Abstract
Background
Dicer-like proteins (DCLs) are essential players in RNA-silencing mechanisms, acting in gene regulation via miRNAs and in antiviral protection in plants and have also been associated to other biotic and abiotic stresses. To the best of our knowledge, despite being identified in some crops, cotton DCLs haven’t been characterized until now. In this work, we characterized the DCLs of three cotton species and analyzed their expression profiles during biotic stress.
Results
As main results, 11 DCLs in the allotetraploid cotton Gossypium hirsutum, 7 and 6 in the diploid G. arboreum and G. raimondii, were identified, respectively. Among some DCLs duplications observed in these genomes, the presence of an extra DCL3 in the three cotton species were detected, which haven’t been found in others eudicots. All the DCL types identified by in silico analysis in the allotetraploid cotton genome were able to generate transcripts, as observed by gene expression analysis in distinct tissues. Based on the importance of DCLs for plant defense against virus, responses of cotton DCLs to virus infection and/or herbivore attack using two commercial cotton cultivars (cv.), one susceptible (FM966) and another resistant (DO) to polerovirus CLRDV infection, were analyzed. Both cvs. Responded differently to virus infection. At the inoculation site, the resistant cv. showed strong induction of DCL2a and b, while the susceptible cv. showed a down-regulation of these genes, wherever DCL4 expression was highly induced. A time course of DCL expression in aerial parts far from inoculation site along infection showed that DCL2b and DCL4 were repressed 24 h after infection in the susceptible cotton. As CLRDV is aphid-transmitted, herbivore attack was also checked. Opposite expression pattern of DCL2a and b and DCL4 was observed for R and S cottons, showing that aphid feeding alone may induce DCL modulation.
Conclusions
Almost all the DCLs of the allotetraploide G. hirsutum cotton were found in their relative diploids. Duplications of DCL2 and DCL3 were found in the three species. All four classes of DCL responded to aphid attack and virus infection in G. hirsutum. DCLs initial responses against the virus itself and/or herbivore attack may be contributing towards virus resistance.
Similar content being viewed by others
Background
DICER proteins represent an ubiquitous class of RNAse III enzymes that are vital for the establishment of RNA interference (RNAi). DCLs recognize and cut a long ds-RNA substrate to release 21–24-nt small RNA duplexes, which have 2-nt 3′ overhangs at each end [1,2,3]. These small RNAs are important riboregulators in fungi, plants and animals, negatively regulating the expression of specific target genes by base-pairing. The function of small RNAs is largely described in plants associated with both development and responses to abiotic and biotic stress [4,5,6]. Plant small RNAs are classically separated into short-interfering RNAs (siRNAs) and microRNAs (miRNAs). Different classes of siRNAs have been previously described as natural-antisense siRNAs (natsiRNA) and trans-acting siRNAs (tasiRNAS), which, as miRNAs, are involved in post-transcriptional gene regulation by degrading their mRNA targets or inhibiting their translation. Heterochromatin-associated siRNAs (hcsiRNA) are associated with chromatin modifications and transcriptional repression of their target DNA loci. The long non-translated RNA (lsiRNA) and the viral small RNA (vsiRNA) were generated by DICER cutting of intermediate viral genome structures during viruses infection. Once produced, small RNAs are incorporated into ARGONAUTE (AGO)-containing RNA-induced silencing complexes (RISCs) to confer sequence specificity in the silencing of RNA information [3, 5, 7, 8]. In plants and fungi, cellular RNA-dependent RNA polymerase (RDR) acts to convert aberrant RNAs to dsRNA, leading to small RNA amplification and more intensive RNA silencing [3, 9, 10].
DICERs, or DICER-like (DCLs), as these proteins are called in plants, present six domains: DEAD, Helicase-C and DUF283 domains at the N-terminus, a Piwi/Argonaute/Zwille (PAZ) domain in the middle and a dual RNAse III domain followed by one or two dsRNA-binding domains in the C-terminal half [11, 12]. In lower eukaryotes, one or more of these domains may be absent [4]. The helicase domain serves to recruit co-factor regulatory proteins [13,14,15]. ATP hydrolysis is used to achieve progressive cleavage of the long dsRNA substrate. The DEAD domain acts as an ATP-binding domain [16]. The PAZ domain in turn mediates the recognition of the dsRNA substrate terminus, and the distance between PAZ and the RNAse III catalytic center determines the size of the small RNAs produced [17, 18]. Each of the two RNase III domains cuts one of the dsRNA strands, leaving a characteristic 2-nt overhang at the 3′-end of the product [19,20,21], and the C-terminal dsRNA-binding domains (dsRBD or DSRM) act as a protein–protein interaction interface and nuclear localization signals, in addition to having dsRNA-binding functions. dsRBD mediates the discrimination of different RNA substrates and subsequent incorporation of effector complexes [22,23,24]. Nevertheless, multiple dsRBD may be able to act in combination by recognizing secondary structures of specific RNAs [13].
In Arabidopsis, 4 genes (DCL1–4) encoding DCL proteins have been found [12, 25]. Each of the four AtDCLs is involved in the biogenesis of specific small RNA species. However, they may play redundant and hierarchical roles in the production of distinct sRNAs [26]. AtDCL1 is responsible for miRNA biogenesis. AtDCL2 generates 22-nt siRNAs and viral siRNAs (vsiRNAs) from endogenous inverted-repeats, integrated viruses, transgenes and RDR-amplified virus dsRNAs [26,27,28]. AtDCL3 is associated with transcriptional silencing, producing 24-nt length siRNAs involved in the establishment and maintenance of the heterochromatin state through RNA-dependent DNA methylation and histone modification [29, 30]. These 24 siRNAs are mostly generated from repetitive DNA loci and transposons, as well as retrotransposon insertion of DNA regions. AtDCL4 responds to the biogenesis of primary vsiRNAs that has 21-nt and are generated by dicing of viral genomes replicative intermediates. This DCL is also responsible for the production of phased siRNAs, ta-siRNAs and virus-activated siRNAS (vasiRNAs, a new class of endogenous RDR1-dependent siRNAs induced by virus infection) [31,32,33]. DCL4 is very important in viral resistance, initiating virus silencing in primary infected cells [33]. Primary vsiRNAs may further initiate secondary siRNA production, under the action of AtDCL2 and AtDCL4, and other genes [25, 34, 35]. So, DCL2 and 4 are key control virus replication levels even in susceptible plants. The production of the secondary vsiRNAs enables the increase of the amplitude of viral defense, making RNAi crucial for anti-virus defense in plants.
In contrast to the number of reports about DCL characterization and functions in Arabidopsis, only a few reports have characterized them in other plant species. In general, these reports show the presence of 4 classes of DCLs, with more than one member for each class. Seven DCLs have been identified in the tomato Solanum lycopersicum [36], eight in rice [37], five in maize [38], five in poplar [12, 39], four in grapevine [40], eight in Brassica napus [41], three in Archis duranensis, and four in chickpea, pigeonpea, A. ipaensis [42] and four in pepper [43]. Thus far, cotton DCLs have only been identified in the G. raimondii genome [44], but they have not been investigated by comparative evolutionary and expression analyses in cotton species.
Cotton crop is important to the economies of more than 30 countries and represent the principal source of natural fiber in the textile industry worldly. Besides cotton fiber, cotton oil is widely used for human consumption. The evolution of the Gossypium genus is marked by two important events. First, a divergence occurred 5–10 million years ago (MYA), when A and D diploid genomes form two separated branches. Subsequently, around 1–2 MYA, allopolyploidization events occurred by interspecific hybridization between A and B-genome ancestors resembling G. arboreum and G. raimondii, respectively, generating new Gossypium species, as the cultivated G. hirsutum (AADD) between others [44, 45]. As a consequence of the allopolyploidization, thousands of genes were duplicated and showed different expression levels, explaining the drastically phytomorphology changes observed in the allopolyploid cotton, compared to the diploid species G. arboreum and G. raimondii [44].
Here, we identify and characterize the DCL family of the allotetraploid G. hirsutum and two diploids, G. arboreum and G. raimondii, cotton species. As observed earlier in G. raimondii [44], an extra DCL3 is also present in G. arboreum and G. hirsutum, where it is currently expressed. Due to the importance of DCL in viral defense, we studied the expression patterns of G. hirsutum DCLs in two commercial cotton cultivars (cv.) during biotic stress induced by CLRDV (Cotton leafroll dwarf virus) infection and/or herbivore attack by Aphis gossypii. Comparison of a CLRDV-resistant and susceptible cotton accession or cv. during infection showed that the modulation of DCL expression, especially DCL2a, 2b, and DCL4, might explain the contrasting viral responses exhibited by each accession. Interestingly, both cotton DCL3 were also differentially modulated during viral infection.
Results
Identification of cotton Dicer-like gene family and chromosomal localization
Using Arabidopsis and rice DCLs as query sequences, we identified the Dicer-like genes of two diploid cottons, G. raimondii and G. arboreum, and of the allotetraploid G. hirsutum acc. TM-1 were identified by searching the cotton genome database [46,47,48]. Based on these queries and Blast tool analyses, we identified 6 genes encoding DCL proteins in G. raimondii, 7 in G. arboreum and 11 in the allotetraploid G. hirsutum, respectively (Table 1).
DCL genes were named according to the closest orthologs in A. thaliana. Among 4 DCL genes in A. thaliana, AtDCL1, AtDCL2, AtDCL3, and AtDCL4 have orthologs in cotton. AtDCL1 has only one ortholog in G. raimondii and G. arboreum, and two in G. hirsutum. AtDCL2 has 2 orthologs in G. raimondii, 3 in G. arboreum, and 4 in G. hirsutum. AtDCL3 has 2 orthologs in the two diploid genomes and 3 in G. hirsutum. All three cotton species present two DCL3. It seems that this duplication could be relevant for cotton evolution as it is present in the three species studied here; however, we cannot exclude the possibility that it is neutral. AtDCL4 has 2 orthologs in tetraploid cotton, but only 1 ortholog in diploid cottons. Cotton DCLs distinct paralogs were coded as a and b, according to their order on the homologous chromosomes.
Detailed information for these genes is listed in Table 1, including the chromosome location, ORF and protein lengths, molecular weight and theoretical isoelectric point. The newly identified Dicer-like loci showed coding potentials of 1209 to 2009 amino acid polypeptides, with predicted molecular weights (MW) of 132.99 to 220.99 kDa, respectively. A very small DCL putative polyprotein was identified in G. arboreum for DCL2a (Cotton_A_34031), which were 770 amino acids in length (84.7 KDa). It represents an extra DCL2a, that is present only in the G. arboreum genome.
The physical location of the three cotton species DCL genes is shown in Fig. 1. A total of eleven GhDCL genes were distributed on 10 G. hirsutum chromosomes. All the chromosomes (A01, A04, A05, A06, A07, D01, D06, D07, and D13) contained a single representative of GhDCL, with the exception of chromosome D05, which contained GhDCL2a and GhDCL4. Curiously, almost all the GhDCLs were correlated with chromosomes inherited from each parental-related species. G. raimondii presented 6 DCL genes distributed in 5 chromosomes, and G. arboreum 7 had DCLs distributed in 5 chromosomes and in a scaffold region (scaffold3086), that had not yet been incorporated into the physical map of chromosomes. G. arboreum presented two DCL2a located in very close proximity on chromosome 10, separated by approximately 9 kb. These two DCL2as (named herein as GaDCL2a31 and 32) were highly similar, sharing 93.4% identity at the amino acid level. The predicted GaDCL2a32 was shorter than its orthologs and seemed to have lost nucleotides/amino acids at the 5′ extremity/N-terminus.
Chromosomal distributions of DCL genes in Gossypium. G. hirsutum DCLs (GhDCLs) are distribute between chromosomes (Chr) A01, A04–07, D01, D05–07 and D13 (a); G. raimondii DCLs (GrDCLs) between chromosomes 01, 02, 09, 10 and 13 (b), and G. arboreum DCLs (GaDCLs) between chromosomes 04, 07, 10, 12 and 13 (c), respectively
The intron/exon (I/E) distribution as well as intron numbers, are shown in Table 1 and Fig. 2. GaDCL1 (Cotton_A_14097) showed the longest ORF of 6027 bp and coding potential for a polypeptide of 2009 amino acids. The maximum number of introns, 25, was found in DCL3a and b of G. raimondii. DCL4 of all three distinct species showed a very similar intron/exon distribution. The same could be observed for DCL3a of G. arboreum, G. raimondii, and G. hirsutum from subgenome D. GhDCL3a from subgenome A, however, showed a distinct pattern at the 5′ end of the gene. It seemed to have lost part of the nucleotides from this region. A very similar distribution of introns and exons was also observed for DCL2b of G. raimondii and G. hirsutum subgenomes A and D. GaDCL2b, on the other hand, showed a very different distribution of I/E.
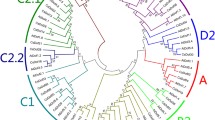
Phylogenetic tree, gene structure and domain compositions of cotton DCLs. The phylogenetic tree was constructed using MEGA 7.0. Exon/intron structures of DCL genes are represented by blue boxes and gray lines, respectively. Protein domain analysis are represented by different colors. Sequence used are listed at Additional file 1
Cotton DCL phylogenetic and domain composition analysis
The phylogenetic relationships of the amino acid sequence of the three cotton species (G. raimondii, G. arboreum, and G. hirsutum) were used to construct a neighbor-joining (N-J) phylogenetic tree, using MEGA 7.0 software (Fig. 2). These analyses indicated that the DCL genes clustered in two separate clades, one composed of DCL1, 2, and 4 and the other of DCL3s. The two GhDCL subfamilies were further divided into many subclades that clearly grouped the DCLs from the parental/ancestral diploid species with each corresponding subgenome in the allotetraploid cotton. The extra DCL3, named herein as DCL3b, was present in all three species, but the allotetraploid cotton presented only one member of this gene on the genome, showing the closest relationship to GrDCL3b. We can hypothesize that the DCL3b gene, that was acquired from G. arboreum during the G. hirsutum evolution, had been lost. Alternately, it may have been acquired independently by the three species after allotetraploid hybridization. The two DCL2a from G. arboreum formed a group together with GhDCL2a from subgenome A, showing that they probably duplicated after tetraploid hybridization. The constructed tree suggested a high level of sequence conservation for DCL sequences in the diploid species and the allotetraploid G. hirsutum during evolution.
SMART was used to identify the DCL domains in all Dicer-like genes from the three species (Fig. 2). All DCLs contained a DEAD, helicase-C, DUF283 and PAZ domain, excluding GaDCL2a31 and GaDCL2b, which did not present any DEAD or helicase-C domains. In fact, GaDCL2a31 was a truncate DCL because it also did not present a DUF283 domain. Two RNase III domains were present in all DCLs, and at least one DSRM domain was present in 15 out of the 22 cotton DCL proteins. All 7 cotton DCL3s had a dsRB domain, which is a characteristic of DCL3 plant proteins. Interestingly, GhDCL1 from subgenome D seemed to have lost a DSRM domain, while DCL1 of subgenome A maintained 2 DSRB domains.
A comparison of cotton DCL proteins with those from Arabidopsis, rice, poplar, grapevine and Medicago sp. revealed that cotton DCL1s shared a common ancestor with grapevine and poplar DCL1s (Fig. 3). DCL2a and 2b from cotton, however, were more related to poplar DCL2s. Grapevine and poplar DCL3s showed the closest relationship to cotton DCL3 from all species analyzed. DCL3 duplication in rice seemed to occur over a long time before cotton DCL3 duplication, but after dicot/monocot divergence. The duplication of DCL3 in cotton probably occurred before cotton allotetraploid hybridization and therefore more than 1.2 MYA. DCL4 seemed to be the most conserved DCL among the eudicots analyzed herein.
Phylogenetic relationships of DCL proteins from cotton G. arboreum, G. raimondii and G. hirsutum, A. thaliana (At), O. sativa (Os), V. vinífera (Vv), Medicago truncatula (Mt), Populus sp. (Po) and Physcomitrella patens (Pp). The unrooted NJ tree was constructed using MEGA 7, and the bootstrap test was performed with 1000 replicates. Sequence used are listed at Additional file 1 and Additional file 2: Table S1
DCL gene expression profiles in different G. hirsutum organs
To evaluate if the in silico-identified DCLs in cotton were able to generate transcripts, we collected tissue samples from root, leaf, stem and flower from greenhouse-grown cotton of two distinct commercial G. hirsutum cv., Fibermax 966 (FM966) and Delta Opal (DO), at 60 days post germination (60 dpg) and analyzed gene expression by quantitative real-time PCR (RT-qPCR). These two cultivars were especially selected because they show contrasting phenotypes against an important worldwide distributed cotton virus disease, the cotton blue disease (CBD). Fibermax is susceptible to CBD, while Delta Opal is resistant.
As shown in Fig. 4, transcripts of all 6 DCLs types were identified in G. hirsutum plants. DCL1 was expressed in all analyzed tissues from both cv. FM and DO, as well as DCL2a, DCL3a, and DCL4. DCL2b was not detected in leaves from the DO cv. and flowers of cv. FM, while DCL3b was almost undetectable in stems from FM and leaves from both cultivars. DCL4, which is essential for intracellular antiviral silencing, was expressed at the same levels in FM and DO plants, as well as DCL2a. However, DCL2b showed slightly contrasting basal expression levels between them. The extra DCL3 identified in cotton seemed to be important, especially in flower and root tissues from healthy plants.
Real-time PCR expression profiling of Dicer-like genes in different cotton tissues. DCL expressions levels of all cotton DCLs were analyzed in stem, flower, leaf and root of two commercial cotton cv. Fibermax 966 (FM) and Delta Opal (DO) at 60 dag by qRT-PCR. The heat map was generated by a log transformation of the real-time PCR data presented as ΔCt (CtDCL – CtGhmiR390/GhPP2A). The expression values are detailed at Additional file 2: Table S3
DCL gene expression is modulated in response to herbivore attack and virus infection
Plant DCLs initiate the RNAi innate defense system against invading viruses because they recognize and process incoming viral and transposon nucleic acids into small siRNAs of 21, 22, and 24 nts. Thus, we were interested in shedding some light on how cotton DCL expression is modulated during RNA viral infection using virus-resistant and virus-susceptible contrasting cotton cvs.: Delta Opal and FM966, respectively. Cotton leafroll dwarf virus (CLRDV) (genus, Polerovirus; family, Luteoviridae), which is transmitted only by an aphid vector (Aphis gossypii), is the causal agent of cotton blue disease [49]. CLRDV is phloem-restricted, and its genome consists of a single-strand, positive-sense, non-polyadenylated RNA (5.8 kb) containing six open reading frames (ORFs) [50].
As CLRDV is only transmitted by its aphid vector, another important point is to understand the aphid vector component in the DCL modulation. Consequently, we evaluated cotton DCL expression patterns during aphid herbivore attack, mediated by Aphis gossypii and/or CLRDV infection.
To analyze the influence of herbivore attack on DCL expression, 30 dag cotton plants were inoculated in the greenhouse with virus-free aphids. Aphid were restricted to one basal leave (inoculated leave) per plant and 24 h after inoculation, the aphids were eliminated by insecticide application. The expression levels of all cotton DCLs were evaluated in young systemic leaves (3–4 leaves above the inoculated leaves) at 24 hpi, 5, 15, and 25 dpi after contact with the aphids (Fig. 5). For CLRDV infection, a similar biological approach was applied using viruliferous aphids harboring CLRDV.
Expression profile of cotton DCL analysis by RT-qPCR during herbivore attack. Fibermax 966 and Delta Opal cotton plants were submitted to contact with 10 aphids for 24 h. Systemic leaves were collected at 24 hpi and 5, 15 and 25 dpi for evaluation of each DCL level over time. The fold change was calculated using the 2-ΔΔCt method as described by [51]. The standard deviation is indicated by the error bars, and “*” indicates significant differences, calculated using the GraphPad Prism program. * P < 0.1 ** P < 0.01 *** P < 0.001. hpi - hours post-aphid inoculation, dpi - days post-aphid inoculation
In general, all DCLs showed an induction of their expression levels in the virus-resistant DO plants after aphid contact (Fig. 5). When these plants were subjected to the aphid and virus simultaneously, in the case of viruliferous aphid contact, DO DCL levels showed an otherwise repression pattern. In the virus-susceptible cv, the inverse was observed (Fig. 6).
Expression profiles of cotton DCLs analyzed by RT-qPCR after CLRDV virus infection. Cotton plants from the cv. Fibermax that is susceptible to CLRDV infection and Delta Opal that is resistant to CLRDV infection, were inoculated with CLRDV vectors. Vectors were eliminated 24 h after inoculation. a Systemic leaves of Fibermax 966 and Delta Opal plants were collected at 24 hpi and 5, 15 and 25 dpi for the evaluation of each DCL level over time. The fold change was calculated using the 2-ΔΔCt method as described by [52]. The standard deviation is indicated by the error bars, and “*” indicates significant differences, calculated using the GraphPad Prism program. * P < 0.1 ** P < 0.01 *** P < 0.001. hpi - hours post-virus infection, dpi - days post-virus infection. b A Nested RT-PCR for CLRDV capsid protein (CP) gene amplification was performed in all systemic leaves to check virus presence in virus-free aphid inoculated (A), CLRDV infected (V) and uninoculated plants (C). The number 1 and 2 represent independent pools of treated plants. FM – CLRDV susceptible plant and DO – CLRDV resistant plant
Interestingly, it was observed that after the first 24 hpi a systemic modulation of all DCLs mRNAs occurred in both cotton cvs., meaning that aphid feed in inoculated leaves is inducing a systemic response. For FM plants, this systemic response is more pronounced than for DO plants. At 24 hpi, FM plants showed strong down regulation of the three cotton DCLs involved in virus defense, DCL2a, 2b and 4, with reductions of approximately 5, 10 and 15-fold, respectively. DO plants, in contrast, showed a systemic upregulation of these DCLs. This contrasting modulation of DCLs by aphid feeding may predispose the CBD susceptible FM cv. to be more vulnerable against virus infection, as they have less DCL2 and 4 accumulation in the incoming virus tissues. Whereas for DO plants, when the virus is trying to spread from the local infection site, it faces a strong antiviral silencing pre-activated in systemic tissues that present 3–5 higher levels of DCL2a, 2b and 4 than healthy plant tissues. The presence of previously systemic accumulation of these DCL induced distally by aphid feeding can help these plants to block the virus infection cycle establishment in new cells far from local infection site.
DCL1 mRNA expression was also modulated, been induced in systemic leaves of DO cv. 24 hpi and 5 days after aphid contact. At 15 and 25 dpi, DCL1 expression was reduced in both FM and DO cvs. in comparison to healthy control plants (Fig. 5).
DCL3a expression was induced by aphid feeding in both DO and FM cvs. at all time points analyzed. Important exceptions were noted for FM at 24 hpi and for DO at 25 dpi, at which time this DCL was downregulated. In contrast, the cotton extra DCL3 (DCL3b) showed strong repression during the first 24 hpi to 5 dpi in both cvs.
When DO and FM plants were infected with viruliferous aphids (Fig. 6), cotton DCLs showed a distinct expression pattern in systemic leaves, compared with those inoculated with virus-free aphids, showing that the presence of virus also modulate DCL expression (Fig. 6 and Additional file 2: Figure S2). The fold change analysis between FM CLRDV-infected and FM mock plants (virus-free aphid inoculum) showed that the presence of virus induced an additional systemically down-regulation of almost all their DCLs during the initial stages of virus infection (24 hpi), with the exception of DCL2a. Five days later, however, the levels of all FM DCLs markedly increased, showing almost 60, 10, 47, 17, 28 and 28-fold change variations for DCL1, DCL2a, DCL2b, DCL3a, DCL3b, and DCL4, respectively. In contrast, the DCL transcript levels from DO plants at all time points were lightly induced or repressed, maintaining levels that were very similar to uninfected mock plants. Even with the strong DCL modulation observed in the susceptible plant FM, CLRDV could be easily detected in systemic leaves from 24 hpi, showing that even with such extensive efforts of the antiviral machinery, the virus was replicating and spreading throughout the plant (Fig. 6b). This strong modulation of DCL expression was not observed in DO plants, in which DCLs were only slightly induced or reduced up to 25 dpi, and the virus was not detected in any systemic leaves between 24 hpi and 25 dpi (Fig. 6b). Absence of virus accumulation in DO plants may explain why DO DCLs were almost no induced at these leaves.
Deep sequencing of viral small RNA from CLRDV FM-infected plants, previously performed by our group at this same time point on systemic leaves, showed that the most abundant viral siRNAs were 22 nt [53], highlighting the importance of DCL2 in combating the spread and/or replication of the virus in aerial parts far from inoculated leaves. These efforts are insufficient for the blockade of virus infection. Both DCL2s probably participate in the processing/dicing of viral dsRNA for the generation of these second viral siRNAs of 22 nts. However, as DCL2b levels are more than 4 times higher than DCL2a levels at the systemic infected leaf cells 5 dpi, we can hypothesize that DCL2b is most important for 22-nt viral siRNA generation.
Modulation of DCL2 and DCL4 expression by virus/aphid infection at local infection sites
It has been already shown for Arabidopsis that DCL4 is an essential component of intracellular antiviral silencing, whereas both DCL4 and DCL2 are necessary for the inhibition of systemic infection. Our results indicated that DCL4 was almost not modulated by CLRDV infection in aerial parts of virus-resistant cotton during infection, while DCL2a and 2b were downregulated (Fig. 6). In susceptible plants, however, DCL4 was strongly downregulated systemically at the beginning of infection. Thus, the next step was to examine how these DCLs were expressed at the infection site. Consequently, we collected samples from inoculated leaves 24 h after virus infection and analyzed DCL mRNA expression profiles by RT-qPCR (Fig. 7).
Profiles of cotton DCL expression at inoculation sites. a DCL expression profiles in aphid-inoculated leaves at 24 hpi. b Cotton DCL expression profiles at 24 hpi with CLRDV. c Identification of CLRDV replication in inoculated leaves. Virus replication was assayed by CLRDV coat protein detection using nested RT-PCR in aphid inoculated (A), CLRDV infected and uninoculated plants (C). d Profiles of viral small RNAs from cotton plants infected with distinct species of polerovirus. CLRDV sviRNA profile was obtained previously by [54] and is shown here just for illustrative purpose. Numbers 1 and 2 represent independent pools of treated plants. FM – CLRDV susceptible plant and DO – CLRDV resistant plant. CAV – Cotton anthocyanosis virus
Aphid contact induced DCL2a and DCL4 expression at similar levels in both FM and DO cvs. (Fig. 7a). DCL2b in turn was 6 times more induced in DO than in FM plants. Local aphid attack modulation responses seems to be the same in the resistance/susceptibility phenotype for DCL4 as both plants showed similar level of induction of this DCL at the inoculated leaves.
The presence of the virus, in contrast, induces stronger modulations of almost all DCLs. At virus infection sites, DCL2a, DCL2b, and DCL3b were strongly induced (approximately 250, 70, and 100-fold change, respectively) in the resistant cv. at 24 hpi (Fig. 7b). The strong DCL2a and 2b induction seemed to be relevant for virus resistance because systemic spread of the virus was completely inhibited in DO plants (Fig. 6b). Virus replication is inhibit even in the virus inoculated leaves, as observed by high sensitivity nested RT_PCR for CLRDV detection (Fig. 7c). The susceptible cv. FM did not respond to virus presence in the same way as the DO cv., as only a very slight induction of DCL2a and b was observed at 24 hpi in inoculated leaves. In these plants virus accumulation is observed in systemic and inoculated leaves since 24 hpi (Figs. 6b and 7c, respectively).
An induction of FM DCL4 6x higher than that of DO DCL4 was also observed. This finding indicated that both susceptible and resistant plants produced more DCL4 to combat virus invasion; however, this overexpression alone did not seem to be sufficient to inhibit the spread of infection, since FM plants that produce more DCL4 than DO are completely susceptible. So, the two cotton DCL2s seem to be very important to avoid virus dissemination and accumulation at inoculation sites while DCL4 may have a secondary paper. However, we cannot say with these results that the strong induction of DCL2 is the responsible for DO CLRDV resistance phenotype as unrelated resistance mechanism may be acting also. Curiously, DCL3a and 3b were highly induced in the FM cv. (11 and 260-fold greater expression than in mock-infected plants and more than 2-fold in DO plants, respectively), while only DCL3b was induced in DO cv. (approximately 100-fold greater expression than in the mock). These results showed that DCL3b seemed to be important during the initial virus defense activation.
Taken together, our results suggested that the contrasting CLRDV susceptibility phenotype demonstrated by FM and DO plants might be related to their distinct DCL modulation mediated both by aphid feed and virus infection.
Corroborating the importance of cotton DCL2 in the polerovirus infection, a profile of the vsiRNAs produced by DCL dicing was obtained for CLRDV and another polerovirus infected cotton plants by deep sequencing. As observed in Fig. 7d, in the plants infected with both CLRDV and Cotton anthocyanosis virus (CAV), 22–nt sviRNAs accumulated in major levels than 21-nt sviRNAs showing the relevant paper of cotton DCL2s in the virus defense.
Biotic and abiotic stress-responsive cis-acting regulatory elements are present in the promoters of upland cotton DCL genes
Promoter sequences 1.5 kb upstream of the translation start of all G. hirsutum DCL genes were obtained from the cotton genome project to attempt to understand how cotton DCLs are modulated by both virus infection and herbivore attack. Transcriptional responsive cis-elements of DCL gene promoters were analyzed using PlantCare. Analysis of the promoter region of all 11 upland cotton DCL genes revealed the presence of various biotic and abiotic stress-responsive cis-acting regulatory elements, including the TCA-element, ERE and ABRE. Light stress-responsive elements were relatively the most abundant in the promoters of the upland cotton DCL genes, specifically Box 1, Box 4 and GT1-motif (Fig. 8 and Additional file 3: Figure S3, and Additional file 4), indicating that all DCL proteins might have an important functional role in light stress responses. All DCL promoters displayed the development of cis elements, especially HD-Zip1 and 2, and almost all the elements that are responsive to drought (especially MBS), salicylic acid (TCA) and other biotic stresses (as WUN, box-W1, ELI-box 3, TC-rich repeats, Box S, JERE, and GCC boxes), revealing possible mechanisms mediated by almost all DCLs in drought tolerance and biotic stress responses in the upland cotton G. hirsutum. Surprisingly, GhDCL4A did not show typical biotic stress or ethylene-responsive cis elements, but they were present in GhDCL4D. In general, there were significant differences in the average proportions of the promoter elements detected within the different DCL gene families, as well as between the same DCLs originating from a distinct parental diploid cotton (Fig. 8). However, abiotic stress elements presented the highest average proportions in all DCLs. Phytohormone-responsive elements, especially those associated with ethylene and gibberellin, were found in DCL1, DCL2a and 2b, DCL3b, and DCL4, while those correlated to Me-jasmonate were predominant in DCL2b, DCL3, and DCL4-A. A large number of enhancer elements were found in all DCLs (Additional file 3: Figure S3), suggesting that all the DCLs from the two subgenomes were able to generate transcripts.
Analysis of G. hirsutum DCL promoter sequences. The 1.5-kb upstream promoter sequence of the transcription start site of each DCL gene was retrieved and analyzed for the presence of putative cis-elements. Different cis-elements with the same or similar functions are shown in the same color. Enhancer cis elements are shown in Additional file 3: Figure S3
Discussion
In the present study, we first characterized the DCLs of 3 cotton species (the commercial allotetraploid G. hirsutum and its parental-like G. arboreum and G. raimondii) at the genomic level. Our results showed that cotton carried extra copies of DCL2 and DCL3. The extra DCL2 has been previously reported in poplar, rice and maize between other plants. Our phylogenic analysis corroborated other findings, showing that DCL2 duplication seemed to have evolved independently in mono- and eudicots, after their genetic separation. The DCL3 duplication shown here is a unique event in eudicots [12]. The extra DCL3, denoted herein as DCL3b, showed a complete DCL3 structure in the allotetraploid G. hirsutum and in the diploids G. arboreum and G. raimondii. Furthermore, quantitative RT-PCR showed that it was expressed in distinct cotton tissues/organs such as flowers and roots. The importance of this extra DCL3 for cotton must be studied, but we can hypothesize that it may be associated with the large number of transposons detected in the cotton genome. G. raimondii, G. arboreum and G. hirsutum genome account approximately to 53, 67 and 62% of retrotransposons, respectively [44]. Curiously, in allotetraploid cotton, we found only one set of DCL3b, which probably derived from its G. raimondii-like parental species. DCL3b from the parental G. arboreum seemed to have been missed during evolution.
Analyzing expression of these cotton extra DCL transcripts during virus infection and herbivore attack, we could observe that they seem to be functional and are modulated in both stresses. However, a further characterization of all the cotton DCL2s and DCL3s in heterologous system will be necessary to understand the paper of these DCLs extra copies in RNA silencing machinery.
Previous studies from our group have shown that CLRDV infection in G. hirsutum impacts small RNA accumulation both quantitatively and qualitatively [54, 55]. Our deep sequencing identified several cotton miRNAs that were down- or upregulated during CLRDV infection of the susceptible FiberMax 966 G. hirsutum cv [56]. All these miRNA families participate in distinct and overlapping networks, such as plant disease resistance (miR393, 403 and 472), leaf development (miR159, 164, 319 and 393), flowering time (miR172, 156 and 157), stress nutrition (miR395, 397, 398 and 408), and miRNA pathways (miR403) [55]. In addition to the global alteration of Gh-miRNAs during CLRDV infection, we observed a decrease in the frequency of 24-nt sRNA in the infected plant with an enrichment of gypsy- and copia-like retrotransposon transcripts [56]. In contrast, the CLRDV viral siRNA profile during infection revealed a greater abundance of the 22-nt vsiRNAs [54]. Thus, cotton DCLs are anticipated to play an important role in the mediation of CLRDV:cotton interactions during this compatible infection.
Here, the analysis of the expression of the 6 cotton DCLs in two contrasting virus-susceptible commercial cotton cvs., during two types of biotic stress, revealed that almost all DCLs were modulated during these stresses. The CLRDV-susceptible cv. FM966 showed a strong downregulation of DCL2a, 2b, 3a, 3b, and 4 in the aerial part of the plant during the initial hours after aphid contact. This downregulation was maintained until 25 dpi for DCL2b, DCL2b, and DCL4 at the majority of the analyzed time points. For the CLRDV-resistant cv. Delta Opal, this downregulation was not observed, except for DCL3b, which was repressed almost 15-fold compared with the mock-infected control plants at 24 hpi (Fig. 5). So, sensing of aphid sucking is able to distinctly modulate DCL expression in aerial parts of these two cvs. That were not in contact with the aphid. However, at the inoculated leaves (Fig. 7) DCL2a and DCL4 modulation by herbivore attack was very similar in both cotton cvs. Thus, virus resistance and/or susceptibility did not seem to be a consequence of differential responses to aphid attack at the initial site of aphid contact but was more likely a consequence of the virus versus cv. interaction. Bozorov et al. [57] studied the effects of N. attenuata DCL silencing on the performance of Manduca sexta larvae. They found that NaDCL2, NaDCL3, and NaDCL4 are involved in the regulation of a number of different genes, leading to signaling and defense metabolite activation in response to herbivore attack. Thus, like N. attenuata DCLs, cotton DCLs are apparently important in anti-herbivore defense activation, as they are clearly modulated locally and especially systemically by aphid sucking.
Antiviral activity of DCLs was already intensively reported ([58,59,60,61], among others). Although DCL4 is the major producer of 21-nt antiviral siRNA, DCL2 is essential for secondary 22-nt siRNA-mediated transitive virus silencing and can compensate the loss of DCL4 in dcl4 mutants [62,63,64,65]. During potyvirus Zucchini yellow mosaic virus (ZYMV) infection of N. benthamiana, for example, DCL4 has been observed to restrict the systemic movement of the virus [66]. The authors observed an enhancement of systemic virus accumulation in double dcl2 and 4 and, particularly, in the triple knockdown mutant dcl2/3/4. Examination of the modulation of DCL expression during virus infection by CLRDV showed that the virus induced strong systemic repression of DCL1, 2b, 3b, and 4 in the susceptible cv. FM966 during the initial hours after infection. This drastic repression was not observed in the resistant cv., in which the expression of all these DCLs remained almost similar to that of mock infected plants. Investigation of DCL expression modulation at infection sites showed that even FM DCL4 was expressed 6x higher than DO DCL4, and this high induction of DCL4 was insufficient for virus restriction to the initially infected cells. In contrast, both FM DCL2a and b were almost not induced in the inoculated leaves. Others authors have reported previously that DCL2 and DCL4 may have different antiviral activities in inoculated and systemic leaves [33, 58, 61]. In these studies it was observed that DCL4 alone responded to the antiviral silencing in inoculated leaves. In systemic leaves, however, both DCL2 and DCL4 are necessary to trigger virus silencing. It has been also previously demonstrated that DCL4 can downregulate DCL2 expression [58]. FM DCL4 overexpression may impair DCL2a and b expression at the inoculation site in the susceptible cotton cv., allowing the cell-to-cell spread of the virus and establishment of systemic infection due to the noneffective intercellular antiviral activity of DCL2 at these sites. In DO-resistant plants, the high levels of DCL2a and b induced by the presence of virus may efficiently activate intercellular antiviral silencing, resulting in restriction of the virus to the first cell-autonomous or inoculated cells during the initial hours after infection and dramatically reducing virus replication and aborting infection spread completely.
DCL2 acts at intercellular VIGS, generating the second front line against viral infection in virus-neighboring recipient cells [58]. CLRDV, similar to all poleroviruses, is able to replicate only in companion cells of the phloem, and the presence of DCL2 at high levels in the neighborhood of companion cells may impose strong antiviral silencing mediated by the 22-nt virus small RNAs, blocking the spread of the virus in subsequent tissues in resistant DO plants.
Donaire et al. [67] analyzed the vsiRNAs derived from nine viruses belonging to eight different genera. The predominant class of vsiRNAs were 21 nt for most viruses, with the exception of tombusvirus Cymbidium ring spot virus-infected plants that accumulated higher levels of 22-nt vsiRNAs. Recently, Brassica yellows virus (BrYV) vsiRNA accumulation was analyzed in single-infected N. benthamiana plants, and the 22-nt class represented the dominant class of BrYV vsiRNAs, followed by the 21-nt class [68]. These results are in agreement with our previous findings [53, 54] and the present studies in which 22-nt vsiRNAs were the most abundant class of vsiRNAs in two other virus species in the polerovirus genus. The accumulation of 22-nt vsiRNAs appears to be characteristic of polerovirus infections, highlighting the important role of DCL2 in antiviral defense against these viruses, which may exceed that of DCL4.
In addition to classical DCL4 and DCL2 antiviral functions, several authors have reported the importance of DCL3 in virus defense via the production of high levels of 24-nt siRNAs from invading viruses [26, 69, 70], guiding AGO family members to cleave viral RNA or recruit cellular machinery to methylate viral DNA. In A. thaliana, DCL3 are also responsible for the directed cleavage of positive-sense RNA viruses, including Cucumber mosaic virus (a cucumovirus), Oilseed rape mosaic virus (a tobamovirus) and Turnip crinkle virus (a carmovirus) [26, 71]. Thus, DCL3 also plays an important role in virus silencing. In CLRDV-infected cotton, GhDCL3a and b were induced on infected leaves (with high levels of expression), as well as systemic leaves, suggesting a possible contribution of these DCLs in viral defense.
It should take in account, however, that ours results to not exclude that other CLRDV-resistance mechanisms not directly related to DCL2/4 modulation should be active in DO plants. These resistance mechanisms may turn down virus replication consequentially decreasing virus imposed stress levels in these plants. One way to deeply check the importance of the DCL modulation observed here by itself in the CLRDV resistance in DO plants would be induce the silencing of each cotton DCL genes individually and/or in combinations in DO cotton plants by VIGS and CLRDV-infect plants the plants further.
Conclusions
We identified G. raimondii, G. arboreum and G. hirsutum cotton DCLs and found duplication of DCL2 and DCL3. Additionally, we found that all G. hirsutum DCLs are involved in polerovirus infection responses, especially the DCL2 and DCL3 paralogs and DCL4, and that modulation of the expression of these DCLs by viruses may be crucial for virus susceptibility and/or resistance response.
Methods
Identification of the DCL family in Gossypium
The genome sequences of three cotton species, G. arboreum (BGI-CGB v2.0 assembly genome), G. raimondii (JGI assembly v2.0 data) and G. hirsutum acc. TM-1 (NAU-NBI v1.1 assembly genome), were downloaded from the Phytozone (http://www.phytozone.net/) and CottonGen (https://www.cottongen.org/) databases. Dicer-like protein sequence data were obtained for A. thaliana and O. sativa from the General Feature Format (GFF) le Arabidopsis Information Resource (TAIR release 10, http://www.arabidopsis.org) and from the Rice Genome Annotation Project Database (RGAP release 7, http://rice.plantbiology.msu.edu/index.shtml). Gene names and IDs are listed in Additional file 1 and in Additional file 2: Table S1).
The physico-chemical properties of cotton DCL proteins were predicted using the ExPASy Compute pI/Mw tool (http://au.expasy.org/tools/pi_tool.html; Bjellqvistetal Bjellqvistetal, 1993).
Chromosomal location analysis and phylogenetic tree construction
The locations of the DCLs in chromosomes were assessed using Mapinspect software (http://www.softsea.com/review/MapInspect.html) using start and end position of each open read frame obtained from the genome database. .
A phylogenetic tree was constructed using MUSCLE (Multiple Sequence Comparison by Log-Expectation) alignment and the neighbor-Joining (NJ) method in MEGA 7.0 software [72], with the 1000-replicate bootstrap test. A keyword search of the Phytozome v12.1 database (https://phytozome.jgi.doe.gov/pz) and National Center for Biotechnology Information database - NCBI (https://www.ncbi.nlm.nih.gov) was further performed to obtain the DCL genes in different plant organisms.
Intron–exon and domain analysis of the DCL family
The Gene Structure Display Server (http://gsds.cbi.pku.edu.cn/index.php) was used to analyze the intron–exon structure by comparing the CDS of cotton DCL genes with their corresponding genomic sequences [45, 73]. The conserved domains in the DCLs were identified by SMART (http://smart.embl-heidelberg.de).
Plant materials
Two upland cotton G. hirsutum cultivars were used for the DCL expression assays and viral infection: acc. FiberMax966 (FM966) (Aventis Crop Science, Australia) and acc. Delta Opal (DO) (Delta and Pine Land Co., United States). Seeds were kindly provided by IMA, Instituto Matogrossense do Algodão, Primavera do Leste, Mato Grosso state, Brazil. Plantlets were grown under greenhouse conditions at 28 +/− 2 °C as previously described [49].
For cotton organ DCL expression, samples of leaves, shoots, flowers, and roots from independent plants at 30 days after germination (dag) were collected from each cotton acc. FM and DO. Samples were immediately frozen in liquid N2 and stored at − 80 °C until RNA extraction.
Plant aphid inoculation and virus infection
Cotton (Gossypium hirsutum) plants of cultivars acc. FM966 (susceptible to Cotton blue disease) and acc. DO (resistant to Cotton blue disease) grown in the greenhouse and at the 30 dag stage were infected with Cotton leafroll dwarf virus (CLRDV, polerovirus, Luteoviridae family) by viruliferous aphids (Aphis gossypii Glover) as described previously [56]. Full-developed leaves of plants with the same age from both cultivars were inoculated with non-viruliferous aphids (virus free aphids). Aphids were restricted at the inoculation site by double-side adhesive tapes (3MM Co.) and killed 24 h after inoculation with insecticide. Aphids were restricted to the inoculation sites at the inoculated leaves by surrounding the inoculation site with a double face tap.
Systemic leaves, localized 3–4 leaves above the inoculated leaf, were collected at 24 h post-infection (hpi) and 5, 15 and 25 days post-infection (dpi) with CLRDV aphids or non-viruliferous aphids. Leaves from the same position of uninfected plants were used as uninoculated controls. Leaves from 3 to 5 independent inoculated plants composed each biological replicate. Samples were stored at − 80 °C until RNA isolation and expression analysis.
All the samples recovered from aphid-free inoculated, CLRDV infected and uninoculated were assayed for CLRDV detection by a Nested RT-PCR assay that amplifies viral coat protein sequence following [53]. CLRDV susceptible infected plants were used as control.
Real-time quantitative RT-PCR (RT-qPCR)
Total RNA was isolated using the Invisorb Spin Plant RNA Kit (Invitek Molecular Co., Germany). The quality and concentration of each RNA samples was determined by agarose gel electrophoresis and using a NanoDrop 2000 (Thermo Fisher Scientific Co.) spectrophotometer. Only RNA samples with a 260/280 nm ratio between 1.8–2.1 and 260/230 ratio ≥ 2.0 were used. The c-DNA first-strand reverse transcription was conducted with the Revertaid First Strand cDNA, Synthesis Kit (Thermo Fisher Scientific Co.) in accordance with the manufacturer’s instructions. One microgram of total RNA from each sample was treated with DNAse I (Fermentas Co.), and cDNAs were synthetized by adding 100 μM of oligo dT24V primer. G. hirsutum DCL reverse and forward primers were designed using NCBI primer-BLAST (Additional file 2: Table S2). Maxima SYBR Green/ROX qPCR Master Mix (Thermo Fisher Scientific Co.) was used to perform RT-qPCR in a 7500 Fast Real-Time PCR System (Applied Biosystems), in accordance with the manufacturer’s instructions. The cycling conditions were 10 min at 95 °C for initial denaturation, followed by 40 cycles of denaturation at 95 °C for 15 s and annealing/extension at 60 °C for 30 s. Cotton GhmiR390 and GhPP2A (catalytic subunit of phosphatase 2A) were used as reference genes for the analysis of DCL expression in different organs, the results were obtained by △CT method and the 2−△△CT method was used to analyze DCL expression during viral infection and/or aphid inoculation [51, 52]. Cotton GhmiR390 and GhPP2A (PROTEIN PHOSPHATASE 2), which were previously identified as the best reference genes for CLRDV infected cotton studies [74], were used to normalize cDNA expression levels. Reactions were prepared in a total volume of 20 μL, which contained 10 μL of SYBR green master mix, 2 μL of cDNA template, 6 μL of ddH2O, and 2 μL of each primer to make a final concentration of 10 μM. Three biological replicates were performed, and three technical replicates were assayed per cDNA sample.
In order to show DCL expression in each organ, a heat-map of the DCLs expression pattern was established by complete clustering method analysis using Euclidean distance and calculated in the R software environment. The DCL Cts results are shown at Additional file 2: Table S3.
Sequencing of CAV sviRNAs by deep sequencing
Leaves of cotton infected with Cotton anthocyanosis virus (CAV) obtained in Mato Grosso state, Brazil, were used for total RNA extraction and small RNA purification and sequencing by Illumina plataform following procedures described by [54].
Statistical analysis
DCL relative expression levels determined for the different samples under herbivore attack and/or virus infection were compared with these controls: untreated plants (control) x aphid inoculated plants for herbivore expression analysis, and mock (virus-free aphids inoculated plants) x CLRDV-aphid inoculated plants for viral infection using the parametric one-way ANOVA test at P ≤ 0.05 and P ≤ 0.01. For checking if the different relative expression levels of DCLs, from FM and DO, are statistical significant T-student test were used.
Promoter region cis-acting element analysis
For the identification of cis-regulatory elements present in G. hirsutum DCLs, upstream sequences of 1500 nucleotides from ATG star codon were downloaded from the CottonGen website (https://www.cottongen.org) and cis elements predicted with PlantCARE software (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/).
Availability of data and materials
All data generated or analysed during this study are included in this published article and in its supplementary information files.
Abbreviations
- CLRDV:
-
Cotton leafroll dwarf virus
- DCL:
-
Dicer-like protein
- DO:
-
Delta Opal cotton cultivar
- FM:
-
Fibermax966 cotton cultivar
- GaDCL:
-
Dicer-like gene or protein from Gossypium arboreum
- GhDCL:
-
Dicer-like gene or protein from Gossypium hirsutum
- GrDCL:
-
Dicer-like gene or protein from Gossypium raimondii
- RNAi:
-
RNA interference
- vsiRNA:
-
Viral small interfering RNAs
References
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. https://doi.org/10.1016/S0092-8674(04)00045-5.
Henderson IR, Zhang X, Lu C, Johnson L, Meyers BC, Green PJ, Jacobsen SE. Dissecting Arabidopsis thaliana DICER function in small RNA processing, gene silencing and DNA methylation patterning. Nat Genet. 2006;38:721–5. https://doi.org/10.1038/ng1804.
Borges F, Martienssen RA. The expanding world of small RNAs in plants. Nat Rev Mol Cell Biol. 2015;16(12):727–41. https://doi.org/10.1038/nrm4085.
Liu QP, Feng Y, Zhu ZJ. Dicer-like (DCL) proteins in plants. Funct Integr Genomics. 2009;9(3):277–86. https://doi.org/10.1007/s10142-009-0111-5.
Aliyari R, Ding SW. RNA-based viral immunity initiated by the Dicer family of host immune receptors. Immunol Rev. 2009;227:176–88. https://doi.org/10.1111/j.1600-065X.2008.00722.x.
Khraiwesh B, Zhu JK, Zhu J. Role of miRNAs and siRNAs in biotic and abiotic stress responses of plants. Biochim Biophys Acta. 2012;1819:137–48. https://doi.org/10.1016/j.bbagrm.2011.05.001.
Hall TM. Structure and function of argonaute proteins. Structure. 2005;13(10):1403–8. https://doi.org/10.1016/j.str.2005.08.00.
Rivas FV, Tolia NH, Song JJ, Aragon JP, Liu J, Hannon GJ, Joshua-Tor L. Purified Argonaute2 and an siRNA form recombinant human RISC. Nat Struct Mol Biol. 2005;12(4):340–9. https://doi.org/10.1038/nsmb918.
Dalmay T, Hamilton A, Rudd S, Angell S. Baulcombe DC An RNA-dependent RNA polymerase gene in Arabidopsis is required for posttranscriptional gene silencing mediated by a transgene but not by a virus. Cell. 2000;101:543–53.
Wang X, Wu Q, Ito T, Cillo F, Li WX, Chen X, Yu JL, Ding SW. RNAi-mediated viral immunity requires amplification of virus-derived siRNAs in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2010;107:484–9. https://doi.org/10.1073/pnas.0904086107.
Bernstein E, Caudy AA, Hammond SM, Hanoon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–6. https://doi.org/10.1038/35053110.
Margis R, Fusaro AF, Smith NA, Curtin SJ, Watson JM, Finnegan EJ, Waterhouse PM. The evolution and diversification of Dicers in plants. FEBS Lett. 2006;580:2442–50. https://doi.org/10.1016/j.febslet.2006.03.072.
Lee Y, Hur I, Park SY, Kim YK, Suh MR, Kim VN. The role of PACT in the RNA silencing pathway. EMBO J. 2006;25:522–32. https://doi.org/10.1038/sj.emboj.7600942.
Ye XC, Paroo Z, Liu QH. Functional anatomy of the Drosophila MicroRNA- generating enzyme. J Biol Chem. 2007;282:28373–8. https://doi.org/10.1074/jbc.M705208200.
Welker NC, Pavelec DM, Nix DA, Duchaine TF, Kennedy S, Bass BL. Dicer’s helicase domain is required for accumulation of some, but not all, C. elegans endogenous siRNAs. RNA. 2010;16(5):893–903. https://doi.org/10.1261/rna.2122010.
Cenik ES, Fukunaga R, Lu G, Dutcher R, Wang YM, Hall TMT, Zamore PD. Phosphate and R2D2 restrict the substrate specificity of Dicer-2, an ATP-driven ribonuclease. Mol Cell. 2011;42:172–84. https://doi.org/10.1016/j.molcel.2011.03.002.
MacRae IJ, Zhou K, Li F, Repic A, Brooks AN, Cande WZ, Adams PD, Douna JA. Structural basis for double-stranded RNA processing by Dicer. Science. 2006;311:195–8. https://doi.org/10.1126/science.1121638.
MacRae IJ, Doudna JA. Ribonuclease revisited: structural insights into ribonuclease III family enzymes. Curr Opin Struct Biol. 2007;17:138–45. https://doi.org/10.1016/j.sbi.2006.12.002.
Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–8. https://doi.org/10.1038/35078107.
Takeshita D, Zenno S, Lee WC, Nagata K, Saigo K, Tanokura M. Homodimeric structure and double-stranded RNA cleavage activity of the c-terminal RNase III domain of human Dicer. J Mol Biol. 2007;374:106–20. https://doi.org/10.1016/j.jmb.2007.08.069.
Zhang H, Kolb FA, Jaskiewicz L, Westhof E, Filipowicz W. Single processing center models for human Dicer and bacterial RNase III. Cell. 2004;118:57–68. https://doi.org/10.1016/j.cell.2004.06.017.
Doyle M, Badertscher L, Jaskiewicz L, Güttinger S, Jurado S, Hugenschmidt T, Kutay U, Filipowicz W. The double-stranded RNA binding domain of human Dicer functions as a nuclear localization signal. RNA. 2013;19:1238–52. https://doi.org/10.1261/rna.039255.113.
Wostenberg C, Lary JW, Sahu D, Acevedo R, Quarles KA, Cole JL, Showalter SA. The role of human Dicer-dsRBD in processing small regulatory RNAs. PLoS One. 2012;7(12):e51829. https://doi.org/10.1371/journal.pone.0051829.
Kini HK, Walton SP. In vitro binding of single-stranded RNA by human Dicer. FEBS Lett. 2007;581:5611–6. https://doi.org/10.1016/j.febslet.2007.11.010.
Moissiard G, Parizotto EA, Himber C, Voinnet O. Transitivity in Arabidopsis can be primed, requires the redundant action of the antiviral Dicer-like 4 and Dicer-like 2, and is compromised by viral-encoded suppressor proteins. RNA. 2007;13:1268–78. https://doi.org/10.1261/rna.541307.
Deleris A, Gallego-Bartolome J, Bao J, Kasschau KD, Carrington JC, Voinnet O. Hierarchical action and inhibition of plant DICER-like proteins in antiviral defence. Science. 2006;313:68–71. https://doi.org/10.1126/science.1128214.
Kurihara Y, Watanabe Y. Arabidopsis micro-RNA biogenesis through Dicer-like 1 protein functions. Proc Natl Acad Sci. 2004;101:12753–8. https://doi.org/10.1073/pnas.0403115101.
Mlotshwa S, Pruss GJ, Peragine A, Endres MW, Li J, Chen X, Poethig RS, Bowman LH, Vance V. DICER-LIKE2 plays a primary role in transitive silencing of transgenes in Arabidopsis. PLoS One. 2008;3:e1755. https://doi.org/10.1371/journal.pone.0001755.
Pontes O, Li CF, Costa Nunes P, Haag J, Ream T, Vitins A, Jacobsen SE, Pikaard CS. The Arabidopsis chromatin-modifying nuclear siRNA pathway involves a nucleolar RNA processing center. Cell. 2006;126:79–92. https://doi.org/10.1016/j.cell.2006.05.031.
Xie ZX, Johansen LK, Gustafson AM, Kasschau KD, Lellis AD, Zilberman D, Jacobsen SE, Carrington JC. Genetic and functional diversification of small RNA pathways in plants. PLoS Biol. 2004;2:642–52. https://doi.org/10.1371/journal.pbio.0020104.
Xie Z, Allen E, Wilken A, Carrington JC. DICER-LIKE 4 functions in trans-acting small interfering RNA biogenesis and vegetative phase change in Arabidopsis thaliana. Proc Natl Acad Sci U S A. 2005;102:12984–9. https://doi.org/10.1073/pnas.0506426102.
Cao M, Dua P, Wanga X, Yua Y-Q, Qiua Y-H, Lia W, Gal-Onc A, Zhoud C, Lib Y, Ding S-W. Virus infection triggers widespread silencing of host genes by a distinct class of endogenous siRNAs in Arabidopsis. PNAS. 2014;111(40):14613–8. https://doi.org/10.1073/pnas.1407131111.
Dunoyer P, Himber C, Voinnet O. DICER-LIKE 4 is required for RNA interference and produces the 21-nucleotide small interfering RNA component of the plant cell-to-cell silencing signal. Nat Genet. 2005;37:1356–60. https://doi.org/10.1038/ng1675.
Wang XB, Jovel J, Udomporn P, Wang Y, Wu Q, Li WX, Gasciolli V, Vaucheret H, Ding SW. The 21-nucleotide, but not 22- nucleotide, viral secondary small interfering RNAs direct potent antiviral defense by two cooperative argonautes in Arabidopsis thaliana. Plant Cell. 2011;23:1625–38. https://doi.org/10.1105/tpc.110.082305.
Parent J-S, Bouteiller N, Elmayan T, Vaucheret H. Respective contributions of Arabidopsis DCL2 and DCL4 to RNA silencing. Plant J. 2015;81:223–32. https://doi.org/10.1111/tpj.12720.
Bai M, Yang GS, Chen WT, Mao ZC, Kang HX, Chen GH, Yang YH, Xie BY. Genome-wide identification of Dicer-like, Argonaute and RNA-dependent RNA polymerase gene families and their expression analyses in response to viral infection and abiotic stresses in Solanum lycopersicum. Gene. 2012;501:52–62. https://doi.org/10.1016/j.gene.2012.02.009.
Kapoor M, Arora R, Lama T, Nijhawan A, Khurana JP, Tyagi AK, Kapoor S. Genome-wide identification, organization and phylogenetic analysis of Dicer-like, Argonaute and RNA-dependent RNA Polymerase gene families and their expression analysis during reproductive development and stress in rice. BMC Genomics. 2008;9:451. https://doi.org/10.1186/1471-2164-9-451.
Qian Y, Cheng Y, Cheng X, Jiang H, Zhu S, Cheng B. Identification and characterization of Dicer-like, Argonaute and RNA-dependent RNA polymerase gene families in maize. Plant Cell. 2011;30:1347–63. https://doi.org/10.1007/s00299-011-1046-6.
Zhao K, Zhao H, Chen Z, Feng L, Ren J, Cai R, Xiang Y. The Dicer-like, Argonaute and RNA-dependent RNA polymerase gene families in Populus trichocarpa: gene structure, gene expression, phylogenetic analysis and evolution. J Genet. 2015;94:317–21.
Zhao H, Zhao K, Wang J, Chen X, Chen Z, Cai R, Xiang Y. Comprehensive analysis of Dicer-like, Argonaute, and RNA-dependent RNA polymerase gene families in grapevine (Vitis vinifera). J Plant Growth Regul. 2015;34:108–21. https://doi.org/10.1007/s00344-014-9448-7.
Cao JY, Xu YP, Li W, Li SS, Rahman H, Cai XZ. Genome-wide identification of Dicer-like, Argonaute, and RNA-dependent RNA polymerase gene families in Brassica species and functional analyses of their Arabidopsis homologs in resistance to Sclerotinia sclerotiorum. Front Plant Sci. 2016;7:1614. https://doi.org/10.3389/fpls.2016.01614.
Garg V, Agarwal G, Pazhamala LT, Nayak SN, Kudapa H, Khan AW, Doddamani D, Sharma M, Kishor PB, Varshney RK. Genome-wide identification, characterization, and expression analysis of small RNA biogenesis purveyors reveal their role in regulation of biotic stress responses in three legume crops. Front Plant Sci. 2017;8:488. https://doi.org/10.3389/fpls.2017.00488.
Qin L, Mo N, Muhammad T, Liang Y. Genome-wide analysis of DCL, AGO, and RDR gene families in pepper (Capsicum Annuum L.). Int J Mol Sci. 2018;19:e1038. https://doi.org/10.3390/ijms19041038.
Paterson AH, Wendel JF, Gundlach H, Guo H, Jenkins J, Jin D, Llewellyn D, Showmaker KC, Shu S, Udall J, Yoo M-J, Byers R, Vaslin MF, et al. Repeated polyploidization of Gossypium genomes and the evolution of spinnable cotton fibres. Nature. 2012;492:423–7. https://doi.org/10.1038/nature11798.
Li F, Fan G, Lu C, Xiao G, Zou C, Kohel RJ, Ma Z, Shang H, Ma X, Wu J, Liang X, Huang G, Percy RG, Liu K, Yang W, Chen W, Du X, Shi C, Yuan Y, Ye W, et al. Genome sequence of cultivated Upland cotton (Gossypium hirsutum TM-1) provides insights into genome evolution. Nature. 2015;3:524–30. https://doi.org/10.1038/nbt.3208.
Wang K, Wang Z, Li F, Ye W, Wang J, Song G, Yue Z, Cong L, Shang H, Zhu S, Zou C, Li Q, Yuan Y, Lu C, Wei H, et al. The draft genome of a diploid cotton Gossypium raimondii. Nat Genet. 2012;44:1098–103. https://doi.org/10.1038/ng.2371.
Li F, Fan G, Wang K, Sun F, Yuan Y, Song G, Li Q, Ma Z, Lu C, Zou C, Chen W, Liang X, Shang H, Liu W, Shi C, Xiao G, Gou C, Ye W, et al. Genome sequence of the cultivated cotton Gossypium arboreum. Nat Genet. 2014;46:567–72. https://doi.org/10.1038/ng.2987.
Zhang T, Hu Y, Jiang W, Fang L, Guan X, Chen J, Zhang J, Saski CA, Scheffler BE, Stelly DM, Hulse-Kemp AM, Wan Q, Liu B, Liu C, Wang S, Pan M, Wang Y, Wang D, Ye W, Chang L, et al. Sequencing of allotetraploid cotton (Gossypium hirsutum L. acc. TM-1) provides a resource for fiber improvement. Nat Biotechnol. 2015;33:531–7. https://doi.org/10.1038/nbt.3207.
Correa RL, Silva TF, Simões-Araujo JL, Barroso PA, Vidal MS, Vaslin MF. Molecular characterization of a virus from the family Luteoviridae associated with cotton blue disease. Arch Virol. 2005;150:1357–67. https://doi.org/10.1007/s00705-004-0475-8.
Distefano AJ, Bonacic Kresic I, Hopp HE. The complete genome sequence of a virus associated with cotton blue disease, cotton leafroll dwarf virus, confirms that it is a new member of the genus Polerovirus. Arch Virol. 2010;155:1849–54. https://doi.org/10.1007/s00705-010-0764-3.
Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative Ct method. Nat Protoc. 2008;3(6):1101–8.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (−Delta Delta Ct) method. Methods. 2001;25:402–8. https://doi.org/10.1006/meth.2001.1262.
Silva TF, Romanel EAC, Andrade RRS, Farinelli L, Osteras M, Deluen C, Correa RL, Scharago CEG, Vaslin MFS. Profile of small interfering RNAs from cotton plants infected with the polerovirus Cotton leafroll dwarf vírus. BMC Mol Biol. 2011;12:1471–2199. https://doi.org/10.1186/1471-2199-12-40.
Andrade RSR, Vaslin MFS. Search small RNA: a graphical interface tool for the assemblage of viral genomes using small RNA libraries data. Virol J. 2014;7:11–45. https://doi.org/10.1186/1743-422X-11-45.
Mallory AC, Vaucheret H. Functions of microRNAs and related small RNAs in plants. Nat Genet. 2006;38:S31–6. https://doi.org/10.1038/ng1791.
Romanel E, Silva TF, Corrêa RL, Farinelli L, Hawkins JS, Schrago CE, Vaslin MF. Global alteration of microRNAs and transposon-derived small RNAs in cotton (Gossypium hirsutum) during Cotton leafroll dwarf polerovirus (CLRDV) infection. Plant Mol Biol. 2012;80(4–5):443–60. https://doi.org/10.1007/s11103-012-9959-1.
Bozorov TA, Pandey SP, Dinh ST, Kim SG, Heinrich M, Gase K, Baldwin IT. DICER-like proteins and their role in plant-herbivore interactions in Nicotiana attenuate. J Integr Plant Biol. 2012;54:189–206. https://doi.org/10.1111/j.1744-7909.2012.01104.x.
Qin C, Li B, Fan Y, Zhang X, Yu Z, Ryabov E, Zhao M, Wang H, Shi N, Zhang P, Jackson S, et al. Roles of Dicer-like proteins 2 and 4 in intra- and intercellular antiviral silencing. Plant Physiol. 2017;174:1067–81. https://doi.org/10.1104/pp.17.00475.
Diaz-Pendon JA, Li F, Li W-X, Ding S-W. Suppression of antiviral silencing by cucumber mosaic virus 2b protein in Arabidopsis is associated with drastically reduced accumulation of three classes of viral small interfering RNAs. Plant Cell Online. 2007;19:2053–63. https://doi.org/10.1105/tpc.106.047449.
Zhang X, Zhang X, Singh J, Li D, Qu F. Temperature-dependent survival of turnip crinkle virus-infected Arabidopsis plants relies on an RNA silencing-based defense that requires DCL2, AGO2, and HEN1. J Virol. 2012;86:6847–54. https://doi.org/10.1128/JVI.00497-12.
Garcia-Ruiz H, Takeda A, Chapman EJ, Sullivan CM, Fahlgren N, Brempelis KJ, Carrington JC. Arabidopsis RNA-dependent RNA polymerases and dicer-like proteins in antiviral defence and small interfering RNA biogenesis during Turnip mosaic virus infection. Plant Cell. 2010;22:481–96. https://doi.org/10.1105/tpc.109.073056.
Gasciolli V, Mallory AC, Bartel DP, Vaucheret H. Partially redundant functions of Arabidopsis DICER-like enzymes and a role for DCL4 in producing trans-acting siRNAs. Curr Biol. 2005;15:1494–500. https://doi.org/10.1016/j.cub.2005.07.024.
Schauer SE, Jacobsen SE, Meinke DW, Ray A. DICER-LIKE1: blind men and elephants in Arabidopsis development. Trends Plant Sci. 2002;7:487–91. https://doi.org/10.1016/S1360-1385(02)02355-5.
Bouche N, Lauressergues D, Gasciolli V, Vaucheret H. An antagonistic function for Arabidopsis DCL2 in development and a new function for DCL4 in generating viral siRNAs. EMBO J. 2006;25:3347–56. https://doi.org/10.1038/sj.emboj.7601217.
Qu F, Ye X, Morris TJ. Arabidopsis DRB4, AGO1, AGO7, and RDR6 participate in a DCL4-initiated antiviral RNA silencing pathway negatively regulated by DCL1. Proc Natl Acad Sci U S A. 2008;105:14732–7. https://doi.org/10.1073/pnas.0805760105.
Cordero T, Cerdán L, Carbonell A, Katsarou K, Kalantidis K, Daròs JA. Dicer-like 4 is involved in restricting the systemic movement of Zucchini yellow mosaic virus in Nicotiana benthamiana. Mol Plant-Microbe Interact. 2017;30:63–71. https://doi.org/10.1094/MPMI-11-16-0239-R.
Donaire L, Wang Y, Gonzalez-Ibeas D, Mayer KF, Aranda MA, Llave C. Deep-sequencing of plant viral small RNAs reveals effective and widespread targeting of viral genomes. Virology. 2009;392:203–14. https://doi.org/10.1016/j.virol.2009.07.005.
Zhou C-J, Zhang X-Y, Liu S-Y, Wang Y, Li D-W, Yu J-L, Han C-G. Synergistic infection of BrYV and PEMV 2 increases the accumulations of both BrYV and BrYV-derived siRNAs in Nicotiana benthamiana. Sci Rep. 2017;7:45132. https://doi.org/10.1038/srep45132.
Fusaro AF, Matthew L, Smith NA, Curtin SJ, Dedic-Hagan J, et al. RNA interference-inducing hairpin RNAs in plants act through the viral defence pathway. EMBO Rep. 2006;7:1168–75. https://doi.org/10.1038/sj.embor.7400837.
Llave C. Virus-derived small interfering RNAs at the core of plant-virus interactions. Trends Plant Sci. 2010;15(12):701–7. https://doi.org/10.1016/j.tplants.2010.09.001.
Blevins T, Rajeswaran R, Shivaprasad PV, Beknazariants D, Si-Ammour A, Park HS, Vazquez F, Robertson D, Meins F, et al. Four plant Dicers mediate viral small RNA biogenesis and DNA virus induced silencing. Nucleic Acids Res. 2006;34:6233–46. https://doi.org/10.1093/nar/gkl886.
Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–4. https://doi.org/10.1093/molbev/msw054.
Hu G, Koh J, Yoo MJ, Chen S, Wendel JF. Gene-expression novelty in allopolyploid cotton: a proteomic perspective. Genetics. 2015;1:91–104. https://doi.org/10.1534/genetics.115.174367.
Fausto AKS, Silva TF, Romanel E, Vaslin M. MicroRNAs as reference genes for quantitative PCR in cotton. PLoS One. 2017;12:e0174722. https://doi.org/10.1371/journal.pone.0174722.
Acknowledgements
We thank Sarah M. Nardeli for help and grateful discussions.
Funding
This research was financially supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil), Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ/Brazil) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP/Brazil). MOM and AKSF received grants from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES/Brazil) for master and PhD development, respectively. None of the funding bodies was involved in the design of the study, the collection, analysis, and interpretation of the data, nor in the writing of the manuscript, which was made entirely by the authors.
Author information
Authors and Affiliations
Contributions
MFSV and ER conceived and designed the experiments. MOM and AKSF performed the experiments. TFS, MOM, AKSF, AF and ER carried out bioinformatics analyses and analyzed data. FAFG performed transcriptome analysis. MOM and MFSV wrote the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1.
Cotton DCL c-DNA and protein sequences.
Additional file 2: Table S1.
Gene name and gene ID of DCLs in Arabidopsis, Medicago, rice, Populus, Physcomitrella and grapevine. Table S2. List of primers and amplicon characteristics of DCL genes. Table S3. DCL expression in organs. Figure S1. DCL amplification melting curves. Figure S2. Expression pattern of G. hirsutum acc. FM DCL genes based on transcriptome sequencing data.
Additional file 3: Figure S3.
Cis-elements distribution in DCL promoters with enhancer elements. Figure S4. Promoter sequences of Gossypium hirsutum DCLs.
Additional file 4.
Positions of all cis elements found in G. hirustum DCLs promoters.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Moura, M.O., Fausto, A.K.S., Fanelli, A. et al. Genome-wide identification of the Dicer-like family in cotton and analysis of the DCL expression modulation in response to biotic stress in two contrasting commercial cultivars. BMC Plant Biol 19, 503 (2019). https://doi.org/10.1186/s12870-019-2112-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-019-2112-4