Abstract
Background
Phloem protein 2 (PP2) proteins play a vital role in the Phloem-based defense (PBD) and participate in many abiotic and biotic stress. However, research on PP2 proteins in cotton is still lacking.
Results
A total of 25, 23, 43, and 47 PP2 genes were comprehensively identified and characterized in G.arboretum, G.raimondii, G.barbadense, and G.hirsutum. The whole genome duplication (WGD) and allopolyploidization events play essential roles in the expansion of PP2 genes. The promoter regions of GhPP2 genes contain many cis-acting elements related to abiotic stress and the weighted gene co-expression network analysis (WGCNA) analysis displayed that GhPP2s could be related to salt stress. The qRT-PCR assays further confirmed that GhPP2-33 could be dramatically upregulated during the salt treatment. And the virus-induced gene silencing (VIGS) experiment proved that the silencing of GhPP2-33 could decrease salt tolerance.
Conclusions
The results in this study not only offer new perspectives for understanding the evolution of PP2 genes in cotton but also further explore their function under salt stress.
Similar content being viewed by others
Background
The phloem is a bidirectional active transport system with a diverse array of molecular contents, and the phloem contains a large and diverse collection of molecule types involved in nutrient transport, inter-organ signaling, and adaptation to stress [1]. Phloem-based defense (PBD) is induced by multiple kinds of stresses, including wounding, oxidative conditions, and insect attacks [2,3,4,5]. Proposed components of PBD include the phloem protein 1 (PP1) and phloem protein 2 (PP2), which represent a type of the most abundant proteins in the phloem sap [6].
PP2 proteins play a vital role in the establishment of PBD. In previous studies, it was found that PP2 proteins could play a vital role in responding the biotic and abiotic stress. In Arabidopsis, the overexpression of AtPP2-A1 could improve the resistance to the Myzus persicae [7]. After the mite feeding, the AtPP2-A5 overexpressing lines showed less quantified damage while the pp2a5 lines showed greater quantified damage [8]. The AtPP2-B11 could play a positive and negative role in salt stress and drought stress, respectively [9, 10]. The further study found that AtPP2-B11 could interact with AtSnRK2.3 and degrade the AtSnRK2.3 to play as a negative regulator in ABA signaling [11]. Similarly, the AtPP2-B1 could also negatively regulates ABA signaling by taking part in the process of the downregulation of many ABA-inducible genes, such as AtABI4 and AtABI5 [12]. In Cucumis sativus, the overexpression of CsPP2-A1 could play a positive role in preventing aphid attacks and enhancing salt tolerance [13, 14]. In Solanum habrochaites, the overexpression of ShPP2-1 could decrease cold tolerance [15]. In Brassica napus, the BnPP2-6 plays a positive role in regulating Sclerotinia disease resistance caused by Sclerotinia sclerotiorum. However, the knowledge related to the PP2 proteins in cotton is still limited.
The genus Gossypium contains 45 diploid species and 7 tetraploid species, it was found that all of the Gossypium species shared a whole genome duplication (WGD) event around 60 million years ago (MYA) compared with the Theobroma cacao [16]. In addition, more than 95% fiber production is produced by two cultivated tetraploids species G. hirsutum [(AD)1, 2n = 4x = 52], G.barbadense [(AD)2, 2n = 4x = 52], and it was hypothesized that they from allopolyploidization of G.arboreum (A2, 2n = 4x = 26) and G.raimondii (D5, 2n = 4x = 26) at approximately 1–1.5 MYA [16, 17]. The Gossypium species play an indispensable role in the textile industry because it could provide natural fibers to meet around 35% of the world’s annual fiber demand. However, the production and quality of cotton are critically limited by abiotic stress, thus it is essential to mine the genes with abiotic stress resistance functions. Fortunately, the increasingly releasing genome and transcriptome sequencing data in cotton made it possible to explore the genes with resistance to abiotic stress and their evolution progress [17,18,19].
In this study, for the first time, the PP2 genes were systematically identified and characterized in Gossypium species, and the phylogenetic, gene structure, duplication events, selective pressure, and cis-elements analysis were comprehensively analyzed. The further evolution analysis showed that the PP2 genes in Gossypium species mainly expanded by the WGD and allopolyploidization events, while their structure and function might largely conserve during these evolutionary processes. The weighted gene co-expression network analysis (WGCNA) and the quantitative RT-PCR (qRT-PCR) assays showed that many GhPP2s could be responding to salt stress. Based on the above analyses, we chose GhPP2-33 to perform the virus-induced gene silencing (VIGS) experiment to determine its function in the salt stress, we found that the GhPP2-33-silenced plants decreased the salinity tolerance compared with control plants. Together, these results would provide new insights into the evolutionary history and functions of GhPP2 genes and lay benefit to the process of salt-resistant varieties in cotton breeding.
Results
Identification of PP2 proteins
A total of 25, 23, 43 and 47 PP2 genes were identified in G.arboretum (A2, 2n = 2x = 26), G.raimondii (D5, 2n = 2x = 26), G.barbadense ((AD)2, 2n = 4x = 52) and G.hirsutum ((AD)1, 2n = 4x = 52), respectively (Table S1). The 138 identified cotton PP2 proteins ranged from 105 to 562 amino acids (aa) in length with an average of 283 aa, the molecular weight varied from 12.17 to 59.42 kDa, and theoretical isoelectric point (pI) ranged from 4.46 to 9.50. The grand average of hydropathy (GRAVY) in this family showed that all proteins had negative values (-0.72–0.02), indicating that all PP2 proteins were hydrophilic in cotton (Table S1).
Phylogenetic analysis and structure analysis of PP2 proteins
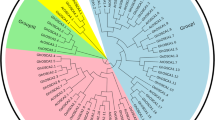
Together with the AtPP2s obtained from the previous study, a phylogenetic tree including 168 PP2 proteins was generated. As clearly shown in the phylogenetic tree, these PP2 proteins can be divided into six distinct subfamilies, namely subfamily I-VI. The members in the subfamily I and subfamily VI constituted the largest number (31.36% and 27.21%), whereas the subfamily III had the lowest number (3.55%) and this subfamily might be specific to the Gossypium species (Fig. 1). Within each subfamily, the members from the G. hirsutum and G. barbadense cotton were almost twice that of the diploid cotton.
The domain analysis showed that all PP2 proteins contain one or two PP2 domains located in the C-terminal. Besides the PP2 domains, F-box domains were found in the members of subfamily V and subfamily VI, respectively (Fig. S1). Most GhPP2s contain three exons, and only a few members harbored 4–6 exons (Fig. 2B). In addition, A total of 20 motifs were detected in GhPP2 proteins, and the motif pattern was conserved in each subfamily. The motif 2, 4, and 7 were detected in most PP2 proteins. However, we also found that motif 14 and 5 only found in the subfamily I, and motif 19 exclusively belong to the subfamily IV (Fig. 2A). The gene structure and distribution of motifs varied in different subfamilies, while they were conserved in each subfamily (Fig. 2).
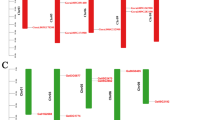
Gene structures and conserved motifs of PP2 members in G. hirsutum. A Distribution of conserved motifs in PP2 proteins. 20 putative motifs are indicated by colored boxes, and their motif name is displayed in the box in the upper right corner. B Exon/intron organization of PP2 genes. Green boxes represent exons and black lines represent introns
Gene duplication and selection pressure of PP2 genes
To further explore the expansion pattern during the evolution, we performed a comprehensive identification in 35 other plant species and identified a total of 1118 PP2 genes in 39 species (Fig. 3). We found that PP2 genes were widespread in monocotyledonous and dicotyledonous plants, and the number of PP2 genes varied greatly in different plants. By performing McScanX, four duplication types were detected in cotton’s PP2 genes, including WGD, tandem, dispersed, and transposed duplication events. Obviously, the WGD events played the main role in the expansion of PP2 genes, followed by dispersed and tandem duplication. Similarly, the WGD is also the driving force in the expansion of PP2 genes in Salix purpurea, Glycine max. However, in chosen species in this study (51.82%), PP2 genes were mainly affected by the tandem duplication events, for instance, 64.29%, 61.11%, and 60.00% of PP2 genes in C.sativus, V.vinifera, and C.clementina were expanded by tandem duplication. Additionally, dispersed duplication events are also an essential factor in expanding the PP2 genes in many species, such as C.papya and O.sativa.
The number of identified PP2 genes and the proportion of genes derived from different modes of duplication events in each plant species. The schematic diagram of the phylogeny of different plant species is retrieved from the TimeTree database (http://timetree.org/)
For allotetraploid species G.hirsutum and G.barbadense, the PP2 genes conserved well during the allotetraploidization event. PP2 genes distribute on nine chromosomes in each Gossypium genome or subgenome, the distribution of PP2 in allotetraploid species is similar to their parental diploid species (Fig. 4, Fig. S2). Moreover, the number of PP2 genes in each allotetraploid is almost the sum of the two diploid species. Similarly, the number of PP2 genes in Brassica napus was almost the sum of the two diploid species Brassica oleracea and Brassica rapa (Fig. 3). These results indicate that the allotetraploidization events also play a vital role in the expansion of the PP2 genes, and they could be conserved during this evolution process.
The chromosome distribution and collinearity analyses of PP2 genes. GaA01-GaA13 and GrD01-GrD13 correspond to the chromosome of G.arboreum and G.raimondii, respectively; GhA01–GhA13, GhD01-GhD13 represent chromosomes of the At sub-genome, and the Dt sub-genome of G. hirsutum. The collinearity gene pairs between the allotetraploid and the diploid species are linked by blue and orange lines
We also analyzed the non-synonymous (Ka) to synonymous (Ks) substitution rates of PP2 gene pairs detected in each diploid Gossypium species and each subgenome of allotetraploid species. All of the Ka/Ks ratios of gene pairs were lower than 1, which indicated that all the PP2 genes might experience purifying selection pressure during evolution (Table S2).
Analyses of cis-regulatory elements, expression profiles, and co-expression of GhPP2s
The 2000-bp upstream sequences of the GhPP2s were uploaded to the PlantCARE website to detect the cis-elements, many cis-elements were identified related to the stress response, light response, hormone response, growth and development were detected. Among these cis-elements, the MYB, MYC, and STRE elements were widely found in the promoter of GhPP2s, indicating that abiotic stress might induce the expression of GhPP2 genes (Fig. S3).
A WGCNA analysis was employed to further explore the function of GhPP2s. The results showed that GhPP2-33, GhPP2-32, GhPP2-41, GhPP2-44, and GhPP2-46 were detected in a network related to salt stress. Many transcription factors were also found in this network, such as GhWRKY47 (GH_A11G1091), GhTFIIS (GH_A09G2334), and GhMYB6 (GH_D05G2774), which suggests that many GhPP2s might co-expression with transcription factors to respond to salt stress (Fig. 6, Table S3).
Next, the qRT–PCR assays were performed to investigate the relative expression of GhPP2s at 0, 2, 4, 6, 8, 10, 12, and 24 h after the salt treatments. The results displayed that GhPP2s were upregulated after NaCl treatments at specific time points. For instance, GhPP2-7 and GhPP2-43 were upregulated after 1 h and 3 h salt treatment, while downregulated in the following hours. GhPP2-15 was downregulated at 1 h-12 h while upregulated at 24 h. The GhPP2-33 showed higher expression at all of the time points compared with the 0 h, especially the expression at 3 h and 24 h were 23–26 times higher than the 0 h (Fig. 6). These results indicate that GhPP2s could be involved in salt tolerance in plants, and we speculated that the GhPP2-33 might play a vital role in responding to salt stress.
Silencing of GhPP2-33 attenuates salt tolerance in Gossypium hirsutum
Based on the above results, we select GhPP2-33 as the candidate gene to perform the VIGS assay to measure its function in the salt treatment. The photobleaching phenotype of pTRV2:PDS plants and the qRT-PCR experiments confirmed the efficiency of the VIGS experiment (Fig. 7B and C). The pTRV2:GhPP2-33 and pTRV2:00 plants showed similar phenotypes, and their MDA and proline content showed no significant difference before the salt treatment. However, after the 400mM NaCl treatment for two days, we observed that the pTRV2:GhPP2-33 plants showed more wilt phenotype than the control plants (pTRV2:00) (Fig. 7A). Additionally, after the salt treatment, the content of malondialdehyde (MDA) and proline in pTRV2: GhPP2-33 plants were noticeably higher and lower than the control plants, respectively (Fig. 7D and E). Therefore, the salt tolerance of the GhPP2-33 silencing plant is weaker than the control plants, and we hypothesize that GhPP2-33 could play a role in the salt stress tolerance.
Discussion
The phloem-based defense (PBD) plays an essential role in various biological processes in the plant, and the PP2 proteins are one of the most critical factors in the PBD [2,3,4,5]. However, research on PP2 proteins in cotton is still lacking. In this study, the PP2 proteins were comprehensively characterized, including their phylogenetic relationship, gene expansion, selection pressure analysis, and expression analysis. Moreover, the WGCNA analysis displayed that the GhPP2-33 might co-expression with many TFs and other GhPP2s in a network related to salt stress. The VIGS assays further confirmed that could play a positive role in responding to the salt stress in cotton. This study will provide essential information for further investigation of the function of cotton PP2 proteins.
The evolution of PP2 genes in cotton
In the present study, we identified PP2 proteins in four cotton species and analyzed their properties. A total of 138 PP2 genes were identified in different cotton varieties (G.arboreum, G.raimondii, G.hirsutum, and G.barbadense), and these genes could be classified into six subfamilies, the structure of PP2 genes is relatively conserved in each subfamily (Figs. 1 and 2).
The Gossypium genus is a perfect model to research polyploidization [16, 20, 21]. In this study, many types of duplication events were detected in the expansion of PP2 genes, and the WGD plays the most critical role in the expansion of PP2 genes in Gossypium species. The tandem duplication plays a more vital role in the expansion of PP2 genes in many species, such as B.rapa and B.napus, although the Brassica species experienced many rounds of WGD events [22]. Besides, the dispersed duplication event also plays an essential role in the expansion of PP2 genes in many species, such as C.papya and O.sativa (Fig. 3). Therefore, the expansion pattern of PP2 genes is very diverse in different species. In addition, the Ka/Ks ratio of gene pairs in Gossypium species generated by multiple duplication events was calculated, and all of the results were lower than 1, indicating that all of the PP2 members might underwent purifying selection pressure during evolution (Table S2).
On the other hand, in each allotetraploid cotton species G.hirsutum and G.barbadense, the number of identified PP2 genes was almost the sum of the number of PP2 genes in diploid cotton varieties G.arboreum and G.raimondii (Fig. 4 and Table S1). The Brassica species are also an ideal model in the research of WGD and allotetraploidization events [22]. Similarly, the number of PP2 genes in allotetraploid species B.napus is almost the sum of diploid species B.rapa and B.olerecea (Fig. 3). Besides, the physical and chemical characteristics of the allotetraploid cotton were also similar with the diploid species (Table S1). These results showed that polyploidization events, including WGD and allotetraploidization events, play a significant role in the expansion of the PP2 gene family in Gossypium species, and strengthen the previous conclusion that the allotetraploid cotton species originated from the natural hybridization of the two diploid progenitors.
The expression and function analysis of GhPP2s
Previous studies show that PP2 genes play significant roles in response to salt stress in other plant species. For instance, AtPP2-B11 could play a positive role in salt stress in Arabidopsis [10]. In Cucumis sativus, the overexpression of CsPP2-A1 could play a positive role in preventing aphid attacks and enhancing salt tolerance [14].
In this study, many stress-responsive cis-elements for the plant were found in the promoter region of almost all PP2 genes (Fig. S3). Next, we further found that many PP2 genes, such as GhPP2-33, were played as hub genes in a salt-stress-related co-expression network by performing WGCNA analysis (Fig. 5). And many TFs were also detected in this network, including the members from the WRKY, TFIIS, and MYB gene family, which might play a role in salt tolerance base on the previous studies [23,24,25]. In addition, by performing the qRT-PCR assays, many GhPP2 genes could express higher under the salt stress, including GhPP2-33 (Fig. 6). Thus, we speculate GhPP2-33 might alter the salt stress in cotton.
A co-expression network related to salt stress of GhPP2-33 All the gene networks are constructed by the weighted gene co-expression network analysis (WGCNA), each node represents a gene. The information regarding genes displayed in the network is listed in the Table S3
To further confirm the role of GhPP2-33 in salt tolerance, we performed VIGS assays. After salt treatment, the silencing of GhPP2-33 in cotton by VIGS experiment showed more wilted leaves in pTRV2:GhPP2-33 plants than that in control plants (Fig. 7A). The measurement of MDA and proline are often used as the marker in displaying plant’s responses to abiotic and biotic stresses. MDA could represent the degree of lipid oxidative damage, while proline is a protective agent against osmotic stress [26, 27]. pTRV2:GhPP2-33 plants possessed higher MDA, whereas the proline contents showed lower content than the control plant, suggesting that the GhPP2-33 plays a positive role in responding to salt stress (Fig. 7D and E).
Silencing GhPP2-33 via VIGS decreases salt tolerance in cotton. A Phenotype of pTRV2:GhPP2-33 plants under 400 mM NaCl treatment. B Albino phenotype of pTRV2:PDS plants. C Relative expression of GhPP2-33 in pTRV2:00 and silencing pTRV2:GhPP2-33 plants via qRT-PCR analysis. D MDA contents. E Proline contents. Error bars represent the standard deviation of three independent biological replicates. (*p < 0.05; ***p < 0.001 when compared with the pTRV:00 group)
Conclusions
In this study, a comprehensive analysis was performed to identify the PP2 proteins in the four Gossypium species. These PP2 proteins could be divided into six subfamilies, and the WGD and allotetraploidization events were the driving forces to expand the PP2 members in cotton. The analysis of cis-elements and the qRT-PCR experiments revealed that GhPP2s could participate in salt stress tolerance. Additionally, the WGCNA analysis revealed that GhPP2-33 might play a role in salt stress tolerance. Finally, the VIGS assays displayed that the silencing of GhPP2-33 could decrease salt tolerance. This study provides a comprehensive understanding of the PP2 proteins.
Materials and methods
The data retrieval and identification of PP2 proteins
A total of 39 species were chosen in this study, and the detail of their genome and annotation was listed in Table S4. By performing the hmmsearch with hidden markov model file of phloem protein 2 (PF14299.9) with the default parameter, the PP2 proteins were identified [28]. The protein sequences with the PP2 domain were further confirmed by the InterProscan program [29]. The protein properties of identified PP2 proteins were predicted by the ProtParam module in Biopython [30].
Phylogenetic analyses
The identified PP2 proteins in Arabidopsis thaliana were obtained from a previous study [6]. Then, the PP2 proteins in Gossypium spp. and Arabidopsis were aligned using MAFFT (v7.310) [31]. Next, the BMGE was used to remove gaps in the alignment with the BLOSUM62 matrix and the gap rate cut-off of value is 50% [32]. The aligned protein was then used to construct a phylogenetic tree using the FastTree program with the LG model and finally visualized in evolview (http://www.evolgenius.info/evolview/) website [33, 34].
Identification of gene collinearity and specific duplication events
To analyze gene collinearity, MCScanX software was performed to search all collinearity gene pairs between the different species and subgenomes with default parameters, and the circos (version 0.69–9) software was used to visualize the results [35, 36]. The duplication events were detected by the duplicate_gene_classifier program of the MCScanX software [35]. The gene pairs in the above duplication events were identified and classified by Dupgen_finder (https://github.com/qiao-xin/DupGen_finder) [37].
The calculation of selective pressure
The detected gene pairs were further aligned by performing MAFFT software and formatted into an AXT format using the ParaAT pipeline [31, 38]. Next, the synonymous rate (Ks), nonsynonymous rate (Ka), and their ratio (Ka/Ks) of each gene pair were calculated by Kaks_calculator (v2.0) [39].
The gene structure and conserved motifs analyses
The exon/intron position information in Gossypium spp. was extracted from the GFF/GTF files. In addition, the full-length protein sequences were submitted to the MEME website (http://meme.sdsc.edu/meme/itro.html) to detect motifs [40]. The results were displayed by TBtools software (version 0.1098765) [20].
The identification of cis-elements
The 2000bp upstream genomic DNA sequences of PP2 genes were submitted to the PlantCARE website (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) to predict the cis-acting elements [41].
RNA-seq and WGCNA analysis
The transcriptome data were retrieved from a previous study (Accessions: PRJNA490626), which included seedlings treated with salt and drought at 1 h (hour), 3 h, 6 h, 12 h, and 24 h [17]. To generate clean reads, the raw RNA-seq reads were filtered by Trimmomatic with the default parameter (v0.3.9) [42]. Next, by performing HISAT2 (v2.1.0), the clean reads were mapped to reference genome (ZJU2.1) to produce SAM (Sequence Alignment/Map) format data and then converted to BAM (Binary Alignment/Map) format data using Samtools (v 1.9) [17, 43, 44]. The BAM files were assembled into transcripts and generated FPKM (Fragments Per Kilobase of transcript per Million mapped reads) using StringTie (v2.0) [45].
In this study, the genes with | logFC |> 1 and p-value 0.05 were chosen as DEGs and identified by edgeR (R version 3.10) [46]. Next, the weighted gene co-expression network, relevant modules, and hub genes were constructed by the WGCNA (version 1.69) package [47]. The weight value was calculated = pickSoftThreshold module in the WGCNA package, and β = 18 was chosen to execute power processing to obtain a scale-free adjacency matrix on the original scaled relationship matrix. The gene clustering and the module division were processed by the topological disparity matrix (dissTOM = 1-TOM) and the dynamic shearing algorithm, respectively. The minimum number of genes in the module was set as 30 (minModuleSize = 30), and the merge threshold of similar modules is 0.25 (cutHeight = 0.25). The final networks were visualized by Cytoscape software (v3.7.2) [47, 48].
Plant cultivation, RNA isolation, and RT-qPCR analysis
The sterilized seeds of G.hirsutum L. (TM-1) were grown in an environment of 16/8 h day/night and 24/16 ◦ C. The three weeks old seedlings were uniformly selected and treated with 400 mM NaCl. Three biological replicates plant samples at 0, 1, 3, 6, 12, and 24 h were freshly collected and frozen in liquid nitrogen and stored at − 80 ◦ C till further analyses. The method of isolation of RNA, RNA reverse transcription, and the qRT-PCR experiments is as previously described [49, 50]. The results were analyzed with the 2−∆∆Ct method [51]. The primers used in RT-qPCR are listed in Table S5.
Virus-induced gene silencing and stresses treatment
The genetic standard TM-1 cultivar was chosen for VIGS assays in this study. A 300bp fragment of GhPP2-33 (291–590bp) was cloned into the pTRV2(pYL156) vector. The vectors pTRV2: 00, pTRV2:GhPP2-33, pTRV2: PDS, pTRV1 (pYL192, helper vector) were transformed into A.tumefaciens strain LBA4404. The experiments of the culture of A.tumefaciens and injection are the same as our previous studies [50, 52]. When the pTRV2: PDS plants showed an albino phenotype, the silencing efficiency of the pTRV2: GhPP2-33 and pTRV2: 00 plants were further determined by qRT-PCR experiments. Next, the pTRV2: GhPP2-33 and pTRV2: 00 plants were treated with 400mM salt for two days. The contents of MDA and Proline were determined using Malondialdehyde (MDA) Assay Kit and Proline Assay Kit according to the standard methods (Solarbio, Beijing, China), respectively.
Availability of data and materials
Genome files of Arabidopsis thaliana, Oryza sativa, Gossypium species, and Brassica species were obtained from TAIR (https://www.arabidopsis.org/), Rice Genome Annotation Project (http://rice.uga.edu/), Cottongen (https://www.cottongen.org/), BRAD (https://brassicadb.org/) respectively. The genome files of the rest species mentioned in the article were retrieved from the Phytozome website (https://phytozome-next.jgi.doe.gov/). The RNA-seq data used in this study were retrieved from the NCBI Sequence Read Archive (SRA) database (https://www.ncbi.nlm.nih.gov/sra) under the accession code PRJNA490626.
References
Koenig AM, Hoffmann-Benning S. The interplay of phloem-mobile signals in plant development and stress response. Biosci Rep. 2020;40(10):BSR20193329.
Will T, Furch AC, Zimmermann MR. How phloem-feeding insects face the challenge of phloem-located defenses. Front Plant Sci. 2013;4:336.
Tjallingii WF. Salivary secretions by aphids interacting with proteins of phloem wound responses. J Exp Bot. 2006;57(4):739–45.
Kehr J. Phloem sap proteins: their identities and potential roles in the interaction between plants and phloem-feeding insects. J Exp Bot. 2006;57(4):767–74.
Lewis JD, Knoblauch M, Turgeon R. The phloem as an arena for plant pathogens. Annu Rev Phytopathol. 2022;60:77–96.
Dinant S, Clark AM, Zhu Y, Vilaine F, Palauqui JC, Kusiak C, Thompson GA. Diversity of the superfamily of phloem lectins (phloem protein 2) in angiosperms. Plant Physiol. 2003;131(1):114–28.
Zhang C, Shi H, Chen L, Wang X, Lu B, Zhang S, Liang Y, Liu R, Qian J, Sun W, et al. Harpin-induced expression and transgenic overexpression of the phloem protein gene AtPP2-A1 in Arabidopsis repress phloem feeding of the green peach aphid Myzus persicae. BMC Plant Biol. 2011;11:11.
Santamaria ME, Martinez M, Arnaiz A, Rioja C, Burow M, Grbic V, Diaz I. An arabidopsis TIR-Lectin two-domain protein confers defense properties against Tetranychus urticae. Plant Physiol. 2019;179(4):1298–314.
Li YZ, Jia FJ, Yu YL, Luo L, Huang JG, Yang GD, Wu CG, Zheng CC. The SCF E3 ligase AtPP2-B11 plays a negative role in response to drought stress in arabidopsis. Plant Mol Biol Rep. 2014;32(5):943–56.
Jia F, Wang C, Huang J, Yang G, Wu C, Zheng C. SCF E3 ligase PP2-B11 plays a positive role in response to salt stress in arabidopsis. J Exp Bot. 2015;66(15):4683–97.
Cheng C, Wang Z, Ren Z, Zhi L, Yao B, Su C, Liu L, Li X. SCFAtPP2-B11 modulates ABA signaling by facilitating degradation in Arabidopsis thaliana. PLoS Genet. 2017;13(8):e1006947.
Kim H, Song E, Kim Y, Choi E, Hwang J, Lee JH. Loss-of-function of Arabidopsis F-BOX protein hypersensitive TO ABA 1 enhances drought tolerance and delays germination. Physiol Plant. 2021;173(4):2376–89.
Li Y, Fan H, Si Y, Guo Y, Wu Z, Du C: The phloem lectin PP2-A1 enhances aphid resistance by affecting aphid behavior and maintaining ROS homeostasis in cucumber plants. Int J Biol Macromol 2022.
Du CX, Si YY, Wang Z, Guo YT, Li YP, Liu C, Fan HF. Overexpression of CsPP2-A1 in cucumber enhanced salt tolerance by participating ABA-JA signaling pathway and antioxidant system. Environ Exper Botany. 2022;204:105095.
Song J, Shang L, Chen S, Lu Y, Zhang Y, Ouyang B, Ye Z, Zhang J. Interactions between ShPP2-1, an F-box family gene, and ACR11A regulate cold tolerance of tomato. Hortic Res. 2021;8(1):148.
Paterson AH, Wendel JF, Gundlach H, Guo H, Jenkins J, Jin D, Llewellyn D, Showmaker KC, Shu S, Udall J, et al. Repeated polyploidization of Gossypium genomes and the evolution of spinnable cotton fibres. Nature. 2012;492(7429):423–7.
Hu Y, Chen J, Fang L, Zhang Z, Ma W, Niu Y, Ju L, Deng J, Zhao T, Lian J, et al. Gossypium barbadense and Gossypium hirsutum genomes provide insights into the origin and evolution of allotetraploid cotton. Nat Genet. 2019;51(4):739–48.
Wang K, Wang Z, Li F, Ye W, Wang J, Song G, Yue Z, Cong L, Shang H, Zhu S, et al. The draft genome of a diploid cotton Gossypium raimondii. Nat Genet. 2012;44(10):1098–103.
Du X, Huang G, He S, Yang Z, Sun G, Ma X, Li N, Zhang X, Sun J, Liu M, et al. Resequencing of 243 diploid cotton accessions based on an updated A genome identifies the genetic basis of key agronomic traits. Nat Genet. 2018;50(6):796–802.
Chen C, Chen H, Zhang Y, Thomas HR, Frank MH, He Y, Xia R. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant. 2020;13(8):1194–202.
Wendel JF, Flagel LE, Adams KL: Jeans, Genes, and Genomes: Cotton as a Model for Studying Polyploidy. In: Polyploidy and Genome Evolution. 2012: 181–207.
Chalhoub B, Denoeud F, Liu S, Parkin IA, Tang H, Wang X, Chiquet J, Belcram H, Tong C, Samans B, et al. Plant genetics. Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science. 2014;345(6199):950–3.
Gu L, Li L, Wei H, Wang H, Su J, Guo Y, Yu S. Identification of the group IIa WRKY subfamily and the functional analysis of GhWRKY17 in upland cotton (Gossypium hirsutum L.). PLoS One. 2018;13(1):e0191681.
Gu L, Wang H, Wei H, Sun H, Li L, Chen P, Elasad M, Su Z, Zhang C, Ma L, et al. Identification, expression, and functional analysis of the group IId WRKY subfamily in upland cotton (Gossypium hirsutum L.). Front Plant Sci. 2018;9:1684.
Ma R, Liu BW, Geng X, Ding X, Yan N, Sun X, Wang WL, Sun XZ, Zheng CS. Biological function and stress response mechanism of MYB transcription factor family genes. J Plant Growth Regul. 2023;42(1):83–95.
Morales M, Munne-Bosch S. Malondialdehyde: facts and artifacts. Plant Physiol. 2019;180(3):1246–50.
Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A. Role of proline under changing environments: a review. Plant Signal Behav. 2012;7(11):1456–66.
Finn RD, Clements J, Eddy SR: HMMER web server: interactive sequence similarity searching. Nucleic Acids Res 2011, 39(Web Server issue):W29–37.
Jones P, Binns D, Chang HY, Fraser M, Li W, McAnulla C, McWilliam H, Maslen J, Mitchell A, Nuka G, et al. InterProScan 5: genome-scale protein function classification. Bioinformatics. 2014;30(9):1236–40.
Cock PJ, Antao T, Chang JT, Chapman BA, Cox CJ, Dalke A, Friedberg I, Hamelryck T, Kauff F, Wilczynski B, et al. Biopython: freely available Python tools for computational molecular biology and bioinformatics. Bioinformatics. 2009;25(11):1422–3.
Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30(4):772–80.
Criscuolo A, Gribaldo S. BMGE (Block Mapping and Gathering with Entropy): a new software for selection of phylogenetic informative regions from multiple sequence alignments. BMC Evol Biol. 2010;10:210.
Price MN, Dehal PS, Arkin AP. FastTree: computing large minimum evolution trees with profiles instead of a distance matrix. Mol Biol Evol. 2009;26(7):1641–50.
Subramanian B, Gao S, Lercher MJ, Hu S, Chen WH. Evolview v3: a webserver for visualization, annotation, and management of phylogenetic trees. Nucleic Acids Res. 2019;47(W1):W270–5.
Wang Y, Tang H, Debarry JD, Tan X, Li J, Wang X, Lee TH, Jin H, Marler B, Guo H, et al. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40(7):e49.
Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19(9):1639–45.
Qiao X, Li Q, Yin H, Qi K, Li L, Wang R, Zhang S, Paterson AH. Gene duplication and evolution in recurring polyploidization-diploidization cycles in plants. Genome Biol. 2019;20(1):38.
Zhang Z, Xiao J, Wu J, Zhang H, Liu G, Wang X, Dai L. ParaAT: a parallel tool for constructing multiple protein-coding DNA alignments. Biochem Biophys Res Commun. 2012;419(4):779–81.
Wang D, Zhang Y, Zhang Z, Zhu J, Yu J. KaKs_calculator 2.0: a toolkit incorporating gamma-series methods and sliding window strategies. Gen Proteo Bioinform. 2010;8(1):77–80.
Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Research. Nucleic Acids Res. 2009;37(Web Server):W202–8.
Lescot M, Dehais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, Rouze P, Rombauts S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002;30(1):325–7.
Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–20.
Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12(4):357–60.
Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R. Genome project data processing S: the sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25(16):2078–9.
Pertea M, Pertea GM, Antonescu CM, Chang TC, Mendell JT, Salzberg SL. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat Biotechnol. 2015;33(3):290–5.
Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–40.
Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9:559.
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–504.
Wei F, Chen P, Jian H, Sun L, Lv X, Wei H, et al. A comprehensive identification and function analysis of Serine/Arginine-Rich (SR) proteins in cotton (Gossypium spp.). Int J Mol Sci. 2022;23(9):4566. https://doi.org/10.3390/ijms23094566.
Chen P, Wei F, Jian H, Hu T, Wang B, Lv X, et al. A comprehensive gene co-expression network analysis reveals a role of GhWRKY46 in responding to drought and salt stresses. Int J Mol Sci. 2022;23(20):12181. https://doi.org/10.3390/ijms232012181.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–8.
Lv X, Wei F, Lian B, Yin G, Sun M, Chen P, An L, Jian H, Wang H, Fu X, et al. A comprehensive analysis of the DUF4228 gene family in gossypium reveals the role of GhDUF4228–67 in salt tolerance. Int J Mol Sci. 2022;23(21):13542.
Acknowledgements
The experiment was performed at the State Key Laboratory of Cotton Biology at the Institute of Cotton Research of the Chinese Academy of Agricultural Sciences.
Funding
This research was supported by the China Agriculture Research System (Grant No. CARS-15–06) and Agricultural Science and Technology Innovation Program of Chinese Academy of Agricultural Sciences.
Author information
Authors and Affiliations
Contributions
Fei Wei and Pengyun Chen conceived and designed the experiments; Fei Wei, Hongliang Jian, Xiaohao Guo, Xiaoyan Lv, Boying Lian, Mengxi Sun and Li An performed experiments; Xinyu Dang, Miaoqian Yang, Hongmei Wu, Nan Zhang and Aimin Wu analyzed the data; Fei Wei took the lead in writing the manuscript with input from all authors; Hantao Wang, Liang Ma, Xiaokang Fu and Jianhua Lu provided critical feedback and helped shape the research, analysis and manuscript. Shuxun Yu and Hengling Wei made the final confrmation of the manuscript. The author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
In this study, the cotton varieties were from the lab of early maturity breeding, Institute of Cotton Research, Chinese Academy of Agricultural Science. These plant materials do not include any wild species at risk of extinction. No specific permits were required for sample collection in this study. We complied with relevant institutional, national, and international guidelines and legislation for plant study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Table S1. Basic information and biophysical properties of the predicted DUF4228 proteins in cotton.
Additional file 2: Table S2.
The Ka and Ks values for duplicated gene pairs.
Additional file 3: Table S3.
The detailed information on the co-expression genes of the WGCNA analysis in this study.
Additional file 4
: Table S4. The plant genome annotation and their version used in this research.
Additional file 5:
Table S5. List of primers used for qRT-PCR analysis.
Additional file 6:
Fig. S1. Domain analysis of GhPP2 proteins. Fig. S2. The chromosome distribution and collinearity analyses of PP2 genes between G.barbadense and its parental diploid species. Fig. S3. cis-element analysis of the promoter of GhPP2s.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wei, F., Chen, P., Jian, H. et al. A systematic analysis of the phloem protein 2 (PP2) proteins in Gossypium hirsutum reveals that GhPP2-33 regulates salt tolerance. BMC Genomics 24, 467 (2023). https://doi.org/10.1186/s12864-023-09546-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-023-09546-4