Abstract
Background
Angelica dahurica belongs to the Apiaceae family, whose dry root is a famous traditional Chinese medicine named as “Bai zhi”. There are two cultivars (A. dahurica cv. ‘Hangbaizhi’ and A. dahurica cv. ‘Qibaizhi’), which have been domesticated for thousands of years. Long term artificial selection has led to great changes in root phenotypes of the two cultivars, and also decreased their adaptability to environment. We proposed hypothesis that the cultivars may have lost some of the genetic diversity found in the wild species and may be highly differentiated from the latter during the domestication process. However, few studies have been carried out on how domestication affected the genetic variation of this species. Here, we accessed the levels of genetic variation and differentiation within and between wild A. dahurica populations and two cultivars using 12 microsatellite markers.
Results
The results revealed that the genetic diversity of the cultivars was much lower than that of wild A. dahurica, and A. dahurica cv. ‘Qibaizhi’ had lower genetic diversity compared to A. dahurica cv. ‘Hangbaizhi’. AMOVA analysis showed significant genetic differentiation between the wild and cultivated A. dahurica populations, and between A. dahurica cv. ‘Hangbaizhi’ and A. dahurica cv. ‘Qibaizhi’. Results from Bayesian, UPGMA, NJ and PcoA clustering analysis indicated that all 15 populations were assigned to two genetic clusters corresponding to the wild and cultivated populations. Bayesian clustering analysis further divided the cultivated populations into two sub-clusters corresponding to the two cultivars.
Conclusions
Our study suggests that the domestication process is likely the major factor resulting in the loss of genetic diversity in cultivated A. dahurica populations and in significant genetic differentiation from the wild populations due to founder effect and/or artificially directional selections. This large-scale analysis of population genetics could provide valuable information for genetic resources conservation and breeding programs of Angelica dahurica.
Similar content being viewed by others
Background
Medicinal plants have been used as important sources of medicine to prevent and treat many human diseases in traditional cultures all over the world for thousands of years [1, 2]. However, given the dramatically increasing demand for plant-derived medicine, many medicinal plants are under over-exploitation, increasing their risk of extinction in the wild [3, 4]. Generally, wild resource contains great genetic variations and beneficial genes before domestication and artificial selection, providing a reservoir of genetic variation for exploiting in breeding efforts [2]. Therefore, effective measures for the conservation need to be taken to further protect the wild resources of medicinal plants. Genetic diversity underlies adaptation and evolution of plants, which allows for dealing with various biotic and abiotic stresses in changing environments [5, 6]. It is also the basis of the plasticity of secondary metabolism, and thus the production of medicinal compounds [7, 8]. Therefore, investigating the genetic variation of medicinal plants in the wild is vital to plan conservation strategies for preserving medicinal plants, as well as breeding programs [9, 10].
Plant domestication is an episode in which human-mediated selection favors phenotypic modification of wild resources to meet human needs, mainly through artificial selection [11, 12]. Domestication of plants not only modifies their phenotypes but also has major impacts on the genetic variation [13,14,15]. One common genetic effect of domestication is the decrease of genetic diversity compared to their wild resources [16]. The loss of genetic diversity may lead to the reduction of the ability to long-term survival and evolution in changeable environments [17, 18]. It is reported that the extent of the loss of genetic diversity may differ considerably among domesticated plants due to various life-history traits and evolutionary history [19]. For example, about one-third of genetic diversity was lost in soybean [20] and maize [21] compared to their wild relatives, while a majority of genetic diversity was lost in wheat [22]. The difference in extent of the loss of variation lies on the initial population size and the duration of that period [13]. Thus, knowledge of how domestication affects genetic diversity and structure across the range of both wild and cultivated populations is critical for the management and improvement of cultivars of medicinal plants in the future [15, 23]. Up to date, most studies on the effect of domestication on genetic diversity have focused on agronomic crops [12,13,14, 19, 24], with less attention being paid to medicinal crops. Although a small number of all known medicinal plants have been studied on their population genetics, e. g. Scrophularia ningpoensis [8], Atractylodes macrocephala [25] and Cannabis species [24], numerous ongoing domestication processes have yet to be studied, and these have been of interest to medicinal plant genetic resources conservation and breeding programs.
Angelica dahurica (Hoffm.) Benth. et Hook. f. ex Franch. & Sav., is a perennial herb belonging to the genus Angelica of the family Apiaceae, mainly distributed in North and Northeast China, Japan, Korea, Russia (Siberia) [26]. Dichogamy has been detected in A. dahurica, which is an effective mechanism to encourage outcrossing and avoid selfing [27]. The pollination mechanisms of the Umbelliferae plants were always thought to be wind or insect pollination [28]. Such mechanisms have been observed in the Angelica species, such as A. biserrata [29]. Therefore, the mating system of A. dahurica is considered to be outcrossing. The dry root of the species, named as “bai zhi” (Angelicae Dahuricae Radix), is a famous traditional Chinese medicine, which has been used as a food additive as well as a folk medicinal therapy of headache, rhinitis, cold and toothache amongst others in East Asian countries (Korea, China, and Japan) for thousands of years [30,31,32]. Two cultivars, A. dahurica cv. ‘Hangbaizhi’ and A. dahurica cv. ‘Qibaizhi’, widely cultivated in China, have gone through evolution by artificial selection for more than 1000 and 200–400 years, respectively [26, 30, 33]. Long term artificial selection has led to great changes in the root phenotypes of the two cultivars compared with their wild species, and also decreased the adaptability (disease and insect resistances) to environment [26, 34]. Herein, we supposed that the cultivated A. dahurica (the two cultivars) may have lost some of the genetic diversity found in the wild species and may be highly differentiated from the latter during the domestication process in the past decades. However, so far, few studies have concerned on how domestication affects the genetic variation of cultivars of A. dahurica. There is still lack of studies on genetic diversity and population structure of A. dahurica and it culitvars.
In a previous study, we developed highly polymorphic SSR markers by transcriptome sequencing for A. dahurica [35], providing efficient molecular markers to conduct population genetics on A. dahurica. In this study, we aim to: (1) access the levels of genetic variation and differentiation within and between wild A. dahurica and its cultivars; (2) clarify how the domestication process influence on genetic variation of this species. Hopefully, the outcomes of this study could provide valuable information for genetic resource conservation and breeding programs of A. dahurica.
Results
Allele frequency distribution patterns and genetic diversity
A total of 336 individuals from 15 populations were genotyped with 12 SSR markers, resulted in 108 alleles, of which 90 and 80 were detected in wild and cultivated populations, respectively (Table 1). On average, 9.0 ± 2.7 (range 6–16) alleles per locus were observed. There were 24 private alleles in 11 populations, including 13 alleles in six wild populations and 11 alleles in five cultivated populations (Table 1). The allele frequency distribution patterns are shown in Fig. 1. At species level, rare allele (allele frequency ≤ 5%) accounted for the highest proportion (61.11%). Medium-to-high frequency allele (50% < allele frequency < 100%) were much less than low-to-medium frequency allele (5% < allele frequency ≤ 50%) (5.56% vs 33.33%). There was no common allele (i.e. found in all individuals within A. dahurica, allele frequency = 100%) in A. dahurica. Wild and cultivated A. dahurica displayed the same allele frequency distribution patterns. Among all populations except for two cultivated populations (ZC and YZ), low-to-medium frequency allele accounted for the highest proportion (41.30–80.49%), followed by rare allele (4.88–38.46%) and medium-to-high frequency allele (12.96–25.00%). Common alleles were the least prevalent one (0–13.33%).
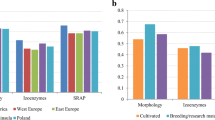
At species level, the average values of observed heterozygosity (Ho) and Shannon’s index (I) were 0.412 and 0.735, respectively (Table 1). The wild A. dahurica had, on average, significantly higher estimates of genetic diversity than did the cultivated one (Ho: 0.523 ± 0.077 vs 0.316 ± 0.063, p = 0.00; I: 0.933 ± 0.121 vs 0.562 ± 0.120, p = 0.00) (Table 1). Compared to populations of A. dahurica cv. ‘Haibaizhi’, populations of A. dahurica cv. ‘Qinaizhi’ showed slightly lower genetic diversity (Ho: 0.301 ± 0.038 vs 0.340 ± 0.097, p = 0.08; I: 0.514 ± 0.083 vs 0.643 ± 0.145, p = 0.19). For each population analyzed, genetic diversity parameters varied widely among populations (Table 1). The highest level of genetic diversity was found in the wild population DH (Ho = 0.605, I = 1.110), and the lowest in the cultivated population JN (Ho = 0.249, I = 0.437). Four populations, including one wild population (BJ) and three cultivated populations (SN, AG and YZ), had significantly positive FIS values (Table 1), suggesting their heterozygote deficiency. The remainder showed negative FIS values, indicating they presented an excess of heterozygotes.
Divergence between wild and cultivated Angelica dahurica, and between two cultivars
Nonhierarchical AMOVA indicated that 34.53% (Fst = 0.345) of the total variation was partitioned among populations of A. dahurica (Table 2). That is, of total genetic variation, most (65.47%) was due to individual differences within populations (Table 2). Only 19.41% of total variation resided among populations in wild A. dahurica, but variation distributed among populations and individuals were almost equal (48.58% vs 51.42%) in cultivated A. dahurica (Table 2). That is, population differentiation in cultivated A. dahurica is much stronger than that in wild A. dahurica (Fst: 0.486 vs 0.194) (Table 2).
Hierarchical AMOVA revealed that 26.99% of the total variation was distributed between wild and cultivated A. dahurica, with 15.89 and 57.13% explained by variation among and within populations, respectively (Table 2). In the two cultivars, 14.78% of the total variation was found between A. dahurica cv. ‘Hangbaizhi’ and A. dahurica cv. ‘Qibaizhi’ (Table 2). Significant genetic divergence was found both between wild and cultivated A. dahurica (Fct = 0.270, p = 0.00), and between A. dahurica cv. ‘Hangbaizhi’ and A. dahurica cv. ‘Qibaizhi’ (Fct = 0.148, p = 0.00) (Table 2).
Genetic structure
Genetic admixture analysis performed by STRUCTURE revealed that both the maximum value of delta K and the highest log likelihood were observed at K = 2 (Fig. 2A-B), indicating that all populations were assigned to two genetic clusters (Fig. 2C). All individuals in wild populations were assigned to the same genetic cluster, while all individuals in the cultivated populations were assigned to a second genetic cluster. There was a high degree of admixture of two gene pools in most individuals of BJ population and some individuals of KS population. With K = 3, substructure appeared in the cultivated populations, which were divided into two genetic cluster corresponding to the two cultivars (A. dahurica cv. ‘Hangbaizhi’ and A. dahurica cv. ‘Qibaizhi’), whereas the wild populations remained relatively uniform (Fig. 2C). When K = 4, substructure appeared in the wild populations. All individuals of BJ and KS populations were assigned into the same genetic cluster, with some degree of admixture of three gene pools in most individuals (Fig. 2C).
Bayesian clustering results of the STRUCTURE analysis for Angelica dahurica and its two cultivar, A. dahurica cv. ‘Hangbaizhi’ and cv. ‘Qibaizhi’ assessed with 12 microsatellite loci. A Estimates of ΔK with respect to K; B Plot of the probability of the data (LnP(D)) values; C Genetic group structure with K = 2, 3 and 4
The UPGMA dendrogram (Fig. 3A) was broadly consistent with the unrooted neighbor-joining (NJ) tree (Fig. 3B). Fifteen populations were classified into two clusters (I, II), which corresponded to cultivated and wild A. dahurica, respectively. PCoA (Fig. 4) largely confirmed the partitioning results of the UPGMA dendrogram, the NJ tree and STRUCTURE analysis, and showed some mixture of populations in both wild (BJ, BX and KS) and cultivated A. dahurica.
Discussion
Genetic diversity of wild A. dahurica
Wild progenitors are always an important genetic resource for plant breeding [36]. However, genetic diversity and population genetic structure of wild populations of A. dahurica is poorly understood. In this study, we therefore revealed the genetic diversity and population genetic structure of this species, providing important basic information for the further conservation and breeding effort. The breeding system is always considered to have a major effect on genetic diversity of plant species [6, 37, 38]. Many studies have shown that outcrossing plants tend to maintain a higher genetic diversity compared to selfing plants [39,40,41]. Our results revealed that genetic diversity of A. dahurica was higher than that of selfing species summarized by previous meta-analyses [37, 42]. As an Umbelliferae and dichogamous plant, A. dahurica may cross-pollinate by wind or insects [28, 29], which will promote gene exchange between individuals, thus preventing loss of diversity caused by genetic drift. This can be evident by the absence of inbreeding signs in most of wild A. dahurica populations. Moreover, the common allele is extremely rare, while the low-to-medium frequency allele is the most prevalent within A. dahurica populations, indicating that the populations are heterogeneous. In addition, A. dahurica harbors greater genetic diversity within population and a low genetic differentiation among populations as many outcrossing plants [37,38,39,40,41,42]. However, when compared to other Apiaceae species, with Ho values ranging from 0.361 to 0.85 [43,44,45,46,47], wild A. dahurica showed moderate genetic diversity (Ho = 0.523). Anthropic activities also have a huge impact on genetic diversity of wild plants as they are usually accompanied with dramatically increasing loss and damage of natural habitats [48, 49]. Theoretically, loss of habitat can cause a decrease of population genetic diversity due to the reduction of population size, increased random genetic drift and inbreeding [6]. During our 6 years (2015–2020) of fieldwork, we observed habitat erosion in A. dahurica due to anthropic activities caused by urbanization, agricultural and silvicultural practices. In addition, the wild resources are in decline due to over-exploitation. Thus, over-exploitation of the wild resources and habitat destruction are the most likely factors responsible for the moderate level of genetic diversity in A. dahurica. Among the seven wild populations, DH, AS and BX showed relatively high genetic diversity, which could provide abundant genetic variation for breeding. In addition, most of populations preserve private alleles, which are also valuable genetic resources for breeding.
Genetic consequence of domestication
Many experiential studies have revealed that domestication processes caused a massive loss of genetic diversity in domestication crop plants [12, 50]. However, the extent of the loss of genetic diversity in domesticated medicinal plants have been poorly understood. Here, we assessed this reduction by comparing the levels of genetic diversity between wild and cultivated populations. Our results demonstrated that the average population genetic diversity of cultivated A. dahurica was approximately reduced by a third compared to the wild resource, respectively. The findings of our study suggested that domestication had a negative effect on genetic diversity of cultivated A. dahurica, and the extent was comparable to many crop plants, e. g. maize and soybean [20, 21].
It has been demonstrated that founder effect or bottleneck effect, selective sweeps and/or directional selection, and intensive breeding are the main factors causing the reduction of genetic diversity [12, 13, 23, 51]. During the initial domestication, the population size is extremely small (founder effect), which will result in enormous decrease of genetic diversity [12]. This might be the cause of A. dahurica, which was most likely descended from a limited number of individuals from the wild since more than 1000 years ago [30,31,32]. Furthermore, directional selection for desirable phenotypes during domestication processes would cause a more drastic loss of diversity [13]. During domestication processes of A. dahurica, the phenotype of thick and unbranched root might be continuously selected, resulting in further loss of diversity by the reduction of the effective population size and the increasing genetic drift. Moreover, inbreeding caused further genetic erosion, which is a recent phenomenon caused by the decrease of the efficient population size after domestication [15, 52]. Obvious signs of inbreeding were observed in some cultivated populations (i. e. populations SN, AG and YZ, see Table 1), suggesting that inbreeding may be also partly responsible for the diversity decline.
For the two cultivars, a reduction of genetic diversity from A. dahurica cv. ‘Hangbaizhi’ to A. dahurica cv. ‘Qibaizhi’ was observed. STRUCTURE cluster analysis showed that the two cultivars clustered into a genetic group, indicating that cultivated A. dahurica have possibly originated from the same genetic resource. According to the time of initial cultivation, A. dahurica cv. ‘Hangbaizhi’ (more than 1000 years) has a longer domestication history compared to A. dahurica cv. ‘Qibaizhi’ (about 200–400 years) [26, 30, 33]. Thus, A. dahurica cv. ‘Qibaizhi’ may have originally being introduced from A. dahurica cv. ‘Hangbaizhi’. Our result is likely consistent to the fact that genetic diversity decreased along the diffusion history [12, 53, 54]. Unfortunately, as there is no record of domestication origin of the two cultivars, the reason behind this diversity decline is still unclear.
Besides the loss of genetic diversity, genetic divergence between wild plants and their cultivars is another major genetic footprint during plant domestication process [25, 50]. Morphological differences in root had been found between wild and cultivated A. dahurica, and between A. dahurica cv. ‘Hangbaizhi’ and cv. ‘Qibaizhi’ [26], however, few investigations have been carried out on their genetic divergence. Our results of NJ tree, UPGMA dendrogram and Bayesian genetic structure revealed a distinct divergence between the wild and cultivated A. dahurica. PCoA analysis almost confirmed the partitioning results of NJ tree, UPGMA dendrogram and Bayesian genetic structure. Significant genetic differentiation was also detected between the wild and cultivated A. dahurica (Fct = 0.270, p = 0.000) in the AMOVA analysis. Furthermore, the Bayesian genetic structure analysis also indicated an obvious genetic clustering between the two cultivars (A. dahurica cv. ‘Hangbaizhi’ and A. dahurica cv. ‘Qibaizhi’). Also, AMOVA analysis showed significant genetic differentiation between them (Fct = 0.148, p = 0.000). Artificial selection as a driving force of divergence between wild and domesticated plants has been documented [55,56,57,58]. In this study, the divergence between wild A. dahurica and its cultivars are most probably derived from artificial selection of root characteristics during the domestication process. Although the two cultivars possibility originated from the same genetic resource, they are cultivated in different regions since initial cultivation. Therein, A. dahurica cv. ‘Hangbaizhi’ was mainly cultivated in Zhejiang and Sichuan Provinces, China, while A. dahurica cv. ‘Qibaizhi’ was planted in Henan, Hebei and Anhui Provinces, China (see Fig. 5). Introduction of one cultivar into the growing areas of the other cultivar hardly ever occurred, which may have prevented gene flow between the two cultivars, promoting their genetic differentiation.
Geographic distribution of sampled populations of Angelica dahurica and its two cultivar, A. dahurica cv. ‘Hangbaizhi’ and cv. ‘Qibaizhi’. The map was drawn by the authors with reference to Google Maps. The map can be found at https://maps.google.com/
Conservation strategies and utilization in breeding
Our results suggested that the long time artificial selection has resulted in the loss of genetic diversity, and this may lead to the decline of disease and insect resistance in cultivated A. dahurica. Generally, wild resources are always recognized as a critical resource for breeding efforts [13]. The significant genetic differentiation observed between wild and cultivated A. dahurica (two different gene pools) indicated that the wild resources could be used as a source of novel alleles for improvement of the future genetic improvement of cultivated A. dahurica. However, the wild resources are in decline. Based on the knowledge of genetic diversity and structure of A. dahurica, an appropriate conservation and management strategy can be formulated here. Conservation of genetic diversity should concentrate on the maintenance of large populations in outcrossing plants due to the fact that most genetic diversity reside within populations [6, 10]. In this case, maintenance of large and diversified populations of A. dahurica (i. e. DH, AS and BX) is a key to preserve diversity due to lower differentiation among populations and greater variation within population. These populations with high levels of diversity could provide abundant genetic variation for future breeding program.
Conclusions
In the present study, we used 12 SSR markers to access the levels of genetic variation and differentiation within and between wild A. dahurica and its cultivars from 15 locations. Our results revealed that cultivated A. dahurica harbored lower genetic diversity, and showed significantly high genetic differentiation from wild A. dahurica. The domestication process through artificial selection is the major factor resulting in the loss of genetic diversity in cultivated A. dahurica, considering that significant genetic divergence has been found in the wild resources. Our results could provide genetic insight to improve conservation and management strategies for A. dahurica, and advance understanding of effects of domestication on genetic diversity of medicinal plants.
Methods
Plant samples collections
Between July 2017 and August 2019, we sampled Angelica dahurica wild populations from 7 locations which covered all the species natural distribution area in China. Specimens of A. dahurica cv. ‘Hangbaizhi’ and A. dahurica cv. ‘Qibaizhi’ were collected from 3 and 5 locations, respectively, which included almost all the main cultivated area (Table 3, Fig. 5). Samples collection protocols are as follow: in each population, the distance between each collected individual plant was over 20 m for both wild and cultivated populations, which aimed to avoid multiple samples from the same clone [35]. We obtained a total of 336 plant individual specimens (fresh leaves) from 15 populations, an average of 22.4 individuals for each population (ranging from 17 to 24 individuals). All the fresh specimens were preserved in gel-dried silica for DNA extraction. For the wild species A. dahurica and two cultivars, as so far they have not been listed in national key protected plants, we collected the samples without any required permissions. Associate professor Enwei Tian from the School of Traditional Chinese Medicine, Southern Medical University (SMU), morphologically identified all the voucher specimens mentioned above, which then were deposited in the herbarium of SMU (Table 3). Our field study and Experimental research complied with local legislation, national and international guidelines. The authors also complied with the Convention on the Trade in Endangered Species of Wild Fauna and Flora.
DNA extraction, SSR-PCR amplification and genotyping
Total genomic DNA was extracted from the dried leaves of each sample using a modified CTAB method [59]. DNA concentration and quality of the exacted DNA were assessed using a NanoDrop 1000 UV/Vis spectrophotometer (Thermo Scientific, Wilmington, DE, USA) and gel electrophoresis in 1.5% agarose, respectively.
Twelve microsatellite markers, previously developed for A. dahurica [35], were selected to determine the genetic diversity and population structure of A. dahurica and its cultivars. Attributes of the 12 SSR primers are shown in Table 4. The forward primers (5′ end) were labeled with one of the following the forward dyes: TAMRA or FAM. PCR amplification was performed in a volume of 20 μL consisting of 20 ng genomic DNA, 0.2 mM each dNTP, 0.4 μM each primer, 10 × PCR buffer (Mg2+ free), 2.5 mM Mg2+, 1 unit Taq DNA polymerase (Takara, Dalian, China) with the following procedure: initial denaturation at 95 °C for 5 min, 35 cycles of denaturation at 94 °C for 30 s, annealing at 50 to 55 °C for 60 s, extension at 72 °C for 45 s and a final extension of 72 °C for 8 min. The PCR products were visualized using capillary electrophoresis on an ABI PRISM 3100 Genetic Analyser (Applied Biosystems, Foster City, CA). The size of all alleles was determined on Genotyper 4.0, with LIZ 500 (Applied Biosystems, Foster City, CA) as an internal product size standard.
Data analysis
To explore the level of genetic diversity, number of observed alleles (Na), effective alleles (Ne) and private alleles (Ap), Shannon’s information index (I), observed heterozygosity (Ho), expected heterozygosity (He) over loci were calculated using GenAlEx v6.502 [60]. GenAlEx was also used to calculate allele frequency. Wright’s F-statistics inbreeding coefficient (FIS) [61] was calculated using ARLEQUIN v3.5 [62], with significance determined by permutation (1000 replicates). Subsequently, an analysis of molecular variance (AMOVA) was also implemented in ARLEQUIN to quantify the partitioning of genetic variation and coefficient of genetic differentiation with 999 permutations used for tests of significance.
To understand the genetic relationship among all populations, Bayesian cluster analysis was conducted in the program STRUCTURE v2.1 [63] to assign a certain individual to K genetic clusters. K was set as 1 to 15. Five runs for each K were performed under the admixture model, with a burn-in length of 100,000 and a run length of 1,000,000 Markov chain Monte Carlo (MCMC) replications. The optimal value of K was determined by Evanno test using Structure Harvester [64]. Nei’s genetic distances between populations were calculated in GenAlEx, and used as input for a cluster analysis using both the unweighted pair-group method of arithmetic averages (UPGMA) and neighbour-joining (NJ) method using MEGA X [65], respectively. Principal coordinate analysis (PCoA), as an alternative mean, was performed in GenAlEx to detect and visualize the genetic relationship among populations.
Availability of data and materials
Raw sequence information are available in National Center for Biotechnology Information (NCBI) Sequence Read Archive (RNA sequence information, SRA: SRP162120 BioProject ID PRJNA490770). Sequence information for the developed EST-SSR primer pairs has been deposited at GenBank (Accession numbers are provided in Table 4). The other datasets supporting the findings of this study are available in the Dryad Digital Repository (https://doi.org/10.5061/dryad.d51c5b050).
Abbreviations
- SSR:
-
Simple Sequence Repeat
- Na:
-
Number of observed alleles
- Ne:
-
Effective alleles
- Ap:
-
Private alleles
- I :
-
Shannon’s information index
- Ho:
-
Observed heterozygosity
- He:
-
Expected heterozygosity
- F IS :
-
Inbreeding coefficient
- MCMC:
-
Markov chain Monte Carlo
- UPGMA:
-
Unweighted pair-group method of arithmetic averages
- NJ:
-
Neighbour-joining method
- PCoA:
-
Principal coordinate analysis
- d.f.:
-
Degree of freedom
- F ct :
-
Genetic differentiation between groups
- Fst:
-
Genetic differentiation among populations within group
References
Hosseinzadeh S, Jafarikukhdan A, Hosseini A, Armand R. The application of medicinal plants in traditional and modern medicine: a review of Thymus vulgaris. Int J Clin Med. 2015;6:635.
Shams R, Azizi A, Hamzei J, Noroozisharaf A, Moghadam S, Kordrostami M. Genetic structure and diversity of Iranian Cannabis populations based on phytochemical, agro-morphological and molecular markers. Ind Crop Prod. 2020;158:112950.
Vidyarthi S, Samant SS, Sharma P. Dwindling status of Trillium govanianum wall. Ex D. Don-A case study from Kullu district of Himachal Pradesh, India. J Med Plant Res. 2013;7:392–7.
Dhyani P, Sharma B, Singh P, Masand M, Seth R. Genome-wide discovery of microsatellite markers and, population genetic diversity inferences revealed high anthropogenic pressure on endemic populations of Trillium govanianum. Ind Crop Prod. 2020;154:112698.
Govindaraj M, Vetriventhan M, Srinivasan M. Importance of genetic diversity assessment in crop plants and its recent advances: an overview of its analytical perspectives. Genet Res Int. 2015;2015:1–14.
Huang R, Zhang ZD, Wang Y, Wang YQ. Genetic variation and genetic structure within meta-populations of two closely related selfing and outcrossing Zingiber species (Zingiberaceae). Aob Plants. 2021;13:1–15.
Hartmann T. From waste products to ecochemicals: fifty years research of plant secondary metabolism. Phytochemistry. 2007;68:2831–46.
Chen C, Li P, Wang RH, Schaal BA, Fu CX. The population genetics of cultivation: domestication of a traditional Chinese medicine, Scrophularia ningpoensis Hemsl. (Scrophulariaceae). PLoS One. 2014;9:e105064.
Ghorbani S, Esmaeili H, Ebrahimi SN, Palazón J, Sonboli A, Mirjalili MH. Genetic structure, molecular and phytochemical analysis in Iranian populations of Ruscus hyrcanus (Asparagaceae). Ind Crop Prod. 2020;154:112716.
Wang SQ. Genetic diversity and population structure of the endangered species Paeonia decomposita endemic to China and implications for its conservation. BMC Plant Biol. 2020;20:510.
Parra F, Casas A, Peñaloza-Ramírez JM, Cortés-Palomec AC, Rocha-Ramírez V, González-Rodríguez A. Evolution under domestication: ongoing artificial selection and divergence of wild and managed Stenocereus pruinosus (Cactaceae) populations in the Tehuacán Valley, Mexico. Ann Bot. 2010;106:483–96.
Bourguiba H, Audergon JM, Krichen L, Trifi-Farah N, Mamouni A, Trabelsi S, et al. Loss of genetic diversity as a signature of apricot domestication and diffusion into the Mediterranean Basin. BMC Plant Biol. 2012;12:49.
Doebley JF, Gaut BS, Smith BD. The molecular genetics of crop domestication. Cell. 2006;127:1309–21.
Abbo S, van Oss RP, Gopher A, Saranga Y, Ofner I, Peleg Z. Plant domestication versus crop evolution: a conceptual framework for cereals and grain legumes. Trends Plant Sci. 2014;19:351–60.
Zhang J, Wang X, Yao J, Li Q, Liu F, Yotsukura N, et al. Effect of domestication on the genetic diversity and structure of Saccharina japonica populations in China. Sci Rep. 2017;7:42158.
Kassa MT, Penmetsa RV, Carrasquilla-Garcia N, Sarma BK, Datta S, Upadhyaya HD, et al. Genetic patterns of domestication in Pigeonpea (Cajanus cajan (L.) Millsp.) and wild Cajanus relatives. PLoS One. 2012;7:e39563.
Wang HZ, Feng SG, Lu JJ, Shi NN, Liu JJ. Phylogenetic study and molecular identification of 31 Dendrobium species using inter-simple sequence repeat (ISSR) markets. Sci Hortic. 2009;12:440–7.
Kaki A, Vafaee Y, Khadivi A. Genetic variation of Anacamptis coriophora, Dactylorhiza umbrosa, Himantoglossum affine, Orchis mascula, and Ophrys schulzei in the western parts of Iran. Ind Crop Prod. 2020;156:112854.
Olsen KM, Gross BL. Detecting multiple origins of domesticated crops. Proc Natl Acad Sci U S A. 2008;105:13701–2.
Hyten DL, Song Q, Zhu Y, Choi IY, Nelson RL, Costa JM, et al. Impacts of genetic bottlenecks on soybean genome diversity. Proc Natl Acad Sci U S A. 2006;103:16666–71.
Tenaillon MI, U’Ren J, Tenaillon O, Gaut BS. Selection versus demography: a multilocus investigation of the domestication process in maize. Mol Biol Evol. 2004;21:1214–25.
Haudry A, Cenci A, Ravel C, Bataillon T, Brunel D, Poncet C, et al. Grinding up wheat: a massive loss of nucleotide diversity since domestication. Mol Biol Evol. 2007;24:1506–17.
Diamond J. Evolution, consequences and future of plant and animal domestication. Nature. 2002;418:700–7.
Zhang J, Yan J, Huang S, Pan G, Chang L, Li J, et al. Genetic diversity and population structure of cannabis based on the genome-wide development of simple sequence repeat markers. Front Genet. 2020;11:958.
Chen C, Zheng L, Ma Q, Zhou WB, Lu Y, Zhao YP, et al. Impacts of domestication on population genetics of a traditional Chinese medicinal herb, Atractylodes macrocephala (Asteraceae). J Syst Evol. 2019;57:222–33.
Pan Z, Watson MF. Angelica. In: Wu ZY, Peter HR, editors. Flora of China Beijing and St. Louis: Science Press and Missouri Botanical Garden Press; 2005. p. 169–70.
Chen J, Wu W, Hou K, Xu Y, Shao J, Li J. Pollen viability and stigma receptivity of Angelica dahurica from Sichuan and Hebei province. China J Chin Mater Med. 2011;36:3079–82.
Zych M, Junker RR, Nepi M, Stpiczynska M, Stolarska B, Roguz L. Spatiotemporal variation in the pollination systems of a supergeneralist plant: is Angelica sylvestris (Apiaceae) locally adapted to its most effective pollinators? Ann Bot. 2019;123:415–28.
Liu M, Hu X, Zhang J, Peng X, Hu Z, Liu Y. Constructing a core collection of the medicinal plant Angelica biserrata using genetic and metabolic data. Front Plant Sci. 2020;11:600249.
Ge SP. Shennong bencaojing. Collections of ancient herbal essence series (eight). Shanghai: Shanghai University of Traditional Chinese Medicine press; 1992. p. 3–57.
Seo WD, Kimd JY, Ryu HW, Kim JH, Han SI, Ra JE, et al. Identification and characterization of coumarins from the roots of Angelica dahurica and their inhibitory effects against cholinesterase. J Funct Foods. 2013;5:1421–31.
Li D, Wu L. Coumarins from the roots of Angelica dahurica cause anti-allergic inflammation. Exp Ther Med. 2017;14:874–80.
Wang MY, Xiong Y, Jia MR, Ma YY, Tang SW, Jiang GH. Investigation on production and marketing of Radix Angelica dahuricae. West China. J Pharm Sci. 2002;17:305–6.
Zhao DY, Hao QX, Jin Y, Kang LP, Liu Y, Guo LP. Research advance on the biological characteristics and cultivation technologies of Angelica dahurica. Mod Chin Med. 2015;17:1188–92.
Liu Q, Lu Z, He W, Li F, Chen W, Li C, et al. Development and characterization of 16 novel microsatellite markers by transcriptome sequencing for Angelica dahurica and test for cross-species amplification. BMC Plant Biol. 2020b;20:152.
Muller MH, Poncet C, Prosperi JM, Santoni S, Ronfort J. Domestication history in the Medicago sativa species complex: inferences from nuclear sequence polymorphism. Mol Ecol. 2006;15:1589–602.
Huang R, Chu QH, Lu GH, Wang YQ. Comparative studies on population genetic structure of two closely related selfing and outcrossing Zingiber species in Hainan Island. Sci Rep. 2019;9:1–11.
Nybom H. Comparison of different nuclear DNA markers for estimating intraspecific genetic diversity in plants. Mol Ecol. 2004;13:1143–55.
Reisch C, Bernhardt-Römermann M. The impact of study design and life history traits on genetic variation of plants determined with AFLPs. Plant Ecol. 2014;215:1493–511.
Pettengill JB, Runquist RDB, Runquist DA. Mating system divergence affects the distribution of sequence diversity within and among populations of recently diverged subspecies of Clarkia xantiana (Onagraceae). Am J Bot. 2016;103:99–109.
López-Villalobos A, Eckert CG. Consequences of multiple mating-system shifts for population and range-wide genetic structure in a coastal dune plant. Mol Ecol. 2018;27:675–93.
Nybom H, Bartish IV. Effects of life history traits and sampling strategies on genetic diversity estimates obtained with RAPD markers in plants. Perspect Plant Ecol. 2000;3:93–114.
Vieira ÂF, Dias EF, Moura M. Geography, geology and ecology influence population genetic diversity and structure in the endangered endemic Azorean Ammi (Apiaceae). Plant Syst Evol. 2018;304:163–76.
Jeong DH, Park YM, Kim KY, Park HW, Jeon KS, Kim MJ, et al. Genetic diversity of Angelica gigas Nakai collected in Korea using genome-wide SSR markers. Korean J Med Crop Sci. 2019;27:376–82.
Li W, Su Z, Yang L, Cao Q, Feng Y. Genetic diversity of the critically endangered Ferula sinkiangensis K.M. Shen (Apiaceae) and the implications for conservation. Turk J Bot. 2020;44:145–52.
Garcia-Jacas N, Requena J, Massó S, Vilatersana R, Blanché C, López-Pujol J. Genetic diversity and structure of the narrow endemic Seseli farrenyi (Apiaceae): implications for translocation. PeerJ. 2021;9:e10521.
Yang L, Hisorievd H, Kurbonovad P, Boboeve M, Bobokalonovd K, Feng Y, et al. High genetic diversity and low differentiation of endangered Ferula tadshikorum Pimenov in Tajikistan. Glob Ecol Conserv. 2021;28:e01627.
Young A, Boyle T, Brown T. The population genetic consequences of habitat fragmentation for plants. Trends Ecol Evol. 1996;11:413–8.
Samarasin P, Shuter BJ, Rodd FH. After 100 years: hydroelectric dam-induced life-history divergence and population genetic changes in sockeye salmon (Oncorhynchus nerka). Conserv Genet. 2017;18:1449–62.
Lam HM, Xu X, Liu X, Chen W, Yang G, Wong FL. Resequencing of 31 wild and cultivated soybean genomes identifies patterns of genetic diversity and selection. Nat Genet. 2010;42:12.
Ellegren H, Galtier N. Determinants of genetic diversity. Nat Rev Genet. 2016;17:422–33.
Kilian B, Özkan H, Kohl J, von Haeseler A, Barale F, Deusch O, et al. Haplotype structure at seven barley genes: relevance to gene pool bottlenecks, phylogeny of ear type and site of barley domestication. Mol Gen Genomics. 2006;276:230–41.
Myles S, Boykob AR, Owens CL, Brown PJ, Grassif F, Aradhya MK, et al. Genetic structure and domestication history of the grape. Proc Natl Acad Sci U S A. 2011;108:3530–5.
Yin S, Yan Y, You L, Chen Q, Zhou Y, Chen K, et al. Newly developed genomic SSRs reveal genetic diversity in wild and cultivated Amorphophallus albus germplasms. Plant Mol Biol Report. 2019;37:365–75.
Casas A, Otero-Arnaiz A, Peréz-Negrón E, Valiente-Banuet A. In situ management and domestication of plants in Mesoamerica. Ann Bot. 2007;100:1101–15.
Vargas-Ponce O, Zizumbo-Villarreal D, Martínez-Castillo J, Coello-Coello J, Colunga-GarcíaMarín P. Diversity and structure of landraces of Agave grown for spirits under traditional agriculture: a comparison with wild populations of A. angustifolia (Agavaceae) and commercial plantations of A. tequilana. Am J Bot. 2009;96:448–57.
De Andrés MT, Benito A, Pérez-Rivera G, Ocete R, Lopez MA, Gaforio L, et al. Genetic diversity of wild grapevine populations in Spain and their genetic relationships with cultivated grapevines. Mol Ecol. 2012;21:800–16.
Li Y, Zhu FL, Zheng XW, Hu ML, Dong C, Diao Y, et al. Comparative population genomics reveals genetic divergence and selection in lotus, Nelumbo nucifera. BMC Genomics. 2020;21:146.
Yang JB, Li DZ, Li HT. Highly effective sequencing whole chloroplast genomes of angiosperms by nine novel universal primer pairs. Mol Ecol Resour. 2014;14:1024–31.
Peakall R, Smouse PE. GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research--an update. Bioinformatics. 2012;28:2537–9.
Weir BS, Cockerham CC. Estimating F-statistics for the analysis of population structure. Evolution. 1984;38:1358–70.
Excoffier L, Lischer HEL. Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and windows. Mol Ecol Resour. 2010;10:564–7.
Pritchard JK, Stephens MJ, Donnelly PJ. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–59.
Evanno G, Regnaut S, Goudet J. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol. 2005;14:2611–20.
Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547–9.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China (grant number: 81603226), Natural Science Foundation of Guangdong Province (grant number: 2020A1515010627) and China Postdoctoral Science Foundation (grant number: 2021 M691482).
Author information
Authors and Affiliations
Contributions
ET and ZC conceived the study and ET and RH provided the funding. ET collected samples. YL, JC, ZL, JW and WH conducted the experiment. RH, ET and YL performed the data analysis. RH and ET wrote the manuscript. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Huang, R., Liu, Y., Chen, J. et al. Limited genetic diversity and high differentiation in Angelica dahurica resulted from domestication: insights to breeding and conservation. BMC Plant Biol 22, 141 (2022). https://doi.org/10.1186/s12870-022-03545-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-022-03545-5