Abstract
Background
Sesame charcoal rot caused by Macrophomina phaseolina is one of the most serious fungal diseases in sesame production, and threatens the yield and quality of sesame. WAKL genes are important in the plant response to biotic stresses by sensing and transmitting external signals to the intracellular receptor. However, there is still a lack about the WAKL gene family and its function in sesame resistance to M. phaseolina. The aim of this study was to interpret the roles of WAKL genes in sesame resistance to M. phaseolina.
Results
In this study, a comprehensive study of the WAKL gene family was conducted and 31 WAKL genes were identified in the sesame genome. Tandem duplication events were the main factor in expansion of the SiWAKL gene family. Phylogenetic analysis showed that the sesame SiWAKL gene family was divided into 4 groups. SiWAKL genes exhibited different expression patterns in diverse tissues. Under M. phaseolina stress, most SiWAKL genes were significantly induced. Notably, SiWAKL6 was strongly induced in the resistant variety “Zhengzhi 13”. Functional analysis showed that SiWAKL6 was induced by salicylic acid but not methyl jasmonate in sesame. Overexpression of SiWAKL6 in transgenic Arabidopsis thaliana plants enhanced their resistance to M. phaseolina by inducing the expression of genes involved in the salicylic acid signaling pathway and reconstructing reactive oxygen species homeostasis.
Conclusions
Taken together, the results provide a better understanding of functions about SiWAKL gene family and suggest that manipulation of these SiWAKL genes can improve plant resistance to M. phaseolina. The findings contributed to further understanding of functions of SiWAKL genes in plant immunity.
Similar content being viewed by others
Introduction
The plant cell wall, composed of cellulose, hemicellulose, pectin and a few structural proteins, is not only crucial in cell morphology but is also the first line of defense against pathogens. Receptor-like kinases (RLKs) are an important kinase family in the cell membrane, which contains various extracellular domains that are suitable for recognizing external signals, such as leucine-rich repeats, lectins, lysine motifs, and epidermal growth factor extracellular domains [1, 2]. Under multifarious stresses, RLKs can recognize extracellular signals and transmit them into cells, subsequently activating the expression of downstream transcription factors (TF), pathogenesis-related (PR) genes and plant hormone-related genes [3, 4]. In vascular plants, cell wall-associated receptor kinases (WAKs) containing the wall-associated receptor kinase galacturonan-binding (GUB-WAK-bind) domain and protein kinase domain (PKinase) are a subfamily of the RLK family [5]. WAKL is the only protein known to act as a direct link between the cell wall and the plasma membrane, which enables plants to sense changes in cell wall structure and rapidly initiate intracellular signal transduction processes and defense responses [6, 7].
Pro25, the first member of the WAKLs, was found to be closely related to the cell wall in Arabidopsis thaliana. Therefore, it was renamed cell wall-associated kinase 1 (WAK1), and the concept of a cell wall-associated receptor kinase was proposed [8]. Subsequently, a highly conserved family containing WAK1-5 and 22 WAK-like genes (WAKLs) in A. thaliana was discovered [9].
WAKLs are important in plant responses to environmental stress. AtWAKL10 can be induced by nitric oxide in A. thaliana. AtWAKL10 plays a positive role in basic defense, effect-triggering immunity and salt stress but is negative in response to drought [10]. The cell wall pectin of Craterostigma plantagineum could bind to CpWAK1 and regulate its growth under non-pressure conditions. However, during dehydration, C. plantagineum glycine-rich protein 1 interacts with Ca2+ and pectin to form a complex that can bind to CpWAK1 with high affinity. Finally, pectin perturbations in the cell wall during dehydration were sensed by CpWAK1, leading to activation of downstream dehydration signaling pathways [11]. WAKLs could mediate defense responses induced by chitin. For instance, the wheat TaWAK7D gene can be induced by chitin stimulation, which plays a positive role in plant defense against Rhizoctonia cerealis [12]. The Rlm9 gene in Brassica napus supports its resistance to blackleg, in which the GUB-WAK-bind domain of Rlm9 is involved in the recognition of pathogens [13]. Hurni et al. showed that Htn1, a WAK gene in maize, was closely associated with resistance to maize leaf blight. The pathogen penetrates the cell wall, and produces cell wall fragments that can be recognized and sensed by Htn1, which in turn transmits the signal downstream and activates the expression of PR genes to enhance maize resistance to leaf blight [14]. Similarly, TaWAK6 is involved in wheat resistance to leaf rust [15], and TaWAK2 enhances wheat resistance to Fusarium graminearum by binding to pectin [16], which further highlights the importance of WAKL gene family in plant disease resistance. Nevertheless, some WAKLs negatively regulate plant disease resistance. For example, ScWAK1 can induced hypersensitive response and the expression of ethylene-related genes, negatively regulating the defense against Sporisorium scitamineum in sugarcane [17]. The Snn1 gene encoding WAK in wheat conferred plant susceptibility by responding to the toxin produced by Stagonospora nodorum and triggering cell death, thereby promoting the proliferation of fungi in wheat [18].
Importantly, an increasing number of studies have indicated that WAKLs are the junctions that regulate plant stress signaling and development in plants [19]. Xa4, encoding a WAK gene, can enhance the thickness of the cell wall and thus confer rice durable resistance to Xanthomonas oryzae without affecting rice yield, which is significant in rice breeding [20]. Similarly, it is proposed that ZmWAK is the hub of fine-tuning between maize growth and defense. ZmWAK promotes cell growth under normal conditions but protects plants when maize plants attacked by pathogens [21], suggesting that studying WAKL-mediated mechanisms to balance plant immunity and yield contributes to crop breeding.
Sesame (Sesamum indicum L.), widely cultivated in tropical and subtropical regions, is one of the most nutritious oil crops. Sesame seeds contain unsaturated fatty acids and various natural antioxidants, which are healthy to humans [22, 23]. Sesame charcoal rot caused by Macrophomina phaseolina is one of the most serious fungal diseases in sesame production, and threatens the yield and quality of sesame, which diminishes sesame production of 10–15% or even over 80% in serious cases. Furthermore, Although the WAKL gene family is important in plant biotic stresses, there is still a lack of systematic studies on the roles of WAKL gene family in the interaction between sesame and M. phaseolina. In this study, the WAKL family in sesame was identified and comprehensively analyzed on a genome-wide scale. In addition, this study reported that SiWAKL6 was crucial in plant immunity, acting in the SA signaling pathway and ROS homeostasis. The results not only provided more insights into the classification and biological function of SiWAKLs, but also promoted the application of the SiWAKL genes in the molecular breeding of sesame resistance to M. phaseolina.
Results
Identification and phylogenetic analysis of the SiWAKL gene family
A total of 31 WAKL genes were exhaustively identified based on the Sesamum indicum genome and were named SiWAKL1-SiWAKL31 according to their position on the chromosome (Additional file: Table S1). The prefix “Si” represents the species “S. indicum”. Bioinformatic analysis showed that the SiWAKL proteins in sesame contained 504 to 787 amino acids. Their molecular weights ranged from 57.3 to 86.62 kDa and their isoelectric points ranged from 5.37 to 8.83. Most SiWAKL proteins are stable, and the high aliphatic index implied that they localized to the cell membrane.
To investigate the taxonomic and evolutionary relationships of WAKL proteins in S. indicum and A. thaliana, a phylogenetic tree was constructed based on the aligned sequences of WAKL proteins. The WAKL proteins in S. indicum and A. thaliana were divided into 4 subfamilies, subfamilies I, II, III and IV (Fig. 1). Among them, subfamily IV is the largest group, containing 19 SiWAKL proteins and 4 AtWAKL proteins, followed by subfamily III, which contains 15 AtWAKL proteins and 4 SiWAKL proteins. Subfamily I contained only 7 AtWAKL proteins. Subfamily II contained no AtWAKL proteins, suggesting that WAKL proteins in subfamily II are conserved and unique WAKLs that have developed in S. indicum during evolution.
Sequence analysis and functional prediction of SiWAKL proteins
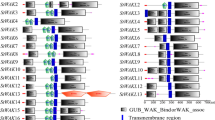
The evolutionary tree of the SiWAKLs proteins was constructed using MEGA7 software (Fig. 2A), which was similar to the results of the phylogenetic tree in Fig. 1, indicating the reliability of the results. Conserved motif analysis of SiWAKL proteins showed that all the SiWAKLs contain Motif 4, Motif 5, Motif 6, Motif 7, Motif 8 and Motif 9 in their C-terminal, indicating that the protein kinase domain was more conserved and important in the SiWAKLs (Fig. 2B). All SiWAKLs have GUB-WAK-bind domain in the N-terminal and protein kinase domain in C-terminal, which further demonstrates the reliability of the SiWAKL members in sesame (Fig. 2C). In addition, 11 members of SiWAKLs, including SiWAKL2, SiWAKL 3, SiWAKL 4, SiWAKL 5, SiWAKL 6, SiWAKL 11, SiWAKL 13, SiWAKL 14, SiWAKL 29, SiWAKL 30, and SiWAKL 31, had WAK or WAK-associated domains. A total of 8 SiWAKLs contained EGF-CA or EGF-3 domains (Fig. 2C). The exon‒intron structure showed that the exons of the SiWAKL genes ranged from 2 to 5, and most SiWAKL genes had 3 exons (Fig. 2D).
To understand the biological roles of SiWAKLs initially, a functional prediction analysis was performed. Gene Ontology (GO) annotation was performed for 31 SiWAKLs based on biological process (BP), cellular component (CC) and molecular function (MF) terms (Fig. 3). In terms of BP, SiWAKL proteins mainly function in protein phosphorylation and the cell surface receptor signaling pathway, which are closely related to cellular perception and transduction of external signals. In terms of MF, it was demonstrated that SiWAKLs could bind polysaccharides, ATP and Ca2+, all of which have been shown to be associated with biotic and abiotic stresses in plants. Based on the GO annotation of SiWAKLs, it was inferred that SiWAKLs exerted protein phosphorylation and cell surface signal transduction by binding polysaccharides, ATP and Ca2+, which might be important for plant resistance to pathogens (Fig. 3).
Chromosome localization and duplication events of SiWAKL genes
Based on the S. indicum genome, 31 SiWAKL genes were mapped unevenly to 8 chromosomes (Chr). SiWAKL genes were distributed on Chr 1, Chr 3, Chr 5, Chr 6, Chr 8, Chr 10, Chr 11 and Chr 12. Chr 12 contained the most SiWAKL genes (6 SiWAKLs, 19.4%), followed by Chr 6 (5 SiWAKLs, 16.1%). Chr 3, Chr 5, Chr 8 and Chr 11 all comprised 4 SiWAKL genes. In contrast, Chr 1 contained only one SiWAKL gene (Fig. 4). Additionally, 9 gene clusters were formed by 25 genes on seven chromosomes (Fig. 4).
Chromosome localization and duplication events of SiWAKL genes. The green boxes represent 13 chromosomes of sesame. The gray lines indicate all the segmentally duplicated gene pairs within sesame genome, while red line highlight segmentally duplicated SiWAKL gene pairs SiWAKL15 and SiWAKL19. SiWAKL genes under orange background represent gene clusters. SiWAKL genes in red font indicate tandemly duplicated SiWAKL genes
Gene duplication events are important in the formation of new genes and plant adaptation. Segmental and tandem duplication are vital drivers in the expansion of gene families, especially plant RLK gene family [24]. To elucidate the mechanism of expansion of the SiWAKL gene family, synteny analysis of SiWAKL genes within the S. indicum genome was performed by MCScanX (Fig. 4). A total of 18 genes in sesame underwent duplication events, including 10 tandem duplication events formed by 18 SiWAKL genes and 1 segmental duplication event formed by 2 SiWAKL genes SiWAKL15 and SiWAKL19 (Fig. 4), indicating that tandem duplication events are the prime driver of SiWAKL gene family expansion.
Evolution analysis of SiWAKL genes in several plants
To infer the syntenic relationship of SiWAKL genes in several plants, seven dicotyledons (Solanum tuberosum, Solanum lycopersicum, Glycine max, Gossypium hirsutum, Vitis vinifera, Medicago truncatula and Arabidopsis thaliana) (Fig. 5A) and seven monocotyledons (Oryza sativa, Setaria italica, Musa acuminata, Hordeum vulgare, Sorghum bicolor, Zea mays and Triticum aestivum) (Fig. 5B) were used for evolution analysis with S. indicum. The WAKL genes are homologous to genes in the dicotyledonous reference plants, and the number of homologous WAKL genes is 8 (S. tuberosum), 7 (S. lycopersicum), 5 (G. max), 5 (G. hirsutum), 6 (V. vinifera), 6 (M. truncatula) and 3 (A. thaliana). Nonetheless, only 2 (O. sativa), 2 (S. italica), 1 (M. acuminata), 1 (H. vulgare), 1 (S. bicolor), 0 (Z. mays) and 0 (T. aestivum) homologous WAKL genes existed in monocotyledons. More homologous WAKL genes were found in dicotyledons than in monocotyledons (Additional file: Table S2). In addition, SiWAKL14 and SiWAKL13 were homologous with 9 and 8 species, respectively, suggesting that they are crucial in the evolution of the WAKL gene family. (Fig. 5).
Synteny analysis of WAKL genes between S. indicum and other plant species. Orange boxes represent chromosomes of sesame while green boxes represent chromosomes of other plant species. The gray lines indicate all the syntenic gene pairs between S. indicum and other plant species while red lines highlight the SiWAKL gene pairs. (A) Synteny analysis of WAKL genes between S. indicum and dicotyledonous plants. (B) Synteny analysis of WAKL genes between S. indicum and monocotyledonous plants
Expression profiles of SiWAKL genes during M. phaseolina Infection
The expression patterns of SiWAKL genes can provide important clues for exploring their potential functions. To gain a broader understanding of the functions of SiWAKLs, the expression profiles of SiWAKL genes in roots, stems, leaves, flowers, capsules and seeds were analyzed using transcriptome data in this investigation. Pearson correlation analyses and principal component analyses showed that the repeatability of samples from diverse sesame tissues was good (Additional file: Figure S1A, S1B). The results revealed that different SiWAKL genes were expressed diversely in different tissues (Fig. 6A, Additional file: Table S3). Most SiWAKL genes were highly expressed in roots, followed by leaves. Among them, SiWAKL22 and SiWAKL24 were constitutively expressed at a high level in all tissues (Additional file: Table S3), suggesting their important role in plant growth and development. Notably, SiWAKL6 was also highly expressed in both leaves and roots (Fig. 6A, Additional file: Table S3).
Expression profiles of SiWAKL genes in sesame based on RNA-seq transcriptomic analysis. FPKM values of SiWAKL genes were normalized. Red boxes mean higher expression level while blue boxes represent lower expression level. (A) Expression profiles of SiWAKL genes in root, stem, leaf, flower, capsule and seed tissues. (B) Expression profiles of SiWAKL genes in response to M. phaseolina stress within 48 h post inoculation. ZZ13, disease-resistant variety Sesamum indicum var. ‘Zhengzhi 13’. J9014, disease-susceptible variety Sesamum indicum var. ‘Ji 9014’
To understand the roles of the SiWAKL genes under M. phaseolina stress, the expression patterns of SiWAKLs were determined using transcriptome data PRJNA706471 of sesame Zhengzhi 13 (ZZ13, disease-resistant variety) and Ji 9014 (J9014, disease-susceptible variety) under M. phaseolina stress. The results showed that different SiWAKL family members responded differently to M. phaseolina stress and the expression of SiWAKLs varied with stress duration (Fig. 6B). SiWAKL8, SiWAKL9, SiWAKL1 and SiWAKL22 were induced by M. phaseolina in both ZZ13 and J9014, indicating that they may contribute to the basal resistance of sesame. In addition, SiWAKL6, SiWAKL11, SiWAKL18, and SiWAKL20 were significantly induced in ZZ13 but not J9014, and their transcripts increased with stress time (Fig. 6B, Additional file: Table S4), implying that they may mediate sesame resistance to M. phaseolina. Although SiWAKL20 was differentially expressed in ZZ13 versus J9014, their expression level was very low (FPKM < 1.5). SiWAKL18 was induced in the ZZ13 within 48 h post inoculation, but it was also induced the same level in the J9014 at 12 h post inoculation. Additionally, SiWAKL11 was induced in ZZ13 within 48 h post inoculation while it was also induced the same level in J9014 at 24 and 48 h post inoculation (Additional file: Table S4). However, the expression of SiWAKL6 gene was induced uniquely in ZZ13 rather than J9014. The expression level of SiWAKL6 gene increased from 1.45 to 9.99 in the resistant cultivar while that of SiWAKL6 gene remained below 1. The overall expression level of SiWAKL6 gene in ZZ13 is much higher than that in J9014. Therefore, we focused on SiWAKL6 gene and cloned it, and found that the coding sequence of SiWAKL6 was different among ZZ13 and J9014 (Additional file: Figure S2). The facts above indicated that SiWAKL6 may be related to sesame resistance to M. phaseolina, hence SiWAKL6 was selected for follow-up functional characterization.
SiWAKL6 was induced by M. phaseolina and SA
To further investigate the function of SiWAKL6 in sesame resistance to M. phaseolina, the complete coding sequence of SiWAKL6 was obtained using root RNA of ZZ13. SiWAKL6 is a 2924-bp gene located on Chr 5 (Fig. 7A) that contains two exons and one intron (Fig. 7B). The SiWAKL6 gene encodes a 729-residue protein with a protein kinase domain in C-terminal (aa 398 to aa 664) and a GUB-WAK-bind domain in the N-terminal (aa 30 to aa 89), which is a characteristic domain of the WAKL family (Fig. 7C). A 22-residue signal peptide was detected at the N-terminal of SiWAKL6 and a transmembrane structure was predicted from aa 323 to aa 342 of SiWAKL6 (Fig. 7D). All these features are consistent with the WAKL identity of SiWAKL6.
Bioinformatics and expression characteristics of SiWAKL6. The chromosomal location (A), gene structure (B), domains (C), signal peptide and transmembrane structure (D) of SiWAKL6. (E) The relative expression level of the SiWAKL6 gene in ZZ13 and J9014 roots post inoculation with M. phaseolina. (F) The relative expression level of the SiWAKL6 gene after SA treatment, with water treatment as a mock. (G) The relative expression level of the SiWAKL6 gene after MeJA treatment, with water treatment as a mock. SiWAKL6 expression were quantified with SiUBQ5 as a normalization control. Student’s t test (*, P < 0.05; **, P < 0.01) was used to analyze the data, with three biological replicates per sample; data are the mean ± SD
The relative expression level of the SiWAKL6 gene in ZZ13 and J9014 roots post inoculation with M. phaseolina was performed by qPCR. The results showed that SiWAKL6 could be significantly induced by M. phaseolina in ZZ13, reaching a peak after 3 h. However, SiWAKL6 was not induced in J9014, suggesting that SiWAKL6 might regulate the high resistance of ZZ13 to M. phaseolina (Fig. 7E). Phytohormones commonly regulate the expression of plant disease-associated proteins [25]. To investigate the potential role of SiWAKL6 in the plant hormonal signaling pathway, the expression pattern of SiWAKL6 in ZZ13 after SA and MeJA treatments was examined, with water treatment as a Mock. The results showed that phytohormones affected SiWAKL6 expression. SiWAKL6 rapidly increased under exogenous SA treatment (Fig. 7F). However, the expression of SiWAKL6 after exogenous MeJA treatment was similar to that after water treatment (Fig. 7G). Taken together, the results demonstrated that SiWAKL6 was induced by M. phaseolina and SA, implying that SiWAKL6 might enhance sesame resistance to M. phaseolina through the SA pathway.
SiWAKL6 enhanced A. thaliana resistance to M. phaseolina through the SA pathway
To further determine the function of SiWAKL6 in resistance to M. phaseolina, transgenic A. thaliana plants overexpression SiWAKL6 (OE-SiWAKL6) were constructed. DNA from three OE-SiWAKL6 transgenic A. thaliana lines (OE-1, OE-2 and OE-3) and WT (Fig. 8A) were used for PCR (Fig. 8B). Eight-week-old A. thaliana were inoculated with M. phaseolina. The results showed that the WT exhibited significant leaf chlorosis, necrosis and growth retardation, while the OE-1, OE-2 and OE-3 lines exhibited milder symptoms (Fig. 8A). Additionally, the disease index (DI) of A. thaliana plants was determined and found that the DI of OE-1, OE-2 and OE-3 decreased by 53%, 44% and 47%, respectively, compared with that of WT (Fig. 8C). Moreover, qPCR was performed with primer pairs specifically targeting species-specific sequence characterized amplified regions of M. phaseolina (MpSyk) and A. thaliana (AtSK11) DNA to compare the relative biomass of M. phaseolina and A. thaliana. The results showed that the relative abundance of M. phaseolina was reduced by 81%, 58% and 72% in the OE1, OE2 and OE3 lines, respectively, compared with the WT (Fig. 8D). The results above all implied that SiWAKL6 can enhance transgenic A. thaliana resistance to M. phaseolina.
SiWAKL6 enhanced transgenic A. thaliana resistance to M. phaseolina. (A) Phenotypes of WT and transgenic A. thaliana plants at 14 days after inoculation by M. phaseolina. (B) Transgenic plants were confirmed by PCR. “-” represents water, while “+” represents recombinant plasmid. (C) Disease index of WT and transgenic A. thaliana plants at 14 days after inoculation by M. phaseolina. (D) Relative biomass of M. phaseolina (MpSyk) and A. thaliana (AtSK11) DNA in WT and transgenic A. thaliana plants at 14 days after inoculation by M. phaseolina. (E) Relative expression of AtNPR1, AtPR1 and AtPR5 genes in the SA pathway at 12 h postinoculation by M. phaseolina, with water treatment as a mock. (F) Relative expression of AtVSP2 and AtPDF1.2 genes in the JA pathway at 12 h postinoculation by M. phaseolina, with water treatment as a mock
When M. phaseolina is challenged, we hypothesized that SiWAKL6 might enhance A. thaliana resistance by regulating the expression of biotic stress marker genes downstream. Thus, the expression of marker genes in the SA and JA hormone signaling pathways was detected, including the AtNPR1, AtPR1 and AtPR5 genes in the SA pathway and the AtVSP2 and AtPDF1.2 genes in the JA pathway. The results showed that the expression of the AtNPR1, AtPR1 and AtPR5 genes was higher in OE-SiWAKL6 plants than in WT plants under M. phaseolina stress (Fig. 8E) while the AtVSP2 and AtPDF1.2 genes showed similar expression patterns in OE-SiWAKL6 and WT plants (Fig. 8F), suggesting that SiWAKL6 could increase A. thaliana resistance by regulating genes in the SA pathway.
SiWAKL6 reconstructed ROS homeostasis in plant immunity
Under biotic stress, plant immunity depends on the production of ROS in plants, but the excess accumulation of ROS causes oxidative damage. Meanwhile, the antioxidant system in plants initiates the synthesis of superoxide dismutase (SOD), catalase (CAT) and peroxidase (POD) to scavenge ROS in plants and inhibit cell death. To investigate whether SiWAKL6 gene-mediated resistance to M. phaseolina was associated with ROS homeostasis, we analyzed the relative ROS levels (hydrogen peroxide (H2O2) and malonaldehyde (MDA) and relative antioxidant enzyme activities (SOD and CAT) in WT and transgenic plants (OE-SiWAKL6) at 14 days after inoculation with M. phaseolina.
We found that the concentrations of H2O2 and MDA were increased in both OE-SiWAKL6 and WT plants under M. phaseolina stress. However, WT accumulated more H2O2 (Fig. 9A) and MDA (Fig. 9B) than OE-SiWAKL6. The high H2O2 and MDA levels in WT could cause hypersensitivity in the M. phaseolina infection process, which provides insight into a potential link between ROS levels and M. phaseolina resistance. In addition, the CAT (Fig. 9C) and SOD (Fig. 9D) activities were enhanced in OE-SiWAKL6 compared with WT plants post inoculation by M. phaseolina, suggesting SiWAKL6 was involved in plant immunity by reconstructing ROS homeostasis, which was regulated by an active antioxidant system.
SiWAKL6 regulated ROS homeostasis in resistance to M. phaseolina. H2O2 (A) and MDA (B) contents in WT and OE-SiWAKL6 plants 14 days after inoculation by M. phaseolina, with water treatment as a mock. CAT (C) and SOD (D) enzyme activity in WT and OE-SiWAKL6 plants 14 days after inoculation by M. phaseolina, with water treatment as a mock
Discussion
WAKL proteins, acting as a link between the cell wall and plasma membrane, enable plants to sense external signals, which is vital for plant growth, development and response to stress. The WAKL gene family has been reported successively in A. thaliana (27 WAKLs) [9], Oryza sativa (130 WAKLs) [26], Gossypium hirsutum (99 WAKLs) [27], Brassica rapa ssp. pekinensis (96 WAKLs) [28] and Populus trichocarpa (175 WAKLs) [29] genomes. However, there is no systematic analysis of the WAKL gene family in sesame, and the functions of the SiWAKL genes are still unclear. The publication of high-quality whole genome sequences of sesame provided the possibility for sesame transcriptome sequencing and gene family identification [30, 31]. In this study, a total of 31 SiWAKL genes were identified within the sesame genome, which are distributed on 8 chromosomes. SiWAKL proteins all comprise GUB-WAK-bind domains and protein kinase domains. Compared to monocotyledons (130 WAKLs in rice, 91 WAKLs in barley and 99 WAKLs in cotton), the WAKL gene family is generally smaller in dicotyledons (31 WAKLs in sesame, 27 WAKLs in Arabidopsis and 21 WAKLs in sweet orange), indicating that WAKL genes have undergone different degrees of expansion during evolution between monocotyledons and dicotyledons.
Within the sesame genome, 18 SiWAKL genes form ten tandem duplication events while 2 SiWAKL genes constitute one segmental duplication event. The tandem duplication events were much more than the segmental duplication events, indicating that tandem duplication events were the principal factor in the expansion of the SiWAKL gene family in this study (Fig. 4). In addition, collinearity analysis showed that the homologous WAKL genes existed much more in dicotyledons than monocotyledons (Fig. 5, Additional file: Table S2), implying that the duplication of the WAKL gene probably occurred after the differentiation of dicotyledons and monocotyledons. Sesame species were evolutionarily more closely related to potato species and tomato species [31]. Interestingly, SiWAKL genes had the most homologous gene pairs with those in potato and tomato, suggesting that WAKL genes in these species may have a common ancestor. The evidence above provided clues to investigate the evolutionary process of WAKL genes in sesame.
The WAKL family is a crucial class of pattern recognition receptors that function in recognizing pathogens in plants. As shown by GO annotation (Fig. 3), SiWAKLs located at the plasma membrane could bind to polysaccharides, ATP and Ca2+. This is consistent with previous studies in other species, such as the OsWAK1 gene in rice [32], the ZmWAK-Hnt1 gene in maize [33] and the TaWAK-6D gene in wheat [34], which are all localized in the plasma membrane. On the one hand, WAKLs can initiate plant immune responses by binding oligogalacturonides (OGs) and pectins. They can perceive exogenous biotic and abiotic stimuli by GUB-WAK-bind domains. On the other hand, WAKLs can transmit signals into the cell to activate downstream cascade responses by their Pkinase domains [35]. AtWAK1 and AtWAK2 have been shown to interact with OGs and pectins in vitro [36, 37]. When treated with OGs, the expression of AtWAK1 could be induced, followed by the initiation of downstream immune responses such as callose accumulation, which enhanced A. thaliana resistance to Botrytis cinerea [38]. In this study, SiWAKL6 responds to M. phaseolina and exogenous SA (Fig. 7E and F), which is similar to previous studies. AtWAK1 also is induced by pathogen infection or exogenous SA treatment [39] to enhance plant resistance. WAKLs generally confer plant resistance against pathogens by regulating biological processes including cell wall reinforcement [20], activation of PR genes [40], SA or JA accumulation [5] and ROS homeostasis [5]. Rice OsWAK14, OsWAK91 and OsWAK92 genes can positively regulate resistance to rice blast fungus by increasing the expression of PR genes. In addition, OsWAK91 is a key gene for H2O2 production in rice, suggesting that OsWAK91 can enhance its resistance to pathogens by re-establishing ROS homeostasis and upregulating PR genes [41]. Additionally, it has been reported that CsWAKL08 in citrus confers resistance to citrus bacterial canker via ROS control and JA signaling [5]. Similarly, the SiWAKL6 gene in this study might also enhance sesame resistance to M. phaseolina through the SA signaling pathway and re-establishment of ROS homeostasis.
WAKL proteins can exert phosphorylation to participate in signal transduction in plants. Studies have reported that AtWAK2 can activate mitogen-activated protein kinases 3 (MPK3) and MPK6 and transmit signals intracellularly in plant innate immunity [36, 42]. Wang et al. showed that cotton GhWAK7A confers high resistance to Verticillium dahliae and Fusarium oxysporum f. sp vasinfectum. GhWAK7A can phosphorylate the chitin receptor complex upon pathogen infestation, which can activate cytoplasmic signaling pathways, including ROS production, activation of MAPK cascades and expression of PR genes. Moreover, silencing of the GhWAK7A gene impaired the activation of GhMPK3 and GhMPK6 genes in cotton and attenuated resistance [43]. However, it is unknown whether SiWAKL6 can mediate downstream signaling pathways through phosphorylation and MAPK cascades, and subsequent studies will continue.
Conclusion
In this study, a total of 31 SiWAKL genes were identified and analyzed for their chromosomal distribution, taxonomy, protein structures, duplication events and expression patterns. The expansion of the SiWAKL gene family was mainly due to tandem duplication events. Transcriptomic and qPCR analyses showed that SiWAKL6 was a potential gene involved in sesame resistance to M. phaseolina, which was induced by M. phaseolina and exogenous SA. Further functional analysis revealed that SiWAKL6 overexpression in transgenic A. thaliana plants enhanced A. thaliana resistance to M. phaseolina. We found that SiWAKL6 conferred higher resistance to transgenic A. thaliana plants by increasing the expression of SA pathway related genes and reconstructing ROS homeostasis. Taken together, the results of this study provide new insight into the mechanisms of SiWAKL6 gene acting in sesame immunity and a basis for the application of SiWAKLs in molecular breeding for sesame resistance to M. phaseolina.
Methods
Identification and bioinformatics analysis of the WAKL gene family in sesame
Gene and protein sequences of all 27 WAKLs of A. thaliana were downloaded from the TAIR website (https://www.arabidopsis.org/). The genome and proteome sequences of sesame were provided by the Sesame Research Center, Henan Academy of Agricultural Sciences [30, 44]. To exhaustively identify WAKLs in sesame, all 27 AtWAKL proteins were used to perform BLASTP with the sesame proteome and all candidate genes with E values less than 10− 10 were screened. The candidate sequences were detected in the InterPro database (https://www.ebi.ac.uk/interpro/) for the presence of both the GUB-WAK-bind domain and PKinase domain. Proteins that met all conditions were considered sesame WAKL proteins.
Multiple sequence alignment of SiWAKL proteins was analyzed using the ClustalW method. Phylogenetic analysis based on the aligned sequences of WAKL proteins was performed by MEGA 7 software [45] with the neighbor Joining (NJ) method (Bootstrap = l000). Additionally, the chromosomal location of the SiWAKL genes was visualized by TBtools [46]. The MCScanX [47] program was used to determine collinear orthologous gene duplications (Tandem and segmental duplications) among the sesame WAKL gene family and syntenic WAKL genes between sesame and other plant species. The genome files and annotation files of S. tuberosum, G. max, S. lycopersicum, M. truncatula, A. thaliana, V. vinifera, G. hirsutum, H. vulgare, Z. mays, T. aestivum, O. sativa, M. acuminata, S. italica and S. bicolor were downloaded from the Phytozome database [48].
The isoelectric point (pI) and molecular weight (MW) of SiWAKL proteins were predicted on the ExPASy website (https://web.expasy.org/compute_pi/). All SiWAKL protein sequences were submitted to the MEME online server (http://meme-suite.org/) to search for conserved motifs with the following parameters: maximum number of motifs limited to 15 and motif size limited between 6 and 50 amino acids. Gene Ontology (GO) functional annotation of SiWAKL proteins were predicted by PANNZER 2 online server (http://ekhidna2.biocenter.helsinki.fi/sanspanz/). Signal peptide and subcellular localization prediction of WAKL proteins were performed at the websites SignalP-5.0 (https://services.healthtech.dtu.dk/services/SignalP-5.0/) and WoLF PSORT (http://psort.hgc.jp/), respectively.
Plant materials and treatment
Seeds of the disease-susceptible genotype J9014 and disease-resistant genotype ZZ13 [49] were disinfected in 5% sodium hypochlorite solution for 15 min and then in 70% alcohol for 30 s. After that, the seeds were rinsed 3–4 times with sterile water, and then dried and planted in mixed nutrient soil (sterile soil: nutrient soil: sterile vermiculite = 3:1:1). The sesame seedlings were cultured under 29 ± 1 °C, 80% relative humidity and a photoperiod of light for 16 h and dark for 8 h. Arabidopsis seeds were sterilized with 5% sodium hypochlorite solution for 10 min and repeatedly rinsed with sterile water 3–5 times. Then the seeds were sown on 1/2 MS medium and placed in a refrigerator at 4 °C for 4 days. After treatment, they were cultured in an incubator at 22 ± 1 °C with 16 h light and 8 h dark conditions. When Arabidopsis grew to two true leaves, the seedlings were transferred to mixed nutrient soil (nutrient soil: vermiculite = 3:1) and continued to be cultured under the same conditions.
The genotype ZZ13 was selected for the tissue-specific RNA-seq of sesame. Sampling methods were referenced to Dossou et al. [50]. ZZ13 was grown under normal culture conditions (16 h light/30°C and 8 h dark/28°C). Flower tissues with consistent growth were randomly sampled and the locations were marked. The capsules at the markers were sampled along with all other tissues (roots, middle stems, middle leaves, fresh capsules and fresh seeds) two weeks later for RNA extraction. When sesame capsules were removed, fresh seeds were separated from the fresh capsules on ice immediately to obtain fresh seeds and capsules. The samples used for RNA-sequencing later.
The method of inoculation with M. phaseolina was performed as described previously [49]. When sesame seedings grew to three pairs of true leaves, pots were irrigated with 167 mL of 200 µmol/L methyl jasmonate (MeJA) and 2 µmol/L salicylic acid (SA) solutions were irrigated to each pot for treatment, respectively, with 167 mL water treatment as a control (Mock). Sesame root tissues were collected at 0 h, 3 h, 6 h, 12 h, 24 and 36 h after treatment and stored at -80 °C.
Total RNA extraction and cDNA library construction
Purity and concentration of total RNA of sesame different tissues of ZZ13 extracted with the TransZol Up Plus RNA Kit were examined by spectrophotometer NanoDrop 2000 while the integrality of total RNA detected by Agient2100/LabChip GX. Then, the cDNA library was constructed. After the library constructed, the initial quantification was performed by the Qubit 3.0 fluorescence quantification instrument. Subsequently, the insert fragment of cDNA library was detected by Qsep400 high-throughput system while the effective concentration of the cDNA library (> 2 nM) was measured by Q-PCR. After quality control of the cDNA library, PE150 sequencing was performed using Illumina NovaSeq6000.
Gene expression analysis
To understand the expression patterns of WAKL genes involved in sesame resistance to M. phaseolina stress, the tissue-specific transcriptome data PRJNA892254 of ZZ13 and the transcriptome data PRJNA706471 [49] of sesame and M. phaseolina interactions were used in this study. “PRJNA892254” is the transcriptome data of root, stem, leaf, flower, capsule and seed tissue of ZZ13 under normal conditions. “PRJNA706471” is the transcriptome data of ZZ13 and J9014 at 0 h, 12 h, 24 h, 36 and 48 h postinoculation with M. phaseolina.
To standardize the gene expression levels of each sample, the clean reads were converted into fragments per kilobase of exon model per million mapped reads (FPKM) [51]. The number of reads of each gene was counted by StringTie (version: 1.3.0) and then clean reads were mapped to the sesame genome with HISAT2 (version: 2.0.4) [52, 53]. Finally, the FPKM value of each gene was calculated by the trimmed mean of M values method [54].
The sesame reference genome is provided by the Sesame Research Center, Henan Academy of Agricultural Sciences [30, 44].
Construction of the overexpression plasmids
The full length SiWAKL6 coding sequence was cloned from the varieties ZZ13 and J9014. SiWAKL6 in ZZ13 was inserted into pCambia2301 plasmids using homologous recombination to construct a 35 S::SiWAKL6 overexpression vector. The 35 S::SiWAKL6 recombinant vector was transformed into wild-type (WT) A. thaliana plants (Col-0) mediated by Agrobacterium tumefaciens GV3101. The transgenic plants were screened on 1/2 MS medium containing 50 mg/L kanamycin, and the forward primer was designed based on the vector sequence upstream of the promoter and reverse primers were designed downstream of the SiWAKL6 gene for PCR identification of the transgenic plants. Three independent T3 transgenic lines were used for subsequent experiments. The primers were 35 S-F (GACGCACAATCCCACTATCC) and SiWAKL6-R (TTGGTTCATGGATGTGTCGG).
Analysis of M. phaseolina resistance in transgenic A. thaliana plants
Strain M. phaseolina was inoculated in PDA solid medium and incubated at 30 °C for 7 days until the mycelium covered the petri dishes. Then, the mycelium was divided into blocks, and each 1/2 petri dish of strains was inoculated evenly into 300 mL of PD liquid medium and incubated at 30 °C and 200 r/min for 5 days. Subsequently, the mycelial suspension was obtained by breaking up the solution with a tissue masher. Each 20 mL mycelium suspension was mixed with 100 mL sterilized water and 120 g sterilized stroma (nutritional soil: vermiculite = 3:1). The WT and transgenic A. thaliana were transplanted to the fungal soil when they had grown for eight weeks. The leaf tissue was taken 12 h after inoculation and stored at -80 °C.
Plant disease classes were classified using a scale of 0–5 based on the phenotypes of leaf chlorosis and necrosis with reference to criteria from a previous study [55]. And the formula of the disease index (DI) is as follows.
Disease index (DI) = Σ (Number of diseased plant each level × The value of each level) / (Total number of the investigated plants × The value of the highest level) × 100.
Analysis of qPCR and ROS contents
In sesame, RNA extraction, cDNA synthesis and qPCR were performed on sesame root tissues after different treatments. In A. thaliana, RNA extraction, cDNA synthesis and qPCR were performed on leaf tissues post inoculation with M. phaseolina. Relative expression levels of genes in sesame and A. thaliana were quantified by the CFX 384™ real-time system made in Singapore and the 2× ChamQ Universal SYBR qPCR Master Mix (Vazyme, Nanjing, China) with the 2−ΔΔCt method. Each sample had 3 replicates. The relative expression levels of sesame genes were normalized to that of the SiUBQ5 gene. The relative expression levels of A. thaliana genes were normalized to that of the AtUBQ10 gene. The primers for qPCR are shown in Additional file: Table S5.
Fourteen days post inoculation with M. phaseolina, the leaf tissues of A. thaliana plants were taken. The contents of H2O2 and MDA as well as the activities of CAT and SOD were detected using kits (Grace Biotechnology, Suzhou, China).
Data availability
Data is available at NCBI SRA accession: PRJNA892254 and PRJNA706471.
References
Dardick C, Schwessinger B, Ronald P. Non-arginine-aspartate (non-RD) kinases are associated with innate immune receptors that recognize conserved microbial signatures. Curr Opin Plant Biol. 2012;15(4):358–66.
Macho AP, Zipfel C. Plant PRRs and the activation of innate immune signaling. Mol Cell. 2014;54(2):263–72.
Chandran D, Rickert J, Huang Y, Steinwand MA, Marr SK, Wildermuth MC. Atypical E2F transcriptional repressor DEL1 acts at the intersection of plant growth and immunity by controlling the hormone salicylic acid. Cell Host Microbe. 2014;15(4):506–13.
Cui H, Tsuda K, Parker JE. Effector-triggered immunity: from pathogen perception to robust defense. Annu Rev Plant Biol. 2015;66:487–511.
Li Q, Hu A, Qi J, Dou W, Qin X, Zou X, Xu L, Chen S, He Y. CsWAKL08, a pathogen-induced wall-associated receptor-like kinase in sweet orange, confers resistance to citrus bacterial canker via ROS control and JA signaling. Hortic Res. 2020;7(1):42.
Anderson CM, Wagner TA, Perret M, He ZH, He D, Kohorn BD. WAKs: cell wall-associated kinases linking the cytoplasm to the extracellular matrix. Plant Mol Biol. 2001;47(1–2):197–206.
Verica JA, Chae L, Tong H, Ingmire P, He ZH. Tissue-specific and developmentally regulated expression of a cluster of tandemly arrayed cell wall-associated kinase-like kinase genes in Arabidopsis. Plant Physiol. 2003;133(4):1732–46.
Kohorn BD, Lane S, Smith TA. An Arabidopsis serine/threonine kinase homologue with an epidermal growth factor repeat selected in yeast for its specificity for a thylakoid membrane protein. Proc Natl Acad Sci U S A. 1992;89(22):10989–92.
Verica JA, He ZH. The cell wall-associated kinase (WAK) and WAK-like kinase gene family. Plant Physiol. 2002;129(2):455–9.
Bot P, Mun BG, Imran QM, Hussain A, Lee SU, Loake G, Yun BW. Differential expression of AtWAKL10 in response to nitric oxide suggests a putative role in biotic and abiotic stress responses. PeerJ. 2019;7:e7383.
Giarola V, Krey S, Von Den Driesch B, Bartels D. The Craterostigma plantagineum glycine-rich protein CpGRP1 interacts with a cell wall-associated protein kinase 1 (CpWAK1) and accumulates in leaf cell walls during dehydration. New Phytol. 2016;210(2):535–50.
Qi H, Zhu X, Guo F, Lv L, Zhang Z. The wall-associated receptor-like kinase TaWAK7D is required for defense responses to Rhizoctonia Cerealis in wheat. Int J Mol Sci. 2021;22(11):5629.
Larkan NJ, Ma L, Haddadi P, Buchwaldt M, Parkin IP, Djavaheri M, Borhan MH. The Brassica napus wall-associated kinase-like (WAKL) gene Rlm9 provides race-specific blackleg resistance. Plant J. 2020;104(4):892–900.
Hurni S, Scheuermann D, Krattinger SG, Kessel B, Wicker T, Herren G, Fitze MN, Breen J, Presterl T, Ouzunova M, Keller B. The maize disease resistance gene Htn1 against northern corn leaf blight encodes a wall-associated receptor-like kinase. Proc. Natl. Acad. Sci. U. S. A. 2015;112(28): 8780–8785.
Dmochowska-Boguta M, Kloc Y, Zielezinski A, Werecki P, Nadolska-Orczyk A, Karlowski WM, Orczyk W. TaWAK6 encoding wall-associated kinase is involved in wheat resistance to leaf rust similar to adult plant resistance. PLoS ONE. 2020;15(1):e0227713.
Gadaleta A, Colasuonno P, Giove SL, Blanco A, Giancaspro A. Map-based cloning of QFhb.mgb-2A identifies a WAK2 gene responsible for Fusarium head blight resistance in wheat. Sci Rep. 2019;9(1):6929.
Wang D, Qin L, Wu M, Zou W, Zang S, Zhao Z, Lin P, Guo J, Wang H, Que Y. Identification and characterization of WAK gene family in saccharum and the negative roles of ScWAK1 under the pathogen stress. Int J Biol Macromol. 2023;224:1–19.
Shi G, Zhang Z, Friesen TL, Raats D, Fahima T, Brueggeman RS, Lu S, Trick HN, Liu Z, Chao W, Frenkel Z, Xu SS, Rasmussen JB, Faris JD. The Hijacking of a receptor kinase-driven pathway by a wheat fungal pathogen leads to Disease. Sci Adv. 2016;2(10):e1600822.
Ning Y, Liu W, Wang G-L. Balancing immunity and yield in crop plants. Trends Plant Sci. 2017;22(12):1069–79.
Hu K, Cao J, Zhang J, Xia F, Ke Y, Zhang H, Xie W, Liu H, Cui Y, Cao Y, Sun X, Xiao J, Li X, Zhang Q, Wang S. Improvement of multiple agronomic traits by a Disease resistance gene via cell wall reinforcement. Nat Plants. 2017;3:17009.
Zhang N, Zhang B, Zuo W, Xing Y, Konlasuk S, Tan G, Zhang Q, Ye J, Xu M. Cytological and molecular characterization of ZmWAK-mediated head-smut resistance in maize. Mol Plant Microbe Interact. 2017;30(6):455–65.
Makinde FM, Akinoso R. Comparison between the nutritional quality of flour obtained from raw, roasted and fermented sesame (Sesamum indicum L.) seed grown in Nigeria. Acta Sci Pol Technol Aliment. 2014;13(3):309–19.
Nagendra Prasad MN, Sanjay KR, Deepika S, Prasad, Vijay N, Kothari R, Nanjunda SS. A review on nutritional and nutraceutical properties of sesame. J Nutr Food Sci. 2012;2:1–6.
Lehti-Shiu MD, Shiu SH. Diversity, classification and function of the plant protein kinase superfamily. Philos Trans R Soc Lond B Biol Sci. 2012;367(1602):2619–39.
He Y, Jia R, Qi J, Chen S, Lei T, Xu L, Peng A, Yao L, Long Q, Li Z, Li Q. Functional analysis of citrus AP2 transcription factors identified CsAP2-09 involved in citrus canker Disease response and tolerance. Gene. 2019;707:178–88.
De Oliveira LFV, Christoff AP, De Lima JC, De Ross BCF, Sachetto-Martins G, Margis-Pinheiro M, Margis R. The wall-associated kinase gene family in rice genomes. Plant Sci. 2014;229:181–92.
Zhang Z, Ma W, Ren Z, Wang X, Zhao J, Pei X, Liu Y, He K, Zhang F, Huo W, Li W, Yang D, Ma X. Characterization and expression analysis of wall-associated kinase (WAK) and WAK-like family in cotton. Int J Biol Macromol. 2021;187:867–79.
Zhang B, Li P, Su T, Li P, Xin X, Wang W, Zhao X, Yu Y, Zhang D, Yu S. Comprehensive analysis of wall-associated kinase genes and their expression under abiotic and biotic stress in Chinese Cabbage (Brassica rapa ssp. pekinensis). J Plant Growth Regul. 2020;39:72–86.
Tocquard K, Lafon-Placette C, Auguin D, Muries B, Bronner G, Lopez D, Fumanal B, Franchel J, Bourgerie S, Maury S. Silico study of wall-associated kinase family reveals large-scale genomic expansion potentially connected with functional diversification in Populus. Tree Genet Genomes. 2014;10(5):1135–47.
Zhang H, Miao H, Wang L, Qu L, Liu H, Wang Q, Yue M. Genome sequencing of the important oilseed crop Sesamum indicum L. Genome Biol. 2013;14(1):401.
Wang L, Yu S, Tong C, Zhao Y, Liu Y, Song C, Zhang Y, Zhang X, Wang Y, Hua W, Li D, Li D, Li F, Yu J, Xu C, Han X, Huang S, Tai S, Wang J, Xu X, et al. Genome sequencing of the high oil crop sesame provides insight into oil biosynthesis. Genome Biol. 2014;15(2):R39.
Li H, Zhou SY, Zhao WS, Su SC, Peng YL. A novel wall-associated receptor-like protein kinase gene, OsWAK1, plays important roles in rice blast Disease resistance. Plant Mol Biol. 2009;69(3):337–46.
Yang P, Praz C, Li B, Singla J, Robert CM, Kessel B, Scheuermann D, Lüthi L, Ouzunova M, Erb M, Krattinger SG, Keller B. Fungal resistance mediated by maize wall-associated kinase ZmWAK-RLK1 correlates with reduced benzoxazinoid content. New Phytol. 2019;221(2):976–87.
Qi H, Guo F, Lv L, Zhu X, Zhang L, Yu J, Wei X, Zhang Z. The wheat wall-associated receptor-like kinase TaWAK-6D mediates broad resistance to two fungal pathogens Fusarium Pseudograminearum and Rhizoctonia Cerealis. Front Plant Sci. 2021;12:758196.
Kohorn BD, Kohorn SL. The cell wall-associated kinases, WAKs, as pectin receptors. Front. Plant Sci. 2012;3:88.
Kohorn BD, Johansen S, Shishido A, Todorova T, Martinez R, Defeo E, Obregon P. Pectin activation of map kinase and gene expression is WAK2 dependent. Plant J. 2009;60(6):974–82.
Kohorn BD, Kobayashi M, Johansen S, Friedman HP, Fischer A, Byers N. Wall-associated kinase 1 (WAK1) is crosslinked in endomembranes, and transport to the cell surface requires correct cell-wall synthesis. J Cell Sci. 2006;119(11):2282–90.
Brutus A, Sicilia F, Macone A, Cervone F, De Lorenzo G. A domain swap approach reveals a role of the plant wall-associated kinase 1 (WAK1) as a receptor of oligogalacturonides. Proc. Natl. Acad. Sci. U. S. A. 2010;107(20): 9452–9457.
He ZH, He D, Kohorn BD. Requirement for the induced expression of a cell wall associated receptor kinase for survival during the pathogen response. Plant J. 1998;14(1):55–63.
Harkenrider M, Sharma R, De Vleesschauwer D, Tsao L, Zhang X, Chern M, Canlas P, Zuo S, Ronald PC. Overexpression of rice wall-associated kinase 25 (OsWAK25) alters resistance to bacterial and fungal pathogens. PLoS ONE. 2016;11(1):e0147310.
Delteil A, Gobbato E, Cayrol B, Estevan J, Michel-Romiti C, Dievart A, Kroj T, Morel JB. Several wall-associated kinases participate positively and negatively in basal defense against rice blast fungus. BMC Plant Biol. 2016;16:17.
Kohorn BD, Kohorn SL, Todorova T, Baptiste G, Stansky K, Mccullough M. A dominant allele of Arabidopsis pectin-binding wall-associated kinase induces a stress response suppressed by MPK6 but not MPK3 mutations. Mol Plant. 2012;5(4):841–51.
Wang P, Zhou L, Jamieson P, Zhang L, Zhao Z, Babilonia K, Shao W, Wu L, Mustafa R, Amin I, Diomaiuti A, Pontiggia D, Ferrari S, Hou Y, He P, Shan L. The cotton wall-associated kinase GhWAK7A mediates responses to fungal wilt pathogens by complexing with the chitin sensory receptors. Plant Cell. 2020;32(12):3978–4001.
Miao H, Zhang H, Kole C. The sesame genome. Springer; 2021.
Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–4.
Chen C, Chen H, Zhang Y, Thomas HR, Frank MH, He Y, Xia R. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant. 2020;13(8):1194–202.
Wang Y, Tang H, Debarry JD, Tan X, Li J, Wang X, Lee TH, Jin H, Marler B, Guo H, Kissinger JC, Paterson AH. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40(7):e49.
Goodstein DM, Shu S, Howson R, Neupane R, Hayes RD, Fazo J, Mitros T, Dirks W, Hellsten U, Putnam N, Rokhsar DS. Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res. 2012;40(Database issue):D1178–1186.
Yan W, Ni Y, Liu X, Zhao H, Chen Y, Jia M, Liu M, Liu H, Tian B. The mechanism of sesame resistance against Macrophomina Phaseolina was revealed via a comparison of transcriptomes of resistant and susceptible sesame genotypes. BMC Plant Biol. 2021;21(1):159.
Dossou SSK, Xu F, Cui X, Sheng C, Zhou R, You J, Tozo K, Wang L. Comparative metabolomics analysis of different sesame (Sesamum indicum L.) tissues reveals a tissue-specific accumulation of metabolites. BMC Plant Biol. 2021;21(1):352.
Mortazavi A, Williams BA, Mccue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-seq. Nat Methods. 2008;5(7):621–8.
Pertea M, Pertea GM, Antonescu CM, Chang TC, Mendell JT, Salzberg SL. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat Biotechnol. 2015;33(3):290–5.
Pertea M, Kim D, Pertea GM, Leek JT, Salzberg SL. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat Protoc. 2016;11(9):1650–67.
Robinson MD, Oshlack A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010;11(3):R25.
Schroeder MM, Lai Y, Shirai M, Alsalek N, Tsuchiya T, Roberts P, Eulgem T. A novel Arabidopsis pathosystem reveals cooperation of multiple hormonal response-pathways in host resistance against the global crop destroyer Macrophomina Phaseolina. Sci Rep. 2019;9(1):20083.
Acknowledgements
Not applicable.
Funding
The works were supported by the China Agriculture Research System of MOF and MARA (CARS-14), the Key Project of Science and Technology of Henan Province (201300110600), The Key Research Project of the Shennong Laboratory (SN01-2022-04), Key Research and Development Project of Henan Province (221111520400) and Key Technologies R&D Program of Henan Province (232102110214).
Author information
Authors and Affiliations
Contributions
Yunxia Ni, Hui Zhao, Xintao Liu, Baoming Tian and Hongyan Liu assisted in experiment conducting. Hongmei Miao and Hengchun Cao prepared the plant materials and sesame genome files. Min Jia assisted in generalizing the data. Peilin Hu and Wenqing Yan performed experiment, data analysis and manuscript writing. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All methods were performed in accordance with the relevant guidelines and regulations. We have obtained permissions to collect plant material and seedlings.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yan, W., Hu, P., Ni, Y. et al. Genome-wide characterization of the wall-associated kinase-like (WAKL) family in sesame (Sesamum indicum) identifies a SiWAKL6 gene involved in resistance to Macrophomina Phaseolina. BMC Plant Biol 23, 624 (2023). https://doi.org/10.1186/s12870-023-04658-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-023-04658-1