Abstract
Soil salinization is a growing issue that limits agriculture globally. Understanding the mechanism underlying salt tolerance in halophytic grasses can provide new insights into engineering plant salinity tolerance in glycophytic plants. Seashore paspalum (Paspalum vaginatum Sw.) is a halophytic turfgrass and genomic model system for salt tolerance research in cereals and other grasses. However, the salt tolerance mechanism of this grass largely unknown. To explore the correlation between Na+ accumulation and salt tolerance in different tissues, we utilized two P. vaginatum accessions that exhibit contrasting tolerance to salinity. To accomplish this, we employed various analytical techniques including ICP-MS-based ion analysis, lipidomic profiling analysis, enzyme assays, and integrated transcriptomic and metabolomic analysis. Under high salinity, salt-tolerant P. vaginatum plants exhibited better growth and Na+ uptake compared to salt-sensitive plants. Salt-tolerant plants accumulated heightened Na+ accumulation in their roots, leading to increased production of root-sourced H2O2, which in turn activated the antioxidant systems. In salt-tolerant plants, metabolome profiling revealed tissue-specific metabolic changes, with increased amino acids, phenolic acids, and polyols in roots, and increased amino acids, flavonoids, and alkaloids in leaves. High salinity induced lipidome adaptation in roots, enhancing lipid metabolism in salt-tolerant plants. Moreover, through integrated analysis, the importance of amino acid metabolism in conferring salt tolerance was highlighted. This study significantly enhances our current understanding of salt-tolerant mechanisms in halophyte grass, thereby offering valuable insights for breeding and genetically engineering salt tolerance in glycophytic plants.
Similar content being viewed by others
Introduction
Salt accumulation in arable soils is a growing concern for global agriculture [1]. High soil salinity can significantly impede plant growth and reduce crop yield, as most crops are highly sensitive to saline conditions [2]. To address this pressing issue, halophytes are increasingly recognized as essential genetic models for studying the molecular mechanisms behind salt tolerance, with potential to develop plant germplasm that can thrive in saline conditions and enable salinized agricultural land use [1].
Halophytes, equipped with various physiological and biochemical mechanisms, can achieve salt tolerance [3]. However, excess Na+ from saline soil can lead to osmotic and oxidative stress, resulting in salt-induced changes in metabolic status, membrane remodelling, and gene and protein expression [1, 4]. To prevent toxic Na+ accumulation in plant tissues, halophytes efficiently regulate soil Na+ uptake [5].
Due to the high energy cost of adaptation, the antioxidant response, also known as oxidative stress tolerance, is of increasing interest for genetic engineering of salt-tolerant crops [6,7,8], as halophytes have evolved a complex antioxidant system to combat the toxic effects of reactive oxygen species (ROS) induced by salt stress, with hydrogen peroxide (H2O2) being the most stable form of ROS. ROS-scavenging enzymes, such as superoxide dismutase (SOD), ascorbate peroxidase (APX), and catalase (CAT) [9, 10], as well as non-enzyme antioxidants, including amino acids (e.g., ascorbate, proline, glutathione), flavonoids, polyamines, and polyols, are necessary for exceptional salinity tolerance [11, 12], although the type and quality of ROS players vary among different halophyte species and even within various individual plant tissues [13]. For example, under high salinity conditions, the non-enzymatic antioxidant glutathione was significantly decreased in the halophytic grass Desmostachya bipinnata and Cakile maritima [14, 15], but significantly increased in halophyte Salicornia brachiata [16]. Thus, mechanistic details of the salt-induced antioxidant response in halophyte species remain largely unclear.
Salt stress is a well-known cause of oxidative damage to the biological membrane, and can lead to changes in plant membrane properties [17]. Membrane lipids, which include sphingolipids, sterols, and glycerolipids, play a crucial role in regulating plant membrane fluidity and permeability in response to abiotic stress [18, 19]. Despite some studies showing salt-induced changes in membrane lipid composition in halophytes, the relationship between specific lipid species and the mechanisms underlying salt tolerance remains poorly understood [19,20,21]. Therefore, further research is necessary to fully explore this correlation.
Seashore paspalum (Paspalum vaginatum Sw.), a halophytic turf species, has excellent potential for improving saline soils in tropical and subtropical regions worldwide due to its salt tolerance capacity [22, 23]. However, there is significant genotypic variation in salt tolerance across P. vaginatum species [24], underscoring the need to comprehend its molecular basis of salt tolerance. Although the molecular mechanisms underlying its salt tolerance have received significant attention recently [10, 24], our understanding of the mechanisms involved in halophyte tolerance in this grass is still limited. While a recent study suggested that Na+ uptake may confer an advantage to seashore paspalum, the correlation between Na+ accumulation and salt tolerance remains unclear. Therefore, we compared salt-tolerant and salt-sensitive P. vaginatum accessions to elucidate tissue-specific salt tolerance mechanisms based on the antioxidant system, metabolic adaptation, and lipid remodeling. This knowledge has the potential to enhance salinity tolerance of other crops through genetic engineering or selective breeding.
Materials and methods
Plant growth conditions and salt treatment
Two contrasting P. vaginatum materials, the salt-tolerant accession (UAS 17–18; PI UPG145) and salt-sensitive accession (USA17-26; PI 647910), were previously reported and used for the current study [25]. The materials were acquired from the University of Georgia, and are now being preserved by the Hainan University and the University of Georgia resource nurseries since they have not been deposited in a publicly available herbarium. Stolons from two-month-old P. vaginatum plants were hydroponically cultivated in plastic containers (90-mm top diameter × 57-mm diameter width × 135-mm depth) filled with full-strength Hoagland solution (pH = 6.0) in a growth chamber with a 16 h:8 h light: dark at 30/25 ℃, 50%-60% relative humidity and 1000 µmol m−2 s−1 standard light intensity.
Salt treatments and non-stress treatments were performed on two-week-old plants in the hydroponic plastic containers by supplementing with (and without) 400 mM NaCl. To avoid the undesirable effects of plant salt shock, NaCl concentrations were gradually increased 100 mM each day up to 400 mM NaCl. On the fifth day of salinity stress, the leaves and roots of salt-stressed and non-stressed plants were collected separately. The treatments were each replicated in three hydroponic plastic containers, and six individual plants from each treatment were considered as a biological replicate. Three to six biological replicates were used for the experiments described below. At the beginning and end of the salt stress period, we measured plant weight (in grams), and using the formula (final weight-initial weight) / time period (5 days), we calculated plant growth rate (in grams per day).
ICP-MS based ion measurement
ICP-MS analysis was used to quantify leaf and root Na+ and K+ concentrations. Approximately 50 mg of oven-dried samples were ground and dissolved in 2% HNO3, then diluted to prepare five-point calibration standards for Na+ and K+ concentration. An iCAP Q ICP mass spectrometer from Thermo Fisher Scientific (USA) was used in single KED mode with the following parameters: 1550 W forward power, 14 L/min coolant gas flow, 1.0749 L/min nebulizer gas flow, 0.8 L/min auxiliary gas flow, and 0.02 s dwell time. ST capacity was calculated as ST = (K+/Na+ in leaves) / (K+/Na+ in roots) according to Wang [26].
H2O2 measurement and antioxidant enzymatic assay
Approximately 100 mg of fresh roots or leaves from three to five biological replicates were used for the following measurements. H2O2 concentrations were measured using a commercial kit (Product ID: BC3590, Nanjing Jiancheng Bioengineering Institute, Nanjing, China. Superoxide dismutase (SOD) levels including Mn2+-SOD activity and Cu/Zn-SOD were measured using a SOD Assay kit (Product ID: A001-2–1; Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Catalase (CAT) levels were measured using a CAT Assay kit (Product ID: BC0205; Beijing, China, Solarbio).
Lipidomic profiling analysis
Approximately 100 mg of fresh leaves and roots from three biological replicates were frozen into liquid nitrogen, ground to powder and extracted with 300 µL Methanol/1 mL MTBE/300 µL water. LC–MS analysis was conducted using the Nexera UHPLC LC-30A system (Shimadzu, Kyoto, Japan) coupled to a TripleTOF5600 + (AB SCIEX™) mass spectrometer. The column temperature was set at 40 °C and the mobile phase was 0.1% HCOOH-H2O (A)-acetonitrile (B) at a flow rate of 0.3 mL/min in gradient elution. ESI source conditions included Ion Source Gas1 (Gas 1) and Ion Source Gas2 (Gas 2) at 50, Curtain Gas (CUR) at 25, and Source Temperature at 500 ℃/450 ℃ (positive ion/negative ion). The Ion Sapary Voltage Floating (ISVF) was set at 5500 V/4400 V (positive ion/negative ion), and TOF MS scan range was 100–1200 Da with a 0.2 s scan accumulation time. The Declustering potential (DP) was ± 60 V. Lipid identification was performed using LipidSearch software 4.0 (Thermo Fisher Scientific, CA, USA).
Untargeted metabolomics profiling and identification
For metabolite extraction, approximately 100 mg of freeze-dried leaves and roots from three biological replicates were used. These samples were then analyzed through a UPLC-ESI–MS/MS system with the following conditions: UPLC column was a Waters ACQUITY UPLC HSS T3 C18 (1.8 µm, 2.1 mm*100 mm), and the mobile phase consisted of solvent A (pure water with 0.04% acetic acid) and solvent B (acetonitrile with 0.04% acetic acid). A gradient program was used, starting with 95% A and 5% B and gradually shifting to 5% A and 95% B within 10 min. The column oven was set to 40 °C, and the injection volume was 4 μl.
A triple quadrupole linear ion trap mass spectrometer (Q TRAP), API 4500 Q TRAP UPLC / MS / MS system, equipped with an ESI Turbo Ion-Spray interface, operating in positive and negative ion mode, and controlled by Analyst 1.6.3 software (AB Sciex, USA) was used to acquire LIT and triple quadrupole (QQQ) scans. The ESI source parameters were set to: ion source, turbo spray; source temperature 550 ℃; ion spray voltage (IS) 5500 V (positive ion mode) / 4500 V (negative ion mode); ion source gas I [27], gas II (GSII), curtain gas (CUR) were set at 50, 60, and 30.0 psi, respectively; the collision gas (CAD) was high. The instrument was tuned and mass calibrated with 10 μmol/L and 100 μmol/L polypropylene glycol solutions in the QQQ and LIT modes, respectively. The QQQ scans were acquired as MRM experiments with collision gas (nitrogen) set at 5 psi, and individual MRM transitions were optimized for DP and CE.
The SIMCA 14.1 software (Umetrics, Umea, Sweden) was used for multivariate modelling in metabolic profiling, including orthogonal partial least squares discriminant analysis (OPLS-DA) to identify significantly abundant metabolites. Only identified metabolites with a fold change of 2 or ≤ 0.5 and VIP ≥ 1 were considered significant. Pathway enrichment analysis and visualization of metabolomic data were performed using MetaboAnalyst 5.0 and KEGG Mapper (https://www.genome.jp/kegg/tool/map_pathway2.html) [28].
Transcriptomic profiling and gene expression analysis
Total RNA was extracted using a Plant RNA Kit (Omega Bio-Tek, Norcross, GA, United States) according to the manufacturer’s protocol. Subsequently, cDNA libraries were prepared using the HiSeq 2000 (Illumina Technologies) platform for transcriptome sequencing and de novo assembly. Raw reads were filtered for adapter sequences and low-quality bases with Trimmomatic (v0.32), followed by alignment to the reference genome using HISAT2 (v2.1.0). Gene expression normalization was performed by calculating Reads per Kilobase per Million Mapped Reads (RPKM) based on Mortazavi and colleagues' method. Identification of differentially expressed genes (DEGs) was achieved using the R software DESeq2 package, with a threshold of false discovery rate (FDR) ≤ 0.05 and |log2 FC|≥ 1.5. GO and KEGG pathway analyses were performed to determine DEGs biological functions. The RNA-seq reads were deposited in the NCBI Gene Expression Omnibus (GEO) under GEO accession number: GSE233155, with quality scores evaluated prior to submission.
Statistical analysis
Statistical significance analysis was calculated using single-factor ANOVA followed by Duncan’s test was performed using GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, CA, USA).
Results
In P. vaginatum, genotypic variation in salinity tolerance is associated with Na+ uptake
After 5 days of NaCl treatment, salt-sensitive plants showed clear symptoms of leaf chlorosis and wilting, while salt-tolerant plants were similar to control plants (Fig. 1A). Notably, a substantial growth rate disparity was observed between salt-sensitive and salt-tolerant plants subjected to 400 mM NaCl treatment (Fig. 1B). In response to high salinity, there was a ssignificant difference in Na+ contents of shoots and leaves between salt-sensitive and salt-tolerant plants after NaCl treatment. Both root and shoot Na+ increased noticeably in salt-tolerant plants (Fig. 1D). However, K+ concentration did not significantly differ between the two accessions under salt stress (Fig. 1E). When exposed to high salinity, Na+ ST values did not show significant changes between the two accessions treated with salt stress (Fig. 1C). Our findings suggest that salt-tolerant plants under saline conditions preferentially absorb more Na+ and probably store it in P. vaginatum roots.
A Phenotypical difference of the two P. vaginatum accessions treated with high salinity. B-C Leaf growth rates and ST values of the two P. vaginatum accessions were calculated after 5 days of salt stress. D-E Changes in Na+ and K.+ concentrations in the roots and leaves of salt-tolerant and salt-sensitive plants under salt or non-salted conditions (** significant at p < 0.001; ns, not significant; one-way ANOVA followed with two-sided Student’s t-test)
Na+-induced root-sourced H2O2 activates antioxidant systems in salt-tolerant plants during salt stress
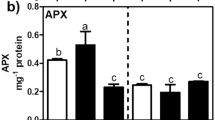
To determine whether such root and shoot Na+ accumulation leads to H2O2 production in salt-tolerant plants after salt treatment, we measured H2O2 levels and activities of H2O2-scavenging enzymes in non-salted and treated roots and shoots of the two accessions. A significant increase in Na+-induced root-sourced H2O2 was detected in the tolerant plants, compared with the sensitive plants (Fig. 2A). CAT and Mn2+-SOD activities significantly increased in salt-tolerant plant roots, unlike in the salt-sensitive plants (Fig. 2B and 2D). There was no significant accumulation of leaf-derived H2O2 or Cu/Zn-SOD activity observed in treated leaves following salt treatment, in comparison to either the salt-sensitive plants nor their corresponding controls. (Fig. 2A and 2C). These results suggest that Na+-induced H2O2 is tissue-specific and confers salt tolerance to P. vaginatum plants in response to salt stress.
A-D Effect of high salinity (400 mM NaCl) on H2O2 levels and activities of enzymatic players (Mn2+-SOD, Cu/Zn-SOD and CAT) in roots and leaves of salt-sensitive and -tolerant plants. (* significant at p < 0.05; ** significant at p < 0.001; ns, not significant; one-way ANOVA followed with two-sided Student’s t-test). Abbrev: ‘R’: salt-tolerant accession; ‘S’: salt-sensitive accession; ‘T’: salt treatment; ‘C’: unsalted treatment; ‘L’: leaf; ‘R.◦’: root)
Metabolome profiling highlights difference between non-enzymatic metabolites from different Na+-induced H2O2
We profiled and compared the metabolic status of non-enzymatic antioxidants in the roots and shoots of treated plants of the two accessions (Fig. 3 and Table. S1). After 5 days of NaCl treatment, salt-tolerant plant roots increased abundance of amino acids (such as proline and ornithine), phenolic acids (such as caffeic acid and vanillylmandelic acid) and polyols (such as mannitol and inositol) (Fig. 3C). Instead of phenolic acids and polyols, high salinity resulted in a greater abundance of amino acids (such as proline and pipecolic acid), flavonoids (such as quercitrin, orientin and luteolin), and alkaloids (such as putrescine, agmatine, and spermine) in salt-tolerant plant leaves. These results indicate that Na+ induced H2O2 could confer tissue-dependent metabolic changes in P. vaginatum plants.
A Scatter plots of O2PLS-DA model of different samples for profiling metabolomics. Each symbol represents an independent sample in the score scatter plot and an independent annotated peak in the loading plot; (B) Effects of salinity (400 mM NaCl) on metabolic changes in salt-tolerant plants compared to salt-sensitive plants. Heat maps indicate the log2 transferred value of the fold change between salt-tolerant plants and salt-sensitive plants treated with or without 400 mM NaCl. C The top enriched KEGG terms of DEGs from leaves in salt-treated tolerant plants of P. vaginatum relative to salt-sensitive plants. Abbrev: ‘R’: salt-tolerant accession; ‘S’: salt-sensitive accession; ‘T’: salt treatment; ‘C’: unsalted treatment; ‘L’: leaf; ‘R.◦’: root)
High salinity induces lipidome adaptation in P. vaginatum plant roots
To investigate tissue-specific lipid metabolism associated with Na+ accumulation in P. vaginatum plants, we detected the changes in salt-induced lipid species in both root and shoot between the two accessions. Salinity stress elevated root lipidomic species accumulation in salt tolerant plants, including two glycolipids (i.e., MDGD, DGDG and SGDGs), two phospholipids (i.e., PCs and PEs), fatty acids (FA), and triacylglycerols (TGs) (Fig. 4 and Table. S2). Conversely, salt-tolerant plant leaf lipid profiling was not affected by salt stress in P. vaginatum plants, as no significant changes in these lipids were observed in leaves of salt-tolerant plants compared to salt-sensitive plants (Fig. 4). These results suggest that Na+ accumulation probably triggers upregulation of lipid metabolism to improve P. vaginatum salt tolerance.
A-C Changes in phospholipids, glycolipids and fatty acids in leaves and roots between salt-tolerant and salt-sensitive accessions of P. vaginatum under salt stress. Asterisks indicate statistical differences between the indicated treatments, calculated using the Student’s t-test with two sides (* significant at p < 0.05; ** significant at p < 0.001; ns not significant)
Transcriptome profiling highlights salt tolerance-associated genes are inversely related to organic acid and amino acid metabolism
To explore the key genes associated with salt tolerance in P. vaginatum plants, we conducted a comprehensive analysis of differentially expressed genes (DEGs) in the roots and shoots of both P. vaginatum accessions under salt stress or non-stress conditions (Fig. 5). In P. vaginatum plants, compared to treated leaves, salt-treated roots exhibited a higher number of regulated DEGs (Fig. 5A and Fig. 5B). Gene enrichment analyses revealed that DEGs from salt-treated roots were involved in more biological process pathways related to salt tolerance (Fig. 5C). Under non-stressed conditions, both leaves and roots showed upregulation of DEGs enriched in aromatic and organic compound metabolic processes (Fig. S1). Upon exposure to high salinity stress, DEGs from salt-treated roots were significantly and positively enriched in organic acid metabolic processes and aromatic and organic cyclic biosynthetic processes, while showing a downregulation in amino acid transport (Fig. 5C). Conversely, DEGs identified from leaves were positively related to organic substance metabolic processes in leaves, while negatively enriched in processes related to amino acid import and transport (Fig. 5C).
A Venn diagram and (B) Volcano plot presenting the number and regulation of differentially expressed genes (DEGs) identified from comparative transcriptome analyses of the two P. vaginatum accessions under salt stress and non-stress conditions. C GO graphs showing the GO terms most enriched involved by DEGs of compound metabolic process in salt-treated leaves and roots. Abbrev: ‘R’: salt-tolerant accession; ‘S’: salt-sensitive accession; ‘T’: salt treatment; ‘C’: unsalted treatment; ‘L’: leaf; ‘R.◦’: root)
Integrated analysis of transcriptome and metabolome highlights amino acid metabolism is essential to plant salt tolerance
Initially, the correlation between important metabolites and their corresponding DEGs on the gene-metabolite regulatory network was assessed using the Partial Least Squares (PLS) regression method (Fig. 6A). Leaf and root individual metabolomes and transcriptomes were well separated based on the sPLS-DA model. An integrated pathway-level analysis was performed to combine the most significant metabolites with their corresponding DEGs in the metabolic networks. Under salt stress in salt-tolerant plants, activation of most metabolic pathways associated with amino acid metabolism played an important switching role in phenolic acids and flavonoids biosynthesis (Fig. 6B and 6C). These findings suggest that during salt stress, amino acids in the shoot may be induced for conversion to phenolic acids and flavonoids for transfer to the roots.
A Scatter plots of the sparse PLS-DA model on the first two components using significant metabolites and DEGs based on comparison analysis. B-C Complex dynamic regulatory networks of important metabolites involved in amino acid metabolism and their corresponding genes in roots of salt-tolerant P. vaginatum accession (The KEGG figures produced under the permission from Kanehisa Laboratories). Solid red or green circles represent either increased or decreased changes in metabolite abundance and gene expression. Abbrev: ‘R’: salt-tolerant accession; ‘S’: salt-sensitive accession; ‘T’: salt treatment; ‘C’: unsalted treatment; ‘L’: leaf; ‘R.◦’: root)
Discussion
Na+ is required for maintaining salt tolerance in seashore Paspalum
Globally, soil salinization is a growing challenge limiting agriculture. Improved knowledge of salt-tolerant mechanisms can be applied to the development and use of salt-tolerant crop plants. Plants that can tolerate repeated exposure to seawater are considered quite rare. The Seashore Paspalum (Paspalum vaginatum Sw.) is one of such ‘rare’ plants. Nevertheless, the precise mechanisms by it deals with high salt concentrations have remained unclear until now.
Recently, Wu et al., (2020) found that higher salinity tolerance of a P.vaginatum cultivar was associated with higher Na+, which triggered our interest in understanding how Na+ accumulation aids its enhanced tolerance[10]. Our study highlighted tissue-specific adaptations as perhaps common among halophytes. High ST values are not necessarily related to a high salt tolerance in a few halophytes such as Puccinellia tenuiflora [29], and our results suggest that P.vaginatum might be one of those.
As active Na+ uptake is efficient and cheaper than osmolyte biosynthesis, it is the principal strategy of some halophytes for osmotic homeostasis under saline conditions [30]. This enables halophytes to maintain cellular turgor pressure and sustain growth even in saline environments. Salt-tolerant P. vaginatum accession tends to accumulate Na+ to maintain high osmotic pressure for water leaf adsorption [10, 31]. With a combination of physiological and omics evidence, we found that the Na+ uptake contributes to osmotic adjustment, lipid adaptation and signalling processes, thereby enhancing salt tolerance in P. vaginatum plants.
Na+ accumulation induces root-sourced H2O2 and subsequently activates the antioxidant system
Extensive studies have shown that hydrogen peroxide (H2O2) can lead to harmful effects in unfavourable environmental conditions [32, 33]. Nevertheless, several reports highlighted the involvement of root-sourced NADPH-mediated H2O2 in increasing plant salt tolerance [34,35,36,37]. In P. vaginatum plants, Na+ uptake caused the accumulation of root-organised H2O2 in salt-tolerant plant roots (Fig. 2A). Our results confirm for the first time that root-sourced H2O2 plays a vital role in halophyte plant tolerance to high salinity.
SOD and CAT are essential defense mechanisms against oxidative stress caused by salt stress [38]. These two enzymes have been identified as crucial regulators for achieving optimal H2O2 levels, contributing to plant salinity tolerance in glycophytic plants [39, 40]. However, whether H2O2 is important for halophytes remains debatable, as there is limited evidence of its importance so far. In our study, increased root-derived H2O2 did trigger higher amounts of CAT and Mn2+-SOD (Fig. 2B and 2D) enzymes suggesting that it induced their activity in roots, therefore contributing to the enzymatic antioxidant system of salt-tolerant seashore paspalum in response to salt stress.
With increasing H2O2, some amino acids that are important as efficient antioxidants for plants to control the appropriate amounts of H2O2 under saline conditions were not significantly abundant (Fig. 3B), such as tryptophan, phenylalanine, and phenylpropanoids [41]. Interestingly, we observed that such amino acids were involved in conferring salt tolerance to P. vaginatum leaves (Fig. 3B). This result is consistent with the observation that Na+ accumulation triggers a slight rise in H2O2 generation although it was not significantly different between the two accessions (Fig. 1B and Fig. 2A). Our evidence supports that Na+ accumulation plays an important role in triggering the enzymatic and non-enzymatic antioxidant system to obtain salinity tolerance in this halophytic species, especially for roots.
Na+ uptake is associated with tissue-specific lipid metabolism
Na+ accumulation is commonly thought to decrease lipid contents [24] through lipid peroxidation caused by Na+-induced ROS in glycophytic plants under salt stress [42, 43], while studies have provided evidence that membrane lipid alterations are essential for maintaining membrane homeostasis in halophytes under salt stress [19, 20].
In agreement with previous reports, we found that salt-tolerant P. vaginatum plants are prone to increase their lipid contents to improve salt tolerance (Fig. 4). The increase was particularly noticeable for galactolipids (DGDGs) and phospholipids (PCs and PEs), and their overall increase has been linked to membrane fluidity and permeability maintenance during osmotic stress [44]. Furthermore, the importance of increased DGDGs, PCs, and PEs contents to plant stress tolerance has been confirmed [24, 45]. We observed an obvious upregulation of DGDGs, PCs and PEs in salt-tolerant plants in response to salt stress, indicating that Na+-induced lipid metabolism is required for improved plant tolerance to salt stress in the halophytic grass P. vaginatum (Fig. 4).
Na+-induced upregulation of amino acid metabolism is the central hub for root-shoot coordination
As mentioned, several amino acids act as antioxidants in the homeostasis of root-dependent H2O2 in salt tolerant accession (Fig. 3B). Additionally, some amino acids, such as arginine, are essential precursor molecules for the synthesis of secondary metabolites, including polyamines, phenolic compounds, and alkaloids associated with salt tolerance [46]. For example, the strongly decreased arginine could contribute to polyamines synthesis during salt stress in the leaves of salt-tolerant P. vaginatum plants (Fig. 3B), and phenylalanine can serve as precursors for phenolic compounds and alkaloids synthesis to minimise salt stress-induced oxidative stress [47, 48]. This result is consistent with our transcriptome profiling study, in which the gene-metabolite regulatory network has an essential role for amino acids in important secondary metabolites synthesis. Additionally, we identified several DEGs in leaves mapped into GO terms called ‘Amino acid transport” and “Amino acid import’, indicating that amino acids produced in leaves may act as important contributors for polyamines and phenolic compounds required for root salt tolerance.
Conclusion
Overall, variation in salinity tolerance among different P. vaginatum genotypes was closely linked to Na+ accumulation in the roots. Under saline conditions, salt-tolerant plants exhibit preferential Na+uptake, which is likely stored in their roots. During salt stress, the presence of Na+-induced root-sourced H2O2 activates antioxidant systems in salt-tolerant plants. This activation is tissue-specific and plays a crucial role in conferring salt tolerance. Furthermore, Na+ accumulation likely serves as a trigger for the upregulation of lipid metabolism, thereby enhancing P. vaginatum salt tolerance. Additionally, DEGs related to salt tolerance are inversely related to organic acid and amino acid metabolism. Overall, this study provides insights into the molecular mechanisms underlying P. vaginatum salt tolerance.
Availability of data and materials
The datasets generated and analyzed for this work were deposited in the NCBI Gene Expression Omnibus under the GEO accession number: GSE233155, available from https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE233155. Other datasets used in this study are included in this published article and its supplementary information files.
References
Flowers TJ, Colmer TD. Plant salt tolerance: adaptations in halophytes. Ann Bot. 2015;115(3):327–31.
Mishra A, Tanna B. Halophytes: potential resources for salt stress tolerance genes and promoters. Front Plant Sci. 2017;8:829.
Flowers TJ, Colmer TD. Salinity tolerance in halophytes*. New Phytol. 2008;179(4):945–63.
Rozema J, Schat H. Salt tolerance of halophytes, research questions reviewed in the perspective of saline agriculture. Environ Exp Bot. 2013;92:83–95.
Muchate NS, Nikalje GC, Rajurkar NS, Suprasanna P, Nikam TD. Plant salt stress: adaptive responses, tolerance mechanism and bioengineering for salt tolerance. Bot Rev. 2016;82(4):371–406.
Marco F, Bitrián M, Carrasco P, Rajam MV, Alcázar R, Tiburcio AF. Genetic engineering strategies for abiotic stress tolerance in plants. In: Bahadur B, Venkat Rajam M, Sahijram L, Krishnamurthy KV, editors. Plant biology and biotechnology: Volume II: plant genomics and biotechnology. New Delhi: Springer India; 2015. p. 579–609.
Vinocur B, Altman A. Recent advances in engineering plant tolerance to abiotic stress: achievements and limitations. Curr Opin Biotechnol. 2005;16(2):123–32.
Zulfiqar F, Ashraf M. Bioregulators: unlocking their potential role in regulation of the plant oxidative defense system. Plant Mol Biol. 2021;105(1):11–41.
Adachi S, Yamamoto T, Nakae T, Yamashita M, Uchida M, Karimata R, et al. Genetic architecture of leaf photosynthesis in rice revealed by different types of reciprocal mapping populations. J Exp Bot. 2019;70(19):5131–44.
Wu PP, Cogill S, Qiu YJ, Li ZG, Zhou M, Hu Q, et al. Comparative transcriptome profiling provides insights into plant salt tolerance in seashore paspalum (Paspalum vaginatum). Bmc Genomics. 2020;21(1):131.
Liu J, Fu C, Li G, Khan MN, Wu H. ROS homeostasis and plant salt tolerance: plant nanobiotechnology updates. Sustainability. 2021;13(6):3552.
Bose J, Rodrigo-Moreno A, Shabala S. ROS homeostasis in halophytes in the context of salinity stress tolerance. J Exp Bot. 2013;65(5):1241–57.
Choudhary A, Kumar A, Kaur N. ROS and oxidative burst: Roots in plant development. Plant Divers. 2020;42(1):33–43.
Asrar H, Hussain T, Qasim M, Nielsen BL, Gul B, Khan MA. Salt induced modulations in antioxidative defense system of Desmostachya bipinnata. Plant Physiol Bioch. 2020;147:113–24.
Amor NB, Jiménez A, Megdiche W, Lundqvist M, Sevilla F, Abdelly C. Response of antioxidant systems to NaCl stress in the halophyte Cakile maritima. Physiol Plantarum. 2006;126(3):446–57.
Parida AK, Jha B. Antioxidative defense potential to salinity in the Euhalophyte Salicornia brachiata. J Plant Growth Regul. 2010;29(2):137–48.
de AzevedoNeto AD, Prisco JT, Enéas-Filho J, Abreu CEBd, Gomes-Filho E. Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ Exp Bot. 2006;56(1):87–94.
Guo Q, Liu L, Barkla BJ. Membrane lipid remodeling in response to salinity. Int J Mol Sci. 2019;20(17):4264.
Guo Q, Liu L, Rupasinghe TWT, Roessner U, Barkla BJ. Salt stress alters membrane lipid content and lipid biosynthesis pathways in the plasma membrane and tonoplast. Plant Physiol. 2022;189(2):805–26.
Lin H, Wu L. Effects of salt stress on root plasma membrane characteristics of salt-tolerant and salt-sensitive buffalograss clones. Environ Exp Bot. 1996;36(3):239–54.
Salama KHA, Mansour MMF, Ali FZM, Abou-hadid AF. NaCl-induced changes in plasma membrane lipids and proteins of Zea mays L. cultivars differing in their response to salinity. Acta Physiologiae Plantarum. 2007;29(4):351–9.
Duncan RR. Seashore paspalum (Paspalum vaginatum Swart). Casler RD, Duncan RR, editors. Turfgrass Biology, Genetics, and Breeding. Hoboken: Wiley; 2003. p. 295–307
Duncan RR, Carrow RN. Seashore paspalum : the environmental turfgrass. Hoboken, N.J: Wiley; 1999.
Gao Y, Li M, Zhang X, Yang Q, Huang B. Up-regulation of lipid metabolism and glycine betaine synthesis are associated with choline-induced salt tolerance in halophytic seashore paspalum. Plant Cell Environ. 2020;43(1):159–73.
申晴, 韦海燕, 卞华, 王志勇, 丁西朋. 海雀稗种质资源的耐盐性评价. 热带生物学报. 2020;11(1):11–19.
Wang S, Zheng W, Ren J, Zhang C. Selectivity of various types of salt-resistant plants for K+ over Na+. J Arid Environ. 2002;52(4):457–72.
Kim J, Woo HR, Nam HG. Toward systems understanding of leaf senescence: an integrated multi-omics perspective on leaf senescence research. Mol Plant. 2016;9(6):813–25.
Kanehisa M, Furumichi M, Sato Y, Kawashima M, Ishiguro-Watanabe M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 2023;51(D1):D587-d592.
Han Q-Q, Wang Y-P, Li J, Li J, Yin X-C, Jiang X-Y, et al. The mechanistic basis of sodium exclusion in Puccinellia tenuiflora under conditions of salinity and potassium deprivation. Plant J. 2022;112(2):322–38.
Shabala S. Learning from halophytes: physiological basis and strategies to improve abiotic stress tolerance in crops. Ann Bot. 2013;112(7):1209–21.
Wu JQ, Liu WK, Jahan MS, Shu S, Sun J, Guo SR. Characterization of polyamine oxidase genes in cucumber and roles of CsPAO3 in response to salt stress. Environ Exp Bot. 2022;194:104696.
Gechev TS, Minkov IN, Hille J. Hydrogen peroxide-induced cell death in Arabidopsis: transcriptional and mutant analysis reveals a role of an oxoglutarate-dependent dioxygenase gene in the cell death process. IUBMB Life. 2005;57(3):181–8.
Chang CJ, Kao CH. H2O2 metabolism during senescence of rice leaves: changes in enzyme activities in light and darkness. Plant Growth Regul. 1998;25(1):11–5.
Ben Rejeb K, Benzarti M, Debez A, Bailly C, Savouré A, Abdelly C. NADPH oxidase-dependent H2O2 production is required for salt-induced antioxidant defense in Arabidopsis thaliana. J Plant Physiol. 2015;174:5–15.
Ma L, Zhang H, Sun L, Jiao Y, Zhang G, Miao C, et al. NADPH oxidase AtrbohD and AtrbohF function in ROS-dependent regulation of Na+/K+homeostasis in Arabidopsis under salt stress. J Exp Bot. 2012;63(1):305–17.
Qiu QS, Guo Y, Dietrich MA, Schumaker KS, Zhu JK. Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc Natl Acad Sci U S A. 2002;99(12):8436–41.
Huang Y, Cao H, Yang L, Chen C, Shabala L, Xiong M, et al. Tissue-specific respiratory burst oxidase homolog-dependent H2O2 signaling to the plasma membrane H+-ATPase confers potassium uptake and salinity tolerance in Cucurbitaceae. J Exp Bot. 2019;70(20):5879–93.
van Zelm E, Zhang Y, Testerink C. Salt tolerance mechanisms of plants. Annu Rev Plant Biol. 2020;71:403–33.
Hu T, Chen K, Hu L, Amombo E, Fu J. H(2)O(2) and Ca(2+)-based signaling and associated ion accumulation, antioxidant systems and secondary metabolism orchestrate the response to NaCl stress in perennial ryegrass. Sci Rep. 2016;6:36396.
Bartoli CG, Gómez F, Martínez DE, Guiamet JJ. Mitochondria are the main target for oxidative damage in leaves of wheat (Triticum aestivum L.). J Exp Bot. 2004;55(403):1663–9.
Batista-Silva W, Heinemann B, Rugen N, Nunes-Nesi A, Araújo WL, Braun HP, et al. The role of amino acid metabolism during abiotic stress release. Plant Cell Environ. 2019;42(5):1630–44.
Biswas MS, Mano Ji. Lipid peroxide-derived short-chain carbonyls mediate hydrogen peroxide-induced and salt-induced programmed cell death in plants. Plant Physiol. 2015;168(3):885–98.
Yalcinkaya T, Uzilday B, Ozgur R, Turkan I, Mano Ji. Lipid peroxidation-derived reactive carbonyl species (RCS): Their interaction with ROS and cellular redox during environmental stresses. Environ Exp Bot. 2019;165:139–49.
Castillo JM, Mancilla-Leytón JM, Martins-Noguerol R, Moreira X, Moreno-Pérez AJ, Muñoz-Vallés S, et al. Interactive effects between salinity and nutrient deficiency on biomass production and bio-active compounds accumulation in the halophyte Crithmum maritimum. Sci Hortic. 2022;301: 111136.
Narayanan S, Tamura PJ, Roth MR, Prasad PV, Welti R. Wheat leaf lipids during heat stress: I. High day and night temperatures result in major lipid alterations. Plant Cell Environ. 2016;39(4):787–803.
Winter G, Todd CD, Trovato M, Forlani G, Funck D. Physiological implications of arginine metabolism in plants. Front Plant Sci. 2015;6:534.
Oliva M, Guy A, Galili G, Dor E, Schweitzer R, Amir R, et al. Enhanced production of aromatic amino acids in tobacco plants leads to increased phenylpropanoid metabolites and tolerance to stresses. Front Plant Sci. 2021;11:604349.
Tyagi K, Shukla P, Rohela GK, Shabnam AA, Gautam R. Plant phenolics: their biosynthesis, regulation, evolutionary significance, and role in senescence. Plant Phenolics Sustain Agri. 2020;1:431–49.
Acknowledgements
The authors thank Paul Raymer (UGA Griffin Campus) for providing the Paspalum genotypes used in this study.
Funding
This work was supported by the Project funded by Hainan Provincial Natural Science Foundation of China [grant number 322QN248] and National Natural Science Foundation of China [grant number 32060409].
Author information
Authors and Affiliations
Contributions
Conceptualization, L.P. and Z.W.; funding acquisition, Z.W. and L.L; investigation, L.P., M.T.and Z.C.; writing—original draft, L.P., X.H. and Q.S.; writing—review and editing, L.P. and X.H. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
In this study, experimental research and field studies on plants, including the collection of plant material, comply with all relevant institutional, national, and international guidelines and legislation.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
A Lists of raw data of root metabolomes. Table S1. B Lists of raw data of leaf metabolomes. Table S1. C Lists of differentially abundanced metabolites from root metabolomes. Table S1. D Lists of differentially abundanced metabolites from leaf metabolomes.
Additional file 2: Table S2.
A Lists of raw data of root lipidomes under different treatments. Table S2. B Lists of differential lipids in roots with or without salt stress (p-value<0.05).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Pan, L., Hu, X., Liao, L. et al. Lipid metabolism and antioxidant system contribute to salinity tolerance in halophytic grass seashore paspalum in a tissue-specific manner. BMC Plant Biol 23, 337 (2023). https://doi.org/10.1186/s12870-023-04358-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-023-04358-w