Abstract
Background
Primary trisomy is a powerful genetic tool in plants. However, trisomy has not been detected in Populus as a model system for tree and woody perennial plant biology.
Results
In the present study, a backcross between Populus alba × Populus glandulosa ‘YXY 7#’ (2n = 2x = 38) and the triploid hybrid ‘Beilinxiongzhu 1#’ (2n = 3x = 57) based on the observation of microsporogenesis and an evaluation of the variations in pollen was conducted to create primary trisomy. Many abnormalities, such as premature migration of chromosomes, lagging of chromosomes, chromosome bridges, asymmetric separation, micronuclei, and premature cytokinesis, have been detected during meiosis of the triploid hybrid clone ‘Beilinxiongzhu 1#’. However, these abnormal behaviors did not result in completely aborted pollen. The pollen diameter of the triploid hybrid clone ‘Beilinxiongzhu 1#’ is bimodally distributed, which was similar to the chromosomal number of the backcross progeny. A total of 393 progeny were generated. We provide a protocol for determining the number of chromosomes in aneuploid progeny, and 19 distinct simple sequence repeat (SSR) primer pairs covering the entire Populus genome were developed. Primary trisomy 11 and trisomy 17 were detected in the 2x × 3 x hybrid using the SSR molecular markers and counting of somatic chromosomes.
Conclusions
Nineteen distinct SSR primer pairs for determining chromosomal number in aneuploid individuals were developed, and two Populus trisomies were detected from 2x × 3 x hybrids by SSR markers and somatic chromosome counting. Our findings provide a powerful genetic tool to reveal the function of genes in Populus.
Similar content being viewed by others
Background
Diploid with one extra chromosome in the genome is called trisomy and is a type of aneuploidy. Liu and Qin [1] divided plant trisomy into primary trisomy, secondary trisomy, tertiary trisomy, and terminal trisomy based on differences in the extra chromosome [2]. Among them, primary trisomy is the most widely used genetics tool. Target chromosomes can be replaced and separated by primary trisomy. Primary trisomy in plants has been used to locate genes and linkage groups to specific chromosomes to construct a physical map of genes [3, 4]. In addition, hybridizing plants with primary trisomy creates many genetic variations [5]. Therefore, primary trisomy genes are crucial genetic material, and more attention should be paid to establishing and identifying primary trisomy in plants.
Primary trisomy sets have been established for many plants. For example, primary trisomy in Nicotiana sylvestris Speg. & Comes [6] and Datura stramonium L. [7] were selected from natural mutations, although it is difficult for plants to spontaneously produce primary trisomy. Physical and chemical factors have been explored to induce primary trisomy. Hu et al. [8] treated Gossypium hirsutum L. seeds with γ-rays and detected five trisomies from self-crossing progeny. Chromosome non-or ahead-of-time-disjunction induced by irradiation may be why trisomic plants are produced. Another way to obtain primary trisomy is to screen the offspring of 3x × 2 x or 2x × 3 x hybrids, taking advantage of the irregular allocation of chromosomes during triploid meiosis. Primary trisomy in some essential crops, such as tomato [9], Zea mays [10], indica [11], sugar beets [12], barley [13], canola [14], and millet [15], were generated using this method. Additionally, autotetraploid microspore culture is another method to select primary trisomy quickly and efficiently. A set of primary trisomies has been obtained from isolated microspore-cultured plantlets of tetraploid Chinese cabbage [16]. Some previous studies [1, 17] reported that a significant number of primary trisomies can be obtained during anther culture of homologous tetraploid rice. Liu and Qin [1] detected 272 primary trisomies by culturing tetraploid rice anthers. Primary trisomy has also been obtained in diploids that have undergone asynapsis or desynapsis [6].
The methods used to identify primary trisomy in plants include morphological identification [7, 18], karyotyping of somatic mitosis [12], karyotyping of meiocytes during pachytene [19, 20], karyotyping of chromosome banding [21], and identification of a trisomic translocation line [8]. The first three methods have not been widely used because of the operation difficulties or errors.
Populus is a model system for tree and woody perennial plant biology. Populus trees are widely cultivated throughout the northern hemisphere as a source of fiber, biomass, and lumber [22]. However, to our knowledge, trisomy has not been detected in Populus. Some important poplar varieties, including ‘Beilinxiongzhu 1#’, ‘Beilinxiongzhu 2#’, and ‘Zhonglin 46’ were found to be triploids after the development of the Populus triploid breeding program in China [23, 24]. Therefore, it is possible to generate primary trisomies by crossing a triploid with a diploid in Populus.
In the present study, we obtained 393 backcross progeny by crossing the pollen of the triploid hybrid clone ‘Beilinxiongzhu 1#’ with the female gametes of P. alba × P. glandulosa ‘YXY 7#’. Then, a protocol for determining the number of chromosomes in aneuploid individuals is provided, and two real trisomies were detected using simple sequence repeat (SSR) markers and counting of the somatic chromosomes. Our findings provide new insight into Populus breeding with new genetic material.
Results
Meiotic analysis of the pollen mother cells (PMCs) in the triploid hybrid clone ‘Beilinxiongzhu 1#’
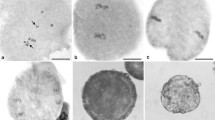
Meiosis of the PMCs in the triploid hybrid clone ‘Beilinxiongzhu 1#’ was a consecutive and asynchronous process (Table 1). Similar to other diploid Populus species, more than one meiotic stage was found in each male bud because of the asynchronous development of the PMCs in the different anthers. Abnormal phenomena often occur in the triploid hybrid poplar clone ‘Beilinxiongzhu 1#’ compared with diploid Populus species (Fig. 1).
Meiosis of the PMCs in the triploid hybrid clone ‘Beilinxiongzhu 1#’. a Leptotene. b Zygotene. c Pachytene. d Diplotene. e Diakinesis. f Metaphase I arrow indicates a chromosome that migrated prematurely. g Lagging chromosomes at anaphase I (arrow). h Chromosome bridge at anaphase I (arrow). i Telophase I. j Micronuclei at telophase I (arrow). k Prophase II. l Metaphase II. m Parallel spindles at metaphase II. n Fused spindles at metaphase II. o Lagging chromosomes at anaphase II (arrow). p Telophase II. q Tetrad. r Dyad. s Triad. t Polyad. Bars, 10 μm
Meiosis of the PMCs in the triploid hybrid clone ‘Beilinxiongzhu 1#’ started 142 h after culture in the greenhouse. About 84% of the PMCs had fine filamentous chromosomes, which were at leptotene (Fig. 1a). The dominant meiotic stage changed from zygotene to diplotene at 168–188 h of culture (Fig. 1b–d). Monovalents, divalents, and trivalents were observed at diakinesis (Fig. 1e), indicating complex chromosome pairing in this triploid hybrid clone. Metaphase I became the dominant stage when male floral branches were cultured for 196 h (25.7%). Chromosomes of most PMCs were arranged on the equatorial plate at metaphase I, while a few PMCs had chromosomes free from the equatorial plate (Fig. 1f), which led to the unequal separation of chromosomes during anaphase I (30.2%; Fig. 1g–h), resulting in lagging chromosomes (Fig. 1g) and a chromosome bridge (Fig. 1h). After 208 h of culture, telophase I (Fig. 1i) was detected under light microscopy, indicating that the first cell division was complete. PMCs with a micronucleus (Fig. 1j) were observed during telophase I. In total, about 66 h were required for the PMCs to complete the first division.
Most of the PMCs directly initiated the second division without cytokinesis. Prophase II (Fig. 1k) became the dominant stage after 212 h of culture (26.80%). Some other meiotic stages were also observed, such as metaphase I, anaphase I, telophase I, and metaphase II, as shown in Fig. 1l–n, anaphase II as shown in Fig. 1o, telophase II as shown in Fig. 1p, and tetrads as shown in Fig. 1q. The PMCs contained parallel (Fig. 1m) fused spindles at metaphase II (Fig. 1n) besides the normally oriented spindles. Lagging chromosomes appeared during anaphase II (Fig. 1o). The dominant meiotic stage was telophase II (35.6%, Fig. 1p) and 24.8% of the PMCs developed into tetrads after 236 h of culture (Fig. 1q). Dyads (Fig. 1r), triads (Fig. 1s), and polyads (Fig. 1t) were observed at the tetrad stage. The second division was much shorter than the first division, taking only 36 h in total.
Cytokinesis analysis of the PMCs in the triploid hybrid clone ‘Beilinxiongzhu 1#’
Meiotic cytokinesis of the PMCs has been divided into successive and simultaneous types [25]. Meiotic cytokinesis of the PMCs in Populus is the simultaneous type, which only occurs at the end of the second division. The cell plate did not form in the cytoplasmic domain during the second meiotic division (Fig. 1k–p). However, some triploid microsporocytes underwent premature cytokinesis following the first meiotic division as a result of the cell plate forming to divide the two daughter nuclei into distinct cytoplasmic domains (Fig. 2a). Chromosomes of the different domains are located in the equatorial region during the second meiosis, and separated into two different poles to form daughter nuclei (Fig. 2b–d). Premature cytokinesis was observed at different meiotic stages in the triploid PMCs (Table 2). The percentages of PMCs with premature cytokinesis in prophase II, metaphase II (Fig. 2b), anaphase II (Fig. 2c), and telophase II (Fig. 2d) were 45.0%, 62.0%, 68.3%, and 69.1%, respectively. This increased percentage of PMCs that were undergoing premature cytokinesis indicates that premature cytokinesis also occurred during the second meiotic division.
Premature cytokinesis during the second meiotic division of the PMCs in the triploid hybrid ‘Beilinxiongzhu 1#’. a PMCs undergoing premature cytokinesis at prophase II. b PMCs undergoing premature cytokinesis at metaphase II. c PMCs undergoing premature cytokinesis at anaphase II. d PMCs undergoing premature cytokinesis at telophase II. Bars, 10 μm
Variations in pollen viability and diameter
Abnormal meiotic chromosomal behaviors are responsible for pollen viability in PMCs [26]. The pollen grains were stained with 2% aceto-carmine solution and the deeply stained pollen grains were regarded as fertile pollen. Unstained pollen grains were considered aborted (Fig. 3a–b). The percentages of aborted pollen grains by the diploid hybrid ‘84 K’ and the triploid hybrid clone ‘Beilinxiongzhu 1#’ are presented in Fig. 3e. The percentage of aborted pollen grains in the triploid hybrid clone ‘Beilinxiongzhu 1#’ was 30.44 ± 0.88%, which was significantly higher (P < 0.05) than that of diploid hybrid ‘84 K’. Furthermore, in vitro pollen germination experiments were carried out to evaluate pollen viability. As shown in Fig. 3f, the pollen germination rate of diploid hybrid ‘84 K’ increased gradually over time, and the highest pollen germination rate reached 18.30 ± 1.29% after 6 h of culture (Fig. 3c). However, a few germinated pollen grains were observed in the triploid hybrid clone ‘Beilinxiongzhu 1#’, and the pollen germination rate was 4.09 ± 0.22% after 6 h of culture (Fig. 3d), suggesting that pollen viability of the triploid hybrid clone ‘Beilinxiongzhu 1#’ was much lower than that of diploid hybrid ‘84 K’.
Variations in the pollen between the diploid hybrid ‘84 K’ and the triploid hybrid ‘Beilinxiongzhu 1#’. a ‘84 K’ pollen. Bars, 20 μm. b ‘Beilinxiongzhu 1#’ pollen. Bars, 20 μm. c Germinated ‘84 K’ pollen grains after 6 h of culture. Bars, 50 μm. d Germinated ‘Beilinxiongzhu 1#’ pollen grains after 6 h of culture. Bars, 50 μm. e The proportion of aborted pollen. f The pollen germination rates. Arrow indicates aborted pollen
The diameter of the pollen grains was closely correlated with the pollen genomic DNA content. The diameters of the pollen from the diploid hybrid ‘84 K’ and the triploid hybrid clone ‘Beilinxiongzhu 1#’ are presented in Fig. 4. The diameter of ‘84 K’ pollen ranged from 20.0 to 41.8 μm and the average diameter was 27.24 μm. The pollen diameters were normal distribution, and most pollen grains were 25–28 μm in diameter. The diameter of pollen in the triploid hybrid clone ‘Beilinxiongzhu 1#’ ranged from 22.5 to 55.0 μm, and the average diameter was 33.7 μm. Pollen diameters between 22 and 40 μm accounted for 85.85% of the total. In addition, some enlarged pollen grains (> 50 μm) were observed in the triploid hybrid clone ‘Beilinxiongzhu 1#’, suggesting that they may have higher ploidy levels than haploid pollen [27]. As shown in Fig. 4, the distribution of pollen diameter in the triploid hybrid clone ‘Beilinxiongzhu 1#’ was bimodal in the 37–40 μm range.
Aneuploid progeny generated by crossing pollen from the triploid hybrid clone ‘Beilinxiongzhu 1#’
Although triploid plants are generally sterile due to their unbalanced chromosome segregation, a few pollen grains were fertile. To explore these fertile pollen grains in the triploid hybrid clone ‘Beilinxiongzhu 1#’, we crossed the pollen with female gametes from diploid hybrid ‘YXY 7#’ to produce a group of aneuploid progeny. A total of 4,510 seeds were obtained and 1,151 seeds germinated. The seed germination rate was 25.52%. A total of 393 seeds were sown and developed into young seedlings. Based on a pollen viability test and hybridization experiments, we concluded that triploid plants produced a small number of fertile gametes following complex meiosis.
Detection of primary trisomy in the aneuploid progeny
Nineteen pairs of distinct SSR markers covering all 19 chromosomes screened from the 184 pairs of SSR markers were used to determine the number of chromosomes in aneuploid individuals. All polymorphic SSR markers were BLAST searched against the publicly available genome sequence of P. trichocarpa v4.1 (https://phytozome-next.jgi.doe.gov/info/Ptrichocarpa_v4_1) to determine the physical positions of the SSR loci. All 19 SSR primer pairs with two different alleles in the female parent and three distinct alleles in the male parent were screened out for genotyping of the aneuploid progeny. Detailed information on the 19 SSR primers is listed in Table 3. The SSR sequence, the chromosome number, the melting temperature (Tm), the motif sequences, and the physical position of each SSR pair in the parents are described.
Based on the number of shared alleles between the female parent ‘YXY 7#’ and the male parent ‘Beilinxiongzhu 1#’, all 19 SSR primer pairs were divided into three types. In the first type, both parents were heterozygous and had distinct alleles. Using primer 12 as an example, the parents ‘YXY 7#’ and ‘Beilinxiongzhu 1#’ were heterozygous with ‘ab’ and ‘cde’ genotypes, respectively (Fig. 5a). The number of distinct alleles (size in bp) at the 12 loci was equal to the number of chromosome 6 for each progeny. The allelic configurations found in aneuploids A1, A2, A3, and A4 were “ac”, “acde”, “bcde”, and “be”. Therefore, we determined that A1 and A4 had 2 chromosome 6 s, whereas A2 and A3 had 4 chromosome 6 s, respectively. In the second type, both parents were heterozygous and shared one distinct allele PTSSR646 is an example of this type (Fig. 5b). The parents ‘YXY 7#’ and ‘Beilinxiongzhu 1#’ were heterozygous, with ‘ab’ and ‘bcd’ genotypes, and they shared one distinct allele at the PTSSR646 locus (194.0 bp). The allelic configurations of aneuploids A1, A2, A3, and A4 were “bb”, “abcd”, “bbcd”, and “bcd”. These results show that the number of chromosome 9 s was 2, 4, 4, and 3 for A1, A2, A3, and A4, respectively. In the third type, both parents were heterozygous and shared two distinct alleles (Fig. 5c). For example, at the Ptr-12-SSR53 locus, the female parent ‘YXY 7#’ had heterozygous genotype ‘ab’, and the male parent ‘Beilinxiongzhu 1#’ was heterozygous with the genotype ‘abc’. The allelic configurations of aneuploids A1, A2, A3, and A4 were “aa”, “abc”, “aabc”, and “bbc”. Thus, we inferred that the number of chromosome 12 s was 2, 3, 4, and 3 for A1, A2, A3, and A4, respectively.
Capillary electrophoresis was used for the SSR markers to infer the chromosomal composition of the backcross progeny. a Genotypic analyses of the parents and some of the progeny based on capillary electrophoresis of locus 12. b Genotypic analyses of the parents and some of the progeny based on capillary electrophoresis of locus PTSSR646. c Genotypic analyses of the parents and some of the progeny based on capillary electrophoresis of locus Ptr-12-SSR53. Letters a–e represent the alleles at those loci. Green letters indicate the allelic composition of the female parent, red letters indicate the allelic composition of the male parent, and blue indicates the allelic composition of the progeny
Among all 393 progeny, the female parent ‘YXY 7#’ only contributed one allelic locus, and the other allelic locus was derived from the male parent ‘Beilinxiongzhu 1#’. According to the relative allelic dosage estimate from MAC-PR analysis at all loci for each sample, the number of chromosomes in the 393 backcross progeny determined by the 19 pairs of SSR primers covered the whole Populus genome. As shown in Fig. 6, the number of chromosomes of each aneuploid progeny ranged from 38 to 74. Similar to the diameter of the pollen grains, the total chromosome number of aneuploid progeny was bimodally distributed with a trough at 59. The chromosome number of most aneuploids varied between 63 and 71. Based on the estimated number of chromosomes, two diploids, 152 hyper-diploids, 23 hypo-triploids, one hyper-triploid, and 215 hypo-tetraploids were found.
Among the 152 hyper-diploids, two putative primary trisomies derived from chromosome 11 (Fig. 7) and chromosome 17 (Fig. 8) were revealed by the allelic configurations of the 19 primer pairs. Trisomy 11 had three allelic loci on chromosome 11. Similarly, trisomy 17 had three allelic loci on chromosome 17. Subsequently, the two putative trisomies were confirmed by counting the number of somatic chromosomes. The chromosome number in the diploids was 38 (2n = 2x = 38, Fig. 9a), and the number of chromosomes in the trisomies was 39 (2n + 1 = 39, Fig. 9b–c), indicating that the two putative primary trisomies were real trisomies.
Discussion
Triploids normally contain three copies of each chromosome. Therefore, complex chromosome pairing and chromosome segregation occur during meiosis. In this study, high frequencies of meiotic abnormalities were detected in triploid poplar, including univalents, premature migration of chromosomes, lagging chromosomes, chromosome bridges, and micronuclei, indicating a high genetic imbalance in this triploid. Microspores with aneuploid chromosome sets formed as a result of unbalanced chromosome segregation and elimination of chromosomes. The results were consistent with studies on other triploid poplar species, such as (Populus tomentosa × P. bolleana) × P. tomentosa [28] and (P. tomentosa × P. bolleana) × (P. alba × P. glandulosa) [27]. Furthermore, this meiotic behavior is similar to other triploid species, such as Glycine max L. [29], Cucumis sativus L. [30], and Lilium pumilum DC [26].
Studies have shown that cytokinesis of PMCs in Populus are of the simultaneous cytokinesis type [31,32,33]. Premature cytokinesis often occurs in Populus tomentosa and other triploid hybrids [27, 34, 35]. In the present study, premature cytokinesis was observed in the triploid PMCs during the second meiotic division (Fig. 2), and the frequency of PMCs with premature cytokinesis was significantly higher than that documented in previous reports. Therefore, we speculated that multiple factors may be partially responsible for premature cytokinesis.
Triploid cytogenetics studies have shown that low triploid fertility is associated with a high frequency of abnormal chromosomal behavior during meiosis [36,37,38]. In this study, the pollen of the triploid hybrid clone ‘Beilinxiongzhu 1#’ was fertile, and the pollen staining and germination rates were 69.5% and 2.9%. In addition, 4,510 seeds were obtained by pollinating ‘YXY 7#’ with ‘Beilinxiongzhu 1#’. A total of 1,151 seeds germinated and 393 seeds developed into young seedlings. These results indicate that lower fertility also occurred in the triploid hybrid ‘Beilinxiongzhu 1#’. Therefore, it was possible to generate primary trisomy with the 2x × 3 x hybrid.
Pollen grain size has been used as an indicator of pollen ploidy level, as cell size increases with DNA content [39]. The range of pollen size in triploids tends to be widely distributed, indicating that pollen grains with different chromosome numbers are produced due to irregular chromosome pairing and unbalanced separation of chromosomes [40, 41]. Most pollen grains produced by a hybrid triploid are aneuploid. The diameter of the pollen of the triploid hybrid clone ‘Beilinxiongzhu 1#’ varied significantly, ranging from 22.5 to 55.0 μm. In addition, the distribution of the pollen diameter was a bimodal curve with the through at 37–40 μm, which was similar to the chromosome distribution number of aneuploid progeny. However, the distribution of pollen diameter was clustered at the first peak compared with the distribution of the chromosome number.
In this study, we identified two primary trisomies using 19 pairs of SSR primers distributed on 19 chromosomes of Populus and somatic chromosome counted from 393 progeny. Although it is common for breeders to generate primary trisomy using a 2x × 3 x hybrid [9, 12, 13], this was the first time primary trisomy was obtained in a tree species. Compared with other methods to identify primary trisomy, this experimental method accurately identified the number of chromosomes [7, 20, 21], because this method required that all of the SSR markers were polymorphic between the hybrid parents and included all 19 Populus chromosomes. Additionally, the number of chromosomes for a large number of samples can be determined quickly using our method. However, a drawback of this approach is a long time needed to screen suitable polymorphic SSR markers.
Conclusion
Many meiotic abnormalities, including premature migration of chromosomes, lagging chromosomes, chromosome bridges, asymmetrical separation, micronuclei, and premature cytokinesis were discovered during meiosis of the triploid hybrid clone ‘Beilinxiongzhu 1#’. However, these abnormal behaviors did not result in completely aborted pollen. The pollen diameter of the triploid hybrid clone ‘Beilinxiongzhu 1#’ was bimodally distributed, which was similar to the number of chromosomes. We provide a protocol for determining the number of chromosomes in aneuploid progeny and 19 distinct SSR primer pairs, including the entire Populus genome, were developed. Two primary trisomies were detected from 2x × 3 x hybrids using the SSR molecular markers and counting of the somatic chromosomes. Our findings provide a powerful genetic tool to reveal the function of Populus genes.
Materials and methods
Plant materials
The collection of flower branches were carried out in late January every year. Male floral branches of ‘Beilinxiongzhu 1#’ were collected from the Huayang Forest Tree Nursery, Hebei Province, China. Female branches of P. alba × P. glandulosa ‘YXY 7#’ (2n = 2x = 38) were collected from the Guan Country Nursery, Shandong Province. All branches were trimmed and cultured in tap water in a greenhouse (10–20 °C) at the Beijing Forestry University to force flower development. No extra nutrients were supplied to the tap water. Male buds of P. alba × P. glandulosa ‘84 K’ were used as the control group. Remarkably, we have permission to collect plant material used in the study. Kang Xiangyang, professor of National Engineering Research Center of Tree breeding and Ecological Restoration, undertook the formal identification of the plant material used in our paper. At present, all materials has been deposited in the Huayang Forest Tree Nursery, Hebei Province, China.
Meiotic and cytokinesis analyses of the PMCs
The flower branches of ‘Beilinxiongzhu 1#’ and ‘84 K’ were cultured in a greenhouse. Two to three flower buds were randomly sampled every 2 h and fixed in Carnoy’s fluid (ethanol: acetic acid, 3:1) until tetrads appeared. After 24 h, the fixed buds were transferred in 70% ethanol solution and stored at 4 °C. PMCs were dissected from the anthers of fixed flower buds using forceps and were crushed in a droplet of acetocarmine solution (2%). Examine developing microsporocytes under fluorescence microscope with 10 × eyepiece and 100 × objective lens (model BX61; Olympus, Tokyo, Japan). And a CCD camera (model DP70; Olympus) were used to take pictures. This meiotic analysis of the PMCs was conducted following the method of Kang et al. [42]. Approximately 200–300 PMCs were counted in each sample to determine the dominant meiotic stages. A meiotic analytical method was used to observe cytokinesis of the PMCs.
Measurement of pollen grain diameter
When the catkins of ‘Beilinxiongzhu 1#’ and ‘84 K’ matured, pollen samples were collected and stored in centrifuge tubes with allochronic silica gel at –20℃. The ‘Beilinxiongzhu 1#’ and ‘84 K’ pollen grains were stained with acetocarmine solution to prepare temporary smears. The pollen grains that were dyed red with the acetic acid magenta were viable, while the empty and unstained pollen grains were unviable [43]. The images of the pollen grains were taken using a CCD camera. In total, 300 viable pollen grains were randomly selected to determine the pollen diameter using ImageJ software (Version 1.53c). The experiment was repeated three times.
Evaluation of pollen viability
Pollen was germinated in vitro to assess viability. The in vitro pollen germination medium of Populus tomentosa was prepared with reference to [44]. Fresh pollen grains of ‘Beilinxiongzhu 1#’ and ‘84 K’ were sampled and spread on slides containing the medium. The slides were placed in a Petri dish containing moist filter paper and cultured in a light incubator at 26℃, with an illumination level of 4 (1500 lx) and humidity of 40%. After 2, 4, and 6 h of culture, the pollen grains were fixed in Carnoy’s fluid (ethanol: acetic acid, 3:1), respectively. The germination rate of all samples was calculated using an eyepiece micrometer. The test was repeated three times, and at least 50 pollen grains were counted for each repetition.
Aneuploid production by crossing ‘YXY 7#’ with ‘Beilinxiongzhu 1#’
When the stigmas of the ‘YXY 7#’ female flower buds were receptive, they were pollinated with fresh pollen from ‘Beilinxiongzhu 1#’. The female floral branches were further hydroponically cultured. The seeds matured after approximately 4 weeks of cultivation. They were harvested and sown in 54 × 28 × 10 cm nutrition plates in the greenhouse.
Detection of primary trisomy
The genomic DNA of all samples was extracted from young leaves using a DP320 DNA Secure Plant Kit (Tian Gen, Beijing, China), following the manufacturer’s instructions. A photometer was used to assess the purity and concentration of the stock DNA. The quality and concentration of the stock DNA were checked with the NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA). The SSR markers used for this study were polymorphic between the parents and included all chromosomes. According to this principle, SSR primers were selected from (1) the SSR database (https://web.ornl.gov/sci/ipgc/ssr_resources.htm) (beginning with ‘GCPM’) released by the International Populus Genome Consortium; (2) SSRs developed previously from an mRNA sequence in our lab (beginning with ‘PTSSR’ and ‘MB’), and (3) SSR primers developed from the P. trichocarpa genomic sequence (beginning with ‘LG’) [45]. The screened primers were applied to the number of aneuploid progeny.
The fluorescently labeled TP-M13-SSR method [46] was employed. The 5′-forward primer was attached with a universal primer M13 tail (5′-TGTAAAACGACGGCCAGT-3′) and labeled with four fluorescent substances (6-carboxy-X-rhodamine, 6-carboxy-fluorescein, tetramethyl-6-carboxyrhodamine, or 5-hexachloro-fluorescein). All primers were synthesized by Sangon Biotech Co. Ltd. (Shanghai, China). The PCR amplification protocol was 5 min at 94℃, 10 cycles of 30 s at 94℃, 30 s at the optimal annealing temperature for each SSR marker, and 30 s at 72℃; 25 cycles of 30 s at 94℃, 30 s at 53℃, and 30 s at 72℃, with a final extension of 8 min at 72℃. The PCR products were used for the capillary electrophoresis fluorescence-based SSR analysis with the ABI 3730XL DNA Analyzer (Applied Biosystems, Foster City, CA, USA), and fragment sizes and peak areas were analyzed using GeneMarker 1.75 software [47] to determine the number of chromosomes in each individual.
Somatic chromosome counts
The number of chromosomes in each plantlet was ultimately confirmed by counting the number of somatic chromosomes. The stem tips were cut from the seedlings and pretreated in a saturated solution of paradichlorobenzene for 3 h. The pretreated samples were washed once and fixed in Carnoy’s fluid (ethanol: acetic acid, 3:1) for at least 24 h at 4℃. The fixed stem tips were hydrolyzed for 25 min at room temperature in 38% HCl, followed by three rinses in distilled water for 10 min. The hydrolyzed samples were stained with Carbol fuchsin, crushed with a coverslip, and examined under an Olympus BX61 microscope at 100 × under an oil lens.
Statistical analysis
Analysis of variance was conducted using the UNIVARIATE procedure in SPSS software (SPSS for Windows, version 20; SPSS Inc., Chicago, IL, USA). Excel software (version 2206 Build 16.0.15330.20260, Microsoft Inc., Redmond, WA, USA) was used to assist with the statistical analysis and drawing. A P-value < 0.05 was considered significant.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
References
Liu ZX, Qin RZ. Selection of primary trisomics from anther culture of autotetraploid rice (Oryza sativa L.). Acta Bot Sin. 1995;37:125–33.
Shen SX. A set of primary trisomics obtained and its characteristics studies in Chinese cabbage. Jiangsu: Dissertation, Nanjing Agricultural University. 2007. https://doi.org/10.7666/d.Y1388312.
Wagenvoort M. Spontaneous structural rearrangements in solanum phureja Juz. et Buk. 2. meiotic behavior and identification of interchange chromosomes using primary trisomics. Genome. 1995;38:140–7.
Miura H, Sugawara A. Dosage effects of the three Wx genes on amylose synthesis in wheat endosperm. Theor Appl Genet. 1996;93:1066–77.
Park SM, Zhang CH, Wakana A, Karigar CS. Frequencies of aneuploid seedlings obtained from aneuploid apple accessions (Malus × domestica) pollinated with diploid “Hongro.” Q I R. 2012;57:67–72.
Goodspeed TH, Avery P. Trisomic and other types in Nieotiana sylvestris. J Genet. 1939;38:381–458.
Blackslee AF, Avery BT. Mutations in jimson weed. J Hered. 1987;74:346–60.
Hu BM, Zhang TZ, Pan JJ. Studies of trisomic plants in upland cotton. origin, cytological identification and their phenotypes. Acta Agr Sin. 1996;22:147–51+257-258.
Rick CM, Barton DW. Cytological and genetical identification of the primary trisomics of the tomato. Genet. 1954;39:640–66.
Mcclintock B. Chromosome morphology of Zeamays. Sci. 1929;69:629–30.
Zhang TB, Zhu H, Shu LH. Studies on aneuploids form a Chinese indica variety of rice I. plant morphological part. Acta Genet Sin. 1987;14:255–61.
Guo DD, Li SY, Bai QW, Jin BH, Lou ZW, Zhu HB, et al. A preliminary report on establishing and identification of the trisomic series in sugar beets. Acta Genet Sin. 1996;13:27–34.
Tsuchiga T. Cytogenetic studies of trisomics in barley. Jpn J Bot. 1960;17:177–213.
Tang K, Hill CB, Williams PH. Development of trisomics of rapid-cycling Brassicarapa. Eucarpla Cruciferae Newsl. 1998;13:60–1.
Wang RQ, Gao JH, Wang ZX, Wang ZM. Establishment of trisomic series of millet ( Setaria Italica L. Beauv.). Acta Bot Sin. 1994;9:690–5+743-745.
Shen SX, Hou XL, Zhang CH. A study on obtaining primary trisomics by the isolated microspore culture of autotetraploid Chinese cabbage. Acta Hortic Sin. 2006;6:1209–14.
Chu QR, Zhang CM, Zheng ZL. Anther culture of rice tetraploid pollen plants and chromosome variation in regenerated plants. Chinese Bull Bot. 1985;3:40–3.
Nobuo I, Takeshi O, Masahiro N. Studies on the trisomics in rice plants (Oryzasativa L.) I. morphological classification of trisomics. Jpn J Breed. 1970;20:230–6.
Sharstry SNS, Ranga RDK, Misra RN. Pachytene analysis in Oryza. I. chromosome morphology in Oryzasativa L. India J Genet Plant Breed. 1960;20:15–21.
Cheng ZK, Li X, Yu HX, Gu MH. A new set of primary trisomics in indica rice, its breeding and cytological investigation. Acta Genet Sin. 1996;5:363–71.
Justus I, Kevin BJ, David H. Production and identification of primary trisomics in diploid Agropyron cristatum (crested wheatgrass). Genome. 1994;7:469–76.
Wang J, Huo BB, Liu WT, Li DL, Liao L, Prigent C. Abnormal meiosis in an intersectional allotriploid of Populus L. and segregation of ploidy levels in 2x × 3x progeny. PloS One. 2017;12:e0181767.
Kang XY, Zhu ZT, Zhang ZY. Breeding of triploids by the reciprocal crossing of Populus alba × P.glandulosa and P.tomentosa × P.bolleana. J B For Univ. 2000;22:8–11.
Zhang SG, Qi LW, Han SY. A report of triploid Populus of the section Aigeiros. Silvae Genet. 2004;53:69–75.
Storme De, Danny G. Cytokinesis in plant male meiosis. Plant Signaling Behav. 2013;8:e23394.
Zhang W, Wang C, Xue L, Zheng Y, Lei JJ. Production of pollenless triploid lily hybrids from Lilium pumilum DC. × 'Brunello'. Euphytica. 2018;214:171. https://doi.org/10.1007/s10681-018-2248-6.
Xu CY, Wang J, Ye S, Kang XY. Meiosis of microsporocyte and variation of pollen in an allotriploid white poplar. Acta Bot. 2011;31:2454–8.
Kang XY, Zhu ZT, Zhang ZY. Morphology and meiosis of Chinese white poplar. J B For Univ. 1999;1:5–9.
Chen LF, Palmer RG. Cytological studies of triploids and their progeny from male-sterile (ms1) Soybean. Theor Appl Genet. 1985;71:400–7.
Diao WP, Cui L, Jiang B, Bao SY, Chen JF. Creation and meiosis studies of autotriploid in Cucumber. Acta Bot Boreal -Occident Sin. 2009;9:36–42.
Wang J, Kang XY, Li DL, Jing YC, Zhang ZH. Meiosis and chromosome behavior of pollen mother cell in PopulussimoniiCarr. × P.nigra L. “Tongliao.” Acta Bot. 2006;26:2231–8.
Zhang ZH, Kang XY, Wang SD, Li DL, Chen HW. Pollen development and multi-nucleate microspores of Populus bolleana Lauche. For stud China. 2008;10:107–11.
Li DL, Shang J, Tian J, Song LJ, Liu CH, Li YC, et al. Meiotic chromosome behavior of pollen mother cells and pollen variation in triploid hybrid between section tacamahaca and sect. Aigeiros of Populus. J B For Univ. 2019;41:75–82.
Zhang ZH, Kang XY, Zhang PD, Li YH, Wang J. Incidence and molecular markers of 2n pollen in Populus tomentosa Carr. Euphytica. 2007;154:145–52.
Zhang JF, Wei ZZ, Li D, Li B. Using SSR markers to study the mechanism of 2n pollen formation in Populus × euramericana (Dode) Guinier and P. × popularis. Ann For Sci. 2009;66:506–506.
Lange W, Wagenvoort M. Meiosis in triploid Solanum tuberosum L. Euphytica. 1973;22:8–18.
Dweikat IM, Lyrene PM. Production and viability of unreduced gametes in triploid interspecific blueberry hybrids. Theor Appl Genet. 1988;76:555–9.
Zhang J, Wu XJ, Wang XD, Zhou KD, Peng H. Cytological study on a special autotriploid rice SAR3. Acta Agron Sin. 2002;28:704–8.
Bretagnolle F, Thompson JD. Gametes with the somatic chromosome number: mechanisms of their formation and role in the evolution of autopolyploid plants. New Phytol. 1995;129:1–22.
Wang J, Kang XY, Zhu Q. Variation in pollen formation and its cytological mechanism in an allotriploid white poplar. Tree Genet Genomes. 2010;6:281–90.
Xi X, Zha Q, Jiang AL, Tian YH. Meiotic chromosome behavior of pollen mother cells and gamete fertility in triploid grape ‘Summer Black.’ Acta Bot. 2015;35:2422–7.
Kang XY, Zhu ZT, Zhang ZY. Meiosis and its stages of pollen mother cells in Chinese white poplar. J B For Univ. 2000;6:5–7.
Feng Z, Wei C, Jia GX. Meiotic chromosome behavior in allotriploid cultivar of Lilium hybrids. J B For Univ. 2012;34:118–24.
Zhao CG, Tian MD, Li YJ, Zhang PD. Slow-growing pollen-tube of colchicine-induced 2n pollen responsible for low triploid production rate in Populus. Euphytica. 2017;213:94.
Yin TM, Zhang XY, Gunter LE, Li SX, Wullschleger SD, Huang MR, et al. Microsatellite primer resource for Populus developed from the mapped sequence scaffolds of the Nisqually-1 genome. New Phytol. 2009;181:498–503.
Schuelke M. An economic method for the fluorescent labeling of PCR fragments. Nat Biotechnol. 2000;18:233–4.
Hulce D, Li X, Snyderleiby T, Liu J. GeneMarker® genotyping software: tools to increase the statistical power of DNA fragment analysis. J Biomol Tech JBT. 2011;22(Suppl):S35.
Acknowledgements
Not applicable.
Research involving plants
The authors confirm that all the experimental methods and plants complied with relevant institutional, national, and international guidelines and legislation.
Funding
This work was supported by the National Key R&D Program of China during the 14th Five-year Plan Period (2022YFD2200301 -02).
Author information
Authors and Affiliations
Contributions
P.Z. contributed to the study conception and design. Material preparation were performed by L.S. and J.W.. Data collection and analysis were performed by Y.S., Y.Z. and Q.Z.. The first draft of the manuscript was written by Y.S. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All the experimental methods and plants complied with relevant institutional, national, and international guidelines and legislation.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sang, Y., Kong, B., Do, P.U. et al. Microsporogenesis in the triploid hybrid ‘Beilinxiongzhu 1#’ and detection of primary trisomy in 2x × 3 × Populus hybrids. BMC Plant Biol 23, 177 (2023). https://doi.org/10.1186/s12870-023-04189-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-023-04189-9