Trichoderma sp. and Bipolaris sp. interacted (the interaction zones) were observed. Characteristics used for differentiating between the hyphae of Trichoderma sp. and Bipolaris sp., were studied according to Rifai [117] and Wiese [118] using a light microscope (Leitz WETZLAR, Wetzlar, Germany).
Antagonistic effects of Trichoderma sp. culture filtrate (Antibiosis)
Trichoderma sp. was grown on PDA plates for 7 days at 28°C. A fungal disc of 5 mm in diam. of Trichoderma sp. mycelial growth was transferred from the periphery of mycelial growth and inoculated on Erlenmeyer flasks (250 mL) containing 100 mL of PDB. The flasks were then incubated in a shaking incubator at 150 rpm at 28°C. Mycelial growth was harvested on the 5th day of incubation. The culture was filtered through Whatman filter paper no. 1 and re-filtered through a Millipore syringe filter (0.22 μm).
A Petri dish (9 cm in diameter) containing 25 mL of PDA was supplied with 1 mL of Bipolaris sp. spore suspension and allowed to harden. After that, three wells with a diameter of 10 mm were made using a sterile cork-borer on each agar plate. The wells were subsequently filled with a cell-free culture of Trichoderma sp. (150 µL) using a well-diffusion technique described by Soliman et al. [124] with slight modifications. The plates were then placed in a refrigerator for 5h before being incubated at 28°C for 5 days. At the end of the incubation period, the inhibition zones were assessed.
Biosynthesis and characterization of TiO 2 NP
In our latest work, TiO2 NPs were prepared by an eco-friendly green synthesis method using titanium tetrachloride (TiCl4) solution and an aqueous extract of Aloe vera plant leaves, which were obtained from the Horticulture Department, Faculty of Agriculture, Zagazig University, Egypt after permission from Zagazig University. In brief, 100 mL of A. vera leaf extract was added dropwise to a 100 mL 1N TiCl4 solution in deionized water under continuous stirring. The pH of the mixture was adjusted to 9, and the stirring continued at room temperature for 4h. The formed NPs were filtered, washed with double-distilled water, and finally dried at 100°C overnight. The obtained dry powder was further calcined at 400°C for 4h. The prepared NPs were characterized by UV–Visible spectrophotometry, Fourier transform infrared (FTIR), X-ray diffraction (XRD), scanning electron microscopy (SEM), and transmission electron microscopy (TEM) as latest described [60].
TiO2 NPs suspension preparation and in vitro assessment of its antifungal activity and growth inhibition against Bipolaris sp.
Suspension of 50 mg/L of these NPs is prepared by dissolving NPs in distilled water and sonicating for 25–30 min using Elma (E15H Elmasonic) for equal distribution of NPs in water.
The antifungal activity of green synthesized TiO2 NPs was carried out using the well diffusion method [125]. One mL of Bipolaris sp. spore suspension prepared from 5-day-old culture was added to 25 mL of PDA and poured in Petri dish. After that, three wells with a 10 mm diam. were made. The wells were filled with 150 µL of two different concentrations of TiO2 NPs (25 and 50 mg/L) individually in triplicate. The culture plates were incubated at 28°C for 5 days, and the zones of inhibition were observed.
Inoculum preparation
PDA plates with 7-day-old pure cultures of Trichoderma sp. and Bipolaris sp. were used. After that, sterile water was added to the cultures, and the mycelium was scraped gently with a sterile glass rod. The spore suspension concentrations of Trichoderma sp. and Bipolaris sp. were adjusted to 2.5 × 107 and 1.7 × 107 conidia/ mL; respectively.
In vivo experiments
Antagonistic activity of Trichoderma sp. on barley plants infected with Bipolaris sp. under filed conditions
Planting, growth conditions, and treatments
To assess Trichoderma sp.'s and the biosynthesized TiO2 NPs' capability for biocontrol against Bipolaris sp. on barley plants, a field experiment was carried out (Fig. 7). Barley (Hordeum vulgare L.) grains were acquired after permission from the Department of Plant Breeding and Genetics, Agricultural Research Center, Giza, Egypt, and were planted in the sandy clay soil of Zagazig, El-Sharkia Governorate. After one month of planting, a spore suspension of Trichoderma sp. was used as a foliar spray and drenched the soil near the stem region of Trichoderma sp.-treated plants. A concentration of 50 mg/L of TiO2 NPs was applied twice to the barley plant leaves, i.e. once before Bipolaris sp. application and then 7 days after inoculation. Ten days after Trichoderma sp. application, a spore suspension of Bipolaris sp. was used for infection by directly spraying the suspension using an atomizer on the leaves of the barley plants. Plants sprayed and irrigated with tap water only were used as a negative control. Six treatments were directed and arranged in a factorial design (2 × 3). Each treatment had 3 replicates. Four weeks after the Bipolaris sp. infection, disease symptoms appear. Bipolaris sp. was re-isolated from symptomatic tissues, and its identity was confirmed. The harvested samples were either immediately used or rapidly stored and frozen at − 20°C for further studies.
Measurements
Growth traits
Morphological traits of barley plants were recorded 4 weeks after Bipolaris sp. infection. The following characteristics were measured for each treatment: shoot height (cm), fresh (fwt) and dry (dwt) weight of shoot (g), root, and spike. Three barley plants were collected for each treatment, and they were gently washed with flowing water to remove soil debris.
Measurement of physio-biochemical indexes
Fresh leaves of barley plants from each treatment were taken separately in order to measure the levels of enzymes linked to plant defense, proline, soluble protein, total carbohydrates, malondialdehyde (MDA), hydrogen peroxide (H2O2), and chlorophyll (Chl).
The quantitative analysis of Chl was done according to the method of Lichtenthaler and Wellburn [126] after the extraction of 100 mg of fresh leaves with acetone, and the absorbance was read at 644, 663, and 452.5 nm wavelengths, and its content was calculated by the following formula:
*OD = Optical density, E.V = Extraction volume of sample, fwt = Fresh weight of sample.
The total soluble protein content of both infected and healthy barley leaves was measured [127] using a Folin–Ciocalteu reagent at 700 nm using bovine serum albumin as a standard. Fwt of barley leaves (250 mg) were ground in potassium phosphate buffer (50 mM pH 7.0) and centrifuged for 7 min at 6000 rpm (4°C) (MIKRO 200R Hettich Zentrifugen, Germany). Then 1 mL of the resultant supernatant was mixed with 5 mL of an alkaline copper solution. Total carbohydrate content was estimated by comparison with a glucose standard curve, as described by Dubois et al. [128]. Barley dried leaves (100 mg) were heated with 10 mL of 2.5N HCl in a boiling water bath for 3 h. The extract (0.1 mL) was then taken, and 1 mL of phenol was added. Sulfuric acid (2.5 mL) was added after 1 h of mixed properly, and the absorbance was measured at 490 nm.
The Bates et al. [129] method was used to estimate the proline content in barley leaves. In brief, 250 mg fwt of barley leaves were extracted and centrifuged in 3% sulphosalicylic acid. Then, 2 mL of filtrate was reacted with 2 mL of ninhydrin reagent and 2 mL of glacial acetic acid and then placed in a boiling water bath. Four mL of toluene were added, and the upper colored layer was separated, and its absorbance was read at 520 nm. Cell membrane decomposition of barley leaves was determined as the concentration of total 2-thiobarbituric acid (TBA) reactive ingredients [130]. To summarize, 250 mg of barley leaf tissues were extracted in 5 ml of 0.1% trichloroacetic acid (TCA). The supernatant (500 L), after being centrifuged for 10 min at 6000 rpm, was mixed with 2 mL of 20% TCA containing 0.5% TBA, incubated for 30 min at 95°C, and cooled on ice immediately. Absorbance at 532 and 600 nm was used for the calculation of the MDA equivalent. Based on the extinction coefficient of 155 mM−1 cm−1, the rates of lipid peroxidation were expressed as nmol g−1 fwt of the MDA-TBA complex formed. The MDA equivalent was calculated using the following equation:
To quantify the H2O2 content in barley, leaf samples were collected from infected and treated plants. The reagent ferrous oxidation-xylenol orange was employed [131]. In 0.1% TCA, a known fwt of barley leaves (250 mg) was homogenized. Half mL of 100 mM potassium phosphate buffer (pH 6.8) and 2 mL reagent (1 M KI w/v) were added to 0.5 mL of the barley leaf extract. The reaction was left for 1 h in darkness, and the absorbance was measured at 390 nm.
Plant defense-related enzyme determination
The concentrations of peroxidase (POX), catalase (CAT), phenylalanine ammonia-lyase (PAL), and lipoxygenase (LOX) were measured in order to confirm the effect of T. cf. asperellum and the biosynthesized TiO2 NPs against spot blotch disease in barley plants. To analyze the activities of POX and CAT, fwt of barley leaves (100 mg) were ground in 25 mL of potassium phosphate buffer (50 mM pH 7.0) and centrifuged at 6000 rpm (4°C) for 20 min. The supernatant was collected to measure the activities of POX and CAT, according to Bergmeyer [132] and Aebi [133].
The activity of PAL was determined in barley plant leaves [134] using phenylalanine as the substrate and the absorbance was recorded at 290 nm. According to Axelord et al. [135], the LOX activity was assessed using linoleic acid as a substrate. Linoleic acid (10 μL) and Tween 20 (50 μL) are both included in the test buffer, which is 10 mL of 0.1 M phosphate buffer with a pH of 9.0. By adding 0.1 mL of enzyme extract to 1 mL of freshly made assay buffer, the assay process was started. LOX activity was determined by observing the absorbance at 234 nm.
Statistical analysis
The average and standard errors of 5 replicates (n = 5) are represented in the tables and graphed findings. The analysis of variance (ANOVA) was used to statistically confirm the results and by using Duncan's multiple range test (p < 0.05), it was concluded that there was a significant difference between the control and treatment groups. SPSS® 18.0 was used to perform the calculations. Using SPSS, Pearson's correlation coefficients (r) were carried out to understand the relationship between growth indices and different physio-biochemical parameters. Figures were assembled using OriginPro 8.5 for data analysis and graphing software.
Conclusion
To achieve sustainable food production, agriculture must employ innovative strategies to decrease the use of agrochemicals. Consequently, NPs and fungal biological agents have been suggested as viable strategies with less environmental impact. Our research confirmed that B. sorokiniana causes spot blotch disease in barley plants, which results in morphological and physio-biochemical alterations. Nevertheless, on the host plant side, the application of T. cf. asperellum or green synthesized TiO2 NPs positively increased the host plant's tolerance against this pathogen and played an important role in the activation of a complex defense system that comprises: (1) induction of osmolytes such as proline, protein, and soluble sugars. (2) An increase in antioxidant defense-related enzyme production. All these defense systems neutralize the destructive effects of ROS, decreasing H2O2 and lipid peroxidation and maintaining homeostasis within B. sorokiniana-challenged barley plants. So, the application of T. cf. asperellum or green synthesized TiO2 NPs could be considered as an alternative or eco-friendly approach to protect barley plants from spot blotch disease caused by B. sorokiniana. Further studies with the B. sorokiniana pathogen must be carried out in the field to explore the potential of T. cf. asperellum and green synthesized TiO2 NPs combined applications as a viable strategy, which might be evaluated in the future to obtain better crop yields with less environmental impact, however further studies will be necessary to gain a comprehensive understanding of biological agent-NPs-plant interaction mechanisms.
Availability of data and materials
The relevant datasets supporting the results of this article are included within the article and the [GenBank NCBI] at:
https://www.ncbi.nlm.nih.gov/nuccore/OP108262.1/T. cf. asperellum.
https://www.ncbi.nlm.nih.gov/nuccore/OP714480.1/B. sorokiniana.
References
Blake T, Blake V, Bowman J, Abdel-Haleem H. Barley feed uses and quality improvement. In: Ullrich SE, editor. Barley: production, improvement and uses. Hoboken: Wiley-Blackwell; 2011. p. 522–31.
FAO. FAOSTAT-Agriculture. 2018.
Triticase C, Amicarelli V, Lamonaca E, Rana RL. Economic analysis of the barley market and related uses. In: Zerihun T, editor. Grasses as food and feed. New York: IntechOpen; 2018.
Liu J, Sui Y, Wisniewski M, Droby S, Liu Y. Review: Utilization of antagonistic yeast to manage postharvest fungal diseases of fruits. Int J Food Microbiol. 2013;167:153–60.
Ghorbanpour M, Omidvari M, Abbaszadeh-Dahaji P, Omidvar R, Kariman K. Mechanisms underlying the protective effects of beneficial fungi against plant diseases. Biol Control. 2018;117:147–57.
Almeida FB, Rodrigues LM, Coelho C. The still underestimated problem of fungal diseases worldwide. Front Microbiol. 2019;10:214.
Escrivá L, Front G, Manyes L. In vivo toxicity studies of fusarium mycotoxins in the last decade: A review. Food Chem Toxicol. 2015;78:185–206.
Zhang DD, Wang YX, Chen YJ, Kong QZ, Gui JY, Li YN, Bao MY, Dai X. Identification and characterization of a pathogenicity-related gene VdCYP1 from Verticillium dahliae. Sci Rep. 2016;6:27979.
Krylov BV, Petruk IM, Glushko IN, Khaldeeva VE, Mokeeva LV, Bilanenko NE, Lebedin SY, Eremin AS, Nifantiev EN. Carbohydrate specificity of antibodies against phytopathogenic fungi of the Aspergillus genus. Appl Biochem Microbiol. 2018;54:522–7.
Li H, Bian R, Liu Q, Yang L, Pang T, Salaipeth L, Andika BI, Kondo H, Sun L. Identification of a novel hypovirulence-inducing Hypovirus from Alternaria alternaria. Front Microbiol. 2019;10:1076.
Tyškiewicz R, Nowak A, Ozimek E, Jaroszuk-Sciseł J, ´,. Trichoderma: The current status of its application in agriculture for the biocontrol of fungal phytopathogens and stimulation of plant growth. Int J Mol Sci. 2022;23:2329.
Attia MS, Hashem AH, Badawy AA, Abdelaziz AM. Biocontrol of early blight disease of eggplant using endophytic Aspergillus terreus: improving plant immunological, physiological and antifungal activities. Bot Stud. 2022;63:26.
Wang R, Leng Y, Zhao M, Zhong S. Fine mapping of a dominant gene conferring resistance to spot blotch caused by a new pathotype of Bipolaris sorokiniana in barley. Theor Appl Genet. 2019;132:41–51.
Kaur S, Bhardwaj RD, Kaur J, Kaur S. Induction of Defense-Related Enzymes and Pathogenesis-Related Proteins Imparts Resistance to Barley Genotypes Against Spot Blotch Disease. J Plant Growth Regul. 2021;41:682–96.
Gupta PK, Chand R, Vasistha NK, Pandey SP, Kumar U, Mishra VK, Joshi AK. Spot blotch disease of wheat, the current status of research on genetics and breeding. Plant Pathol. 2018;67:508–31.
Singh UB, Sharma AK. Compatible salt-tolerant rhizosphere microbe-mediated induction of phenylpropanoid cascade and induced systemic responses against Bipolaris sorokinina (Sacc.) Shoemaker causing spot blotch disease in wheat (Triticum aestivum L.). Appl Soil Ecol. 2016;108:300–6.
Daba SD, Horsley R, Brueggeman R, Chao S, Mohammadi M. Genome-wide association studies and candidate gene identification for leaf scald and net blotch in barley (Hordeum vulgare L.). Plant Dis. 2019;103:880–9.
Al-Sadi AM. Variation in resistance to spot blotch and the aggressiveness of Bipolaris sorokiniana on barley and wheat cultivars. J Plant Pathol. 2016;98(1):97–103.
Rios JA, Perez CA, Debona D, Neto LC, Rios VS, Rodriguesa FA. Changes in gas exchange, chlorophyll a fluorescence and antioxidant metabolism within wheat leaves infected by Bipolaris sorokiniana. Ann Appl Biol. 2016;170:189–203.
Chen J, Zhou L, Din IU, Arafat Y, Li Q, Wang J, Wu T, Wu L, Wu H, Qin X, Pokhrel GR, Lin S, Lin W. Antagonistic activity of Trichoderma spp. against Fusarium oxysporum in rhizosphere of Radix pseudostellariae triggers the expression of host defense genes and improves its growth under long-term monoculture system. Front Microbiol. 2021;12:579920.
Matarese F, Sarrocco S, Gruber S, SeidlSeibot V, Vannacci G. Biocontrol of Fusarium head blight: interactions between Trichoderma and mycotoxigenic Fusarium. Microbiol. 2012;158(1):98–106.
Walters DR, Ratsep J, Havis ND. Controlling crop diseases using induced resistance: challenges for the future. J Exp Bot. 2013;64(5):1263–80.
Berg G. Plant–microbe interactions promoting plant growth and health: perspectives for controlled use of microorganisms in agriculture. Appl Microbiol Biotechnol. 2009;84:11–8.
Ferreira FV, Musumeci MA. Trichoderma as biological control agent: scope and prospects to improve efficacy. World J Microbiol Biotechnol. 2021;37:90.
Abdelhameed RE, Metwally RA. Assessment of beneficial fungal microorganism’s bio-efficacy in stimulating morphological and physiological parameters of Allium cepa plants grown in soil amended with fish wastes. BMC Plant Biol. 2022;22:617.
Druzhinina IS, Seidl-Seiboth V, Herrera-Estrella A, Horwitz BA, Kenerley CM, Monte E. Trichoderma: The genomics of opportunistic success. Nat Rev Microbiol. 2011;9:749.
Morais EM, Silva AAR, Sousa FWAd, Azevedo IMBd, Silva HF, Santos AMG, Júnior JEAB, Carvalho CPd, Eberlin MN, Porcari AM, Silva Araújo FDd. Endophytic Trichoderma strains isolated from forest species of the Cerrado-Caatinga ecotone are potential biocontrol agents against crop pathogenic fungi. PLoS ONE. 2022;17(4):e0265824.
Inglis PW, Mello SC, Martins I, Silva JB, Macêdo K, Sifuentes DN, Valadares-Inglis MC. Trichoderma from Brazilian garlic and onion crop soils and description of two new species: Trichoderma azevedoi and Trichoderma peberdyi. PLoS ONE. 2020;15:e0228485.
Mukherjee PK, Hurley JF, Taylor JT, Puckhaber L, Lehner S, Druzhinina I, Schumacher R, Kenerley CM. Ferricrocin, the intracellular siderophore of Trichoderma virens, is involved in growth, conidiation, gliotoxin biosynthesis and induction of systemic resistance in maize. Biochem Bioph Res Commun. 2018;505:606–11.
Galletti S, Paris R, Cianchetta S. Selected isolates of Trichoderma gamsii induce different pathways of systemic resistance in maize upon Fusarium verticillioides challenge. Microbiol Res. 2020;233:126406.
De Palma M, Salzano M, Villano C, Aversano R, Lorito M, Ruocco M, Docimo T, Piccinelli AL, D’Agostino N, Tucci M. Transcriptome reprogramming, epigenetic modifications and alternative splicing orchestrate the tomato root response to the beneficial fungus Trichoderma harzianum. Horticul Res. 2019;6:5.
Pimentel MF, Arnao E, Warner AJ, Subedi A, Rocha LF, Srour AY, Bond JP, Fakhoury AM. Trichoderma isolates inhibit Fusarium virguliforme growth, reduce root rot, and induce defense-related genes on soybean seedlings. Plant Dis. 2020;104:1949–59.
Metwally RA, Al-Amri SM. Individual and interactive role of Trichoderma viride and arbuscular mycorrhizal fungi on growth and pigment content of onion plants. Lett Appl Microbiol. 2019;70:79–86.
Metwally RA. Arbuscular mycorrhizal fungi and Trichoderma viride cooperative effect on biochemical, mineral content, and protein pattern of onion plants. J Basic Microbiol. 2020;60(8):712–21. https://doi.org/10.1002/jobm.202000087.
Metwally RA, Soliman SA, Abdel Latef AA, Abdelhameed RE. The individual and interactive role of arbuscular mycorrhizal fungi and Trichoderma viride on growth, protein content, amino acids fractionation, and phosphatases enzyme activities of onion plants amended with fish waste. Ecotoxicol Environ Saf. 2021;214:112072.
Metwally RA, Abdelhameed RE, Soliman SA, Al-Badwy AH. Potential use of beneficial fungal microorganisms and C-phycocyanin extract for enhancing seed germination, seedling growth and biochemical traits of Solanum lycopersicum L. BMC Microbiol. 2022;22:108.
Yassin MT, Mostafa AA, Al-Askar AA. In vitro antagonistic activity of Trichoderma spp. against fungal pathogens causing black point disease of wheat, J. Taibah Univ Sci. 2022;16:1:57–65.
Natsiopoulos D, Tziolias A, Lagogiannis I, Mantzoukas S, Eliopoulos PA. Growth-promoting and protective effect of Trichoderma atrobrunneum and T. simmonsii on tomato against Soil-Borne fungal pathogens. Crops. 2022;2:202–17.
Hao Y, Fang P, Ma C, White JC, Xiang Z, Wang H, Zhang Z, Rui Y, Xing B. Engineered nanomaterials inhibit Podosphaera pannosa infection on rose leaves by regulating phytohormones. Environ Res. 2019;170:1–6.
Javed B, Mashwani ZUR. Phytosynthesis of colloidal nanosilver from Mentha longifolia and Mentha arvensis: Comparative morphological and optical characterization. Microsc Res Tech. 2020;83(11):1299–307. https://doi.org/10.1002/jemt.23518.
Abu-Elsaad NI, Abdelhameed RE. Copper ferrite nanoparticles as nutritive supplement for cucumber plants grown under hydroponic system. J Plant Nutri. 2019;42:1645–59.
Abdelhameed RE, Abu-Elsaad NI, Abdel Latef AAH, Metwally RA. Tracking of zinc ferrite nanoparticle effects on pea (Pisum sativum L.) plant growth, pigments, mineral content and arbuscular mycorrhizal colonization. Plants (Basel). 2021;10(3):583.
Rodríguez-González V, Terashima C, Fujishima A. Applications of photocatalytic titanium dioxide based nanomaterials in sustainable agriculture. J Photochem Photobiol C Photochem Rev. 2019;40:49–67.
Waghmode MS, Gunjal AB, Mulla JA, et al. Studies on the titanium dioxide nanoparticles: biosynthesis, applications and remediation. SN Appl Sci. 2019;1:310. https://doi.org/10.1007/s42452-019-0337-3.
Satti SH, Raja NI, Ikram M, Oraby HF, Mashwani ZUR, Mohamed AH, Singh A, Omar AA. Plant-based titanium dioxide nanoparticles trigger biochemical and proteome modifications in Triticum aestivum L under. biotic stress of Puccinia striiformis. Molecules. 2022;27:4274.
Ganesan S, Babu IG, Mahendran D, Arulselvi PI, Elangovan N, Geetha N, Venkatachalam P. Green engineering of titanium dioxide nanoparticles using Ageratina altissima (L.) King & HE Robines. medicinal plant aqueous leaf extracts for enhanced photocatalytic activity. Ann Phyomedicine. 2016;5:69–75.
Javed B, Nadhman A, Mashwani Z. Optimization, characterization and antimicrobial activity of silver nanoparticles against plant bacterial pathogens phyto-synthesized by Mentha longifolia. Mater Res Express. 2020;7:085406.
Ikram M, Raja NI, Javed B, Mashwani Z-R, Hussain M, Hussain M, Ehsan M, Rafique N, Malik K, Sultana T, Akram A. Foliar applications of bio-fabricated selenium nanoparticles to improve the growth of wheat plants under drought stress. Green Process Synth. 2020;9:706–14.
Satti SH, Raja NI, Javed B, Akram A, Z-u-R M, Ahmad MS, Ikram M. Titanium dioxide nanoparticles elicited agromorphological and physicochemical modifications in wheat plants to control Bipolaris sorokiniana. PLoS ONE. 2021;16(2):e0246880.
Joshi AK, Chand R. Progress of researches done to understand host-pathogen relationship for spot blotch pathogen of wheat. J Wheat Res. 2011;3:1–7.
Singh PK, He X, Sansaloni CP, Juliana P, Dreisigacker S, Duveiller E, Kumar U, Joshi AK, Singh RP. Resistance to spot blotch in two mapping populations of common wheat is controlled by multiple QTL of minor effects. Int J Mol Sci. 2018;19:4054.
Singh UB, Malviya D, Singh S, Kumar M, Sahu PK, Singh HV, Kumar S, Roy M, Imran M, Rai JP, Sharma AK, Saxena AK. Trichoderma harzianum-and methyl jasmonate-induced resistance to Bipolaris sorokiniana through enhanced phenylpropanoid activities in bread wheat (Triticum aestivum L.). Front Microbiol. 2019;10:1697.
Villa-Rodríguez E, Parra-Cota F, Castro-Longoria E, López-Cervantes J, de los Santos-Villalobos S,. Bacillus subtilis TE3: A promising biological control agent against Bipolaris sorokiniana, the causal agent of spot blotch in wheat (Triticum turgidum L. subsp. durum). Biol Contr. 2019;132:135–43.
Matroudi S, Zamani MR, Motallebi M. Antagonistic effects of three species of Trichoderma sp. on Sclerotinia sclerotiorum, the causal agent of canola stem rot. Egypt J Biol. 2009;11:37–44.
Mukherjee M, Mukherjee PK, Horwitz BA, Zachow C, Berg G, Zeilinger S. Trichoderma-plant-pathogen interactions: Advances in genetics of biological control. Indian J Microbiol. 2012;53:522–9.
McIntyre M, Nielsen J, Arnau J, van der Brink H, Hansen K, Madrid S (eds). Proceedings of the 7th European Conference on Fungal Genetics. Copenhagen, Denmark. 2004.
Moreno-Ruiz D, Lichius A, Turra D, Di Pietro A, Zeilinger S. Chemotropism assays for plant symbiosis and mycoparasitism related compound screening in Trichoderma atroviride. Front Microbiol. 2020;11:601251.
Howell CR. Mechanisms employed by Trichoderma species in the biological control of plant diseases: the history and evolution of current concepts. Plant Dis. 2003;87:4–10.
Javed B. Synergistic effects of physicochemical parameters on bio-fabrication of mint silver nanoparticles: Structural evaluation and action against HCT116 colon cancer cells. Int J Nanomed. 2020;15:3621.
Abdalla H, Adarosy MH, Hegazy HS, Abdelhameed RE. Potential of green synthesized titanium dioxide nanoparticles for enhancing seedling emergence, vigor and tolerance indices and DPPH free radical scavenging in two varieties of soybean under salinity stress. BMC Plant Biol. 2022;22:560.
Burda C, Chen X, Narayanan R, El-Sayed MA. Chemistry and properties of nanocrystals of different shapes. Chem Rev. 2005;105:1025–102.
Subhapriya S, Gomathipriya P. Green synthesis of titanium dioxide (TiO2) nanoparticles by Trigonella foenum-graecum extract and its antimicrobial properties. Microb Pathog. 2018;116:215–20.
Lakshmeesha TR, Murali M, Ansari MA, Udayashankar AC, Alzohairy MA, Almatroudi A, Alomary MN, Asiri SMM, Ashwini BS, Kalagatur NK, Nayak CS, Niranjana SR. Biofabrication of zinc oxide nanoparticles from _Melia azedarach_ and its potential in controlling soybean seed-borne phytopathogenic fungi. Saudi J Biol Sci. 2020;27(8):1923–30.
Ilkhechi NN, Mozammel M, Khosroushahi AY. Antifungal effects of ZnO, TiO2 and ZnO- TiO2 nanostructures on Aspergillus flavus”. Pestic Biochem Phys. 2021;176:104869.
Abdelaziz AM, Salem SS, Khalil AMA, El-Wakil DA, Fouda HM, Hashem AH. Potential of biosynthesized zinc oxide nanoparticles to control Fusarium wilt disease in eggplant (Solanum melongena) and promote plant growth. Biometals. 2022;35:601–16.
Ghorbani F, Gorji P, Mobarakeh MS, Mozaffari HR, Masaeli R, Safaei M. Optimized synthesis of Xanthan gum/ZnO/TiO2 nanocomposite with high antifungal activity against pathogenic Candida albicans". J Nanomater. 2022;2022:10.
Boxi SS, Mukherjee K, Paria S. Ag doped hollow TiO2 nanoparticles as an effective green fungicide against Fusarium solani and Venturia inaequalis phytopathogens. Nanotechnology. 2016;27(8):085103.
Irshad MA, Nawaz R, Zia ur Rehman M, Imran M, Ahmad J, Ahmad S, Inam A, Razzaq A, Rizwan M, Ali S,. Synthesis and characterization of titanium dioxide nanoparticles by chemical and green methods and their antifungal activities against wheat rust. Chemosphere. 2020;258:127352.
Liu Z, Zhang M, Han X, Xu H, Zhang B, Yu Q, Li M. TiO2 nanoparticles cause cell damage independent of apoptosis and autophagy by impairing the ROS-scavenging system in Pichia pastoris. Chem Biol Interact. 2016;252:9–18.
Safaei M, Taran M, Imani MM. Preparation, structural characterization, thermal properties and antifungal activity of alginate-CuO bionanocomposite. Mater Sci Eng C. 2019;101:323–9.
Liao C, Li Y, Tjong SC. Visible-light active titanium dioxide nanomaterials with bactericidal properties”. J Nanomater. 2020;10(1):124.
Khalil MII, Youssef SA, Tartoura KA, Eldesoky AA. Comparative evaluation of physiological and biochemical alteration in tomatoplants infected by Alternaria alternata in response to Trichoderma viride and Chaetomium globosum application. Physiol Mol Plant Pathol. 2021;115:101671.
Yedidia I, Srivastva AK, Kapulnik Y, Chet I. Effect of Trichoderma harzianum on microelement concentrations and increased growth of cucumber plants. Plant Soil. 2001;235:235–42.
Contreras-Cornejo HA, Macı´as-Rodrı´guez L, Corte´ s-Penagos C, Lo´ pez-Bucio J,. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol. 2009;149:1579–92.
Morteza E, Moaveni P, Farahani HA, Kiyani M. Study of photosynthetic pigments changes of maize (Zea mays L.) under nano Tio2 spraying at various growth stages. Springerplus. 2013;2:1–5.
Mishra V, Mishra RK, Dikshit A, Pandey AC. Interactions of nanoparticles with plants: An emerging prospective in the agriculture industry. An emerging prospective in the agriculture industry. Emerg Technol Manag Crop Stress Toler Biol Tech. 2014;1:159–80.
Yang F, Hong FS, You WJ, Liu C, Gao F, Wu C, Yang P. Influences of nano-anatase TiO2 on the nitrogen metabolism of growing spinach. Biol Trace Element Res. 2006;110:179–90.
Jaberzadeh A, Moaveni P, Tohidi Moghadam HR, Zahedi H. Influence of bulk and nanoparticles titanium foliar application on some agronomic traits, seed gluten and starch contents of wheat subjected to water deficit stress. Not Bot Horti Agrobot Cluj-Napoca. 2013;41:201.
Rizwan M, Ali S, Qayyum MF, Ok YS, Adrees M, Ibrahim M, Zia-Ur-Rehman M, Farid M, Abbas F. Effect of metal and metal oxide nanoparticles on growth and physiology of globally important food crops: A critical review. J Hazard Mater. 2017;322:2–16.
El-Tayeb MA. Response of barley grains to the interactive effect of salinity and salicylic acid. Plant Growth Regul. 2005;45(3):215–24.
Sharma P, Jha AB, Dubey RS, Pessarakli M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot. 2012;2012:ID 217037. https://doi.org/10.1155/2012/217037.
Gámez-Arcas S, Baroja-Fernández E, García-Gómez P, Muñoz FJ, Almagro G, Bahaji A, Sánchez-López ÁM, Pozueta-Romero J. Action mechanisms of small microbial volatile compounds in plants. J Exp Bot. 2022;73(2):498–510. https://doi.org/10.1093/jxb/erab463.
Aldinary AM, Abdelaziz AM, Farrag AA, Attia MS. Biocontrol of tomato fusarium wilt disease by a new moringa endophytic aspergillus isolates. materials today: Proceedings. 2021;2021.
Khodakovskaya MV, Lahiani MH. Nanoparticles and plants: From toxicity to activation of growth. In: Sahu SC, Casciano DA, editors. Handbook Nanotoxicology, Nanomedicine Stem Cell Use Toxicol. Wiley online Library; 2014. p. 121–30. https://doi.org/10.1002/9781118856017.ch7.
Rodrìguez-González V, Obrego´n S, Patro´n-Soberano OA, Terashima C, Fujishima A. An approach to the photocatalytic mechanism in the TiO2-nanomaterials microorganism interface for the control of infectious processes. Appl Catal B Environ. 2020;270:118853.
Pelaez M, Nolan NT, Pillai SC, Seery MK, Falaras P, Kontos AG, Dunlop PSM, Hamilton JWJ, Byrne JA, O’Shea K, Entezari MH, Dionysiou DD. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl Catal B Environ. 2012;125:331–49.
Kubeš M, Drážná N, Konrádová H, Lipavská H. Robust carbohydrate dynamics based on sucrose resynthesis in developing Norway spruce somatic embryos at variable sugar supply. In Vitro Cell Dev Biol Plant. 2014;50(1):45–57.
Abdelhameed RE, Metwally RA. Mitigation of salt stress by dual application of arbuscular mycorrhizal fungi and salicylic acid. Agrochimica. 2018;62:353–66.
Ze Y, Liu C, Wang L, Hong M, Hong F. The regulation of TiO2 nanoparticles on the expression of light-harvesting complex II and photosynthesis of chloroplasts of Arabidopsis thaliana. Biol Trace Elem Res. 2011;143:1131–41.
Kumari M, Pandey S, Bhattacharya A, Mishra A, Nautiyal C. Protective role of biosynthesized silver nanoparticles against early blight disease in Solanum lycopersicum. Plant Physiol Biochem. 2017;121:216–25.
Abdel Latef AAH, Srivastava AK, El-sadek MSA, Kordrostami M, Tran LSP. Titanium dioxide nanoparticles improve growth and enhance tolerance of broad bean plants under saline soil conditions. Land Degrad Develop. 2018;29:1065–73.
Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A. Role of proline under changing environments: a review. Plant Signal Behav. 2012;7:1456–66.
Metwally RA, Azab HS, Al-Shannaf HM, Rabie GH. Prospective of mycorrhiza and Beauvaria bassiana silica nanoparticles on Gossypium hirsutum L. plants as biocontrol agent against cotton leafworm. Spodoptera littoralis BMC Plant Biol. 2022;22(1):1–18.
Metwally RA, Soliman SA. Alleviation of the adverse effects of NaCl stress on tomato seedlings (Solanum lycopersicum L.) by Trichoderma viride through the antioxidative defense system. Bot Stud. 2023;64:4.
Mohamed HI, Akladious SA. Changes in antioxidants potential, secondary metabolites and plant hormones induced by different fungicides treatment in cotton plants. Pest Biochem Physiol. 2017;142:117–22.
Metwally RA, Abdelhameed RE. Impact of Ridomil, Bavistin and Agrothoate on arbuscular mycorrhizal fungal colonization, biochemical changes and potassium content of cucumber plants. Ecotoxicology. 2019;28:487–98.
Abdelrhim AS, Mazrou YSA, Nehela Y, Atallah OO, El-Ashmony RM, Dawood MFA. Silicon dioxide nanoparticles induce innate immune responses and activate antioxidant machinery in wheat against Rhizoctonia solani. Plants. 2021;10:2758.
Shimizu N, Hosogi N, Hyon GS, Jiang S, Inoue K, Park P. Reactive oxygen species (ROS) generation and ROS induced lipid peroxidation are associated with plant membrane modifications in host cells in response to AK-toxin from Alternaria alternata Japanese pear pathotype. J Gen Plant Pathol. 2006;72:6–15.
Soliman SA, Abdelhameed RE, Metwally RA. In vivo and In vitro evaluation of the antifungal activity of the PGPR Bacillus amyloliquefaciens RaSh1 (MZ945930) against Alternaria alternata with growth promotion influences on Capsicum annuum L. plants. Microb Cell Fact. 2023;22:70.
Abdelhameed RE, Metwally RA. Alleviation of cadmium stress by arbuscular mycorrhizal symbiosis. Int J Phytoremediation. 2019;21:663–71.
Nasrallah DA, Morsi MA, El-Sayed F, Metwally RA. Structural, optical and electrical properties of copper chloride filled polyvinyl chloride/polystyrene blend and its antifungal properties against Aspergillus avenaceus and Aspergillus terreus. Compos Commun. 2020;22:100451.
Abdelhameed RE, Abdel Latef AAH, Shehata RS. Physiological responses of salinized fenugreek (Trigonella foenum-graecum L.) plants to foliar application of salicylic acid. Plants. 2021;10:657.
Metwally RA, Abdelhameed RE. Synergistic effect of arbuscular mycorrhizal fungi in growth and physiology of salt-stressed Trigonella foenum-graecum plants. Biocatal Agric Biotechnol. 2018;16:538–44.
Ishihara A, Kumeda R, Hayashi N, Yagi Y, Sakaguchi N, Kokubo Y, Ube N, Tebayashi SI, Ueno K. Induced accumulation of tyramine, serotonin, and related amines in response to Bipolaris sorokiniana infection in barley. Biosci Biotechnol Biochem. 2017;81(6):1090–8.
Bagy HMMK, Hassan EA, Nafady NA, Dawood MFA. Efficacy of arbuscular mycorrhizal fungi and endophytic strain Epicoccum nigrum ASU11 as biocontrol agents against blackleg disease of potato caused by bacterial strain Pectobacterium carotovora subsp. atrosepticum PHY7. Biol Control. 2019;134:103–13.
Singla P, Bhardwaj RD, Kaur S, Kaur J, Grewal SK. Metabolic adjustments during compatible interaction between barley genotypes and stripe rust pathogen. Plant Physiol Biochem. 2020;147:295–302.
Kaur S, Bhardwaj R, kaur J, kaur S. Induction of defense-related enzymes and pathogenesis-related proteins imparts resistance to barley genotypes against spot blotch disease. J Plant Growth Regul. 2021;41(2). https://doi.org/10.1007/s00344-021-10333-2.
Naz R, Nosheen A, Yasmin H, Bano A, Keyani R. Botanical-chemical formulations enhanced yield and protection against Bipolaris sorokiniana in wheat by inducing the expression of pathogenesis-related proteins. PLoS ONE. 2018;13(4):e0196194.
Ferrer JL, Austin MB, Stewart C Jr, Noel JP. Structure and function of enzymes involved in the biosynthesis of phenylpropanoids. Plant Physiol Biochem. 2008;46:356–70.
Król P, Igielski R, Pollmann S, Kȩpczyńska E. Priming of seeds with methyl jasmonate induced resistance to hemi-biotroph Fusarium oxysporum f. sp. lycopersici in tomato via 12-oxo-phytodienoic acid, salicylic acid, and flavonol accumulation. J Plant Physiol. 2015;179:122–32.
Abdelhameed R, Metwally R, Soliman S. Prospects of Bacillus amyloliquefaciens (MZ945930) mediated enhancement of Capsicum annuum L. plants under stress of Alternaria alternata in terms of physiological traits, thiol content, antioxidant defense, and phytohormones. J Plant Growth Regul. 2023. https://doi.org/10.1007/s00344-023-11132-7.
Metwally RA, Abdelhamed RE. Co-application of arbuscular mycorrhizal fungi and nano-ZnFe2O4 improves primary metabolites, enzymes and NPK status of Pea (Pisum sativum L.) plants. J Plant Nutr. 2023. https://doi.org/10.1080/01904167.2023.2280121.
Ohta H, Shida K, Peng Y, Furusawa I, Shishiyama J, Aibara S, Morita Y. A Lipoxygenase Pathway Is Activated in Rice after infection with the rice blast fungus Magnaporthe grisea. Plant Physiol. 1991;97(1):94–8.
Nandini B, Hariprasad P, Prakash HS, Geetha N. Trichoderma oligosaccharides priming mediates resistance responses in pearl millet against downy mildew pathogen. J Appl Biol Biotechnol. 2017;5(2):97–103.
Koch E, Meier BM, Eiben HG, Slusarenko A. A lipoxygenase from leaves of tomato (Lycopersicon esculentum Mill.) is induced in response to plant pathogenic pseudomonads. Plant Physiol. 1992;99:571–6.
Rusterucci C, Montillet JL, Agnel JP, Battesti C, Alonso B, Knoll A, Bessoule JJ, Etienne P, Suty L, Blein JP, Triantaphylides C. Involvement of lipoxygenase-dependent production of fatty acid hydroperoxides in the development of the hypersensitive cell death induced by cryptogein on tobacco leaves. J Biol Chem. 1999;274:36446–55.
Kumar S, Kaushik N, Edrada-Ebel R, Ebel R, Proksch P. Isolation, characterization, and bioactivity of endophytic fungi of Tylophora indica. World J Microbiol Biotechnol. 2011;27:571–7.
Rifai MA. A revision of the genus Trichoderma. Mycol Pap. 1969;116:1–56.
Wiese MV. Compendium of Wheat Diseases (St. Paul, MN: APS Press; 1987.
Wu ZH, Wang TH, Huang W, Qu YB. A simplified method for chromosome DNA preparation from filamentous Fungi. Mycosystema. 2001;20:575–7.
Tamura K, Nei M, Kumar S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci USA. 2004;101:11030–5.
Campanile G, Ruscelli A, Luisi N. Antagonistic activity of endophytic fungi towards Diplodia corticola assessed by in vitro and in planta tests. Eur J Plant Pathol. 2007;117:237–46.
Ghildial A, Pandey A. Isolation of cold tolerant antifungal strains of Trichoderma sp. from glacier sites of Indian Himalayan region. Res J Microbiol. 2008;3(8):559–64.
Soliman SA, Khaleil MM, Metwally RA. Evaluation of the antifungal activity of Bacillus amyloliquefaciens and B. velezensis and characterization of the bioactive secondary metabolites produced against plant pathogenic fungi. Biology. 2022;11:1390.
Mohamed AA, Abu-Elghait M, Ahmed NE, Salem SS. Ecofriendly mycogenic synthesis of ZnO and CuO nanoparticles for in vitro antibacterial, antibiofilm, and antifungal applications. Biol Trace Elem Res. 2021;199:2788–99.
Lichtenthaler HK, Wellburn AR. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Analysis. 1983;11(5):591–2.
Lowry OH, Rosbrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–75.
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Calorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–6.
Bates LS, Waldren RP, Teare LD. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–7.
Heath RL, Packer L. Photoperoxidation in isolated chloroplasts: I. kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125(1):189–98.
Alexieva V, Sergiev I, Mapelli S, Karanov E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001;24:1337–44.
Bergmeyer HU. Methods of Enzymatic Analysis 1. New York: Seacond es. Acadimic press; 1974.
Aebi H. Catalase. In: Bergmeyer H, editor. Methods of enzymatic analysis. Weinheim: Weinheim-Verlagchemie; 1983. p. 273–86.
Alunni S, Cipiciani A, Fiorini G, Ottavi L. Mechanisms of inhibition of phenylalanine ammonia lyase by phenol inhibitors and phenol / glycine synergistic inhibitors. Arch Bioch Biophy. 2003;412:170–5.
Axelord B, Cheesbrough TM, Laakso S. Lipoxygenases in soybean. Methods Enzymol. 1981;71:441–51.
Acknowledgements
We greatly appreciate the financial support from the Academy of Scientific Research and Technology, Egypt.
Experimental research and field studies on plants
“All relevant institutional, national and international guidelines and legislation were compiled or adhered to in the production of this study”.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Conceptualization: H.A., R.E.A., R.A.M. and S.A.S.; Methodology: R.E.A., R.A.M., H.A. and S.A.S.; Formal analysis and investigation: R.A.M. and R.E.A.; Writing: R.A.M. and R.E.A.; Review and editing: R.A.M., S.A.S. and R.E.A. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.”
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Metwally, R.A., Soliman, S.A., Abdalla, H. et al. Trichoderma cf. asperellum and plant-based titanium dioxide nanoparticles initiate morphological and biochemical modifications in Hordeum vulgare L. against Bipolaris sorokiniana. BMC Plant Biol 24, 118 (2024). https://doi.org/10.1186/s12870-024-04785-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-024-04785-3