Abstract
Background
Vancomycin-resistant enterococci (VRE) are among the most common causative pathogens for nosocomial infections worldwide. Moreover, strains of VRE have been isolated from several domestic livestock in Egypt.
Methods
This study examined if healthy dogs are a potential source of VRE infection by isolating and characterizing Enterococcus faecium strains from stool samples on a morphological basis and biochemical activities. Subsequently, it was confirmed by genotypic characterization using polymerase chain reaction (PCR), followed by the detection of antibiotic resistance genes, virulence determinants, and genes contributing to enterocin production by PCR. Furthermore, the phylogenetic relationships among vanB and tetL genes were analyzed.
Results
All ten fecal samples were identified as E. faecium and confirmed by PCR. In addition, 90% of the isolates tested were positive for the virulence genes gelE and esp, and all the isolates tested were positive for the antibiotic resistance genes tetL and vanB. Only three of the five enterocin genes examined were detected. Ent As-48, bacteriocin 31, and Ent L50 were identified in 100%, 80%, and 60% of the samples, respectively.
Conclusion
Dogs should be regarded as a reservoir of E. faecium that carries vancomycin resistance and virulence determinants that may affect public health in Egypt, considering a "One Health" task force approach to restrict their spread.
Similar content being viewed by others
Introduction
An ancient relationship between humans and dogs evolved from a working animal to welfare use as a companion animal. Although they can improve their owners' physical and mental health, they can spread disease. The first study in dogs reported the existence of multidrug-resistance Shiga-toxigenic Escherichia coli in Egypt [1]. The One Health effort is a global approach to partnerships in all facets of health care for people, animals, plants, and the environment, providing a basis for combating major public health threats [2]. Enterococci are commensals of the gastrointestinal tract of both humans and animals and are a significant cause of nosocomial infections [3]. Companion animals (such as dogs and cats) may act as asymptomatic reservoirs for virulent and multidrug-resistant (MDR) enterococcal species (E. faecalis and E. faecium) [4]. A high percentage of MDR E. faecalis strains isolated from companion animals have been described [5]. However, intestinal carriage of vancomycin-resistant enterococci (VRE) was rare in healthy dogs [6]. Many studies have focused on detecting VRE in swine, poultry, and food-producing animals, but the epidemiology of E. faecalis and E. faecium in dogs is not well described [7].
Enterococcal strains can produce bacteriocins, and several of these probiotic proteins have been purified and genetically characterized to control pathogenic bacteria [8]. Despite their benefits, certain enterococcal strains are associated with their pathogenic characteristic as opportunistic bacteria. Enterococci possess several virulence genes, including asa1 (encoding aggregating substance), gelE (encoding gelatinase), esp (encoding enterococcal surface protein), and ace (encoding adhesion of collagen). Enterococcal surface proteins (esp), hyaluronidase (hyl), and collagen-binding adhesin (ace) are virulence proteins carried by E. faecium that are linked to host invasion, persistence, biofilm formation, and pathogenicity [9]. Moreover, gelatinase is an extracellularly expressed zinc metalloprotease that hydrolyzes casein, collagen, and gelatin [10]. As a result, enterococcal strains considered for use as probiotics should be thoroughly tested for effectiveness and safety [11].
Emerging multidrug-resistant bacterial infections are a significant problem in developing worldwide public health risks [12]. Vancomycin-resistant E. faecium is the second most common pathogen on the World Health Organization's (WHO) priority list of antibiotic-resistant pathogens that seriously threaten public health [13]. The expression of van operons confers vancomycin resistance. Eight acquired vancomycin-resistance operons (vanA, vanB, vanD, vanE, vanG, vanL, vanM, and vanN) were described and named according to their ligase genes. Besides, the "accessory" genes vanY and vanZ are carried by the transposon Tn1546 and are located on the gene cluster vanA. Vancomycin resistance is expressed through three primary resistance genes: vanH, vanA, and vanX (vanHAX). VanR and vanS are additional essential regulatory genes that govern the primary resistance genes [14, 15]. The vanA-positive and vanB-positive enterococci are the most common European resistance genotypes (14). Enterococci expressing vanA exhibited elevated resistance to vancomycin and teicoplanin, while enterococci expressing vanB showed elevated resistance to vancomycin only [14].
The health sector and veterinary practice must regularly conduct antimicrobial sensitivity tests and utilize antibiotics appropriately [16, 17]. Combining classical and molecular diagnostic assays offers a precise epidemiological tool for pathogen research. Worryingly, multidrug-resistant bacteria are portrayed as a public health issue [18]. Therefore, this study aimed to investigate the potential risk of companion healthy dogs in the transmission of enterococci. Accordingly, antimicrobial-resistant genes, bacteriocin, and virulence genes of enterococci collected in Egypt were identified, besides analyzing the phylogenetic relationships among vanB and tetL genes expressed by E. faecium isolated from different species, including humans.
Results
Isolates
Ten putative E. faecium strains isolated from fecal samples of healthy dogs were characterized by morphology, biochemical tests, and the Api20 Strep system (Biomérieux, France).
Phenotypic characteristics of the recovered isolates
The Gram stain results revealed a single, pair, or chain of gram-positive cocci, ovoid to coccobacillary in shape. Colonies on blood agar are 1 to 2 mm in diameter, non-hemolytic or alpha-hemolytic; colonies on bile esculin media cause blacking of the medium around the growth. Besides, isolates are catalase-negative and resistant to 6.5% sodium chloride (positive).
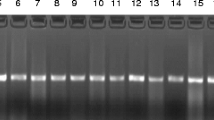
All isolates were confirmed using a species-specific primer for E. faecium by the detection of a specific 658-bp PCR product, as shown in Fig. 1.
Antibiotic sensitivity
The antibiotic sensitivity test showed that 80% of isolates showed phenotypical resistance to vancomycin, and 50% showed resistance to tetracycline, while 30%, 60%, and 10% showed resistance against ceftriaxone, ampicillin, and amoxicillin/clavulanic acid, respectively. All isolates were sensitive to ciprofloxacin.
Detection of antibiotic resistance, virulence, and enterocin genes using PCR
Ninety percent of E. faecium isolates harbored the virulence genes gelE and esp. In addition, all isolates showed 100% resistance against the antibiotic resistance genes tetL and vanB. Furthermore, Ent As-48, bacteriocin 31, and Ent L50 were found to have a prevalence of 100%, 80%, and 60%, respectively, while none of the isolates expressed Ent P or Ent 1071A/1071B. These findings are summarized in Table 1 and Figs. 2, 3, 4, 5, 6, 7 and 8.
Phylogenetic analysis
The tetL sequences of all isolates showed high homology with reference sequences from E. faecium (LR145483), E. faecalis (CP049776), and Strept. suis (MK359989) (Fig. 9).
Moreover, all vanB sequences from dog isolates formed a distinct clade with VanB sequences of E. faecium isolates from humans (KT003971, KT003978, and KT003982) (Fig. 10).
Discussion
Enterococcus opportunistic pathogens are a significant public health concern due to their frequent involvement in nosocomial infections, high antibiotic resistance, and severe morbidity. This study suggests that enterococci carrying antibiotic resistance and virulence genes can spread through asymptomatic pets. Our results reflect that VRE may be spread in households and veterinary hospitals through the feces of healthy dogs. Five E. faecalis and 15 E. faecium isolates were recovered from 20 Enterococcus isolates taken from the library collections of randomly chosen private hospitals in El Qanater El Khayreya, Egypt, suggesting that these strains are common contaminants in some Egyptian hospitals [19]. In addition, 61 E. faecium strains were identified among 110 isolates from clinical specimens [20]. Isolates of E. faecium have previously been found in healthy dogs, although at a lower frequency than in the current study. Said and his colleagues [21] identified 16 E. faecium strains among 39 isolates from healthy dogs. Moreover, Feßler et al. [22] found 37 E. faecium strains among 215 samples collected from dogs and cats. These findings indicate that Enterococcus species distribution varies depending on the host environment.
Various factors, including expression of esp and gelE, control the virulence of enterococci. However, the expression of these virulence genes varies widely among enterococcal species from different hosts. The esp is responsible for increased biofilm formation and colonization potential. At the same time, gelE is essential for resistance against the host's innate immune defense [4]. The esp was detected in 66.4% and the gelE in 33.6% of clinical specimens [20].
On the other hand, gelE and esp were not found in any of the eight E. faecium isolates from healthy dogs in Egypt [23]. The gelE was found in 35.41% of isolates from wild and domestic ruminants in Italy, the highest frequency among tested genes, while esp the least frequent, found in only 0.04% of samples [24]. Besides, gelE was found in the vast majority of samples (91.4%), consistent with our findings, but esp was found less frequently (65.7%) than in the present study (90%) [25]. Similarly, a study conducted at the University Hospital of the Faculty of Veterinary Medicine, Cairo University, and private veterinary clinics in Cairo [26] reported oral colonization of domestic dogs and cats by E. faecalis and E. faecium. However, only two of nine canine ampicillin-resistant E. faecium (AREfm) multidrug-resistant isolates and none of the feline AREfm isolates expressed the esp gene. These findings suggest that virulence genes vary substantially across E. faecium strains. Therefore, screening is recommended for all strains isolated in clinical settings (Table 1).
Regarding phenotypic examination for antibiotic resistance, the results showed that not all the isolates with resistance genes showed phenotypic resistance. These findings confirmed that isolates may carry antibiotic resistance genes without expression (Table 2).
Furthermore, vancomycin-resistant enterococci have been identified as human pathogens in the natural environment, and the spread of opportunistic bacteria with vancomycin resistance outside of the hospital environment poses a severe public health risk because vancomycin is considered the last line of defense. Ulrich and his colleagues [27] have documented 35 VRE outbreaks, with 757 individual infections and 77 deaths. The most common recovery sites were rectal swabs and fecal samples, suggesting that these infections spread due to poor hygiene. Further, previous antibiotic treatment was the most significant risk factor.
Concerning the vanB gene, our result is consistent with studies showing that E. faecium can colonize the gut, although typically without substantial vancomycin resistance (MIC = 3 mg/L). Antibiotic resistance is far more frequent in this study than previously reported. According to El-Tayeb et al. [28], only 24% of 25 VRE isolates collected from diverse regions in Egypt were E. faecium. Moreover, Seputiene et al. [29] identified the tetL gene in only 23% of isolates from E. faecium-infected farm animals (pigs, cattle, and poultry).
Similarly, tetL was found in only 12% of isolates collected from 2 hospitals in Kerman, Iran [30]. In Japan, tetL was found in 30.4% of E. faecium isolates obtained from clinical samples [31]. The high proportion of vanB- and tetL-expressing strains isolated in this study is suggested to be due to the inappropriate use of antibiotics in veterinary medicine. For instance, tetL was identified in 25 out of 31 Enterococcus isolates collected from infected poultry in six regions of Egypt [32]. In turn, these animals could potentially spread the contamination into the surrounding environment, resulting in both animal and human infections. Most of the strains isolated in this study harbored a combination of Ent As-48, bacteriocin 31, and EntL50 genes, while neither Enterocin 1071A/1071B nor Enterocin P was found in any isolate.
Similarly, four strains of eight E. faecium from eight healthy dogs in Egypt carried the Ent AS-48 gene, and one strain carried the EntL50A/B gene [23].
In contrast, enterocin AS-48 was not found among 54 strains from different origins, including animals, while combinations including EntP and EntL50A/B were the most common (44%) [33]. Generated peptides with masses similar to those of enterocins A and B from 3 E. faecium isolates were found in donkey milk [34]. According to the previous study, wild animals are a significant source of bacteriocinogenic enterococci, especially in fecal matter, with enterocin P being the most common in most isolates; however, only a subset of bacteriocin genes is expressed [35].
Sequence alignment of tetL genes from our isolates with various reference sequences, including human isolates, revealed significant homology with E. faecium (LR145483), E. faecalis (CP049776), and Strept. suis (MK359989). Moreover, the vanB expressed by these isolates formed a separate clade with vanB sequences expressed by E. faecium isolated from humans (KT003971, KT003978, and KT003982), which suggests that tetL and vanB resistance genes can be transferred between dogs and their owners.
Conclusion
Companion animals are a potential source of severe VRE infections that can endanger veterinary health and human health. The findings reflected that E. faecium isolates from domestic dogs in Egypt frequently harbor antibiotic resistance genes and virulence factors. Thus, an effective antimicrobial stewardship program and regular surveillance using a transdisciplinary "One Health" approach are recommended to investigate the role of dogs as vectors for vancomycin resistance and prevent its dissemination.
Materials and methods
Sample collection
Fresh fecal samples were obtained from 10 randomly selected healthy domestic dogs admitted to the Faculty of Veterinary Medicine (Cairo University, Egypt), for routine medical checkups or vaccinations from January to November 2021. Sampled dogs were of both sexes. Samples were taken using sterile swabs and delivered in an icebox to the National Research Centre Microbiology and Immunology Laboratory for immediate processing.
Bacterial isolation and identification
Fecal swabs were pre-diluted, added to a 25 mL Ringer's solution containing 0.30 g/L of potassium chloride, 0.33 g/L of calcium chloride dehydrate, and 8.60 g/L of sodium chloride, and shaken vigorously for 30 min. Ten milliliters of the resultant suspension were added to 90 ml of nutrient broth (Merck). Incubation of the inoculated media was performed for 48 h at 37 °C. The inoculum was next streaked over bile esculin agar (Oxoid, Hampshire, UK) and incubated under the same conditions [36]. An analysis of colony morphology on blood agar prepared from tryptic soy agar (Oxoid, Hampshire, UK) with 5% sheep blood was performed. Moreover, catalase expression and resistance to 6.5% sodium chloride were examined. Biochemical characteristics were analyzed using the Api20 Strep system (Biomérieux, France).
Antibiotic susceptibility test
Kirby Bauer's disc diffusion method [37] was used to investigate the antibiotic sensitivity of Enterococcus faecium isolates against different antimicrobial categories such as glycopeptides (Vancomycin 30 µg), tetracycline (Tetracycline 30 µg), fluoroquinolones (Ciprofloxacin 5 µg), cephalosporin (Ceftriaxone 30 µg), beta-lactamase inhibitors (Amoxicillin/Clavulanic acid 30 µg), and penicillins (Ampicillin 10 µg). All antibiotic discs were obtained from HI Media Laboratories (Mumbai, India). 20 µL overnight culture (1 × 105 CFU/ml) was added to 100 mL of Nutritional Broth (NB; Oxoid, UK) medium and incubated for 24 h at 37°C/120 rpm. Using cotton swabs, a bacterial culture (100 µL) was streaked onto Mueller Hinton Agar (MHA; Oxoid, UK) plates and incubated aerobically at 37°C for 18–24 h. The inhibitory zones (mm) were measured after the incubation, and the findings were classified according to the CLSI interpretation criteria [37].
Detection of virulence, antibiotic resistance, and bacteriocin genes using PCR
The QIAamp DNA Mini kit (Qiagen, Germany, GmbH) was used to extract genomic DNA from samples according to the manufacturer's instructions, and the nucleic acid was eluted with 50 µl of elution buffer.
All PCR reactions were conducted in a final reaction volume of 25 μL containing 12.5 μL 2 × cosmo PCR red master mixes (Cat. W1020300X, Willofort Co., UK), 1 μL (10 μM) of each primer (Metabion, Germany), and 1 μL of sample DNA. The PCR products were separated by electrophoresis on 1.5% agarose gels, which were then photographed and analyzed using the InGenius3 gel documentation system (Syngene, UK). The primer sequences and annealing temperatures used for PCR are presented in Table 3.
The genes of the E. faecium confirmation, entertains, and antimicrobial resistance indicated in Table 1 were amplified using the following thermocycling: 94°C for 5 min, 35 cycles of 94°C for 1 min, 53°C, 54°C, and 55°C (according to specific annealing for each gene) for 1 min, and 72°C for 40 s, and a final extension step at 72°C for 7 min.
Phylogenetic tree construction
Ten tetL nucleotide sequences from E. faecium isolates were submitted to GenBank under accession numbers MT295234 to MT295243 (https://www.ncbi.nlm.nih.gov/nuccore/MT295234), and ten VanB nucleotide sequences from E. faecium isolates were submitted to GenBank under accession numbers MT295244 to MT295253 (https://www.ncbi.nlm.nih.gov/nuccore/MT295244).
Both vanB and tetL genes from isolates were sequenced using 3730 L sequencers (Applied Biosystem, USA) at Macrogen (Seoul, Korea), and findings were validated by two-directional sequencing using the same forward and reverse PCR primers listed in Table 3. The gene sequences were analyzed using BioEdit 7.0.4.1 and ClustalW2 (http://www.clustal.org/), and compared to reference sequences of Enterococcus spp. using a neighbor-joining application in CLC Sequence Viewer 6.
Statistical analysis
Data were computerized and analyzed by the SPSS program (2004) [45]. Moreover, significant differences among means were detected by Duncan (1955) [46]:
where Yijk: Observation of i E. faecium isolates, and j phenotypic resistance; µ: General mean; Ei: Fixed effect of E. faecium isolates; Pj: Fixed effect of (Dj) phenotypic resistance; (S × D)ij: Effect of interaction (S × D)ij; and eijk: Residual effect.
Availability of data and materials
Ten tetL nucleotide sequences from E. faecium isolates were submitted to GenBank under accession numbers MT295234 to MT295243 and are publicly available at https://www.ncbi.nlm.nih.gov/nuccore/MT295234. Furthermore, ten VanB nucleotide sequences from E. faecium isolates were submitted to GenBank under accession numbers MT295244 to MT295253 and are publicly available at https://www.ncbi.nlm.nih.gov/nuccore/MT295244.
References
Algammal AM, El-Tarabili RM, Alfifi KJ, Al-Otaibi AS, Hashem MEA, El-Maghraby MM, Mahmoud AE. Virulence determinant and antimicrobial resistance traits of emerging MDR Shiga toxigenic E. coli in diarrheic dogs. AMB Express. 2022;12(1):34.
Overgaauw PAM, Vinke CM, Van Hagen MAE, Lipman LJA. A One health perspective on the human-companion animal relationship with emphasis on zoonotic aspects. Int J Environ Res Public Health. 2020;17(11):3789.
Sykes J. E.: Streptococcal and enterococcal infections. In Canine and Feline Infectious Diseases; Sykes J.E., Ed., Elsevier Inc.: St. Louis, MO, USA. 2014; 334–346, ISBN 9781437707953.
Pillay S, Zishiri OT, Adeleke MA. Prevalence of virulence genes in enterococcus species isolated from companion animals and livestock. Onderstepoort J Vet Res. 2018;85:1–8 ([Google Scholar] [CrossRef] [PubMed]).
Tros’cian’czyk A, Nowakiewicz A, Gnat S, Łagowski D, Osin’ska M. Are dogs and cats a reservoir of resistant and virulent Enterococcus faecalis strains and a potential threat to public health? J Appl Microbiol. 2021;131:2061–71.
Van den Bunt G., Top J., Hordijk J., de Greeff S.C., Mughini-Gras L., CoranderJ.,van Pelt W., Bonten M.J.M., Fluit A.C., Willems R.J.L.: Intestinal carriage of ampicillin- and vancomycin-resistant Enterococcus faecium in humans, dogs and cats in the Netherlands. J. Antimicrob. Chemother. 2018; 73: 607–614.
Donabedian SM, Perri MB, Abdujamilova N, Gordoncillo MJ, Naqvi A, Reyes KC, Reyes KC, Zervos MJ, Bartlett P. Characterization of vancomycin-resistant Enterococcus faecium isolated from swine in three Michigan counties. J ClinMicrobiol. 2010;48:4156–60.
Da Costa RJ, da Silva AP, da Fonseca RN, de Oliveira Hübner S, Nalério ES, de Lima Marques J, et al. Characterization of Enterococcus faecium EO1 isolated from mutton and activity of bacteriocin-like substances in the control of Listeria monocytogenes in fresh mutton sausage. Lebensmittel-Wissenschaft + Technol. 2021;141:110954.
Yang JX, Li T, Ning YZ, Shao DH, Liu J, Wang SQ, Liang GW. Molecular characterization of resistance, virulence and clonality in vancomycin-resistant Enterococcus faecium and Enterococcus faecalis: a hospital-based study in Beijing. China Infect Genet Evol. 2015;33:253–60.
Mohamed JA, Huang DB. Biofilm formation by enterococci. J Med Microbiol. 2007;56(Pt 12):1581–8.
Wang X, Yang Y, Huycke MM. Risks associated with enterococci as probiotics. Food Res Int. 2020;129: 108788. https://doi.org/10.1016/j.foodres.2019.108788. (Epub 2019 Nov 21).
Algammal A, Hetta HF, Mabrok M, Behzadi P. Editorial: Emerging multidrug-resistant bacterial pathogens “superbugs”: A rising public health threat. Front Microbiol. 2023;1(14):1135614. https://doi.org/10.3389/fmicb.2023.1135614. (PMID: 36819057).
World Health Organization. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics.2017.
Bender JK, Cattoir V, Hegstad K, Sadowy E, Coque TM, Westh H, Hammerum AM, Schaffer K, Burns K, Murchan S, Novais C, Freitas AR, Peixe L, Del Grosso M, Pantosti A, Werner G. Update on prevalence and mechanisms of resistance to linezolid, tigecycline and daptomycin in enterococci in Europe: towards a common nomenclature. drug resist. Updates. 2018;40:25–39.
Stogios PJ, Savchenko A. Molecular mechanisms of vancomycin resistance. Protein Sci. 2020;29:654–69. https://doi.org/10.1002/pro.3819.
Algammal AM, Ibrahim RA, Alfifi KJ, Ghabban H, Alghamdi S, Kabrah A, Khafagy AR, Abou-Elela GM, Abu-Elala NM, Donadu MG, El-Tarabili RM. A first report of molecular typing, virulence traits, and phenotypic and genotypic resistance patterns of newly emerging XDR and MDR aeromonas veronii in mugil seheli. Pathogens. 2022;11(11):1262. https://doi.org/10.3390/pathogens11111262. (PMID: 36365013).
Algammal AM, Abo Hashem ME, Alfifi KJ, Al-Otaibi AS, Alatawy M, ElTarabili RM, Abd El-Ghany WA, Hetta HF, Hamouda AM, Elewa AA, Azab MM. sequence analysis, antibiogram profile, virulence and antibiotic resistance genes of XDR and MDR gallibacterium anatis isolated from layer chickens in Egypt. Infect Drug Resist. 2022;9(15):4321–34. https://doi.org/10.2147/IDR.S377797. (PMID: 35971557).
Algammal AM, Eidaroos NH, Alfifi KJ, Alatawy M, Al-Harbi AI, Alanazi YF, Ghobashy MOI, Khafagy AR, Esawy AM, El-Sadda SS, Hetta HF, El-Tarabili RM. oprL gene sequencing, resistance patterns, virulence genes, quorum sensing and antibiotic resistance genes of XDR pseudomonas aeruginosa isolated from broiler chickens. Infect Drug Resist. 2023;13(16):853–67. https://doi.org/10.2147/IDR.S401473. (PMID: 36818807).
Osman K, Zolnikov TR, Badr J, Naim H, Hanafy M, Saad A, Elbehiry A. Vancomycin and florfenicol resistant Enterococcus faecalis and Enterococcus faecium isolated from human urine in an Egyptian urban-rural community. Acta Trop. 2020;201:105209.
Gök SM, Dağı HT, Kara F, Arslan U, Fındık D. Investigation of antibiotic resistance and virulence factors of enterococcus faecium and enterococcus faecalis strains isolated from clinical samples. Mikrobiyol Bul. 2020;54(1):26–39. https://doi.org/10.5578/mb.68810.
Said LB, Dziri R, Sassi N, Lozano C, Ben SK, Ouzari I, Torres C, Klibi N. Species distribution, antibiotic resistance and virulence traits in canine and feline enterococci in tunisia. Acta Vet Hung. 2017;65(2):173–84. https://doi.org/10.1556/004.2017.018.
Feßler AT, Scholtzek AD, Schug AR, Kohn B, Weingart C, Hanke D, Kathrin Schink A, Bethe A, Lübke-Becker A, Schwarz S. Antimicrobial and biocide resistance among canine and feline enterococcus faecalis, enterococcus faecium, escherichia coli, pseudomonas aeruginosa, and acinetobacter baumannii isolates from diagnostic submissions. Antibiotics. 2022;11:152. https://doi.org/10.3390/11020152.
Abd El-Razik KA, Ibrahim ES, Younes AM, Arafa AA, Abuelnaga AS, Hedia RH. Enterococcus faecium isolated from healthy dogs for potential use as probiotics. Bulg J Vet Med. 2020;23:197–205. https://doi.org/10.15547/bjvm.2213.
Smoglica C, Vergara A, Angelucci S, Festino AR, Antonucci A, Marsilio F, Di Francesco CE. Evidence of linezolid resistance and virulence factors in enterococcus spp. isolates from wild and domestic ruminants, Italy. Antibiotics. 2022;11:223 (antibiotics11020223).
Aladarose BE, Said HS, Abdelmegeed ES. Incidence of virulence determinants among enterococcal clinical isolates in Egypt and its association with biofilm formation. Microb Drug Resist. 2019;25(6):880–9. https://doi.org/10.1089/mdr.2018.0320.
Abdel-Moein KA, El-Hariri MD, Wasfy MO, Samir A. Occurrence of ampicillin-resistant enterococcus faecium carrying the esp gene in pet animals: an upcoming threat for pet lovers. J Glob Antimicrob Resist. 2017;9:115–7. https://doi.org/10.1016/j.jgar.2017.02.011.
Ulrich N, Vonberg R, Gastmeier P. Outbreaks caused by vancomycin-resistant Enterococcus faecium in hematology and oncology departments: a systematic review. Heliyon. 2017;3(12):00473. https://doi.org/10.1016/j.heliyon.2017.e00473. (PMID: 29322099; PMCID: PMC5753762).
El-Tayeb WN, Gamil MA, Ashour MSE, Ismail MA. prevalence of vancomycin resistant enterococci in a governmental hospital in Cairo-Egypt .N. Egypt. J. Microbiol.2009; 23.
Seputiene V, Bogdaite A, Ruzauskas M, Suziedeliene E. Antibiotic resistance genes and virulence factors in Enterococcus faecium and Enterococcus faecalis from diseased farm animals: Pigs, cattle and poultry. Polish J Veter Sci. 2012;15(3):431–8.
Ahmadpoor N, Ahmadrajabi R, Esfahani S, Hojabri Z, Moshafi MH, Saffari F. High-level resistance to erythromycin and tetracycline and dissemination of resistance determinants among clinical enterococci in Iran. Med PrincPract. 2021;30(3):272–6. https://doi.org/10.1159/000516216.
Nishimoto Y, Kobayashi N, Alam MM, Ishino M, Uehara N, Watanabe N. Analysis of the prevalence of tetracycline resistance genes in clinical isolates of Enterococcus faecalis and Enterococcus faecium in a Japanese hospital. Microb Drug Resist. 2005;11(2):146–53. https://doi.org/10.1089/mdr.2005,11.146.
Ahmed W, Hotzel H, Neubauer H. antimicrobial resistance genes associated with enterococci from poultry in Egypt, first reporting of mecA in Enterococcus from poultry source. Adv Animal Veteri Sci. 2020;8(6):570.581. https://doi.org/10.17582/journal.aavs/2020/8.6.
Özdemir GB, Oryaşın E, Bıyık HH, Özteber M, Bozdoğan B. Phenotypic and genotypic characterization of bacteriocins in enterococcal isolates of different sources. Indian J Microbiol. 2011;51(2):182–7.
Aspri M, O’Connor PM, Field D, Cotter PD, Ross P, Hill C, Papademas P. Application of bacteriocin-producing Enterococcus faecium isolated from donkey milk, in the bio-control of Listeria monocytogenes in fresh whey cheese. Int Dairy J. 2017;73:1–9. https://doi.org/10.1016/j.idairyj.2017.04.008.
Almeida T, Brandão A, Muñoz-Atienza E, Gonçalves A, Torres C, Igrejas S, Hernandez P, Herranz C, Poeta P, Cintas LM. Identification of bacteriocin genes in enterococci isolated from game animals and saltwater fish. J Food Prot. 2011;74(8):1252–60.
Grudlewska-Buda K, Skowron K, Bauza-Kaszewska J, et al. Assessment of antibiotic resistance and biofilm formation of Enterococcus species isolated from different pig farm environments in Poland. BMC Microbiol. 2023;23:89. https://doi.org/10.1186/s12866-023-02834-9.
CLSI. Performance Standards for Antimicrobial Susceptibility Tests, 13th ed.; CLSI Standard M02; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018.
Cheng S, McCleskey FK, Gress MJ, Petroziello JM, Liu R, Namdari H, Beninga K, Salmen A, DelVecchio VG. A PCR assay for identification of Enterococcus faecium. J Clin Microbiol. 1997;35:1248–50.
Vankerckhoven V, Van Autgaerden T, Vael C, Lammens C, Chapelle S, Rossi R, Jabes D, Goossens H. Development of a multiplex PCR for the detection of asa1, gelE, cylA, esp, and hyl genes in enterococci and survey for virulence determinants among European hospital isolates of Enterococcus faecium. J ClinMicrobiol. 2004;42(10):4473–9.
Eaton TJ, Gasson MJ. Molecular screening of enterococcus virulence determinants and potential for genetic exchange between food and medical isolates. Appl Environ Microbiol. 2001;67:1628–35.
Yousif NM, Dawyndt P, Abriouel H, Wijaya A, Schillinger U, Vancanneyt M, Swings J, Dirar HA, Holzapfel WH, Franz CM. Molecular characterization, technological properties and safety aspects of enterococci from ‘Hussuwa’, an African fermented sorghum product. J Appl Microbiol. 2005;98:216–28.
Ben BZ, Abriouelb H, BenOmarb N, Lucasb R, Martínez-Canamerob M, Gálvezb A, Manai M. Antimicrobial activity, safety aspects, and some technological properties of bacteriocinogenic Enterococcus faecium from artisanal Tunisian fermented meat. Food Control. 2010;21:462–70.
Cancilla MR, Powell IB, Hillier AJ, Davidson BE. Rapid genomic fingerprinting of Lactococcus lactis strains by arbitrarily primed polymerase chain reaction with 32P and fluorescent labels. Appl Environ Microbiol. 1992;58:1772–5.
Patel R, Uhl JR, Kohner P, Hopkins MK, Cockerill FR. Multiplex PCR detection of vanA, vanB, vanC-1, and vanC-2/3 genes in enterococci. J Clin Microbiol. 1997;35(3):703–7.
SPSS Program: User's guide statistic. Release 10.01, Copyright SPSS Inc., USA.2004.
Duncan DB. Multiple ranges and multiple F test. Biometrics. 1955;11:1–42.
Acknowledgements
Great support was provided by Department of Microbiology and Immunology, Department of Animal Reproduction and Department of Hydrobiology Veterinary Research Institute, National Research Centre, Dokki, Egypt.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
K.A. and E. S. designed the study. E. S.,A. A. ,R. H. , preform isolation identification and molecular characterization. A. M.,M.H. carried out detection of antibiotic resistance, virulence , and enterocin genes using PCR. K.A. carried out Phylogenetic tree and prepared Figs. 1, 2, 3, 4, 5, 6, 7 and 8. K. A. and , E. S. wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All methods were performed in accordance with relevant guidelines and regulations. The study was approved by the Animal Ethics Review Committee of National Research Centre, Egypt, under approval number 19153.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
El-Razik, K.A.A., Ibrahim, E.S., Arafa, A.A. et al. Molecular characterization of tetracycline and vancomycin-resistant Enterococcus faecium isolates from healthy dogs in Egypt: a public health threat. BMC Genomics 24, 610 (2023). https://doi.org/10.1186/s12864-023-09708-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-023-09708-4