Abstract
Background
Probiotics have recently been applied in aquaculture as eco-friendly alternatives to antibiotics to improve fish health, simultaneously with the increase of production parameters. The present study aimed to investigate the functional potential of lactic acid bacteria (LAB) isolated from the gut of Tilapia (Oreochromis niloticus) originating from the aquaculture farm of Oceanologic Research Center in Ivory Coast.
Results
Twelve LAB strains were identified by 16 S rDNA gene sequence homology analysis belonging to two genera Pediococcus (P. acidilactici and P. pentosaceus) and Lactobacillus (L. plantarum) with a predominance of P. acidilactici. Several aspects including functional, storage, and safety characteristics were taken into consideration in the selection process of the native LAB isolates as potential probiotics. All LAB isolates showed high antagonistic activity against bacterial pathogens like Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Proteus mirabilis, and Staphylococcus aureus. In addition, the LAB isolates exhibited different degrees of cell surface hydrophobicity in the presence of hexane, xylene, and chloroform as solvents and a good ability to form biofilm. The strong antioxidant activity expressed through the DPPH scavenging capacity of LAB intact cells and their cell-free supernatants was detected. LAB strains survived between 34.18% and 49.9% when exposed to low pH (1.5) and pepsin for 3 h. In presence of 0.3% bile salts, the growth rate ranged from 0.92 to 21.46%. Antibiotic susceptibility pattern of LAB isolates showed sensitivity or intermediate resistance to amoxicillin, cephalothin, chloramphenicol, imipenem, kanamycin, penicillin, rifampicin, streptomycin, tetracycline and resistance to oxacillin, gentamicin, and ciprofloxacin. No significant difference in antibiotic susceptibility pattern was observed between P. acidilactici and P. pentosaceus strains. The non-hemolytic activity was detected. Following the analysis of the enzyme profile, the ability of LAB isolates to produce either lipase or β-galactosidase or both enzymes was highlighted. Furthermore, the efficacy of cryoprotective agents was proved to be isolate-dependent, with LAB isolates having a high affinity for D-sorbitol and sucrose.
Conclusion
The explored LAB strains inhibited the growth of pathogens and survived after exposure to simulated gastrointestinal tract conditions. The safety and preservative properties are desirable attributes of these new probiotic strains hence recommended for future food and feed applications.
Similar content being viewed by others
Background
In West Africa, intensive and semi-intensive systems of aquaculture farming remain the most common among fish farmers [1, 2]. Bamba et al. [3]; Gabriel et al. [4] and Crentsil and Ukpong [5] reported a massive use of agro-industrial by-products of plant origin (wheat bran, corn bran, rice bran, low rice flour) at a lower cost as feed for fish farming on most fish farms in sub-Saharan Africa. However, these agro-industrial by-products have a low protein, nutritional and immune contribution [6]. Furthermore, an inadequate application of antibiotics to boost fish production could lead to adverse disorders such as an imbalance in the gut microbiota, poisoning, immunity reduction as well as predisposition to the development of diseases [7,8,9]. Moreover, using antibiotics could be a potential risk to the health of consumers since the vast majority of antibiotics used are the same used to treat human infections [9]. Currently, probiotics are intensively promoted as healthy alternatives for sustainable aquaculture [10,11,12,13,14].
According to the World Health Organization (WHO) and the Food and Agriculture Organization (2014), probiotics were defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” [15, 16]. Lactic acid bacteria (mainly Lactobacillus sp., Bifidobacterium sp. and Pediococcus sp.) [17,18,19,20], Bacillus sp. [20, 21] and a few yeasts (mainly Saccharomyces boulardii and S. cerevisiae) [22,23,24] are intensively studied as probiotics to improve aquatic life health and performance of fish. However, it was found that the probiotic bacteria isolated from other hosts used in aquaculture do not colonize efficiently the fish gut as the native (indigenous) probiotics [25,26,27]. In their study, Boutin et al. [26] reported that native probiotic strains are a better choice than exogenous probiotics which could cause the homeostatic disturbance of the fish microbiota. Recently, research has focused more on host-associated microorganisms as a source of probiotics, due to the fact that the health beneficial effects could be species-specific, as well as that they adapt much more easily to the aquatic environment (e.g. salinity, temperature) [28,29,30,31,32,33,34]. Microorganisms with potential use as probiotics have been isolated from the gastrointestinal tract of Atlantic cod (Gadus morhua) [31], common carp [32], giant freshwater prawn (Macrobrachium rosenbergii) [33], rainbow trout (Oncorhynchus mykiss) [34], Nile Tilapia (Oreochromis niloticus) [29, 30, 35], Mediterranean trout (Salmo macrostigma) [36]. The choice of inappropriate microbes could have been the cause of the negative results observed in probiotic research [27, 37,38,39]. Different functionality, safety, and storage criteria have been established to investigate the microbial strains with probiotic potential, thus allowing the screening of the most promising strains [27, 37,38,39]. Generally, the criteria for the selection of probiotics are highlighted as antibacterial activity, antibiotic susceptibility, simulation of gastrointestinal conditions [40,41,42,43,44,45,46] biofilm-forming ability [47,48,49,50], hemolytic activity [51], hydrophobicity [52], antioxidant activity [53, 54], and enzymes production [36, 55, 56].
Currently, many commercial probiotics which contain one or more live microorganisms are introduced in fish farming industries mainly to improve the growth performance and boost the health of fish [57,58,59,60]. According to the study of Nimrat and Vuthiphandchai [57], none of the 12 commercial probiotics used in marine shrimp culture in Thailand did offer correct informations about the composition or number of micro-organisms or qualitative extracellular enzymes described on the labels. Furthermore, none of the commercial probiotics could inhibit the growth of the shrimp pathogen V. harveyi [57].
Several studies have proven some beneficial effects linked to the administration of native probiotics on fish species, including high feed conversion efficiency, supply of nutrients and enzymatic input to digestion, increased growth performance and stimulation of the immune system [61,62,63,64,65,66,67,68,69,70,71,72]. overall suggesting that native probiotics could be relevant alternatives to antibiotics to control emerging fish diseases, increase stress resistance and improve water quality [61,62,63,64,65,66,67,68,69,70,71,72]. Fish production may therefore be improved by using indigenous probiotics for the sustainable development of African aquaculture [73].
Thus, the current study aimed to investigate the lactic acid bacterial (LAB) strains isolated from the gut of Tilapia (Oreochromis niloticus) as potential probiotics, by addressing their functional properties (antibacterial activity, biofilm-forming ability, simulation of gastrointestinal conditions, hydrophobicity, antioxidant, and enzymatic activities), safety (antibiotic sensibility and hemolytic activity) and storage (freeze-drying survival).
Results
Molecular identification of LAB isolates
The LAB strains included in the study (Table 1) were isolated from the intestine of Tilapia (Oreochromis niloticus) originating from the aquaculture farm of the Oceanologic Research Center in Ivory Coast. The full-length 16 S rDNA genes of all the LAB isolates were sequenced to identify them at the species level. A BLAST search of the 16 S rDNA gene sequences obtained was performed at NCBI and revealed high similarity values to many bacterial 16 S rDNA sequences deposited in the NCBI database. LAB strains identified belonged to two genera Pediococcus and Lactobacillus. The partial 16 S rDNA gene sequences of nine LAB strains (LB45, LB98, LB100, LB137, LB143, LB156, LB166, LB187, and LB194) were identified as P. acidilactici (showed 96.38 − 98.21% homology to the GenBank sequences). The other two LAB strains (LB82 and LB195) were identified as P. pentosaceus (97.43-99% homology to GenBank sequences). The LB96 strain had 97.66% homology with the known Lactobacillus plantarum sequences. The partial 16 S rDNA sequences of LAB strains were deposited in the NCBI database (the accession numbers are listed in Table 1).
The phylogenetic tree revealed the existence of several groups of LAB species (Fig. 1). Thus, the L. plantarum LB96 (ON141905) was related to Lactobacillus plantarum TMPC 3M613 strain (OM757925), supporting a bootstrap value of 88%. P. pentosaceus LB82 (ON141904) and LB195 (ON141903) were related to P. pentosaceus FB 145 strain (MF945626) with a bootstrap score of 40%. P. acidilactici strains (LB45, LB98, LB100, LB137, LB143, LB156, LB166, LB187, LB194) were related to different P. acidilactici strains.
Functional properties of LAB isolates
Antibacterial activity of the cell-free supernatants (CFS) from the LAB strains
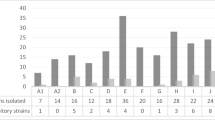
Antibacterial activity against pathogenic bacteria was considered an important criterion for the selection of probiotics. In this research, the antibacterial activity of cell-free supernatants (CFS) from the LAB isolates was assessed against five pathogens including E. coli, K. pneumoniae, P. mirabilis, P. aeruginosa, and S. aureus using agar well diffusion method. The value of the inhibition zone diameter expressed in mm is summarized in Table 2. The LAB isolates showed statistically significant (p < 0.05) inhibition rates regardless of the pathogen. Our results showed that the CFSs obtained from all LAB strains exhibited good antibacterial activity against tested pathogenic bacteria. Moreover, CFS from the LB143 strain seems to exert the highest inhibition effect against S. aureus, P. mirabilis, and K. pneumoniae. A similar inhibitory activity against P. aeruginosa and E. coli was observed for CFS from LB195.
Ability to form a biofilm
All LAB isolates had a high capacity to form a biofilm. The absorbance values were higher than 0.5, ranging from 0.928 ± 00 (LB96) to 3.211 ± 0.01 (LB143). The statistical analyses showed a significant difference (p < 0.05) between the isolates (Table 3).
Hydrophobicity
The hydrophobicity test was carried out in the presence of hexane, xylene, and chloroform as solvents. The results revealed that hydrophobicity rates were 1.53 ± 0.1 and 16.30 ± 0.4% in the presence of hexane for isolates LB195 and LB156, respectively. In the presence of xylene as the solvent, isolate LB137 manifested the highest hydrophobicity (51.10 ± 0.8%), while the lowest was observed at isolate LB82 (1.17 ± 0.8%). The use of chloroform as solvent showed hydrophobicity values that ranged from 9.4 ± 0.14% (LB96) to 87.2 ± 0.14% (LB166). Furthermore, statistical analyses showed a significant difference (p < 0.05) between isolates for the same solvent and between different solvents (Table 3).
Antioxidant activity
In general, antioxidant activity levels were statistically significant (p < 0.05) for both supernatants and LAB intact cells. Overall, the antioxidant activity levels observed in the supernatants were higher than those in the intact cells (Table 3). However, for isolates LB96, LB137, and LB195 antioxidant activity values were highest in intact cells than in the supernatant with 87.28 ± 0.40, 70.40 ± 0.57%, and 57.19 ± 0.27%, respectively (Table 3). For each sample (supernatant and intact cells), statistical analyses showed a significant (p < 0.05) difference between the isolates.
Tolerance to bile salts and resistance to pepsin and acid pH of LAB isolates
A critical step toward the selection of probiotic strains was to survive conditions that mimic the gastrointestinal tract. The LAB isolates bile salts tolerance, as well as the resistance in the presence of pepsin and acid pH evolution tests are shown in Fig. 2. Generally, an adaptation of all LAB isolates was observed after 4 h of exposure to bile salts characterized by cell growth, while exposition after 3 h to 0.3% pepsin and acid pH (1.5) was marked with a decrease in bacterial load. Based on growth rates, three profiles were observed. The most significant growth (p < 0.05) was observed for isolate LB 194, with a growth rate of 27.33%, followed by isolates LB45, LB82, LB98, LB100, and LB166 (growth rate between 15.36 and 16.88%). The least growth rates were observed in isolates LB 143 and LB 195 (0.92 and 1.06%). The growth rates showed a significant difference (p < 0.05) between the three profiles.
Despite the overall decrease in bacterial load, the level of resistance to pepsin and acid pH (1.5) was reflected by a survival rate ranging from 34.18 to 49.9%. The highest survival rate was obtained for the LB166 isolate which was significantly different (p < 0.05), while the lowest rate was observed in the LB96 isolate.
Each value represents the mean value ± standard deviation (SD) (n = 3). Bars with different lower-case letters denoted significantly different (p < 0.05).
In vitro investigation of enzymatic activities of LAB isolates.
To detect amylase, protease, lipase, and β-galactosidase activities the LAB isolates were inoculated into selective media for each enzyme. Our results revealed that the nine LAB strains tested were positive for lipases (pink-orange colony under UV 352 nm) and β-galactosidase (green colony) (Table 4). LB 143 did not exhibit lipase activity, while LB 137 and LB 156 did not exhibit β-galactosidase activity. No activity was detected for the amylases, cellulases, and proteases, respectively (Table 4).
Safety properties of LAB isolates
Antibiotic susceptibility
The antibiotic susceptibility of the LAB isolates was tested using the antibiotic disc diffusion method on MRS agar plates. A total of 12 antibiotics were included in the assay: gentamicin, chloramphenicol, kanamycin, streptomycin, tetracycline as inhibitors of protein synthesis, amoxicillin, cephalothin, oxacillin, penicillin, imipenem as inhibitors of cell wall synthesis, ciprofloxacin as inhibitors of DNA replication and rifampicin as inhibitors of nucleic acids synthesis. All LAB isolates showed variations in antibiotic susceptibility to 9 out of the 12 antibiotics and also showed multidrug resistance for oxacillin, gentamicin, and ciprofloxacin (Table 5). No significant difference in antibiotic susceptibility profile was observed between P. acidilactici and P. pentosaceus strains.
Hemolytic activity
Probiotic strains must be risk-free (γ-hemolysis), which makes them safe for consumption [15, 37]. In our study, all LAB strains showed γ-hemolysis activity (without clearing zones around the colonies on blood agar plates) (Fig. 3), thus ensuring the safety to be used as potential probiotics.
Storage and preservation of LAB isolates: freeze-drying procedures
Preservation of the viability of LAB strains during freeze-drying is a critical challenge. Three cryoprotectants (D-sorbitol, D-glucose, and sucrose) were tested for their ability to protect the LAB cells during freeze drying, while sterile deionized water served as the negative control. The survival rate of freeze-dried LAB isolates is presented in Fig. 4. A strain-dependent relationship existed between the cryoprotectant’s effectiveness. The isolates LB143 and LB98 had over 62% survival rate with all tested cryoprotectants, with maximum of 83.77 ± 0.44% and 87.97 ± 5.13% when sucrose was used as a cryoprotectant. On the contrary, the LB187 isolate had less than 10% survival rate with all cryoprotectants. LB143, LB96, LB137, and LB195 isolates demonstrated a high survival rate, from 53.50 ± 0.33% to 74.52 ± 9.64 with D-sorbitol (2%), whereas in the case of the LB82 and LB143 strains, glucose 2% (w/v) led to survival of 56.60 ± 0.18%, and 66.44 ± 0.14% respectively. Statistical analyses revealed a significant difference (p < 0.05) in the survival rates of different cryoprotectants between isolates. The boxplot presented in Fig. 5 shows that the D-sorbitol (41.48%) offered better protection of the LAB isolates during freeze-drying compared with sucrose (34.17%) and glucose (21.78%).
Hierarchical ascending classification (HAC)
The HAC carried out showed that all the LAB isolates had the same characteristics (Fig. 6). Three groups were generated, the first group included isolates LB 82, LB166, and LB 195. The second group included isolates LB194; LB187; LB156 and LB100. The last group consisted of isolates LB143; LB137; LB98; LB96, and LB45.
Discussion
Today, aquaculture in the Ivory Coast has not yet reached a viable economic dimension, despite immense physical, hydrological (150 000 ha of lagoons, 350,000 ha of lakes, numerous shallows, etc.), climatic, and human resources [1]. The development of Ivorian aquaculture is hampered by several factors, the most significant of which are the availability of high-quality feed at exorbitant prices, a lack of technical skills, and the poor quality and quantity of fish [1]. Thus, the use of functional food (food enriched with probiotics) seems to be an ecological, economical, and sustainable solution.
Several aspects, including functional characteristics (antibacterial activity, biofilm-forming ability, simulation of gastrointestinal conditions, hydrophobicity, antioxidant activity), safety characteristics (molecular identification, antibiotic sensibility, and hemolytic activity), and storage (freeze-drying survival), have been taken into consideration in the selection process of LAB isolates as potential native probiotics.
Careful selection remains the main tool to obtain high quality probiotics. Proper strain identification at the species level is one key criterion to classify a microbial isolate as probiotics, especially for the microorganisms to be used in the food chain [74,75,76]. In this context, the well-known amplification and sequencing of the 16 S ribosomal DNA region are reliable tools to identify species at the expense of classical methods [74, 75]. In the present study, 12 LAB strains isolated from the intestine of Tilapia (Oreochromis niloticus) were identified by 16 S rDNA gene sequence homology analysis and belonged to P. acidilactici (9 strains), P. pentosaceus (2 strains), and L. plantarum (1 strain). The list of probiotics includes mainly members of the genera Lactobacillus and Bifidobacterium [35, 61, 71], but species belonging to the genus Pediococcus, particularly P. acidilactici and P. pentosaceus, have been investigated many times, indicating that newly isolated strains may play a key role in the new generation of functional ingredients [19, 77,78,79,80].
Some bacterial pathogens, such as Escherichia sp., Klebsiella sp., Staphylococcus sp., Proteus sp., and Pseudomonas sp., were isolated from fish and can indicate multiple sources of contamination [81,82,83]. All LAB isolates showed strong growth inhibition of all reference pathogens: P. aeruginosa, E. coli, S. aureus, P. mirabilis, and K. pneumoniae. These results are in agreement with previous studies where Lactobacillus and Pediococcus strains exhibited a broad spectrum of antagonistic activity against fish pathogens [19, 77, 84, 85].
In terms of probiotics, biofilm formation offered a more rapid capacity for metabolite production and resistance to hostile environments [47,48,49,50]. In our study, all the isolates showed a good ability to form biofilm. Lamari et al. [86] selected several LAB strains with the ability to form biofilm and high adherence to polystyrene microplates and hydrocarbon. This characteristic was important for bacteria’s ability to adhere to an abiotic surface, which could be a potential indicator for LAB to colonize the gut and further antagonize the pathogens [86].
Cell surface hydrophobicity is another property considered important for the probiotics’ overall adhesion capacity to various types of surfaces. The hydrophobicity of LAB isolates selected in the presence of three different solvents revealed that values depended on the solvent used. Overall, the hydrophobicity values of LAB isolates with chloroform were higher than those with other solvents. The values vary between 1.53% (LB 195) and 16.30% (LB 166) for hexane, 3.48% (LB 98) and 51.10% (LB 137) for xylene, and 9.4% (LB 96) and 87.2% (LB 166) for chloroform. Yasmin et al. [51] reported high hydrophobicity values for Bifidobacterium strains for xylene; in this work, the highest hydrophobicity values were obtained in the case of chloroform. The results are confirmed in another study on L. fermentum URLP18 and L. lactis URLA2 strains, which showed high aggregation capacities and high affinity towards xylene, followed by chloroform [84].
Free radical-scavenging ability, as a criterion for probiotics, had been studied by several authors [53, 54]. These authors reported that extracellular liquid, intracellular liquid, and intact cells had free radical-scavenging properties. In our study, CFS and intact cells were investigated. Remarkably, the CFS of the nine LAB strains exhibited higher DPPH scavenging activities than the intact cells, as follows: LB45 (90.23%); LB82 (51.92%); LB98 (74.29%); LB100 (84.06%); LB143 (75.57%); LB156 (64.52%); LB166 (63.85%); LB187 (57.84%); and LB194 (76.60%). Yasmin et al. [51] reported that the Bifidobacterium exhibited strong antioxidant activity in cell-free supernatant, whose values varied between 80.72% and 87.72%.
Several studies reported the ability of probiotic microorganisms to produce extracellular enzymes improving the nutrient digestibility, growth performances, and health status of fish [13, 36, 55, 56]. For instance, Iorizzo et al. [36] showed that the LABs isolated from the intestinal tract of the Mediterranean trout (Salmo macrostigma) are producers of extracellular enzymes that help absorb the nutrients in the fish intestine. Our results showed the ability of 9 out of 12 LAB isolates to synthesize both β-galactosidase and lipases. No other enzymatic activity (amylases, cellulases, and proteases) was detected. This finding was in contrast with the results reported by Muñoz-Atienza et al. [42], in which the majority of the LAB strains did not shown lipolytic activity (with few exceptions). On the other hand, the LAB strains isolated from the intestine of freshwater fish species exhibited amylase, lipase, and protease activities [84]. Similarly, Marchwiska and Gwiazdowska [87] reported that different Lactobacillus and Pediococcus isolated from swine faeces for feed additive composition had protease and amylase activities, but no lipase activity. Bacillus species have already been known as enzyme producers, being one of the reasons for their use in aquaculture as probiotics enhancing feed digestibility, digestive enzyme activities, and growth performance [21].
Furthermore, antibiotic susceptibility is a prerequisite from a safety standpoint because probiotic bacteria might transfer antibiotic-resistance genes either directly or indirectly to pathogenic bacteria. This approach requires evidence that the LAB strain does not show resistance to antibiotics used in human and veterinary medicine. In our study, all LAB isolates were susceptible or intermediately resistant to 9 antibiotics and resistant to 3 antibiotics out of the 12 antibiotics used. LAB isolates showed resistance to oxacillin, gentamycin, and ciprofloxacin. Our result was important because LAB isolates showed sensitivity to penicillin, ampicillin, and chloramphenicol, among the most commonly used antibiotics in aquaculture. The results obtained were not consistent with those documented by Diguță et al. [78], which indicated that two Pediococcus strains (L3 and L5) isolated from the Kombucha consortium were found to be resistant to amoxicillin, streptomycin, kanamycin, and tetracycline. Furthermore, antibiotic sensitivity is a variable and strain-dependent property. In the context of probiotic LAB selection, drug-resistant probiotic bacteria are serious health threats. Increasing and abusive use of antibiotics has given rise to resistant bacteria through the transfer of resistance plasmids between bacteria [7,8,9, 88,89,90]. The resistance to an antibiotic may be accompanied by resistance to one or more other antibiotics, even if the bacteria have no contact with these antibiotics. Bacterial strains with transferable antibiotic resistance genes should not be used in animal feeds, fermentation, or probiotic foods for human consumption, according to the European Food Safety Authority (EFSA) [91]. Given the increase of drug-resistant probiotics has been recently developed the online ProbResist database, which centralizes reports of probiotic bacteria that have been demonstrated experimentally to be resistant to antibiotics [92].
The examination of hemolytic activity is strongly recommended by the EFSA as long as the isolated bacteria are intended for use in food products, even if they have “generally recognized as safe” (GRAS) or “quality presumption of safety” (QPS) status. In this study, all the isolates exhibited no hemolytic activity (γ-hemolysis), indicating that they are non-pathogenic and considered safe for animal or human probiotic applications. Similar results were previously obtained with two Pediococcus strains isolated from Kombucha (L3 and L5) [78]. Yasmin et al. [51] shown that eight Bifidobacterium strains isolated from raw camel milk did not exhibit hemolytic activity. Lack of hemolytic activity is significant during the selection of probiotic strains when it comes to probiotic safety because such strains are non-virulent, and the lack of hemolysin assures that virulence will not arise among bacterial strains [15].
The ability of LAB strains to effectively function in the gastrointestinal tract (including bile salts tolerance and low gastric pH resistance) is the most important criterion for their selection as probiotics. Probiotic bacteria must first make it through the stomach, where the pH can be as low as 1.5 to 2 before reaching the intestinal tract [93, 94]. In our study, the resistance tests of these bacteria at low pH levels (ranging from 1 to 3) revealed that all strains are resistant at pH 1.5 for 3 h, while most strains lose viability in 1 h at pH 1.5. The resistance at 0.3% pepsin and low pH (1.5) was characterized by a high level of cell viability. The survival rates of the 12 LAB isolates ranged from 34.8 to 49.9%. Chemlal-Kerrhaz et al. [39] showed that two isolates of LAB from the Nile Tilapia (Oreochromis niloticus) tolerated a concentration of 0.3% bile salts for 4 h and pH 2 for 3 h.
In addition, all strains tolerated the concentration of 0.3% bile salts for 4 h. The growth rate of the 12 LAB isolates varied between 0.92 and 21.46%. The results obtained were lower than in previous studies which reported high survival abilities of different Pediococcus strains [78] and L. fermentum URLP18 isolated from C. mrigala [84] in the presence of high bile salts concentration (until 2%). Our study showed a similar outcome; all isolates tolerated a concentration of 0.3% bile salts for 4 h and exhibited resistance at pH 1.5, however with different intensities.
Freeze-drying, as a LAB conditioning technique, is recognized to cause severe damage to organisms, particularly at the membrane level as well as to their proteins, but the addition of cryoprotective agents may mitigate injury or inactivation by increasing cell survival during freeze-drying [78, 95]. In this study, three cryoprotective agents were assessed for their influence on the LAB isolates’ viability rate at the end of the freeze-drying process. Our results indicate that the LAB isolates showed an important affinity for D-sorbitol and sucrose. This trend has been observed by Diguță et al. [78], where sucrose was responsible for the best viability rate of P. acidilactici and P. pentosaceus at the end of freeze-drying. Considering the strain-dependent variation in response to the stress conditions during the freeze-drying process, cryoprotectant agents must be investigated to choose them for conditioning of LAB strains with high efficiency on cell viability and economically feasible, before being included in functional foods or feeds.
Conclusion
Based on 16 S rDNA gene sequencing, 12 LAB strains isolated from the intestine of Tilapia (Oreochromis niloticus) were identified as belonging to P. acidilactici, P. pentosaceus, and L. plantarum species with a predominance of P. acidilactici. All LAB isolates showed a high antibacterial activity as well as a strong antioxidant activity. Additionally, they showed no hemolytic activity, a typical pattern of antibiotic susceptibility, and a good ability to form a biofilm, respectively. All LAB isolates exhibited either lipase or β-galactosidase or both enzymes production. Some LAB strains showed good survival rates in the simulated gastrointestinal conditions. Conditioning of LAB strains by freeze-drying using D-sorbitol or sucrose as cryoprotectant agents could be used to formulate probiotic products in powdered form. According to these results, P. acidilactici LB137 and P. pentosaceus LB195 present promising probiotic properties and could be applied as health promoters for fish. Further in vivo studies might use these strains in monoculture or co-culture to obtain enriched/supplemented food for fish farming, ultimately ensuring that healthier fish will be part of a healthier human diet.
Materials and methods
Bacterial strains and culture conditions
Twelve LAB strains were isolated from the intestine of Tilapia (Oreochromis niloticus) originating from the aquaculture farm of Oceanologic Research Center in Ivory Coast. Five pathogenic bacteria including P. mirabilis JCM1669 (University Nangui Abrogoua of Ivory Coast), P. aeruginosa ATCC 27853, E. coli ATCC 25922, K. pneumoniae ATCC 43816, and S. aureus ATCC 25913 (American Type Culture Collection (ATCC), Manassas, VA, USA) were used as indicator strains. LAB strains were routinely grown in MRS (De Man, Rogosa, and Sharpe) broth or agar (Oxoid Limited, Hampshire, United Kingdom) for 24–48 h at 37 °C under microaerophilic conditions (5% CO2). The reference pathogenic bacteria were grown in tryptic soy broth (TSB) or agar (TSA) (Scharlab S.L., Barcelona, Spain) for 18–24 h at 37 °C under aerobic conditions. All strains were stored at − 20 °C in an adequate culture medium containing 30% (v/v) glycerol (Scharlab S.L., Barcelona, Spain) and subcultured twice before being used in assays.
Identification of LAB strains
LAB strains were grown in MRS broth for 48 h at 37 °C. Cells were harvested by centrifugation at 5000 x g for 10 min. Genomic DNA extraction was performed using a ZR Fungal/Bacterial DNA kit (Zymo Research, Irvine, CA, USA), according to the manufacturer’s instructions. The DNA concentration and purity were verified with a SpectraMax® QuickDrop™ (Molecular Devices, San Jose, CA, USA). LAB strains were identified by analysis of 16 S rDNA amplified with the universal primers 27 F (AGAGTTTGATCMTGGCTCAG) and 1492R (TACGGYTACCTTGTTACGACTT) (Biolegio B.V. Nijmegen, The Netherlands). The reaction mixture consisted of 50 µl of 10X DreamTaq Green Buffer (contains 20 mM MgCl2), 0.5 µM of each primer, 0.2 mM dNTPs, 0.025 U of DreamTaq DNA Polymerase (Thermo Fisher Scientific, Baltics, UAB, Vilnius, Lithuania), and 10 ng of bacterial DNA. The amplification program cycles started with an initial denaturation at 95 °C for 3 min, followed by 35 cycles (94 °C for 1 min, 55 °C for 1 min, 72 °C for 1 min), and a final extension step at 72 °C for 7 min. The amplification reactions of the 16 S rDNA region were performed using a thermal cycler (MultiGene Thermal Cycler Labnet International, Inc., Edison, NJ, USA). The PCR products were detected by agarose gel electrophoresis (2% (w/v) agarose, 90 V, 60 min), and visualized using a GelDoc-It Imaging System (Analytik Jena, Upland, CA, USA). Sequencing was performed in both directions with the universal primers 27 F and 1492R by Cellular and Molecular Immunological Application, Holland (CEMIA, Greece). The partially obtained nucleotide sequences were aligned with multiple available homologous sequences in the National Center for Biotechnology Information (NCBI) GenBank databases (http://www.ncbi.nlm.nih.gov, accessed on 29 November 2021) to identify at the species level based on high similarity. The phylogenetic tree was constructed via the neighbour-joining method [96] using MEGA (Molecular Evolution Genetic Analysis) software, version X [97].
Functional characterization LAB isolates
Antibacterial activity of LAB isolates
Antagonistic activities of the LAB isolates were recorded against five pathogens indicators (E. coli ATCC 25922, K. pneumoniae ATCC 43816, P. aeruginosa ATCC 27853, P. mirabilis JCM1669, and S. aureus ATCC 25913) by the agar well diffusion method described by Balouiri et al. [98] with some modifications. The LAB isolates were cultured in MRS broth at 37 °C for 48 h. and centrifuged at 10,000 x g at 4 °C, for 5 min. Cell-free supernatants (CFS) were obtained by filtration using sterile 0.22 μm Millipore filters (VWR International, Rosny-sous-Bois, France). 1mL of the overnight pathogen culture (adjusted OD600 nm to 0.2 ± 0.05, representing approximately 107− 108 cfu/ml) was added to a sterile Petri dish (90 mm), overlaid with approximately 20 mL of TSA cooled to 45 °C, and gently homogenized until solidification. Wells with a diameter of 6 mm have been punched aseptically with a sterile tip, filled with 100 µl of CFS tested, and incubated at 37 °C for 24 h. A clear zone of 1 mm or more around each well was considered positive inhibition, which demonstrated the antibacterial activity of the CFS.
Ability to form a biofilm
The LAB isolates were grown in MRS broth at 37 °C for 48 h and bacterial load was adjusted to the same optical density OD600 nm of 0.2 ± 0.05. Bacterial cells were centrifuged at 4000 rpm for 10 min and the pellets were washed three times with NaCl solution (0.9%) and dried at 50 °C for 30 min. The bacterial biofilms were stained with 1 ml of 0.1% crystal violet (Sigma Aldrich, Saint Louis, MO, USA) for 20 min and washed with the NaCl solution until the liquid was clear. The dye was eluted with ethanol (96%). The quantification was performed by measuring absorbance value (OD) at the 595 nm wavelength spectrophotometer (BioBase, Jinan, Shandong, China). The ability to form a biofilm was considered positive for OD ≥ 0.5.
Tolerance of LAB isolates to bile salts
The bile salts tolerance test of the LAB isolates was performed according to the method described by Diguță et al. [78] with some modifications. Test tubes containing MRS broth were supplemented with 0.3% bile salts (Oxoid Limited, Hampshire, United Kingdom), inoculated with each LAB isolate (adjusted to the OD600 nm at 0.2 ± 0.05), and incubated at 37 °C, for 4 h. The cell viability was tested by the plate count method at 0 h and after 4 h of incubation. Tolerance toward bile salts was estimated by\(\text{g}\text{r}\text{o}\text{w}\text{t}\text{h} \text{r}\text{a}\text{t}\text{e}=\left(\frac{\text{l}\text{o}\text{g} \text{C}\text{F}\text{U} \text{N}\text{i} }{\text{l}\text{o}\text{g} \text{C}\text{F}\text{U} \text{N}\text{t}}\right)\times 100\), where Ni and Nt mean the viable cells (CFU/ml) at 0 h and after 4 h of incubation.
Resistance of LAB isolates to pepsin and acid pH
The ability of LAB isolates to survive the presence of pepsin and acid pH was done using the method described by Diguță et al. [78]. After overnight culture, LAB cells were centrifuged at 2000 x g for 10 min and pellets were suspended and washed twice with sterile physiological saline (0.9% NaCl). The pellets were suspended in phosphate-buffered saline (PBS) solution (VWR International, Rosny-sous-Bois, France) previously supplemented with 0.3% pepsin (Sigma-Aldrich, Saint Louis, MO, USA) and pH was adjusted to 1.5 with 1 N HCl. The cell viability was tested by the plate count method at 0 h and after 3 h of incubation. The percentage (%) survival of LAB isolates was calculated by the following formula: \(\% \text{v}\text{i}\text{a}\text{b}\text{i}\text{l}\text{i}\text{t}\text{y}=\left(\frac{\text{l}\text{o}\text{g} \text{U}\text{F}\text{C} \text{N}\text{t} }{\text{l}\text{o}\text{g} \text{U}\text{F}\text{C} \text{N}\text{i}}\right)\times 100\), where Ni and Nt mean the viable cells (CFU/ml) at 0 h and after 3 h of incubation.
Evaluation of LAB isolates hydrophobicity
Overnight LAB cultures were centrifuged at 12000 x g for 5 min at 4 °C. The cell pellets were washed twice using PBS solution (pH 7.2) and adjusted to the optical density of 1 ± 0.05 at 650 nm wavelength (H0). To determine the cell surface hydrophobicity, three solvents were used: hexane (VWR International, Rosny-sous-Bois, France), xylene (Bernd Kraft GmbH, Duisburg, Germany), and chloroform (Bernd Kraft GmbH, Duisburg, Germany). The mixture of 2.4 ml of cell suspension with 0.4 mL of solvent was vigorously vortexed for 2 min. After the phase stabilization and separation period of 30 min at room temperature, the aqueous phase was carefully recovered and the optical density was measured at 650 nm wavelength (H1). The hydrophobicity values were calculated according to the following formula: \(H \%=\left(\frac{\text{H}0 - \text{H}1}{\text{H}0}\right)\times 100\).
DPPH Free Radical Scavenging ability
After the LAB cells were incubated at 37 °C in MRS broth overnight, the cells were harvested by centrifugation at 12000 x g for 5 min at 4 °C. The supernatant samples were collected and cell pellets were washed twice with PBS solution and suspended in the same solution to adjust to OD600 nm 0.2 ± 0.05 and served as intact cells. The 1-diphenyl-2-picrylhydrazyl (DPPH) scavenging activity was determined by the method described by Brand-Williams et al. [99]. A volume of 2 ml of DPPH (Alfa Aesar, Kandel, Germany) (100 µM in methanol) was added to 1 ml of the cell suspension or 1 ml of the supernatant, the mixtures were mixed vigorously and incubated at laboratory temperature in the dark for 30 min. In the case of the intact cell, the absorbance of the resulting solution was measured in triplicate at 517 nm wavelength after centrifugation at 12,000 x g, for 5 min. The deionized water was used as the negative control. The presence of antioxidant activity is shown by the change in color of the mixture from purple to yellow. The scavenging ability was defined as: \(\% \text{A}\text{A}=\left(\frac{\text{O}\text{D} \text{D}\text{P}\text{P}\text{H}-\text{O}\text{D} \text{s}\text{a}\text{m}\text{p}\text{l}\text{e}}{\text{O}\text{D}\text{D}\text{P}\text{P}\text{H}}\right)\times 100\).
Plate screening of enzymes producing LAB isolates
LAB isolates were inoculated in spots on the surface of culture media distributed in Petri dishes. Amylase activity was evaluated on MRS Agar medium supplemented with 1% of soluble starch (VWR International, Rosny-sous-Bois, France). After incubation at 37 °C for 4 days, the positive reaction was indicated by a clear zone surrounding LAB isolates by adding Iodine-potassium iodide solution (Carl Roth GmbH & Co. KG, Karlsruhe, Germany). Cellulase activity was tested on MRS Agar supplemented with 1% carboxymethylcellulose (Sigma-Aldrich, Merck, Darmstadt, Germany). The zone of clearance was visualized after staining with 0.1% Congo red solution (Sigma-Aldrich, Merck, Darmstadt, Germany) and washing the plate with 1 M NaCl. Lipase activity was determined on MRS Agar medium supplemented with 0.25 mL olive oil, 0.01% CaCl2xH2O, and 0.0001% (w/v) rhodamine B (Alfa Aesar, Kandel, Germany). Positive reactions were observed by pink-orange colony under UV 350 nm. Protease activity was detected on skim milk (1%) agar medium (PanReac AppliChem, Darmstadt, Germany). After the incubation period, the LAB isolates showing a clear zone of the degradation of casein were read as positive for protease production. The β-galactosidase activity was determined on MRS agar containing 20 µl of X-Gal (20 mg/ml in DMSO) (PanReac AppliChem, Darmstadt, Germany). The green color colonies were regarded as bacteria producing β-galactosidase enzyme.
Safety characterization of the LAB isolates
Antibiotic susceptibility
The LAB strains were tested for antibiotic susceptibilities by the disc diffusion method described by CLSI [100]. Twelve (12) antibiotics belonging to 8 classes of antibiotics were used, namely Beta-lactams (Penicillin: PEN 6 µg; Amoxicillin: AML 10 µg; Oxacillin: OX 5 µg), Cephalosporins (Cephalothin: CN 30 µg), Aminoglycosides (Gentamicin: GM 10 µg; Kanamycin: KAN 1 mg; Streptomycin: STR 500 µg), Quinolones (Ciprofloxacin: CIP 5 µg), Cyclines (Tetracycline: TE 30 µg), Rifampicin (Rifampicin: RAM 30 µg), Carbapenems (Imipenem: IPM 10 µg), Phenicols (Chloramphenicol: C 30 µg). All antibiotics were provided by Oxoid Limited (Hampshire, United Kingdom). A volume of 100 µl of LAB fresh cultures (adjusted to the OD600 nm at 0.2 ± 0.05) was inoculated into MRS agar plates and dried. Antibiotic discs were placed on the MRS plates agar and incubated at 37 °C, for 48 h. The diameter of the zone of inhibition was measured and classified as sensitive (S); intermediate resistant (IR); or resistant (R) in agreement with the Clinical and Laboratory Standards Institute CLSI [100].
Hemolytic activity of LABs
An aliquot of each LAB culture (5µL) was applied onto Columbia Agar plates containing 5% (w/v) sheep blood (Oxoid Limited, Hampshire, United Kingdom) and cultured at 37 °C, for 48 h. The hemolytic activity was assessed by β-hemolysis (the clear halo around colonies), α-hemolysis (the green halo around colonies), or γ-hemolysis (no halo around colonies). Here, S. aureus ATCC 25913 (β-hemolytic) was used as a positive control strain.
Storage and preservation of LAB isolates: freeze-drying procedures
The LAB cultures left in MRS broth overnight were centrifuged at 4000 x g for 10 min at 4 °C. The pellets were washed twice using saline solution (0.9% NaCl) and suspended in 2 ml of cryoprotectant solutions. Three cryoprotectants (at a final concentration of 2%) were tested: D-sorbitol (Sigma-Aldrich, Saint Louis, MO, USA), D-glucose, and sucrose (PanReac AppliChem, Darmstadt, Germany). After freezing at -20 °C overnight, cell suspensions (prepared as described above) were freeze-dried in a chamber-type freeze-dryer (FreeZone6, LABCONCO, 6 L Benchtop Freeze Dry System, Kansas, MO, USA) at − 55 °C and 0.3 mbar, for 4 h. The cell viability was tested before and after the freeze-drying procedure by the plate count method. Distilled water was used as a control. The survival rate of LAB strains was calculated as:
\(\text{\%} \text{v}\text{i}\text{a}\text{b}\text{i}\text{l}\text{i}\text{t}\text{y}=\left(\frac{\text{l}\text{o}\text{g} \text{C}\text{F}\text{U} \text{N} }{\text{l}\text{o}\text{g} \text{C}\text{F}\text{U} \text{N}0}\right)\times 100\), where N0 and N mean the viable cells (CFU/ml) before and after freeze drying, respectively.
Statistical analysis
All the experiments were carried out in triplicate and the results were expressed as the mean ± standard deviation. The calculations, figures, and boxplots were performed using Excel 2016. For the comparison of the means of the studied parameters, one-way analysis of variance (ANOVA) and Tukey’s test were performed with the XLStat software (Version 2016). For p < 0.05, the means were considered significant. XLSTAT software was used to create a dendrogram to group LAB species with similar characteristics.
Data Availability
All the datasets generated in the present study are included in the manuscript. The partial 16 S rDNA sequences of LAB strains were deposited in the NCBI GenBank. Accession numbers:
https://www.ncbi.nlm.nih.gov/nuccore/ON141894.1/.
https://www.ncbi.nlm.nih.gov/nuccore/ON141895.1/.
https://www.ncbi.nlm.nih.gov/nuccore/ON141896.1/.
https://www.ncbi.nlm.nih.gov/nuccore/ON141897.1/.
https://www.ncbi.nlm.nih.gov/nuccore/ON141898.1/.
https://www.ncbi.nlm.nih.gov/nuccore/ON141899.1/.
https://www.ncbi.nlm.nih.gov/nuccore/ON141900.1/.
https://www.ncbi.nlm.nih.gov/nuccore/ON141901.1/.
https://www.ncbi.nlm.nih.gov/nuccore/ON141902.1/.
https://www.ncbi.nlm.nih.gov/nuccore/ON141903.1/.
References
FAO. Fishing profil by country, the Republic of Côte d’Ivoire. Rome (Italie): FAO. 2008; http://ftp.fao.org/FI/document/fcp/fr/FI_CP_CI.pdf (30/10/2014).
Hecht T. Review of feeds and fertilizers for sustainable aquaculture development in sub-saharan Africa. In: Hasan MR, Hecht T, DeSilva SS, Tacon AGJ, editors. Study and analysis of feeds and fertilizers for sustainable aquaculture development. Rome (Italie): FAO; 2007.
Bamba Y, Ouattara A, Kouassi SDC, Gourène G. Production of Oreochromis niloticus with feed made from agricultural by-products. Sci and Nat. 2008;5(1):89–99.
Gabriel UU, Akinrotimi OA, Bekibele DO, Onunkwo DN, Anyanwu PE. Locally produced fish feed: potentials for aquaculture development in sub-saharan Africa. Afr J Agric Res. 2007;2:287–95.
Crentsil C, Ukpong IG. Economics of fish production in Amansie-West District of Ghana: implication for Food Security in West Africa. Asian J Agric Ext Econ Soc. 2014;3:179–88.
Médale F, Le Boucher R, Dupont-Nivet M, Quillet E, Aubin J, Panserat S. Des aliments à base de végétaux pour les poissons d’élevage. INRA Productions Animales. 2013;26:303–16.
O’Neill J. Antimicrobials in Agriculture and the Environment: Reducing Unnecessary Use and Waste. The Review on Antimicrobial Resistance. Available online: http://amr-review.org/sites/default/files/Antimicrobials%20in%20agriculture%20and%20the%20environment%20-%20Reducing%20unnecessary%20use%20and%20waste.pdf ((accessed on 25 March 2023).)
Chuah L, Effarizah ME, Goni AM, Rusul G. Antibiotic application and emergence of multiple antibiotic resistance (MAR) in global catfish aquaculture. Curr Environ Health Rep. 2016;3:118–27.
Watts JEM, Schreier HJ, Lanska L, Hale MS. The Rising Tide of antimicrobial resistance in aquaculture: sources, sinks and solutions. Mar Drugs. 2017;15(6):158.
Irianto A, Austin B. Probiotics in aquaculture. J Fish Dis. 2002;25:633–42.
Banerjee G, Ray AK. The advancement of probiotics research and its application in fish farming industries. Res Vet Sci. 2017;115:66–77.
Hasan KN, Banerjee G. Recent studies on probiotics as beneficial mediator in aquaculture: a review. JoBAZ. 2020;81:53.
El-Saadony MT, Alagawany M, Patra AK, Kar I, Tiwari R, Dawood MAO, Dhama K, Abdel-Latif HMR. The functionality of probiotics in aquaculture: an overview. Fish Shellfish Immunol. 2021;117:36–52.
Hancz C. Application of probiotics for environmentally friendly and sustainable aquaculture: a review. Sustainability. 2022;14(22):15479.
FAO/WHO. Guidelines for the evaluation of probiotics in food. London, Ontario, Canada: Food and Agriculture Organization of the United Nations and World Health Organization Working Group Report; 2002.
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–14.
Jahangiri L, Esteban M. Administration of Probiotics in the water in finfish aquaculture systems: a review. Fishes. 2018;3:33.
Chauhan A, Singh R. Probiotics in aquaculture: a promising emerging alternative approach. Symbiosis. 2019;77:99–113.
Gong L, He H, Li D, Cao L, Khan TA, Li Y, et al. A new isolate of Pediococcus pentosaceus (SL001) with antibacterial activity against fish pathogens and potency in facilitating the immunity and growth performance of grass carps. Front Microbiol. 2019;10:1384.
Ringø E, Van Doan H, Lee SH, Soltani M, Hoseinifar SH, Harikrishnan R, et al. Probiotics, lactic acid bacteria and bacilli: interesting supplementation for aquaculture. J Appl Microbiol. 2020;129(1):116–36.
Kuebutornye FKA, Abarike ED, Lu Y. A review on the application of Bacillus as probiotics in aquaculture. Fish Shellfish Immunol. 2019;87:820–8.
Navarrete P, Tovar-Ramírez D. Use of yeasts as probiotics in fish aquaculture. Sustain Aquac Tech. 2014;1:57196.
Dumitrache C, Mihai C, Frîncu M. Yeast - sustainable nutrient source for fish feed - review. Sci Papers Ser E Land Reclam Earth Observation Surveying Environ Eng. 2022;XI:464–9.
Diguță CF, Mihai C, Toma RC, Cîmpeanu C, Matei F. In vitro assessment of yeasts strains with probiotic attributes for aquaculture use. Foods. 2023;12:124.
Buntin N, Chanthachum S, Hongpattarakere T. Screening of lactic acid bacteria from gastrointestinal tracts of marine fish for their potential use as probiotics. Songklanakarin J Sci Technol. 2008;30(s1):141–8.
Boutin S, Audet C, Derome N. Probiotic treatment by indigenous bacteria decreases mortality without disturbing the natural microbiota of Salvelinus fontinalis. Can J Microbiol. 2013;59:662–70.
Wanka KM, Damerau T, Costas B, Krueger A, Schulz C, Wuertz S. Isolation and characterization of native probiotics for fish farming. BMC Microbiol. 2018;18:119.
Mladineo I, Bušelić I, Hrabar J, Radonić I, Vrbatović A, Jozić S, Trumbić Ž. Autochthonous bacterial isolates successfully stimulate in vitro peripheral blood leukocytes of the European Sea bass (Dicentrarchus labrax). Front Microbiol. 2016;7:1244.
Ridha MT, Azad IS. Effect of autochthonous and commercial probiotic bacteria on growth, persistence, immunity and disease resistance in juvenile and adult Nile tilapia Oreochromis niloticus. Aquac Res. 2016;47:2757–67.
Van Doan H, Hoseinifar SH, Khanongnuch C, Kanpiengjai A, Unban K, Van Kim V, Srichaiyo S. Host-associated probiotics boosted mucosal and serum immunity, disease resistance and growth performance of Nile tilapia (Oreochromis niloticus). Aquaculture. 2018;491:94–100.
Dhanasiri AKS, Brunvold L, Brinchmann MF, Korsnes K, Bergh Ø, Kiron V. Changes in the intestinal microbiota of wild Atlantic cod Gadus morhua L. upon captive rearing. Microb Ecol. 2011;61:20–30.
Chi C, Jiang B, Yu X-B, Liu T-Q, Xia L, Wang G-X. Effects of three strains of intestinal autochthonous bacteria and their extracellular products on the immune response and disease resistance of common carp. Fish Shellfish Immunol. 2014;36(1):9–18.
Mohamad N, Manan H, Sallehhuddin M, Musa N, Ikhwanuddin M. Screening of lactic acid Bacteria isolated from giant freshwater prawn (Macrobrachium rosenbergii) as potential probiotics. Aquac Rep. 2020;18:100523.
Araújo C, Muñoz-Atienza E, Nahuelquín Y, Poeta P, Igrejas G, Hernández PE, et al. Inhibition of fish pathogens by the microbiota from rainbow trout (Oncorhynchus mykiss, Walbaum) and rearing environment. Anaerobe. 2015;32:7–14.
Chemlal-Kherraz D, Sahnouni F, Matallah-Boutiba A, Boutiba Z. The probiotic potential of lactobacilli isolated from Nile tilapia (Oreochromis niloticus)’s intestine. Afr J Biotechnol. 2012;11(68):13220–7.
Iorizzo M, Albanese G, Testa B, Ianiro M, Letizia F, Succi M, Tremonte P, D’Andrea M, Iaffaldano N, Coppola R. Presence of lactic acid bacteria in the intestinal tract of the Mediterranean trout (Salmo macrostigma) in its natural environment. Life. 2021;11(7):667.
Shewale RN, Sawale PD, Khedkar CD, Singh A. Selection criteria for probiotics: a review. Int J Probiotics Prebiotics. 2014;9:17–22.
de Melo Pereira GV, de Oliveira Coelho B, Magalhães Júnior AI, Thomaz-Soccol V, Soccol CR. How to select a probiotic? A review and update of methods and criteria. Biotechnol. Adv. 2018;36:2060–76.
Byakika S, Mukisa I, Byenkya Y, Muyanja C, Byenkya Byaruhanga Y. A review of criteria and methods for evaluating the probiotic potential of microorganisms. Food Rev Int. 2019;35:427–66.
Verschuere L, Rombaut G, Sorgeloos P, Verstraete W. Probiotic bacteria as biological control agents in aquaculture. Microbiol Mol Biol Rev. 2000;64:655–71.
Defoirdt T, Sorgeloos P, Bossier P. Alternatives to antibiotics for the control of bacterial disease in aquaculture. Curr Opin Microbiol. 2011;14:251–8.
Muñoz-Atienza E, Gómez-Sala B, Araújo C, Campanero C, del Campo R, Hernández PE, Herranz C, Cintas LM. Antimicrobial activity, antibiotic susceptibility and virulence factors of lactic acid bacteria of aquatic origin intended for use as probiotics in aquaculture. BMC Microbiol. 2013;13:15.
Sharifuzzaman S, Austin B. Probiotics for disease control in aquaculture. in Diagnosis and control of Diseases of Fish and Shellfish, Austin B, Newaj-Fyzul A, editors (Hoboken, NJ: John Wiley & Sons Ltd), 189–222. https://doi.org/10.1002/9781119152125.ch8.
Ringø E, Hoseinifar SH, Ghosh K, Doan HV, Beck BR, Song SK. Lactic acid bacteria in finfish—An update. Front Microbiol. 2018;9:1818.
Li X, Ringø E, Hoseinifar SH, Lauzon HL, Birkbeck H, Yang D. The adherence and colonization of microorganisms in fish gastrointestinal tract. Rev Aquac. 2019;11:603–18.
Danilova T, Adzhieva A, Danilina G, Polyakov N, Soloviev A, Zhukhovitsky V. Antimicrobial activity of supernatant of Lactobacillus plantarum against pathogenic microorganisms. Bull Exp Biol Med. 2019;167:751–4.
Salas-Jara MJ, Ilabaca A, Vega M, García A. Biofilm forming Lactobacillus: new challenges for the development of probiotics. Microorganisms. 2016;4(3):35.
Todhanakasem T, Triwattana K, Pom J, Havanapan P, Koombhongse P, Thitisak P. Physiological studies of the Pediococcus pentosaceus biofilm. Lett Appl Microbiol. 2021;72(2):178–86.
Chamignon C, Guéneau V, Medina S, Deschamps J, Gil-Izquierdo A, Briandet R, Mousset PY, Langella P, Lafay S, Bermúdez-Humarán LG. Evaluation of the probiotic properties and the capacity to form biofilms of various Lactobacillus strains. Microorganisms. 2020;8(7):1053.
Kim JH, Lee ES, Song KJ, Kim BM, Ham JS, Oh MH. Development of desiccation-tolerant probiotic biofilms inhibitory for growth of foodborne pathogens on stainless steel surfaces. Foods. 2022;11(6):831.
Yasmin I, Saeed M, Khan WA, Khaliq A, Chughtai MFJ, Iqbal R, Tehseen S, Naz S, Liaqat A, Mehmood T, Ahsan S, Tanweer S. Vitro probiotic potential and safety evaluation (hemolytic, cytotoxic activity) of Bifidobacterium strains isolated from raw Camel milk. Microorganisms. 2020;8:354.
Krausova G, Hyrslova I, Hynstova I. In Vitro evaluation of adhesion capacity, hydrophobicity, and auto-aggregation of newly isolated potential probiotic strains. Fermentation. 2019;5:100.
Mishra V, Shah C, Mokashe N, Chavan R, Yadav H, Prajapati J. Probiotics as potential antioxidants: a systematic review. J Agric Food Chem. 2015;63:3615–26.
Feng T, Wang J. Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: a systematic review. Gut Microbes. 2020;12:1801944.
Bairagi A, Sarkar Ghosh K, Sen SK, Ray AK. Enzyme producing bacterial flora isolated from fish digestive tracts. Aquacult Int. 2002;10:109–21.
Ray AK, Ghosh K, Ringo E. Enzyme-producing bacteria isolated from fish gut: a review. Aquac Nutr. 2012;18(5):465–92.
Nimrat S, Vuthiphandchai V. Vitro evaluation of commercial probiotic products used for marine shrimp cultivation in Thailand. Afr J Biotechnol. 2011;10:4643–50.
Abomughaid MM. Isolation and identification of some probiotic bacteria and their potential role in improving immune response and resistance of Nile Tilapia (Oreochromis niloticus) in comparison with a commercial product. International J Microbiol. 2020; 2020: 8865456.
Melo-Bolívar JF, Pardo RYR, Hume ME, Díaz LMV. Multistrain probiotics use in main commercially cultured freshwater fish: a systematic review of evidence. Rev Aquac. 2021;13:1758–80.
El-Kady AA, Magouz FI, Mahmoud SA, Abdel-Rahim MM. The effects of some commercial probiotics as water additive on water quality, fish performance, blood biochemical parameters, expression of growth and immune-related genes, and histology of Nile tilapia (Oreochromis niloticus). Aquaculture. 2022;546:737249.
Al-Dohail MA, Hashim R, Aliyu-Paiko M. Effects of the probiotic, Lactobacillus acidophilus, on the growth performance, haematology parameters and immunoglobulin concentration in African Catfish (Clarias gariepinus, Burchell 1822) fingerling. Aquac Res. 2009;40(14):1642–52.
Nayak SK. Probiotics and immunity: a fish perspective. Fish Shellfish Immunol. 2010;29:2–14.
Lakshmi B, Viswanath B, Sai Gopal DVR. Probiotics as antiviral agents in shrimp aquaculture. J Pathog. 2013;8:424123.
De B, Meena DK, Behera BK, Das P, Das Mohapatra PK, Sharma AP. Probiotics in fish and shellfish culture: Immunomodulatory and ecophysiological responses. Fish Physiol Biochem. 2014;40:921–71.
Allameh S, Noaman V, Nahavandi R. Effects of probiotic bacteria on fish performance. Adv Tech Clin Microbiol. 2017;1(2):11.
Yi C-C, Liu C-H, Chuang K-P, Chang Y-T, Hu S-Y. A potential probiotic Chromobacterium aquaticum with bacteriocin-like activity enhances the expression of indicator genes associated with nutrient metabolism, growth performance and innate immunity against pathogen infections in zebrafish (Danio rerio). Fish Shellfish Immunol. 2019;93:124–34.
Zabidi A, Yusoff FM, Amin N, Yaminudin NJM, Puvanasundram P, Karim MMA. Effects of probiotics on growth, survival, water quality and disease resistance of red hybrid Tilapia (Oreochromis spp.) fingerlings in a biofloc system. Anim (Basel). 2021;11(12):3514.
Iorizzo M, Albanese G, Letizia F, Testa B, Tremonte P, Vergalito F, Lombardi SJ, Succi M, Coppola R, Sorrentino E. Probiotic potentiality from versatile lactiplantibacillus plantarum strains as resource to enhance freshwater fish health. Microorganisms. 2022;10(2):463.
Nathanailides C, Kolygas M, Choremi K, Mavraganis T, Gouva E, Vidalis K, Athanassopoulou F. Probiotics have the potential to significantly mitigate the environmental impact of freshwater fish farms. Fishes. 2021;6:76.
Silva VV, Salomão RAS, Mareco EA, Pai MD, Santos VB. Probiotic additive affects muscle growth of Nile tilapia (Oreochromis niloticus). Aquac Res. 2021;52:2061–9.
Sahandi J, Jafaryan H, Soltani M, Ebrahimi P. The use of two Bifidobacterium strains enhanced growth performance and nutrient utilization of rainbow trout (Oncorhynchus mykiss) Fry. Probiotics Antimicrob Proteins. 2019;11(3):966–72.
Sîrbu E, Dima MF, Tenciu M, Cretu M, Coadă MT, Țoțoiu A, Cristea V, Patriche N. Effects of dietary supplementation with probiotics and prebiotics on growth, physiological condition, and resistance to pathogens challenge in Nile Tilapia (Oreochromis niloticus). Fishes. 2022;7:273.
Kouhoundé S, Coulibaly WH, Adeoti K, Somda M, Dadié AT, Savadogo A, Fatiou Toukourou F, Baba-Moussa F, Probiotics. A sustainable option for food safety and preservation in Africa In: Studium Press LLC, editor Trends in probiotics applications, edn 1st. Houston 2018; pp. 112–134.
Ben Amor K, Vaughan EE, de Vos WM. Advanced molecular tools for the identification of lactic acid bacteria. J Nutr. 2007;137:741–7.
Mohania D, Nagpal R, Kumar M, Bhardwaj A, Yadav M, Jain S, Marotta F, Singh V, Parkash O, Yadav H. Molecular approaches for identification and characterization of lactic acid bacteria. J Dig Dis. 2008;9:190–8.
Sharma A, Lee S, Park YS. Molecular typing tools for identifying and characterizing lactic acid bacteria: a review. Food Sci Biotechnol. 2020;29:1301–18.
Bajpai VK, Han J-H, Rather IA, Park C, Lim J, Paek WK, Lee JS, Yoon J-I, Park Y-H. Characterization and antibacterial potential of lactic acid bacterium Pediococcus pentosaceus 4I1 isolated from freshwater fish Zacco koreanus. Front Microbiol. 2016;7:2037.
Diguță FC, Nițoi GD, Matei F, Luță G, Cornea CP. The biotechnological potential of Pediococcus spp. isolated from Kombucha microbial consortium. Foods. 2020;9:1780.
Jiang S, Cai L, Lv L, Li L. Pediococcus pentosaceus, a future additive or probiotic candidate. Microb Cell Fact. 2021;20:45.
Matei B, Salzat J, Diguță CF, Cornea CP, Luta G, Utoiu ER, Matei F. Lactic acid bacteria strains isolated from Kombucha with potential probiotic effect. Rom Biotech Lett. 2018;23(3):13592–8.
Ringø E, Løvmo L, Kristiansen M, Bakken Y, Salinas I, Myklebust R, Olsen RE, Mayhew TM. Lactic acid bacteria vs. pathogens in the gastrointestinal tract of fish: a review. Aquacult Res. 2010;41:451–67.
Gufe C, Canaan Hodobo T, Mbonjani B, Majonga O, Marumure J, Musari S, Jongi G, Makaya PV, Machakwa J. Antimicrobial profiling of bacteria isolated from fish sold at informal market in Mufakose, Zimbabwe. Int J Microbiol. 2019;8(7):59–63.
Acharjee M, Hoque R, Shreya SS, Tabassum N, Acharjee MR, Rezanujjaman M, Rahman M, Amin A, Mahmud MR. Antibiotic susceptibility pattern of fish pathogens: a new approach of emerging the bacterial resistance through biofilm formation in in-vitro condition. Saudi J Biol Sci. 2021;28(12):6933–8.
Govindaraj K, Samayanpaulraj V, Narayanadoss V, Uthandakalaipandian R. Isolation of lactic acid bacteria from intestine of freshwater fishes and elucidation of probiotic potential for aquaculture application. Volume 13. Prot: Probiotics & Antimicro; 2021. pp. 1598–610.
Allameh SK, Yusof FM, Daud HM, Ringø E, Ideris A, Saad CR. Characterization of a probiotic Lactobacillus fermentum isolated from snakehead, Channa striatus stomach. J World Aquac Soc. 2013;44(6):835–44.
Lamari F, Sadok K, Bakhrouf A, Gatesoupe F-J. Selection of lactic acid bacteria as candidate probiotics and in vivo test on Artemia nauplii. Aquac Int. 2014;22:699–709.
Marchwińska K, Gwiazdowska D. Isolation and probiotic potential of lactic acid bacteria from swine feces for feed additive composition. Arch Microbiol. 2022;204:61.
Michael CA, Dominey-Howes D, Labbate M. The antibiotic resistance crisis: causes, consequences, and management. Front Public Health. 2014;2:145.
Viswanathan VK. Off-label abuse of antibiotics by bacteria. Gut Microbes. 2014;5(1):3–4.
Daniali M, Nikfar S, Abdollahi M. Antibiotic resistance propagation through probiotics. Expert Opin Drug Metab Toxicol. 2020;16:1207–15.
EFSA. Guidance on the characterization of microorganisms used as feed additives or as production organisms. EFSA J. 2018;16:5206.
Dou W, Abdalla HB, Chen X, Sun C, Chen X, Tian Q, Wang J, Zhou W, Chi W, Zhou X, Ye H, Bi C, Tian X, Yang Y, Wong A. ProbResist: a database for drug-resistant probiotic bacteria. Database (Oxford). 2022: baac064. https://doi.org/10.1093/database/baac064.
Binda S, Hill C, Johansen E, Obis D, Pot B, Sanders ME, Tremblay A, Ouwehand AC. Criteria to qualify microorganisms as ”Probiotic” in foods and dietary supplements. Front Microbiol. 2020;11:1662.
Dunne C, O’Mahony L, Murphy L, Thornton G, Morrissey D, O’Halloran S, Feeney M, Flynn S, Fitzgerald G, Daly C, Kiely B, CO’Sullivan G, Shanahan F, Collins JK. In vitro selection criteria for probiotic bacteria of human origin: correlation with in vivo findings. Am J Clin Nutr. 2001;73:386S–92.
Hubalek Z. Protectants used in the cryopreservation of microorganisms. Cryobiology. 2003;46:205–29.
Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–25.
Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol Biol Evol. 2018;35:1547–9.
Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal. 2016;6(2):71–9.
Brand-Williams W, Cuvelier ME, Berset C. Use of free radical method to evaluate antioxidant activity. Lebensm WissTechnol. 1995;28:25–30.
CLSI. CLSI Supplement M100S. Performance standards for antimicrobial susceptibility testing, 26th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2016.
Acknowledgements
Thanks to the Romanian government and AUF: Agence Universitaire de la Francophonie for a post-doctoral grant by “Eugen Ionescu” 2021 fellowship.
Funding
This work was supported by grants from the Romanian government and AUF: Agence Universitaire de la Francophonie for a post-doctoral “Eugen Ionescu” 2021 fellowship.
Author information
Authors and Affiliations
Contributions
W.H.C: conceptualization, investigation, methodology, validation, writing—original draft. N.R.K: investigation, methodology. C.D: investigation, methodology, writing—original draft, validation. F.C and F.Mi: conceptualization, supervision, writing—review and editing, validation. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The work research complies with the current animal welfare laws in Ivory Coast. The experimental animals’ Tilapia (Oreochromis niloticus) is not an endangered fish; the provisions of the Govt. of Ivorian’s Wildlife Protection Act of 1965 are not applicable for experiments on this Tilapia. All experimental protocols were approved by the University Nangui Abrogoua ethics committee. All methods are reported following ARRIVE guidelines.
Conflict of interest
The authors declare no conflict of interest.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Coulibaly, W.H., Kouadio, N.R., Camara, F. et al. Functional properties of lactic acid bacteria isolated from Tilapia (Oreochromis niloticus) in Ivory Coast. BMC Microbiol 23, 152 (2023). https://doi.org/10.1186/s12866-023-02899-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-023-02899-6