Abstract
Background
Colistin is an antibiotic used as a last-resort to treat multidrug-resistant Gram-negative bacterial infections. Colistin had been used for a long time in veterinary medicine for disease control and as a growth promoter in food-producing animals. This excessive use of colistin in food animals causes an increase in colistin resistance. This study aimed to determine molecular characteristics of colistin-resistant Escherichia coli in broiler chicken and chicken farm environments.
Results
Four hundred fifty-three cloacal and farm environment samples were collected from six different commercial chicken farms in Kelantan, Malaysia. E. coli was isolated using standard bacteriological methods, and the isolates were tested for antimicrobial susceptibility using disc diffusion and colistin minimum inhibitory concentration (MIC) by broth microdilution. Multiplex PCR was used to detect mcr genes, and DNA sequencing was used to confirm the resistance genes. Virulence gene detection, phylogroup, and multilocus sequence typing (MLST) were done to further characterize the E. coli isolates. Out of the 425 (94%; 425/453) E. coli isolated from the chicken and farm environment samples, 10.8% (48/425) isolates were carrying one or more colistin-resistance encoding genes. Of the 48 colistin-resistant isolates, 54.2% (26/48) of the mcr positive isolates were genotypically and phenotypically resistant to colistin with MIC of colistin ≥ 4 μg/ml. The most prominent mcr gene detected was mcr-1 (47.9%; 23/48), followed by mcr-8 (18.8%; 9/48), mcr-7 (14.5%; 7/48), mcr-6 (12.5%; 6/48), mcr-4 (2.1%; 1/48), mcr-5 (2.1%; 1/48), and mcr-9 (2.1%; 1/48) genes. One E. coli isolate originating from the fecal sample was found to harbor both mcr-4 and mcr-6 genes and another isolate from the drinking water sample was carrying mcr-1 and mcr-8 genes. The majority of the mcr positive isolates were categorized under phylogroup A followed by phylogroup B1. The most prevalent sequence typing (ST) was ST1771 (n = 4) followed by ST206 (n = 3). 100% of the mcr positive E. coli isolates were multidrug resistant. The most frequently detected virulence genes among mcr positive E. coli isolates were ast (38%; 18/48) followed by iss (23%; 11/48). This is the first research to report the prevalence of mcr-4, mcr-5, mcr-6, mcr-7, and mcr-8 genes in E. coli from broiler chickens and farm environments in Malaysia.
Conclusion
Our findings suggest that broiler chickens and broiler farm environments could be reservoirs of colistin-resistant E. coli, posing a risk to public health and food safety.
Similar content being viewed by others
Background
Antimicrobial resistance (AMR) is a major threat to global public health. AMR spreads to the community primarily due to the excessive use of antimicrobials in humans and animals [1]. The use of antimicrobials for disease control or growth promoters in animals causes the commensal microflora to acquire antimicrobial resistance genes (ARGs) through horizontal gene transfer from resistant strains [2]. Evidence shows that AMR in humans can be caused by horizontal transfer of food animal-originated ARGs to human pathogens or through direct transfer of resistant bacteria [3].
Escherichia coli (E. coli) is an Enterobacteriaceae that commonly inhabits the guts of animals and humans. However, it is responsible for many life-threatening infections in humans and animals including chickens. Antimicrobial resistant E. coli strains cause a potential risk to public health. Meanwhile, antimicrobial resistant E. coli may function as carriers for antimicrobial resistance determinants to its other strain or other bacteria species [4, 5]. Though colistin was previously avoided from human medicine due to its systemic toxicity, its use has been revived due to its efficacy in the treatment of multi-drug resistant (MDR) Gram-negative bacteria [6]. According to World Health Organization (WHO), colistin serves as a last-resort antibiotic that is critically important to human medicine [7]. Colistin has used for a long time in veterinary medicine for disease control and as a growth promoter in food-producing animals [8]. This excessive use of colistin in animals causes antibiotic resistance in bacteria from animals, leading to the emergence of colistin-resistant bacteria which spreads to humans [9]. Resistant microorganisms in humans could have originated from livestock and food producing animals. Colistin-resistant in bacteria was considered as the result of chromosomal mutation until the discovery of the transferable plasmid-mediated gene (mcr-1) in 2015 [10]. The emergence of mcr related colistin-resistance is a major threat to the treatment of infections. Following the first report of mcr-1 from China, many studies reported continuously the novel mcr genes in Salmonella and E. coli [11,12,13,14].
In addition, following the invention of mcr-1 in China, an increased rate of colistin-resistant bacteria was reported in food animals including poultry worldwide, especially in Asia [15, 16]. Many countries including Malaysia, have banned the use of colistin in food additives as a growth promoter due to increased colistin-resistant strains in animals [17]. Even though colistin-resistance has reduced after the complete ban of colistin in animal production, a significant colistin-resistance is still being reported from food animals mainly from pigs and poultry throughout the world [17]. To date, the common reported colistin-resistance encoding genes are mcr-1 through mcr-10 [18]. Previous studies from Malaysia specifically in Kelantan showed that chicken meat was contaminated with colistin mcr-1 encoded resistant E. coli [19, 20].
Escherichia coli strains are classified into phylogroups of A, B1, B2, C, D, E, F, and clade I/II [21]. Phylogroups B2 and D are associated with virulent extraintestinal pathogenic E. coli (ExPEC), whereas phylogroups A and B1 contain mainly commensal E. coli strains [22, 23]. Multilocus sequence typing (MLST) is important for understanding the molecular evolution and phylogenetic relationship of important bacteria such as E. coli [24]. It helps to detect the emerging E. coli sequence type lineages, which are important in the control of AMR in humans and animals. Commensal and environmental bacteria may serve as a reservoir of ARGs that may be transferred to pathogenic bacteria in farm environments. AMR bacteria may be shed with animal feces and contaminate the farm environment. In previous studies, high rates of AMR gene were reported from broiler chicken litter and sewage [25, 26]. Therefore, the purpose of this study was to identify the molecular characteristics of colistin-resistant E. coli in broiler chickens and farm environments, which is important for understanding the potential reservoir of ARGs in chicken farms in Kota Bharu and nearby located commercial farms.
Methods and materials
Sample collection
Sample size was calculated using the single population formula based on the previous prevalence 52.1% [19]. A total of 453 samples (210 cloacal and 243 environmental samples) were collected from six different farms in Kota Bharu, Malaysia since February-November 2021. Environmental samples collected were drinking water (n = 27), sewage water (n = 14), fresh droppings (feces) (n = 55), feed (n = 32), litter (n = 20) and environmental swabs (a swab from utilities used in the farm) (n = 95). Cloacal and environmental swab samples were collected using Amies transport medium. The water samples were collected using a clean and sterile container. The collected samples were transported to the laboratory in an ice box with an ice pack and the samples were processed within 6 h of sample collection.
Z = Z value (95% CI, z = 1.96)
p = estimated prevalence, p = 0.521 for previous prevalence [19] of colistin resistant
d = margin of error (0.05)
\({\varvec{n}}\) = (1.96)2 × 0.521 x(1–0.521)/0.05 = 383, by adding 10% contingency, the sample size (\({\varvec{n}}\))=421
Isolation and identification of E. coli
Collected samples were enriched in Buffered Peptone Water (Oxoid, Manchester, UK) and incubated at 37 °C for 24 h. Using sterile wire loop, the enriched bacteria were inoculated to MacConkey agar (Oxoid, Manchester, UK). Lactose fermenter colonies were streaked with Eosin Methylene Blue (EMB) (Oxoid, Manchester, UK) agar and incubated at 37 °C for 24 h. The green metallic sheen colonies were presumptively identified as E. coli and the colonies were further tested for biochemical tests such as triple sugar iron agar (TSI), citrate, urea, indole, methyl red and motility. E. coli ATCC® 25922 was used as a positive control strain.
PCR confirmation of isolated E. coli
Genomic DNA was extracted using the boiling method as described previously [27]. Extracted DNA of isolated E. coli were amplified with species-specific Pho A and E coli primers for further PCR confirmation as used in previous studies [19, 28,29,30,31]. The PCR protocol used for the Pho and E coli primers was as previously described [29, 31]. DNA template extracted from E. coli ATCC® 25922 strain was used as a positive control and a PCR tube added nuclease free water instead of the DNA template was used as the negative control. In all PCR reactions in this study, PCR products were analyzed using agarose gel electrophoresis and gel images were analysed using GelDoc© Gel Documentation System (Bio-Rad, USA).
Antimicrobial susceptibility test
Antimicrobial susceptibility testing (AST) of isolated E. coli were conducted using Kirby-Bauer disk diffusion method on Mueller-Hinton agar (MHA) (Oxoid, Manchester, UK). The antimicrobial resistant profile of the isolates were determined against 16 antibiotic discs including aztreonam (30 µg), cefotaxime (30 µg), amoxicillin-clavulanic acid (30 µg), ceftazidime (30 µg), ceftriaxone (30 µg), trimethoprim-sulfamethoxazole (25 µg), chloramphenicol (30 µg), tetracycline (30 µg), imipenem (10 µg), meropenem (10 µg), ciprofloxacin (5 µg), ampicillin (10 µg), streptomycin (10 µg), nalidixic acid (30 µg), cefuroxime (30 µg), and gentamicin (10 µg). All the antibiotics were from Oxoid, UK. The zone of inhibition was interpreted based on CLSI guideline. E. coli ATCC® 25922 was used as a control strain [32].
Colistin minimum inhibitory concentration (MIC)
According to the CLSI recommendation, colistin minimum inhibitory concentration (MIC) was determined by broth microdilution (BMD) elusion using Cation-Adjusted Mueller Hinton Broth (CAMHB) [32]. Briefly, four tubes with 10 ml each of CAMHB and colistin discs were thawed to room temperature. Then aseptically one colistin disc (10 µg) was added to the tube labelled as “1 μg/ml”, two colistin discs to the tube labelled as “2 μg/ml” and 4 colistin discs to the tube labeled “4 μg/ml” and no colistin disc was added to the fourth growth control tube. The tubes were vortexed to precipitate the colistin disc into the broth and elute the colistin from the discs by leaving the mix for at 30 min at room temperature. Then after 3–5 freshly grown colonies were transferred to 4–5 ml of sterile saline, the turbidity of bacterial suspension was adjusted to be equivalent to 0.5 McFarland standard. 50 μl bacterial suspension with an approximate inoculum concentration of 7.5 × 105 CFU/ml was added to each of the four tubes [32]. The minimum inhibitory concentration (MIC) was read as the lowest concentration that inhibits the growth of E coli isolates after incubating for 16–20 h at 35 °C. The isolates were considered resistant with MIC ≥ 4 μg/ml and intermediate for MIC ≤ 2 μg/ml based on CLSI guideline [32]. E. coli ATCC® 25922 was used as a negative control strain.
Molecular detection of colistin resistance encoding genes
The confirmed E. coli isolates were screened for mcr-1 to mcr-9 genes using two separate multiplex PCR. The first multiplex PCR was mcr1-5 following previous protocol [33], and the second multiplex PCR was mcr6-9 as described previously [34]. PCR products were analyzed using agarose gel electrophoresis and gel images were analysed using GelDoc© Gel Documentation System (Bio-Rad, USA). Selected samples with mcr positive E. coli were further confirmed by sequencing. All the primers used in this study are summarized in Table 1.
Multilocus sequence typing (MLST)
Escherichia. coli isolates that were positive for colistin-resistant encoding mcr gene were selected for MLST analysis. MLST was performed by sequencing seven housekeeping genes, adk, fumC, gyrB, icd, mdh, purA, and recA, as described online in, https://enterobase.readthedocs.io/en/latest/mlst/mlst-legacy-info-ecoli.html.
The primers and PCR protocol used are available on the website [36]. The amplified PCR products were sent to Apical 1st base Sequencing service (Apical, Malaysia), to perform a sequence analysis. The alleles and sequence types were assigned from the E. coli database at the MLST website, http://enterobase.warwick.ac.uk/.
Phylogenetic typing of E. coli isolates
Quadruplex PCR was used to classify the isolates into phylogroups A, B1, B2 and D using primers chuA, yjaA, TspE4.C2 and arpA according to the revised protocol of Clermont et al. [35]. The isolates of phylogroup A were separated from phylogroup C by trpAgpC primer which is C-specific primers. Similarly, phylogroup D isolates were differentiated from E using ArpAgpE primer and phylogroup F was separated from phylogroup D in the quadruplex PCR as F does not contain ArpA gene.
PCR detection of virulence genes of E. coli isolates
The mcr positive E. coli isolates were assessed for avian pathogenic E. coli (APEC) associated virulence genes. Multiplex PCR protocol used to determine the presence of papC, iucD, irp2, tsh, vat, astA, iss and cva/cvi virulence genes associated with virulence factors as previously described [37].
Results
A total of 425(94%) E. coli were isolated from the 453 collected samples. Out of these, 203(97%) of E. coli strains were isolated from cloacal swab, 83(87%) from environmental swab, 23(85%) from drinking water, 55(100%) from fecal, 31(97%) from feed and 20(100%) from litter. All the isolated E. coli were confirmed targeting species specific pho and E. coli genes (Fig. 1).
Antimicrobial susceptibility profile
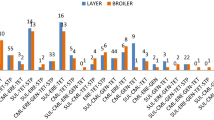
Isolated E. coli was evaluated for antimicrobial susceptibility towards 16 antibiotics of 11 different classes (Fig. 2). All the mcr positive E. coli isolates were resistant to at least three of the tested antibiotic discs belonging to different classes. The result shows that 100% of the mcr positive E. coli isolates were resistant to tetracycline, streptomycin, chloramphenicol, and ampicillin (Fig. 3). It was further revealed that 98% of the mcr positive E. coli strains were susceptible to meropenem. Moreover, compared with mcr negative E. coli, mcr harboring E. coli isolates showed higher resistance rates to nalidixic acid, ciprofloxacin, trimethoprim/sulfamethoxazole, and cefotaxime (Fig. 3). Out of 48 mcr gene positive E. coli isolates 26 (54.2%) of them were with colistin MIC of ≥ 4 μg/ml while the rest, 22 (45.8%) of the mcr gene positive isolates were MIC ≤ 2 μg/ml (Table 4).
Colistin resistance encoding genes
Colistin-resistance encoding genes were detected using multiplex PCR. Out of the PCR confirmed E. coli, 48 (10.8%) isolates were found harboring at least one mcr gene. The most prominent mcr gene detected was mcr-1 (47.9%; 23/48), followed by mcr-8 (18.8%; 9/48), mcr-7 (14.5%; 7/48), mcr-6 (12.5%; 6/48), mcr-4 (2.1%; 1/48), mcr-5 (2.1%; 1/48), and mcr-9 (2.1%; 1/48) genes (Table 2). Four (8.3%) isolates harbored more than one gene, mcr-4 and mcr-6, mcr-1, and mcr-8, mcr-1and mcr-7 and the fourth one was harboring mcr-1and mcr-5. In this study out of the mcr positive isolates the dominant mcr gene detected were mcr-1. Majority of the mcr-1 gene positive E. coli were isolated from cloacal and environmental swab samples. Meanwhile, the mcr-1 positive isolates were also detected in food, fecal, litter and drinking water samples. The mcr-4 and mcr-5 gene positive isolates were detected from freshly passed fecal and food samples respectively. In the current study, 33.3% (16/48) of mcr positive E. coli were from cloacal samples, 29.2% (14/48) from environmental swab,10.4% (5/48) from drinking water,10.4% (5/48) fecal,10.4% (5/48) feed, and 6.3% (3/48) were from litter. The gel electrophoresis image of amplified PCR product with mcr-1, mcr-4, and mcr-5 gene (Fig. 4); mcr-7 gene (Fig. 5); mcr-6,mcr-8, and mcr-9, and ESBL (TEM, SHV, and CTX) genes (Fig. 6); mcr-6 and mcr-8 genes (Fig. 7) are described below.
Phylogenetic typing of E. coli isolates
The majority of the mcr positive E. coli isolates were assigned to phylogroup A, which is 50% (24/48), followed by B1(12.5%) (Table 3). While the rest belonged to phylogroup C (n = 5, 10.4%); D (n = 5, 10.4%); E (n = 2, 4.2%); F (n = 2, 4.2%); Clade I or II (n = 2, 4.2%) and B2 (n = 1, 2.1%). Most isolates with phylogroup A originated from cloacal (n = 6) and poultry environment (n = 18). Figure 8 below shows the gel electrophoresis image of the four genes used to separate the isolates to phylogenetic groups.
Agarose gel electrophoresis image of mcr positive E. coli phylogenetic typing. Amplified PCR products with E. coli phylogrouping genes; arpA (400 bp), chuA (288 bp), yjaA (211 bp) and TspE4C2 (152 bp); lane 1, + - - -, belonging to phylogroup A; lane 2, + - - + , belonging to group B1; lane 3,- + + -, group B2; lane 4, + - + -, group C; lane 5, + + - -, group D; lane 6, + + + -, group E; lane 7, - + - -,group F; lane 8, - - + -, clade I/II
Multilocus sequence typing and virulence genes
Among the forty-eight mcr encoding colistin-resistant isolates, 23 mcr gene and virulence gene positive isolates were selected for MLST sequencing. Tthe MLST result of the isolates shows that the E. coli isolates were widely diverse. The most prevalent STs found were ST1771 (n = 4) followed by ST206 (n = 3). ST 1771 was found from cloacal, food and drinking water source isolates, ST206 was found from cloacal, environmental swab and drinking water sources. In this study among 18STs, the majority belonging to phylogroup A (44.4%) (Table 4). All the ST1771 except one were phylogroup A, one was group C and two of the ST206 were phylogroup A whereas one was phylogroup C. E. coli strains with ST165, ST206, ST1771, ST162, ST398, ST1285 and ST106 were found associated with mcr, CTX and SHV genes originate from fecal, cloacal, environmental swab, water, and litter sources. Moreover, ST165, ST1771, ST155, ST48, ST206, ST162, ST159, ST38 were found positive for ast, iss, irp2, or /and iucD virulence genes, which belongs to phylogroup A, B1, D, C, E and Clade I/II. The most frequently detected virulence genes among mcr positive E. coli isolates were ast (38%; 18/48) followed by iss (23%; 11/48), irp2 (17%; 8/48), iucD (13%; 6/18), papC(6%; 3/48) and tsh (2%; 1/48) genes.
Discussion
In the present study, 425 (94%) E. coli were isolated from 453 cloacal and environmental samples. Forty-eight (10.8%) of E. coli isolates were positive for at least one of the colistin-resistance encoding mcr gene. 100% of the mcr positive isolates were multidrug-resistant, which have the potential to disseminate to human and farm animals.
In this study 100% of the mcr positive E. coli isolates were resistant to tetracycline, streptomycin, chloramphenicol, and ampicillin. This correlation between mcr and resistance of these antibiotics might be due to the coexistence of mcr and ESBLs in the plasmids that could also harbor resistant genes of different classes of antimicrobials and have the trait of being multi-drug resistant [38, 39]. Among the mcr positive strains, 98% were susceptible to meropenem, a carbapenem antibiotic. Moreover, mcr harboring E. coli isolates exhibited higher resistance rates against nalidixic acid, ciprofloxacin, trimethoprim/sulfamethoxazole, and cefotaxime as compared with mcr negative E. coli. High resistance rate to tetracycline and ampicillin was also reported in E. coli isolates from chicken in Malaysia and Vietnam [20, 40]. The susceptibility test for colistin by disc diffusion and E-test is difficult due to polymyxins’ poor agar diffusion [41], and reliable reference break point is not available. Therefore, in this study MIC was used for colistin susceptibility test. This study revealed that 26(54.2%) of the mcr positive isolates have MICs of colistin ≥ 4 µg/ml, suggesting that the isolates are genotypically and phenotypically resistant to colistin. However, 22(45.8%) of genetically resistant isolates were phenotypically susceptible to colistin, with MIC ≤ 2 µg/ml. These discrepancies might be due to sensitivity difference between broth micro dilution and PCR detection methods in colistin resistance. A previous study stated that broth micro dilution has a 71.4% sensitivity rate in the detection of mcr-1 positive Enterobacteriaceae [42]. Nonetheless, PCR is widely considered as the gold standard method in detecting colistin-resistance [10, 33].
An interesting finding of this research is that 48 (10.8%) of the E. coli isolates were positive for at least one colistin-resistance encoding mcr gene. Out of the mcr genes mcr-1 was the prominent gene (47.9%; 23/48). Studies from Malaysia have found 23.08% mcr-1 encoding E. coli from broiler chicken, while 52.1% of E. coli from chicken meat was carrying mcr-1 gene [19, 20, 43]. Similarly, 23.08% mcr-1 harboring Klebsiella pneumonia strains were reported from pigs in Malaysia [44]. Furthermore, mcr-1 gene positive Enterobacteriaceae isolates have been found in food animals, humans, and environment globally, with high prevalence in food animals compared to humans [15, 45,46,47]. This widespread and relatively increased prevalence of mcr-1 gene related colistin-resistance in farm animals and retail meat indicate that food animals could be a potential reservoir for human transmission [48]. Recently, following the ban of colistin use as food additive in food animals in many countries, the prevalence of mcr-1 gene positive bacteria in food animals, including chickens has decreased [49].
Moreover, we found other mcr genes including mcr-4 (2.1%; 1/48), mcr-5 (2.1%; 1/48), mcr-6 (12.5%; 6/48), mcr-7 (14.5%; 7/48), mcr-8 (18.8%; 9/48) and mcr-9 (2.1%; 1/48) for the first time in Malaysia. However, none of the isolates of this research were positive for mcr-2 and mcr-3 genes. A study from China were reported mcr-4 and mcr-5 from chicken origin isolates [50]. In addition, the mcr-5 gene was also detected from chicken origin from Singapore, Brazil, and Paraguay [51, 52]. The mcr-5 and mcr-9 genes were found harbored in E. coli from chickens in Brazil [53]. In addition, mcr-9 genes were detected in Salmonella isolated from chicken meat in Korea and from USA in Salmonella and E. coli, even though the bacteria were not associated with colistin resistance [54, 55]. Meanwhile, mcr-7 and mcr-8 genes were detected from Klebsiella pneumonia from chicken and animal origin respectively in China [14, 56]. The mcr-6 was revealed from Moraxella species of pig origin from Britain [57]. This indicates that mcr genes are widely disseminated among different bacterial species and have spread globally. A review showed that among the colistin-resistance mcr genes, mcr-1 and mcr-9 have become globally spread [58]. In our research, four (8.3%) mcr positive E. coli isolates were harboring more than one mcr genes. One isolate tested positive for both mcr-4 and mcr-6, while another carried mcr-1 and mcr-8. The third harbored mcr-1 and mcr-7 and the fourth E. coli isolate was positive for both mcr-1 and mcr-5. The first two isolates belonged to phylogroup A, while the latter belonged to phylogroup D. These strains were isolated from chicken fecal samples, drinking water and cloacal swab. Majority of the mcr-1 gene positive E. coli were detected from cloacal and environmental swab samples. Meanwhile, mcr-1 gene positive E. coli were also detected in food, feces, litter and drinking water samples. The majority of the mcr genes positive E. coli were detected from chicken farm environment samples. Similarly, mcr-1 encoding E. coli strains were reported from litter, feed and drinking water in study from Lebanon and Indonesia [59, 60]. Shedding of the AMR bacteria and determinants from the feces into the farm environment causes dissemination of AMR to the chicken and animal handlers. As the litter often be used as a fertilizer, it also serve as a reservoir of resistant bacteria and determinants to agriculture and the environment [61]. Few previous studies in Asia showed mcr genes ranging from 10.5%-36.6% of mcr-1 in Bangladesh, Indonesia chicken [46, 60, 62]. To best of our knowledge, mcr-4, mcr-5, mcr-6, mcr-7, mcr-8, and mcr-9 genes were not reported from broiler chicken and farm environment origin in Malaysia.
Most of the isolates in our study were assigned to phylogroup A followed by B1, which is consistent with previous studies based on healthy broilers and environment [20, 63, 64]. In addition, phylogroup D (10.4%), F (4.2%) and B2 (2.1%) were found in the current study. Several studies have shown phylogroups B2 and F from APEC colibacillosis-causing strains that were a causative agent of human ExPEC [63, 65,66,67]. Phylogenetic group B2 and D are the most virulent causes of ExPEC infections in humans and chickens in France and China [22, 68].
In the present study, E. coli ST155, ST48 and ST38 harbored four virulence genes, including ast, iss, irp2 and iucD genes. E. coli ST155 and ST48 were caring mcr-1,while E. coli ST38 were positive for mcr-1 and mcr-7. E. coli ST165 were related to ast, iss and irp2 genes which was positive for mcr-4, mcr-6 and CTX, while E. coli ST206 were carrying ast, tsh and iss genes that was positive for mcr-1. E. coli ST155 isolates were also found in broiler chicken samples from previous research from Malaysia [20, 69]. Previous study reported the presence of E. coli ST155, ST48, and ST398 with MDR trait in human and chicken farm environment in Nigeria [70]. Furthermore E. coli ST155 was detected from APEC [71, 72] and from both APEC and human ExPEC [73] strains in previous studies. ST206 has been reported in China from human clinical samples, harboring both the mcr-1 and carbapenems gene blaNDM-5 genes [74]. E. coli ST206 isolates were also associated with ESBL gene (CTX-M-27) from human and animal sources in Nigeria [75]. ST48 was previously reported in systemic E. coli associated with APEC in UK [76]. E. coli ST38 was isolated from human EXPEC from several studies around the globe [77]. In the present study, E. coli ST155, ST48, ST38, ST398 and ST206 were positive for APEC associated virulence genes. Furthermore, these E. coli STs were reported as potentially zoonotic human ExpEC. We detected several virulence genes, including papC, iucD, irp2, tsh, ast and iss genes from the mcr positive E. coli isolates. The ast (38%,18/48) were the most frequently detected virulence gene followed by iss (23%,11/48), irp2 (17%, 8/48), iucD (13%, 6/18), papC(6%, 3/48) and tsh (2%,1/48) genes. The detection of Inspect and ExPEC virulent genes from apparently healthy broilers and farm environment indicates that healthy chickens and farm environment can be the reservoir of mcr positive virulent APEC. Similarly, intestinal E. coli were found harboring ExPEC including APEC associated genes as reported in previous studies [76, 78, 79]. These virulence genes, including papC, iucD, irp2, tsh, astA and iss genes has been reported from previous study associated with APEC [80].
Conclusion and recommendations
This study found that colistin-resistant E. coli in broiler chickens and chicken farm environment is high, despite a decrease observed in previous studies following the ban of colistin from animal food additives. However, the E. coli isolates harbored a variety of mcr genes and were highly resistant to antibiotics such as tetracycline, aminoglycoside, chloramphenicol, penicillin, quinolone, fluoroquinolone, sulfonamide, and cephalosporin. All the mcr genes positive E. coli isolates displayed resistance against multiple antibiotics. Among E. coli isolates from cloacal and farm environments, mcr-1 was the dominant mcr gene. Furthermore, mcr-4 and mcr-5 genes were found in faecal and feed samples respectively. This is the first study to report the prevalence of mcr-4, mcr-5, mcr-6, mcr-7, mcr-8, and mcr-9 genes in E. coli isolated from Malaysian broiler chickens and farm environments. Our findings suggested that MDR colistin-resistant E. coli strains carry virulence genes could be found in broiler chickens and broiler farm environments. These strains pose a high risk of spreading to humans, animals, and the environment. Based on our findings, we recommend stricter regulation of antibiotic use in farm animals since livestock and environments have a vital role in the transmission of antibiotic-resistant bacteria.
Limitation of the study
This study was limited in scope and did not cover all districts in Kelantan state and other Malaysian states. In addition, samples were collected only from broiler chickens reared under intensive production systems. Diversifying the study by including different poultry production systems including layers, breeders, and backyard poultry farms from representative poultry farms across the country may give a better and more accurate epidemiological information on the resistant bacteria. Moreover, this study did not detect plasmids of the isolated strains.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding authors on reasonable request.
References
Angulo FJ, Nargund VN, Chiller TC. Evidence of an association between use of anti-microbial agents in food animals and anti-microbial resistance among bacteria isolated from humans and the human health consequences of such resistance. J Vet Med B Infect Dis Vet Public Health. 2004;51(8–9):374–9. https://doi.org/10.1111/j.1439-0450.2004.00789.x.
Salinas L, Cardenas P, Johnson TJ, Vasco K, Graham J, Trueba G. Diverse commensal Escherichia coli clones and plasmids disseminate antimicrobial resistance genes in domestic animals and children in a semirural community in Ecuador. mSphere. 2019;4(3):e00316–19. https://doi.org/10.1128/mSphere.00316-19.
Marshall BM, Levy SB. Food animals and antimicrobials: impacts on human health. Clin Microbiol Rev. 2011;24(4):718–33. https://doi.org/10.1128/CMR.00002-11.
Akond MA, Hassan SMR, Alam S, Shirin M. Antibiotic resistance of Escherichia Coli isolated from poultry and poultry environment of Bangladesh. Am J Environ Sci. 2009;5(1):47–52. https://doi.org/10.3844/ajessp.2009.47.52.
Rasheed MU, Thajuddin N, Ahamed P, Teklemariam Z, Jamil K. Antimicrobial drug resistance in strains of Escherichia coli isolated from food sources. Rev Inst Med Trop Sao Paulo. 2014;56(4):341–6. https://doi.org/10.1590/s0036-46652014000400012.
Poirel L, Jayol A, Nordmann P. Polymyxins: antibacterial activity, susceptibility testing, and resistance mechanisms encoded by plasmids or chromosomes. Clin Microbiol Rev. 2017;30(2):557–96. https://doi.org/10.1128/CMR.00064-16.
WHO. Critically important antimicrobials for human medicine. 2019.
Liu Y, Liu JH. Monitoring colistin resistance in food animals, an urgent threat. Expert Rev Anti Infect Ther. 2018;16(6):443–6. https://doi.org/10.1080/14787210.2018.1481749.
Poirel L, Nordmann P. Emerging plasmid-encoded colistin resistance: the animal world as the culprit? J Antimicrob Chemother. 2016;71(8):2326–7. https://doi.org/10.1093/jac/dkw074.
Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. 2016;16(2):161–8. https://doi.org/10.1016/S1473-3099(15)00424-7.
Borowiak M, Fischer J, Hammerl JA, Hendriksen RS, Szabo I, Malorny B. Identification of a novel transposon-associated phosphoethanolamine transferase gene, mcr-5, conferring colistin resistance in d-tartrate fermenting Salmonella enterica subsp. enterica serovar Paratyphi B. J Antimicrob Chemother. 2017;72(12):3317–24. https://doi.org/10.1093/jac/dkx327.
Carattoli A, Villa L, Feudi C, Curcio L, Orsini S, Luppi A, et al. Novel plasmid-mediated colistin resistance mcr-4 gene in Salmonella and Escherichia coli, Italy 2013, Spain and Belgium, 2015 to 2016. Euro Surveill. 2017;22(31):30589. https://doi.org/10.2807/1560-7917.ES.2017.22.31.30589.
Xavier BB, Lammens C, Ruhal R, Kumar-Singh S, Butaye P, Goossens H, et al. Identification of a novel plasmid-mediated colistin-resistance gene, mcr-2, in Escherichia coli, Belgium, June 2016. Euro Surveill. 2016;21(27):30280. https://doi.org/10.2807/1560-7917.ES.2016.21.27.30280.
Wang X, Wang Y, Zhou Y, Li J, Yin W, Wang S, et al. Emergence of a novel mobile colistin resistance gene, mcr-8, in NDM-producing Klebsiella pneumoniae. Emerg Microbes Infect. 2018;7(1):122. https://doi.org/10.1038/s41426-018-0124-z.
Kempf I, Jouy E, Chauvin C. Colistin use and colistin resistance in bacteria from animals. Int J Antimicrob Agents. 2016;48(6):598–606. https://doi.org/10.1016/j.ijantimicag.2016.09.016.
Jeannot K, Bolard A, Plesiat P. Resistance to polymyxins in Gram-negative organisms. Int J Antimicrob Agents. 2017;49(5):526–35. https://doi.org/10.1016/j.ijantimicag.2016.11.029.
Wang Y, Xu C, Zhang R, Chen Y, Shen Y, Hu F, et al. Changes in colistin resistance and mcr-1 abundance in Escherichia coli of animal and human origins following the ban of colistin-positive additives in China: an epidemiological comparative study. Lancet Infect Dis. 2020;20(10):1161–71. https://doi.org/10.1016/S1473-3099(20)30149-3.
Valiakos G, Kapna I. Colistin resistant mcr genes prevalence in livestock animals (swine, bovine, poultry) from a multinational perspective. A systematic review. Vet Sci. 2021;8(11):265. https://doi.org/10.3390/vetsci8110265.
Aklilu E, Raman K. MCR-1 gene encoded colistin-resistant Escherichia coli in raw chicken meat and bean sprouts in Malaysia. Int J Microbiol. 2020;2020:8853582. https://doi.org/10.1155/2020/8853582.
Aklilu E, Harun A, Singh KKB. Molecular characterization of bla(NDM), bla(OXA-48), mcr-1 and bla(TEM-52) positive and concurrently carbapenem and colistin resistant and extended spectrum beta-lactamase producing Escherichia coli in chicken in Malaysia. BMC Vet Res. 2022;18(1):190. https://doi.org/10.1186/s12917-022-03292-7.
Dale AP, Woodford N. Extra-intestinal pathogenic Escherichia coli (ExPEC): disease, carriage and clones. J Infect. 2015;71(6):615–26. https://doi.org/10.1016/j.jinf.2015.09.009.
Clermont O, Bonacorsi S, Bingen E. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl Environ Microbiol. 2000;66(10):4555–8. https://doi.org/10.1128/AEM.66.10.4555-4558.2000.
Johnson JR, Russo TA. Extraintestinal pathogenic Escherichia coli: “the other bad E coli.” J Lab Clin Med. 2002;139(3):155–62. https://doi.org/10.1067/mlc.2002.121550.
Yu J, Sun Z, Liu W, Xi X, Song Y, Xu H, et al. Multilocus sequence typing of Streptococcus thermophilus from naturally fermented dairy foods in China and Mongolia. BMC Microbiol. 2015;15(1):236. https://doi.org/10.1186/s12866-015-0551-0.
Lu J, Sanchez S, Hofacre C, Maurer JJ, Harmon BG, Lee MD. Evaluation of broiler litter with reference to the microbial composition as assessed by using 16S rRNA and functional gene markers. Appl Environ Microbiol. 2003;69(2):901–8. https://doi.org/10.1128/AEM.69.2.901-908.2003.
Mandal AK, Talukder S, Hasan MM, Tasmim ST, Parvin MS, Ali MY, et al. Epidemiology and antimicrobial resistance of Escherichia coli in broiler chickens, farmworkers, and farm sewage in Bangladesh. Vet Med Sci. 2022;8(1):187–99. https://doi.org/10.1002/vms3.664.
Dashti AA, Jadaon MM, Abdulsamad AM, Dashti HM. Heat treatment of bacteria: a simple method of DNA extraction for molecular techniques. Kuwait Med J. 2009;41(2):117–22.
Alnahass R, Khaliel S, Ellakany H, Ibrahim M. Comparison between bacteriological isolation and molecular detection of E. coli from chickens suffering from colibacillosis and/or diarrhea. Alex J Vet Sci. 2017;49(2). https://doi.org/10.5455/ajvs.219057.
Yu KX, Thong KL. Multiplex PCR for simultaneous detection of virulence genes in Escherichia coli. Malays J Sci. 2009;28(1):1–14.
Elmi SA, Simons D, Elton L, Haider N, Abdel Hamid MM, Shuaib YA, et al. Identification of risk factors associated with resistant Escherichia coli isolates from poultry farms in the east coast of Peninsular Malaysia: a cross sectional study. Antibiotics (Basel). 2021;10(2):117. https://doi.org/10.3390/antibiotics10020117.
Aliyu AB, Saleha AA, Jalila A, Zunita Z. Risk factors and spatial distribution of extended spectrum beta-lactamase-producing- Escherichia coli at retail poultry meat markets in Malaysia: a cross-sectional study. BMC Public Health. 2016;16(1):699. https://doi.org/10.1186/s12889-016-3377-2.
CLSI. Performance Standards for Antimicrobial Susceptibility Testing. 31st ed. CLSI supplement M100. Clinical and Laboratory Standards Institute; 2021.
Rebelo AR, Bortolaia V, Kjeldgaard JS, Pedersen SK, Leekitcharoenphon P, Hansen IM, et al. Multiplex PCR for detection of plasmid-mediated colistin resistance determinants, mcr-1, mcr-2, mcr-3, mcr-4 and mcr-5 for surveillance purposes. Euro Surveill. 2018;23(6):29–39. https://doi.org/10.2807/1560-7917.ES.2018.23.6.17-00672.
Borowiak M, Baumann B, Fischer J, Thomas K, Deneke C, Hammerl JA, et al. Development of a novel mcr-6 to mcr-9 multiplex PCR and assessment of mcr-1 to mcr-9 occurrence in colistin-resistant Salmonella enterica isolates from environment, feed, animals and food (2011–2018) in Germany. Front Microbiol. 2020;11(80):80. https://doi.org/10.3389/fmicb.2020.00080.
Clermont O, Christenson JK, Denamur E, Gordon DM. The Clermont Escherichia coli phylo-typing method revisited: improvement of specificity and detection of new phylo-groups. Environ Microbiol Rep. 2013;5(1):58–65. https://doi.org/10.1111/1758-2229.12019.
Wirth T, Falush D, Lan R, Colles F, Mensa P, Wieler LH, et al. Sex and virulence in Escherichia coli: an evolutionary perspective. Mol Microbiol. 2006;60(5):1136–51. https://doi.org/10.1111/j.1365-2958.2006.05172.x.
Ewers C, Janssen T, Kiessling S, Philipp HC, Wieler LH. Rapid detection of virulence-associated genes in avian pathogenic Escherichia coli by multiplex polymerase chain reaction. Avian Dis. 2005;49(2):269–73. https://doi.org/10.1637/7293-102604R.
Bastidas-Caldes C, Cisneros-Vasquez E, Zambrano A, Mosquera-Maza A, Calero-Caceres W, Rey J, et al. Co-harboring of beta-lactamases and mcr-1 genes in Escherichia coli and Klebsiella pneumoniae from healthy carriers and backyard animals in rural communities in Ecuador. Antibiotics (Basel). 2023;12(5):856. https://doi.org/10.3390/antibiotics12050856.
Dominguez JE, Redondo LM, Figueroa Espinosa RA, Cejas D, Gutkind GO, Chacana PA, et al. Simultaneous carriage of mcr-1 and other antimicrobial resistance determinants in Escherichia coli from poultry. Front Microbiol. 2018;9:1679. https://doi.org/10.3389/fmicb.2018.01679.
Nguyen NT, Nguyen HM, Nguyen CV, Nguyen TV, Nguyen MT, Thai HQ, et al. Use of colistin and other critical antimicrobials on pig and chicken farms in southern Vietnam and its association with resistance in commensal Escherichia coli bacteria. Appl Environ Microbiol. 2016;82(13):3727–35. https://doi.org/10.1128/AEM.00337-16.
Hindler JA, Humphries RM. Colistin MIC variability by method for contemporary clinical isolates of multidrug-resistant Gram-negative bacilli. J Clin Microbiol. 2013;51(6):1678–84. https://doi.org/10.1128/JCM.03385-12.
Chew KL, La MV, Lin RTP, Teo JWP. Colistin and polymyxin B susceptibility testing for carbapenem-resistant and mcr-positive Enterobacteriaceae: comparison of sensititre, MicroScan, Vitek 2, and Etest with broth microdilution. J Clin Microbiol. 2017;55(9):2609–16. https://doi.org/10.1128/JCM.00268-17.
Devan SS, Aklilu E, Hamdan RH, Lemlem M, Zakaria Z. Detection of colistin-resistant Escherichia coli isolated from broiler chickens in Kelantan, Malaysia. Trop Biomed. 2022;39(2):197–202. https://doi.org/10.47665/tb.39.2.010.
Mobasseri G, Teh CSJ, Ooi PT, Thong KL. The emergence of colistin-resistant Klebsiella pneumoniae strains from swine in Malaysia. J Glob Antimicrob Resist. 2019;17:227–32. https://doi.org/10.1016/j.jgar.2018.12.015.
Zhong YM, Liu WE, Zheng ZF. Epidemiology and molecular characterization of mcr-1 in Escherichia coli recovered from patients with bloodstream infections in Changsha, central China. Infect Drug Resist. 2019;12:2069–76. https://doi.org/10.2147/IDR.S209877.
Islam S, Urmi UL, Rana M, Sultana F, Jahan N, Hossain B, et al. High abundance of the colistin resistance gene mcr-1 in chicken gut-bacteria in Bangladesh. Sci Rep. 2020;10(1):17292. https://doi.org/10.1038/s41598-020-74402-4.
Timmermans M, Wattiau P, Denis O, Boland C. Colistin resistance genes mcr-1 to mcr-5, including a case of triple occurrence (mcr-1, -3 and -5), in Escherichia coli isolates from faeces of healthy pigs, cattle and poultry in Belgium, 2012–2016. Int J Antimicrob Agents. 2021;57(6):106350. https://doi.org/10.1016/j.ijantimicag.2021.106350.
Skov RL, Monnet DL. Plasmid-mediated colistin resistance (mcr-1 gene): three months later, the story unfolds. Euro Surveill. 2016;21(9):30155. https://doi.org/10.2807/1560-7917.ES.2016.21.9.30155.
Zhang W, Zhang T, Wang C, Liang G, Lu Q, Wen G, et al. Prevalence of colistin resistance gene mcr-1 in Escherichia coli isolated from chickens in central China, 2014 to 2019. J Glob Antimicrob Resist. 2022;29:241–6. https://doi.org/10.1016/j.jgar.2022.03.024.
Chen L, Zhang J, Wang J, Butaye P, Kelly P, Li M, et al. Newly identified colistin resistance genes, mcr-4 and mcr-5, from upper and lower alimentary tract of pigs and poultry in China. PLoS One. 2018;13(3):e0193957. https://doi.org/10.1371/journal.pone.0193957.
Guo S, Tay MYF, Thu AK, Seow KLG, Zhong Y, Ng LC, et al. Conjugative IncX1 plasmid harboring colistin resistance gene mcr-5.1 in Escherichia coli isolated from chicken rice retailed in Singapore. Antimicrob Agents Chemother. 2019;63(11):e01043–19. https://doi.org/10.1128/AAC.01043-19.
Nesporova K, Jamborova I, Valcek A, Medvecky M, Literak I, Dolejska M. Various conjugative plasmids carrying the mcr-5 gene in Escherichia coli isolates from healthy chickens in Paraguay. J Antimicrob Chemother. 2019;74(11):3394–7.
Saidenberg ABS, Stegger M, Price LB, Johannesen TB, Aziz M, Cunha MPV, et al. mcr-positive Escherichia coli ST131-H22 from poultry in Brazil. Emerg Infect Dis. 2020;26(8):1951–4. https://doi.org/10.3201/eid2608.191724.
Cha MH, Woo GJ, Lee W, Kim SH, Woo JH, Kim J, et al. Emergence of transferable mcr-9 gene-carrying colistin-resistant Salmonella enterica Dessau ST14 isolated from retail chicken meat in Korea. Foodborne Pathog Dis. 2020;17(11):720–7. https://doi.org/10.1089/fpd.2020.2810.
Tyson GH, Li C, Hsu C-H, Ayers S, Borenstein S, Mukherjee S, et al. The mcr-9 gene of Salmonella and Escherichia coli is not associated with colistin resistance in the United States. Antimicrob Agents Chemother. 2020;64(8):e00573–e620.
Yang YQ, Li YX, Lei CW, Zhang AY, Wang HN. Novel plasmid-mediated colistin resistance gene mcr-7.1 in Klebsiella pneumoniae. J Antimicrob Chemother. 2018;73(7):1791–5. https://doi.org/10.1093/jac/dky111.
AbuOun M, Stubberfield EJ, Duggett NA, Kirchner M, Dormer L, Nunez-Garcia J, et al. mcr-1 and mcr-2 variant genes identified in Moraxella species isolated from pigs in Great Britain from 2014 to 2015. J Antimicrob Chemother. 2017;72(10):2745–9. https://doi.org/10.1093/jac/dkx286.
Ling Z, Yin W, Shen Z, Wang Y, Shen J, Walsh TR. Epidemiology of mobile colistin resistance genes mcr-1 to mcr-9. J Antimicrob Chemother. 2020;75(11):3087–95. https://doi.org/10.1093/jac/dkaa205.
Dandachi I, Fayad E, Sleiman A, Daoud Z, Rolain JM. Dissemination of multidrug-resistant and mcr-1 Gram-negative Bacilli in broilers, farm workers, and the surrounding environment in Lebanon. Microb Drug Resist. 2020;26(4):368–77. https://doi.org/10.1089/mdr.2019.0137.
Palupi MF, Wibawan IWT, Sudarnika E, Maheshwari H, Darusman HS. Prevalence of mcr-1 colistin resistance gene in Escherichia coli along broiler meat supply chain in Indonesia. Biotropia. 2019;26(2):272126.
Furtula V, Farrell EG, Diarrassouba F, Rempel H, Pritchard J, Diarra MS. Veterinary pharmaceuticals and antibiotic resistance of Escherichia coli isolates in poultry litter from commercial farms and controlled feeding trials. Poult Sci. 2010;89(1):180–8. https://doi.org/10.3382/ps.2009-00198.
Ahmed S, Das T, Islam MZ, Herrero-Fresno A, Biswas PK, Olsen JE. High prevalence of mcr-1-encoded colistin resistance in commensal Escherichia coli from broiler chicken in Bangladesh. Sci Rep. 2020;10(1):18637. https://doi.org/10.1038/s41598-020-75608-2.
Murase T, Ozaki H. Relationship between phylogenetic groups of Escherichia coli and pathogenicity among Isolates from chickens with Colibacillosis and healthy chickens. Poult Sci. 2022;101(9):102007. https://doi.org/10.1016/j.psj.2022.102007.
Coura FM, Diniz SA, Silva MX, Arcebismo TLM, Minharro S, Feitosa ACF, et al. Phylogenetic group of Escherichia coli isolates from broilers in Brazilian poultry slaughterhouse. Sci World J. 2017;2017:5898701. https://doi.org/10.1155/2017/5898701.
Jeong J, Lee JY, Kang MS, Lee HJ, Kang SI, Lee OM, et al. Comparative characteristics and zoonotic potential of Avian Pathogenic Escherichia coli (APEC) isolates from chicken and duck in South Korea. Microorganisms. 2021;9(5):946. https://doi.org/10.3390/microorganisms9050946.
Wang M, Jiang M, Wang Z, Chen R, Zhuge X, Dai J. Characterization of antimicrobial resistance in chicken-source phylogroup F Escherichia coli: similar populations and resistance spectrums between E. coli recovered from chicken colibacillosis tissues and retail raw meats in Eastern China. Poult Sci. 2021;100(9):101370. https://doi.org/10.1016/j.psj.2021.101370.
Zhuge X, Zhou Z, Jiang M, Wang Z, Sun Y, Tang F, et al. Chicken-source Escherichia coli within phylogroup F shares virulence genotypes and is closely related to extraintestinal pathogenic E. coli causing human infections. Transbound Emerg Dis. 2021;68(2):880–95.
Lu Q, Zhang W, Luo L, Wang H, Shao H, Zhang T, et al. Genetic diversity and multidrug resistance of phylogenic groups B2 and D in InPEC and ExPEC isolated from chickens in Central China. BMC Microbiol. 2022;22(1):60. https://doi.org/10.1186/s12866-022-02469-2.
Lemlem M, Aklilu E, Mohammed M, Kamaruzzaman F, Zakaria Z, Harun A, et al. Molecular detection and antimicrobial resistance profiles of Extended-Spectrum Beta-Lactamase (ESBL) producing Escherichia coli in broiler chicken farms in Malaysia. PLoS One. 2023;18(5):e0285743. https://doi.org/10.1371/journal.pone.0285743.
Aworh MK, Kwaga JKP, Hendriksen RS, Okolocha EC, Thakur S. Genetic relatedness of multidrug resistant Escherichia coli isolated from humans, chickens and poultry environments. Antimicrob Resist Infect Control. 2021;10(1):58. https://doi.org/10.1186/s13756-021-00930-x.
Giufre M, Graziani C, Accogli M, Luzzi I, Busani L, Cerquetti M, et al. Escherichia coli of human and avian origin: detection of clonal groups associated with fluoroquinolone and multidrug resistance in Italy. J Antimicrob Chemother. 2012;67(4):860–7. https://doi.org/10.1093/jac/dkr565.
Dissanayake DR, Octavia S, Lan R. Population structure and virulence content of avian pathogenic Escherichia coli isolated from outbreaks in Sri Lanka. Vet Microbiol. 2014;168(2–4):403–12. https://doi.org/10.1016/j.vetmic.2013.11.028.
Maluta RP, Logue CM, Casas MR, Meng T, Guastalli EA, Rojas TC, et al. Overlapped sequence types (STs) and serogroups of avian pathogenic (APEC) and human extra-intestinal pathogenic (ExPEC) Escherichia coli isolated in Brazil. PLoS One. 2014;9(8):e105016. https://doi.org/10.1371/journal.pone.0105016.
Zheng B, Lv T, Xu H, Yu X, Chen Y, Li J, et al. Discovery and characterisation of an escherichia coli ST206 strain producing NDM-5 and MCR-1 from a patient with acute diarrhoea in China. Int J Antimicrob Agents. 2018;51(2):273–5. https://doi.org/10.1016/j.ijantimicag.2017.09.005.
Ayeni FA, Falgenhauer J, Schmiedel J, Schwengers O, Chakraborty T, Falgenhauer L. Detection of blaCTX-M-27-encoding Escherichia coli ST206 in Nigerian poultry stocks. J Antimicrob Chemother. 2020;75(10):3070–2. https://doi.org/10.1093/jac/dkaa293.
Kemmett K, Humphrey T, Rushton S, Close A, Wigley P, Williams NJ. A longitudinal study simultaneously exploring the carriage of APEC virulence associated genes and the molecular epidemiology of faecal and systemic E. coli in commercial broiler chickens. PLoS One. 2013;8(6):e67749. https://doi.org/10.1371/journal.pone.0067749.
Manges AR, Geum HM, Guo A, Edens TJ, Fibke CD, Pitout JDD. Global extraintestinal pathogenic Escherichia coli (ExPEC) lineages. Clin Microbiol Rev. 2019;32(3):e00135–e218. https://doi.org/10.1128/CMR.00135-18.
Stromberg ZR, Johnson JR, Fairbrother JM, Kilbourne J, Van Goor A, Curtiss RR, et al. Evaluation of Escherichia coli isolates from healthy chickens to determine their potential risk to poultry and human health. PLoS One. 2017;12(7):e0180599. https://doi.org/10.1371/journal.pone.0180599.
Ewers C, Antao EM, Diehl I, Philipp HC, Wieler LH. Intestine and environment of the chicken as reservoirs for extraintestinal pathogenic Escherichia coli strains with zoonotic potential. Appl Environ Microbiol. 2009;75(1):184–92. https://doi.org/10.1128/AEM.01324-08.
Subedi M, Luitel H, Devkota B, Bhattarai RK, Phuyal S, Panthi P, et al. Antibiotic resistance pattern and virulence genes content in avian pathogenic Escherichia coli (APEC) from broiler chickens in Chitwan, Nepal. BMC Vet Res. 2018;14(1):113. https://doi.org/10.1186/s12917-018-1442-z.
Acknowledgements
The authors would like to thank the Faculty of Veterinary Medicine, Universiti Malaysia Kelantan for providing research facility to conduct the research. We would like to acknowledge Ministry of Higher Education Malaysia (MOHE) for funding us to the laboratory analysis and consumables of this research.
Funding
The Laboratory reagents and consumables of this research was funded by Ministry of Higher Education Malaysia (MOHE) under the fundamental research grant scheme (FRGS), grant no. FRGS/1/2019/WAB01/UMK/02/6.
Author information
Authors and Affiliations
Contributions
ML conducted the sample collection and laboratory analysis, wrote the main manuscript text, and prepared figures and tables. SMD assists in laboratory analysis and sample collection. EA supervised the laboratory and provided funding for the laboratory consumables. EA, MM, FK, ZZ, and Az supervise and edit the manuscript. INAK, MFHR, and MS review the manuscript. All authors have reviewed the manuscript. ML=Mulu Lemlem. SSD=Susmita Seenu Devan. EA=Erkihun Aklilu. MM=Maizan Mohamed. NFK=Nor Fadhilah Kamaruzzaman. AZ=Azian Harun. INAK=Intan Noor Aina Kamaruzaman. MFHR=Mohd Farhan Hanif Reduan. MS = Muthupandian Saravanan.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The Institutional Animal Care and Use Committee of Universiti Malaysia Kelantan approved this study (UMK/FPV/ACUE/PG/2/2019). The animal subjects (chicken from commercial poultry farms) were only used to collect cloacal swabs, and no invasive or harmful procedures were used to handle the birds. Informed consent was obtained from the farm owners for the use of their animals in the study prior to study commencement. All methods carried out in this manuscript are based on standard guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure1a.
Original gel electrophoresis image of Pho A genes. Figure 1b. Original gel electrophoresis image of Ecoli genes. Figure 4. Original gel electrophoresis image of amplified PCR product with mcr-1, mcr-4, and mcr-5 genes. Figure 5. Original gel electrophoresis image of amplified PCR product mcr-7 gene. Figure 6. Original multiplex PCR6-9, gel image of mcr-6, mcr-8, mcr-9 and ESBL genes. Figure 7. Original gel electrophoresis image of amplified PCR product with mcr-6 and mcr-8 genes. Figure 8. Agarose gel electrophoresis image of mcr positive E. coli phylogenetic typing. Amplified PCR products with E. coli phylogrouping genes; arpA (400bp), chuA (288bp), yjaA (211bp) and TspE4C2 (152bp); lane 1,+ - - -, belonging to phylogroup A; lane 2,+ - - +, belonging to group B1; lane 3,- + + -, group B2; lane 4,+ - + -, group C; lane 5,+ + - -, group D; lane 6,+ + + -, group E; lane 7,- + - - ,group F; lane 8,- - + -, clade I/II.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lemlem, M., Aklilu, E., Mohamed, M. et al. Phenotypic and genotypic characterization of colistin-resistant Escherichia Coli with mcr-4, mcr-5, mcr-6, and mcr-9 genes from broiler chicken and farm environment. BMC Microbiol 23, 392 (2023). https://doi.org/10.1186/s12866-023-03118-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-023-03118-y