Abstract
Background
Babesia caballi is an intraerythrocytic parasite from the phylum Apicomplexa, capable of infecting equids and causing equine piroplasmosis. However, since there is limited genome information available on B. caballi, molecular mechanisms involved in host specificity and pathogenicity of this species have not been fully elucidated yet.
Results
Genomic DNA from a B. caballi subclone was purified and sequenced using both Illumina and Nanopore technologies. The resulting assembled sequence consisted of nine contigs with a size of 12.9 Mbp, rendering a total of 5,910 protein-coding genes. The phylogenetic tree of Apicomplexan species was reconstructed using 263 orthologous genes. We identified 481 ves1-like genes and named “ves1c”. In contrast, expansion of the major facilitator superfamily (mfs) observed in closely related B. bigemina and B. ovata species was not found in B. caballi. A set of repetitive units containing an open reading frame with a size of 297 bp was also identified.
Conclusions
We present a chromosome-level genome assembly of B. caballi. Our genomic data may contribute to estimating gene expansion events involving multigene families and exploring the evolution of species from this genus.
Similar content being viewed by others
Background
Babesia caballi is a protozoan parasite (phylum Apicomplexa) known to infect horses, causing equine piroplasmosis. Horses infected with this parasite experience anemia and systemic illness (i.e., fever, lethargy, anorexia, and peripheral edema) [1]. Equine piroplasmosis has a worldwide distribution, being endemic to tropical, subtropical, and some temperate regions. The main vectors of B. caballi include tick species of Dermacentor, Haemaphysalis, Hyalomma, and Rhipicephalus genera [2]. It is estimated that approximately 90% of the world’s horses are bred in endemic areas [3]. Furthermore, the growing international movement of horses has also raised concerns regarding outbreaks in nonendemic areas.
The Babesia bovis genome sequenced in 2007 was the first whole-genome sequence of a species from the Babesia genus [4], followed by the release of the B. microti genome [5]. Both genomes were sequenced using the whole-genome shotgun Sanger sequencing approach, being well assembled and containing few gaps. Whole-genome sequences of B. bigemina, B. divergens, B. ovata, and Babesia sp. Xinjiang species are nowadays also available [6,7,8]. However, despite next-generation sequencing technologies, assembled genomes in these species showed more fragmentation than B. bovis and B. microti genomes (Table 1).
The phylogenetic relationships between B. caballi and related Babesia species are still inconsistent. In a previous study, based on 18S rRNA and cob sequences, B. bovis and B. bigemina were located outside of B. caballi [9]. In contrast, in another study based on β-tubulin and cox3 sequences, B. caballi and B. bigemina located in the same clade and B. bovis appeared outside of them [9]. Another phylogenetic analysis using cox1 and cytb sequences was also conducted [10] but more genes are required to reach a consensus.
Another inconsistency is observed from a morphological point of view. It is known that there are the ridge structures on the surface of B. bovis-infected erythrocytes and they are involved in cytoadhesion and capillary sequestration [10,11,12]. In contrast, neither ridge structures nor sequestration are observed for B. bigemina [13]. In B. caballi, no ridge structures in parasitized erythrocytes are observed [14], but sequestration is believed to be involved in persistent subclinical infection [1]. Interestingly, tubular structures in infected erythrocytes of B. caballi can be identified by electron microscopy [15]; however, specific molecular characteristics of these structures are unknown. Variant erythrocyte surface antigen 1 (VESA1), initially identified in B. bovis [16], is a heterodimeric protein encoded by ves1α and ves1β genes, which comprise the largest multigene family in B. bovis [17, 18]. Proteins encoded by ves1α and ves1β generally have a cysteine- and lysine-rich domain (CKRD) motif and a c-terminal transmembrane domain [17, 18]. It has been shown that ves1α and ves1β are mutually transcribed at a genomic location referred to as locus of active transcription (LAT) [19]. The VESA1 of B. bovis is known to be involved in cytoadhesion and pathogenicity [12, 20]. Sequence homology and Hidden-Markov-Modeling (HMM) analyses have been applied to identify ves1a, ves1b, and ves2 genes in B. bigemina and B. ovata from the B. bovis ves1 gene [6, 7]. Additionally, ves1 and ves2 in B. divergens and other VESA coding genes in Babesia sp. Xinjiang have been identified [6, 8]. Interestingly, ves-like genes in the B. microti genome have not been detected [5]. In Babesia species, VESA likely functions in immune evasion through antigenic variation and plays a role in pathogenicity, although this hypothesis needs further confirmation.
The multi-transmembrane (mtm) family is another important expanded gene family which has recently been identified to encode proteins with eight or more transmembrane domains [21, 22]. In B. bovis, there are two mtm sub-families, A-type and B-type. Although the function of mtm genes is not yet well understood, it has been found that mtm expression is associated with the resistance to the antibiotic blasticidin S. Therefore, it has been suggested that mtm genes may be involved in the transport of specific substrates [22]. The major facilitator superfamily (mfs) is also an expanded gene family encoding multi-transmembrane proteins. In B. bigemina and B. ovata approximately 40 mfs genes have been identified [7, 21, 22]. Moreover, in B. bovis and B. divergens, two mfs genes have been detected, while no mfs genes have been identified in B. bigemina and B. ovata genomes. Similarly to the mtm family, the function of mfs genes remains to be elucidated.
Long-term cultivation of B. bovis causes quasi species in the population which hamper genome assembly (data not shown). Therefore, we developed a subclone, USDA-D6B2, and applied a hybrid sequencing approach using nanopore long reads and Illumina accurate reads to assemble at the chromosome level. It will contribute to further understanding of evolution, adaptation and parasitic capability of the parasite.
Results
De novo assembly of B. caballi genome
Using Oxford Nanopore and Illumina sequencing, we generated 3.89M (5.14 Gbp) long reads and 8.90M (2.67 Gbp) short paired-end reads, respectively. These reads were assembled into nine contigs covering a total of 12,916,698 bp, which also include apicoplast and a mitochondrial genomes (Table 1). The BUSCO completeness analysis revealed that the genome assembly of B. caballi contained 96.8% (432/446) complete, 1.1% (5/446) fragmented, and 2.1% (9/446) of the missing orthologous genes expected to be present in other Babesia species, supporting the idea that missing regions were limited. We detected a telomeric sequence pattern with (TTTAGGG)n. From all nine contigs, three contigs, BcabD6B2_scf02, 04 and 05, showed the telomeric sequence pattern at both terminal ends, and three contigs displayed this sequence at one end, thereby suggesting that BcabD6B2_scf02, 04 and 05 contained completely sequences chromosomes. Moreover, contigs corresponding to apicoplast and mitochondrial genomes did not show telomeric sequences.
Gene model prediction
In total, 5,910 protein coding genes were predicted with AUGUSTUS and then validated using transcriptomic data (Table 1). The average and median coding sequence (CDS) lengths were 1622.7 bp and 1173 bp, respectively. Approximately 74.8% of the B. caballi genome consists of CDSs. B. caballi contains 47 and 18 tRNA genes scattered on nuclear (chromosomes) and apicoplast genomes, respectively (Table 1). Moreover, nine 5.8S, four 18S, and three 28S rRNA genes were identified (Table 1). We also detected three intact 18S-5.8S-28S rRNA ribosomal loci.
Phylogenetic analyses
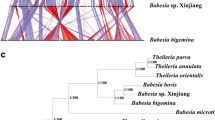
We generated a phylogenetic tree using 263 orthologous genes which were identified in ten different species (i.e., B. bovis, B. bigemina, B. ovata, B. microti, B. divergens, Babesia sp. Xinjiang, T. equi, P. falciparum, T. gondii, and B. caballi). To construct the phylogenetic tree, orthologous gene sequences were translated, and aligned amino acid sequences were concatenated (Fig. 1). The genome-wide phylogenetic tree indicated that B. caballi was closer to B. bigemina and B. ovata than B. bovis and Babesia sp. Xinjiang.
Orthologous-gene-based phylogenetic analysis. The phylogenetic tree was constructed using 263 orthologous genes conserved among B. caballi, B. bovis, B. bigemina, B. ovata, B. microti, B. divergens, Babesia sp. Xinjiang, P. falciparum, and T. gondii. The arrowheads represent estimated expansion events for each gene family and the de novo emergence of repetitive sequences
Subsequently, a phylogenetic analysis based on apicoplast genomes from B. caballi, B. bovis, B. orientaris, B. sp. Xinjiang, B. ovata, B. gibsoni, and B. microti was also performed. First, dotplots were used to verify that no recombination occurred among apicoplast genomes (Fig. S1). The topology of the apicoplast-based phylogenetic tree was inconsistent with the results of the phylogenetic analysis using orthologous genes (Fig. S2).
The V4 regions of the three 18S rRNA genes in the D6B2 B. caballi strain were clustered with representative sequences from Clade A1 (Fig. S3).
We also examined the presence and absence of orthologous genes in the B caballi genome. From this analysis, 33 and 316 lost genes were found (Table S1). The functional enrichment analysis of orthologous genes was performed, but no significant enrichment was found.
Multigene families
The HMM model identified 481 ves1-like genes in the B. caballi genome (Table S2). Homology between ves1-like genes of B. caballi, ves and small open reading frame (smorf) genes of B. bovis, B. bigemina, B. divergens, and Babesia sp. Xinjiang was verified, showing that B. caballi ves1-like genes were clustered separately from other Babesia ves genes (Fig. 2A). Nonetheless, we found certain similarity between B. caballi ves1-like and B. bigemina ves1b genes; therefore, we named B. caballi ves1-like genes as ves1c. These ves1c genes shared sequence similarity with several Babesia sp. Xinjiang ves and B. divergens ves1 genes; however, no significant similarity to B. bovis ves1α, ves1β, and smorf genes or B. bigemina ves1a and ves2 genes was detected in the analysis. For further validation, sequence alignment and phylogenetic analysis were performed for B. caballi ves1-like genes; however, no alignment remained after removing the gap. Transcriptome of ves1c genes showed that the number of mapped read to one of the ves1c genes, BcabD6B2_18120, accounted for 38% of all reads mapped to the 481 ves1c genes. It was located at the terminal of contig 2 (Fig. 2B). The second and third most abundant genes were BcabD6B2_30960 and BcabD6B2_10260, accounting for 9% and 8% of all reads for ves1c genes, respectively.
Characterization of ves1c genes in B. caballi. A Sequence homology cluster analysis based on sequence similarity among ves-related genes of B. caballi, B. bigemina, B. divergens, B. bovis, and Babesia sp. Xinjiang. Each node represents a protein-coding gene in the five parasites species analyzed. The edges represent similarity between nodes. B Distribution of ves1c genes in B. caballi genome. The vertical lines represent ves1c loci. The horizontal lines represent contigs and their corresponding ID. The locus of the exclusively expressed ves1c gene, BcabD6B2_18120, is highlighted in red. Telomeric repeats are indicated by arrowheads
Smorf is another multigene family initially identified in B. bovis. We examined whether there are any homologous genes in the B. caballi genome by using blastp against 44 smorf genes in B. bovis. The most similar gene was BcabD6B2_03440, showing only 22.2% sequence identity and an e-value of 2.37E-05. The HMM model did not identify any B. caballi sequence related to smorf genes of B. bovis. (Fig. 2A).
A total of 29 predicted genes encoding proteins with eight or more predicted transmembrane domains were identified in the B. caballi genome. The number of multi-transmembrane protein-coding genes identified in B. bovis, B. bigemina, B. divergens, and Babesia sp. Xinjiang genomes were 98, 85, 55, and 56, respectively. Therefore, our results suggest that the B. caballi genome has fewer genes encoding multi-transmembrane proteins than other Babesia species. While predicted multi-transmembrane genes of B. bovis, B. bigemina, B. divergens, and Babesia sp. Xinjiang grouped in the same cluster, no specific clustering pattern was observed for B. caballi multi-transmembrane protein-coding genes (Fig. 3).
Other multigene families were searched for similarity against the predicted B. caballi genes (Fig. 4 and Table S2). The biggest cluster was cluster 01, which consisted of most ves1c genes identified. Moreover, Delta-BLAST revealed that 393 out of the 450 genes in cluster 01 have a VESA1 N-terminal (gnl|CDD|315457) (Table S3A). Among the 450 genes, we identified 259 genes similar to a Babesia sp. Xinjiang ves gene, BXIN_1821 (Table S3B). In addition, other Babesia sp. Xinjiang ves genes also shared high similarity levels with ves1c genes and even ves genes of B. bigemina, B. bovis, and B. divergens shared certain sequence similarity (Fig. 2A). Cluster No. 02, 04, 05, 07, and 08 also contained genes with VESA1 N-terminal (gnl|CDD|315457); however, no corresponding clusters in other Babesia species were found (Table S3A), but most genes were not identified as ves-like genes. Cluster 03 contained genes with a putative reverse transcriptase motif (Table S3A). A similar expansion event involving genes encoding a putative reverse transcriptase motif in B. ovata genome has also been reported [7]. Expansion of clusters No. 06, 09, 10, and 11 were unique in B. caballi; although their functions were unclear. For further validation, sequence alignment and phylogenetic analysis were performed for genes in each cluster; however, no alignment remained after removing the gap for clusters 01 to 06 and 11.
Repeat sequences
Tandem repeat sequences of the B. caballi genome were examined using Tandem Repeats Finder. One of the most significant ones was found in the contig, BcabD6B2_scf02, at a position from 2,073,070 bp to 2,095,944 bp (Fig. 5). The repetitive unit consisted of 297 bp with an open reading frame (ORF) encoding 99 amino acids. The largest repeated sequence consisted 17.9 repetitive units, with some having frameshift mutation, while the largest ORF encoded 599 amino acids. Genomic regions with 8.9, 6.9, 5.9, and 4.4 repetitive units were also identified. The analysis of B. bovis, B. bigemina, B. ovata, B. divergens, B. microti and Babesia sp. Xinjiang genomes did not identify homology to this repeat sequence. The repeat unit was found to fall into cluster 11 (Fig. 4 and Table S3). Other repeat sequences scattered throughout the genome were also examined using RepeatScout (Table S4). Most of the identified repeats were ves-related. The tandem repeats found above partially overlapped with R = 122. Sequences potentially coding reverse-transcriptase were found, consistent with cluster 3 observed in the sequence homology cluster analysis (Fig. 4 and Table S3). The most abundant repeat sequence was R = 8, covering 707 Kbp across the genome. It consisted of 4.5 repetitions of approximately 1,750 bp units encoding up to approximately 200 amino acids of unknown function.
Schematic representation of BcRS1. The light gray boxes represent intact ORFs. The dark gray boxes represent disrupted ORFs with frameshift or truncated site. Numbers represent nucleotide positions in the contig, BcabD6B2_scf02. Nucleotide and encoded amino acids of the repetitive unit are shown at the bottom
Discussion
Using a hybrid sequencing approach, we obtained nine contigs capable of reconstructing almost all complete chromosomes of B. caballi (Table 1). Indeed, three contigs exhibited telomeric sequences at both terminal ends, suggesting completed end-to-end sequencing of whole chromosomes (Fig. 2). The BUSCO analysis also supported high integrity in the sequenced and assembled contigs. The long-read sequencing technique likely contributed to this good performance. In addition, sub-cloning might also have helped a successful assembly process since the draft genome of B. ovata prepared without this step seemed to be poorly assembled even though long-read sequencing was also conducted [7]. In this regard, we observed that the genetic diversity of B. bovis progressively increased during in vitro culture, likely due to segmental gene conversion (unpublished data).
Previously, a phylogenetic study based onβ-tublin and cox3 genes inferred that B. caballi and B. bigemina form a clade separated from B. bovis [9]. In contrast, another study using 18S rRNA and cob genes and combined cox1 and cytb sequences indicated B. bigemina and B. bovis form a clade separated from B. caballi [9, 10]. To provide a comprehensive phylogenetic analysis of the relationship between B. caballi and other species from the Babesia genus, we constructed a phylogenetic tree using 263 orthologous genes. Our results indicated that B. caballi was phylogenetically closer to B. bigemina than B. bovis (Fig. 1). We further examined this phylogeny using apicoplast genome sequences. Firstly, we performed a dotplot analysis to confirm the absence of specific structural variation in the B. caballi apicoplast genome (Fig. S1). Subsequently, we observed that the topology of the phylogenetic tree based on apicoplast genomes differed from that using concatenated orthologous gene sequences. However, both phylogenetic trees supported the notion that B. caballi is closer to B. ovata than B. bovis or Babesia sp. Xinjiang (Fig. 1 and Fig. S2). Collectively, our results are not conclusive on the evolutional history of Babesia species, but the phylogenetic tree using orthologous genes seems to be more consistent because it was constructed using 263 gene sequences encoding 118,671 amino acids scattered throughout the genome and phylogenetic tree based on the apicoplast genomes was constructed by much shorter nucleotides alignment [4, 6, 7]. Genetic diversity among B. caballi strains/isolates has been examined using 18S rRNA gene sequences [23,24,25]. Those studies differ in their nomenclature of clusters, namely alphabetical (A, B, C) or numerical (1, 2, 3). Moreover, it has been shown that the V4 hypervariable region can discriminate clades at a high resolution [26]. We followed this approach and found the B. caballi genome encoded three set of 18S rRNA genes clustered together in Clade A1. This is consistent with the observation that Clade A1 is distributed worldwide, including Florida (USA), where the USDA strain used in this study was isolated.
It is known that Apicomplexan parasites have different telomeric sequence patterns [27]. For instance, Cryptosporidium parvum, Theileria annulata, and B. bovis exhibit TTTAGG, TTTTAGGG, and TTTAGGG, respectively. Our our results revealed that telomeric sequences are conserved between B. caballi and B. bovis, suggesting that telomeric patterns may also be evolutionarily conserved in Babesia species; however, more evidence is required to support this hypothesis.
We estimated that the B. caballi genome contains 5,910 protein-coding genes. It was approximately more than 800 genes than closely related B. bigemina and B. ovata [6, 7]. The well-assembled genome sequence obtained in this study likely contributed to this difference. Moreover, the expansion of ves genes in B. caballi genome might also be another factor associated with the high number of protein-coding genes.
The variant erythrocyte surface antigen 1 (VESA1) heterodimeric protein complex is encoded by a multigene family originally identified in B. bovis [16]. It has been shown that other Babesia species, including B. bigemina and B. ovata, B. divergens, and Babesia sp. Xinjiang, encode ves-related genes [6,7,8]. In this study, we identified 481 putative ves-like genes (Table S2). Sequence homology revealed that B. caballi ves-like genes formed a well-differentiated cluster with sequence similarity to B. bigemina ves1b genes (Fig. 2A). Therefore, we named this novel gene family “ves1c”. We further performed alignment and phylogenetic analysis for the ves1c genes; however, no sequence alignment remained after removing gaps. It suggests a high diversity not only among ves-genes of different species, but also among the same species. Our transcriptome analysis revealed that the transcripts of BcabD6B2_18120 gene accounted for 38% of total transcripts of ves1c genes, suggesting that transcriptional regulation of B. caballi ves1c genes is similar to that in ves1 genes of B. bovis and B. bigemina [4, 6]. Interestingly, the most abundantly transcribed ves1c gene, BcabD6B2_18120, were located in the telomeric vicinity of the contig 2 (Fig. 2B).
We identified several repetitive sequences, being the most representative found in the contig, BcabD6B2_scf02, at a position from 2,073,070 bp to 2,095,944 bp. We named this repetitive sequence “B. cabali repetitive sequence 1 (Bcrs1)” (Fig. 5). Bcrs1 has a size of 297 bp encoding 99 amino acids, which are arranged in a tandem array containing almost 44 units, i.e., including both intact and incomplete units. The function of Bcrs1 is unknown since it has homology with neither Apicomplexan nor other species sequences. Therefore, Bcrs1 is also a promising target for species-specific sequence identification.
The occurrence of gene expansions during the evolution of Babesia species could be inferred by integrating phylogenetic information (Fig. 1). The ves comprise a unique gene family present in all member of the Babesia genus except B. microti. The ves1c, ves1b, and Xinjiang ves shared certain similarity (Fig. 2), suggesting that they evolved from the same ancestral genes. On the other hand, ves1a and ves2 genes likely underwent an expansion after the divergence between B. caballi and the ancestral species of B. bigemina and B. ovata. The mtm genes form another gene family characterized to have eight or more transmembrane domains. The mtm gene family includes several sub-family, i.e., A-type and B-type (only identified in B. bovis) and the mfs-expanded-type in B. bigemina and B. ovata. However, mfs genes were absent in B. caballi (Figs. 1 and 3), suggesting gene expansion after the speciation process that originated B. caballi and before the divergence of B. bigemina and B. ovata. It is also suggested that the repetitive expansion of Bcrs1 occurred after the speciation because Bcrs1 is present only in B. caballi but absent in B. bigemina and B. ovata (Fig. 1). We hypothesize that this type of species-specific gene expansion event could be associated with host specific parasite adaptation.
Methods
Obtention of the B. caballi strain and culture
The B. caballi USDA strain was isolated from ticks in Florida, the USA in the 1960s and maintained in horses by serial passage of blood [28]. The B. caballi-infected horse blood was obtained from U. S. Department of Agriculture (Ames, Iowa). The parasite was isolated as previously described [29], and cultured in vitro in RPMI1640 medium (Sigma-Aldrich, Tokyo, Japan) containing 40% horse serum, 13.6 µg/ml hypoxanthine (Sigma-Aldrich), 1% GlutaMAX-I (Sigma-Aldrich), and 10% horse red blood cells (RBCs). We developed the USDA-D6B2 subclone, which derived from two rounds of limiting dilution.
Genomic DNA extraction, library construction, and sequencing
B. caballi-infected RBCs were treated with saponin to remove hemoglobin and the genomic DNA was then extracted by the Wizard Genomic DNA Purification Kit (Promega), following the manufacturer’s instructions. The library was constructed using the Ligation Sequencing Kit, SQK-LSK109 (Oxford Nanopore Technologies) and sequenced using a FLO-MIN107 flowcell (Oxford Nanopore Technologies). The Fast5 files generated by MinION were basecalled with guppy_basecaller: version 3.2.4 + d9ed22f (Oxford Nanopore Technologies). A PCR free library with 350-bp was constructed using the NEBNext Ultra IIDNA Library Prep Kit (NEB) provided by Novogene Ltd and 150 bp paired-end sequences were obtained using the Illumina NovaSeq 6000 platform (Illumina).
RNA-seq analysis
Total RNA was extracted from in vitro cultured B. caballi parasites using TRIzol reagent (Sigma), following the manufacturer’s instructions. The quality and quantity of the purified RNA were assessed using a Bioanalyzer (Agilent). The library was constructed using the TruSeq Stranded mRNA LT Sample Prep Kit (Illumina), and 300 bp paired-end reads were sequenced using Miseq (Illumina). The obtained reads were aligned with TopHat2 v2.1.1 [30] and counted using HTSeq 2.0.2 [31].
De novo genome assembly
The MinION long reads of B. caballi were assembled using Canu version 1.5 based on the following parameters: genomeSize = 10m -nanopore-raw ovsConcurrency = 80 [32]. The resulting contigs were polished using the Illumina reads by pilon version 1.22 [33]. A total of seven iterations were applied until results remained constant. Apicoplast genome was identified using Blast against the B. bovis apicoplast genome. It was visualized by YASS [34] then manually circularized. The mitochondrial genome was not found in the original genome assembly. A second assembly was constructed by abyss-pe assembler from the ABYSS program v2.1.5 [35] using the Illumina reads based on k = 128 parameter. The mitochondrial sequence was identified by Blast against the B. bovis mitochondrial genome.
Gene model estimation and functional annotation
Protein-coding gene models were estimated with AUGUSTUS version 3.3.3 [36] using the RNA-seq data mentioned above. Trained parameters were obtained by webAugustus [37] using a B. bovis genome sequence (PiroplasmaDB-5.1_BbovisT2Bo_Genome.fasta) and a full-length EST set (B.bov.FL-EST.fa) retrieved from PiroplasmaDB and DB-AT, respectively [38, 39]. The tRNAs and rRNAs were annotated using tRNAscan-SE 2.0 [38] and barrnap version 0.8, respectively. The functional annotation of protein-coding genes was performed with blast2go [39]. Gene models for the apicoplast and the mitochondria genomes were predicted with DFAST [40].
Acquisition of publicly available sequences and gene annotation
For comparative genomic analysis among Apicomplexan parasites, we used genome assembly in fasta format and annotation in general feature format (gff) available at the PiroplasmaDB database, i.e., obtained from PiroplasmaDB-37 (B. bovis T2Bo strain), PiroplasmaDB-36 (B. bigemina BOND strain), PiroplasmaDB-40 (B. ovata Miyake strain and B. microti RI strain), PiroplasmaDB-46 (B. divergens 1802A strain), PiroplasmaDB-55 (Babesia sp. Xinjiang), PiroplasmaDB-55 (T. equi), ToxoDB-40 (T. gondii ME49 strain), and PlasmoDB-56 (P. falciparum 3D7 strain).
Phylogenetic analyses
An orthology inference analysis was conducted by the OMA package (v2.5.0) [41] with default parameters. Putative orthologous genes encoded by the genomes of the parasitic species specified before were selected. Amino acid sequences of these orthologous were aligned using MAFFT [42] with default parameters. After the gaps were trimmed, the remaining sequences were concatenated. We then constructed a phylogenetic tree based on the maximum likelihood method with MEGA version 10.0.5 [43]
The structural variation among B. caballi (BPLF01000008), B. bovis (NC 011395), B. orientaris (KT 428643), Babesia sp. Xinjiang (KX881914), B. ovata (BDSA01000044), B. gibsoni (MN481613), and B. microti (LK028575) apicoplast genomes were validated. First, genome positions were sorted using the 50S ribosomal protein L2 gene, and dotplots were generated with YASS [34]. Subsequently, phylogenetic relationships among apicoplast genomes were also examined.
A phylogenetic tree was constructed using three 18S rRNA loci (BcabD6B2_91080, BcabD6B2_91090, and BcabD6B2_91140) and representative sequences were used for intra-species [26] and inter-species analysis [9]. A total of 68 sequences were aligned with MUSCLE [44] in MEGA version 10.0.5 [43], and a conserved region of 247 bp length comparable to the V4 region was selected to construct a phylogenetic tree by the neighbor-joining method [45].
Uniquely gained and lost genes in B. caballi
The uniquely gained genes in the B. caballi genome were defined as those t with no orthologous sequences in B. bovis, B. bigemina, B. ovata, B. divergens, or Babesia sp. Xinjiang genomes. A gene ontology (GO) enrichment analysis of uniquely gained and whole-predicted B. caballi genes was performed using agriGO [46]. The uniquely lost genes were defined as the genes conserved in the five Babesia species mentioned above but with no orthologous in B. caballi. B. bovis genes retrieved from the PiroplasmaDB database were used as representatives in GO enrichment and metabolic pathway enrichment analysis.
Identification of multigene families
The members of ves and smorf multigene families in the B. caballi genome were searched using Hidden Markov Model. The training data set was constructed using publicly available genomes and annotations mentioned above. Specifically, ves1α, ves1β, and smorf genes from B. bovis were retrieved from gff annotation files by searching “variant erythrocyte surface antigen-1, alpha”, “variant erythrocyte surface antigen-1, beta”, and “smorf”, respectively. Babesia divergens ves1 genes were retrieved from the gff by searching “variant erythrocyte surface antigen”. Babesia bigemina ves1a, ves1b, ves1ba, and ves2 genes were retrieved from the Sanger database (https://www.sanger.ac.uk/resources/downloads/protozoa/babesia-bigemina.html). Babesia xinjiang ves were retrieved from a previous study [8]. Hidden Markov Models for each gene set were constructed, and ves-related genes in B. caballi genome were identified using HMMER version 3.3.2 [47]. Babesia caballi genes and ves and smorf genes in B. bovis, B. divergens, B. bigemina, and Babesia sp. Xinjiang genome were aligned using BLASTP [48]. We selected genes showing a bit-score higher than 200, and mutual similarity was visualized using the Gephi by a Fruchterman–Reingold layout [49].
To identify proteins having equal or more than eight predicted transmembrane domains and at least one TM domain per 100 amino acids, the multi-transmembrane gene family in the B. caballi genome was examined using TMHMM version 2.0c [50]. The same methodology was applied in B. divergens and Babesia sp. Xinjiang genome. Genes from the multi-transmembrane family in B. bovis and B. bigemina genomes have been retrieved from a previous study [22]. Sequence similarity among genes was examined and visualized using the method mentioned before.
To find other multiple gene families, genes in the B. caballi genome were aligned to themselves using BLASTP. Genes with a bit-score higher than 200 were selected, and mutual sequence similarity was visualized using Gephi by a Fruchterman–Reingold layout [49]. Clusters were assigned by the modularity algorithm of Gephi. The function of the gene sets were estimated using BLASTP with the NR database and Delta-BLAST for functional motifs in the CDD database provided by NCBI.
Identification of repetitive and repeat sequences
Repetitive sequences in the B. caballi genome were searched with Tandem Repeats Finder version 4.09 based on the parameters: 2 7 7 80 10 50 500 -f -d -m [51]. Repeated sequences were also searched using RepeatScout based on the parameter: -l 14 [52]. Blastn was used to verify if predicted sequences included CDSs. The retrieved DNA sequences translated into amino acids in six frames, and functional motifs were searched using Pfam [53].
Availability of data and materials
The genome sequence and annotation are available at DNA Data Bank of Japan (DDBJ; http://www.ddbj.nig.ac.jp/) under accession numbers BPLF01000001-BPLF01000009. Corresponding BioProject and Biosample ID are PRJDB9184 and SAMD00325088, respectively. Nanopore, Illumina, and RNAseq reads are available from SRA under the following accession numbers, DRR394095, DRR296275, and DRR394096, respectively.
References
Wise LN, Kappmeyer LS, Mealey RH, Knowles DP. Review of equine piroplasmosis. J Vet Intern Med. 2013;27:1334–46.
Scoles GA, Ueti MW. Vector ecology of equine piroplasmosis. Annu Rev Entomol. 2015;60:561–80.
Tirosh-Levy S, Gottlieb Y, Fry LM, Knowles DP, Steinman A. Twenty years of equine piroplasmosis research: global distribution, molecular diagnosis, and phylogeny. Pathogens. 2020;9:1–32.
Brayton KA, Lau AOT, Herndon DR, Hannick L, Kappmeyer LS, Berens SJ, et al. Genome sequence of Babesia bovis and comparative analysis of apicomplexan hemoprotozoa. PLoS Pathog. 2007;3:1401–13.
Cornillot E, Hadj-Kaddour K, Dassouli A, Noel B, Ranwez V, Vacherie B, et al. Sequencing of the smallest Apicomplexan genome from the human pathogen Babesia microti. Nucleic Acids Res. 2012;40:9102–14.
Jackson AP, Otto TD, Darby A, Ramaprasad A, Xia D, Echaide IE, et al. The evolutionary dynamics of variant antigen genes in Babesia reveal a history of genomic innovation underlying host-parasite interaction. Nucleic Acids Res. 2014;42:7113–31.
Yamagishi J, Asada M, Hakimi H, Tanaka TQ, Sugimoto C, Kawazu SI. Whole-genome assembly of Babesia ovata and comparative genomics between closely related pathogens. BMC Genomics. 2017;18:832.
Guan G, Korhonen PK, Young ND, Koehler AV, Wang T, Li Y, et al. Genomic resources for a unique, low-virulence Babesia taxon from China. Parasit Vectors. 2016;9:1–8.
Sivakumar T, Igarashi I, Yokoyama N. Babesia ovata: Taxonomy, phylogeny and epidemiology. Vet Parasitol. 2016;229:99–106.
Wang X, Wang J, Liu J, Liu A, He X, Xiang Q, et al. Insights into the phylogenetic relationships and drug targets of Babesia isolates infective to small ruminants from the mitochondrial genomes. Parasit Vectors. 2020;13:378.
Allred DR. Variable and variant protein multigene families in babesia bovis persistence. Pathogens. 2019;8:76.
O’Connor RM, Allred DR. Selection of Babesia bovis-infected erythrocytes for adhesion to endothelial cells coselects for altered variant erythrocyte surface antigen isoforms. J Immunol. 2000;164:2037–45.
Hutchings CL, Li A, Fernandez KM, Fletcher T, Jackson LA, Molloy JB, et al. New insights into the altered adhesive and mechanical properties of red blood cells parasitized by Babesia bovis. Mol Microbiol. 2007;65:1092–105.
Scudiero L, Mercado-Rojano WDJ, Rudolph A, Wang J, Laughery JM, Suarez CE. Comparisons of the topographic characteristics and electrical charge distributions among Babesia-infected erythrocytes and extraerythrocytic merozoites using AFM. J Microsc. 2018;271:84–97.
Kawai S, Igarashi I, Abgaandorjiin A, Ikadai H, Omata Y, Saito A, et al. Tubular structures associated with Babesia caballi in equine erythrocytes in vitro. Parasitol Res. 1999;85:171–5.
Allred DR, Hines SA, Ahrens KP. Isolate-specific parasite antigens of the Babesia bovis-infected erythrocyte surface. Mol Biochem Parasitol. 1993;60:121–32.
Allred DR, Carlton JM, Satcher RL, Long JA, Brown WC, Patterson PE, et al. The ves multigene family of B. bovis encodes components of rapid antigenic variation at the infected erythrocyte surface. Mol Cell. 2000;5:153–62.
Xiao Y-P, Al-Khedery B, Allred DR. The Babesia bovis VESA1 virulence factor subunit 1b is encoded by the 1beta branch of the ves multigene family. Mol Biochem Parasitol. 2010;171:81–8.
Al-Khedery B, Allred DR. Antigenic variation in Babesia bovis occurs through segmental gene conversion of the ves multigene family, within a bidirectional locus of active transcription. Mol Microbiol. 2006;59:402–14.
Pedroni MJ, Sondgeroth KS, Gallego-Lopez GM, Echaide I, Lau AOT. Comparative transcriptome analysis of geographically distinct virulent and attenuated Babesia bovis strains reveals similar gene expression changes through attenuation. BMC Genomics. 2013;14:763.
Hakimi H, Yamagishi J, Kawazu SI, Asada M. Advances in understanding red blood cell modifications by Babesia. PLoS Pathog. 2022;18:e1010770.
Hakimi H, Templeton TJ, Sakaguchi M, Yamagishi J, Miyazaki S, Yahata K, et al. Novel Babesia bovis exported proteins that modify properties of infected red blood cells. PLoS Pathog. 2020;16:e1008917.
Bhoora R, Franssen L, Oosthuizen MC, Guthrie AJ, Zweygarth E, Penzhorn BL, et al. Sequence heterogeneity in the 18S rRNA gene within Theileria equi and Babesia caballi from horses in South Africa. Vet Parasitol. 2009;159:112–20.
Qablan MA, Oborník M, Petrželková KJ, Sloboda M, Shudiefat MF, Hořín P, et al. Infections by Babesia caballi and Theileria equi in Jordanian equids: Epidemiology and genetic diversity. Parasitology. 2013;140:1096–103.
Braga M do SC de O, Costa FN, Gomes DRM, Xavier DR, André MR, Gonçalves LR, et al. Genetic diversity of piroplasmids species in equids from island of São Luís, northeastern Brazil. Rev Bras Parasitol Vet. 2017;26:331–9.
Nehra AK, Kumari A, Moudgil AD, Vohra S. Phylogenetic analysis, genetic diversity and geographical distribution of Babesia caballi based on 18S rRNA gene. Ticks Tick Borne Dis. 2021;12:101776.
Fulnečková J, Ševčíková T, Fajkus J, Lukešová A, Lukeš M, Vlček Č, et al. A broad phylogenetic survey unveils the diversity and evolution of telomeres in eukaryotes. Genome Biol Evol. 2013;5:468–83.
Knowles RC, Mathis RM, Bryant JE, Willers KH. Equine piroplasmosis. J Am Vet Med Assoc. 1966;148:407–10.
Avarzed A, Igarashi I, Kanemaru T, Hirumi K, Omata Y, Saito A, et al. Improved in vitro Cultivation of Babesia caballi. J Vet Med Sci. 1997;59:479–81.
Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25:1105–11.
Anders S, Pyl PT, Huber W. HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–9.
Koren S, Walenz BP, Berlin K, Miller JR, Bergman NH, Phillippy AM. Canu: scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res. 2017;27:722–36.
Walker BJ, Abeel T, Shea T, Priest M, Abouelliel A, Sakthikumar S, et al. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS One. 2014;9:e112963.
Noe L, Kucherov G. YASS: enhancing the sensitivity of DNA similarity search. Nucleic Acids Res. 2005;33 Web Server issue:W540–3.
Simpson JT, Wong K, Jackman SD, Schein JE, Jones SJM, Birol I. ABySS: a parallel assembler for short read sequence data. Genome Res. 2009;19:1117–23.
Stanke M, Diekhans M, Baertsch R, Haussler D. Using native and syntenically mapped cDNA alignments to improve de novo gene finding. Bioinformatics. 2008;24:637–44.
Hoff KJ, Stanke M. WebAUGUSTUS–a web service for training AUGUSTUS and predicting genes in eukaryotes. Nucleic Acids Res. 2013;41 Web Server issue:W123–8.
Lowe TM, Eddy SR. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25:955–64.
Conesa A, Gotz S, Garcia-Gomez JM, Terol J, Talon M, Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21:3674–6.
Tanizawa Y, Fujisawa T, Kaminuma E, Nakamura Y, Arita M. DFAST and DAGA: web-based integrated genome annotation tools and resources. Biosci Microbiota Food Health. 2016;35:173–84.
Altenhoff AM, Škunca N, Glover N, Train C-M, Sueki A, Piližota I, et al. The OMA orthology database in 2015: function predictions, better plant support, synteny view and other improvements. Nucleic Acids Res. 2015;43 Database issue:D240–9.
Katoh K, Misawa K, Kuma K, Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30:3059–66.
Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547–9.
Edgar RC. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–7.
Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–25.
Tian T, Liu Y, Yan H, You Q, Yi X, Du Z, et al. AgriGO v2.0: A GO analysis toolkit for the agricultural community, 2017 update. Nucleic Acids Res. 2017;45:W122–9.
Mistry J, Finn RD, Eddy SR, Bateman A, Punta M. Challenges in homology search: HMMER3 and convergent evolution of coiled-coil regions. Nucleic Acids Res. 2013;41:e121.
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–10.
Bastian M, Heymann S, Jacomy M. Gephi: An Open Source Software for Exploring and Manipulating Networks. 2009.
Krogh A, Larsson B, von Heijne G, Sonnhammer ELL. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J Mol Biol. 2001;305:567–80.
Benson G. Tandem repeats finder: A program to analyze DNA sequences. Nucleic Acids Res. 1999;27:573–80.
Price AL, Jones NC, Pevzner PA. De novo identification of repeat families in large genomes. Bioinformatics. 2005;21(Suppl 1 SUPPL.):1.
Mistry J, Chuguransky S, Williams L, Qureshi M, Salazar GA, Sonnhammer ELL, et al. Pfam: the protein families database in 2021. Nucleic Acids Res. 2021;49:D412–9.
Acknowledgements
We thank Ms. Naoko Kawai for conducting sequencing.
Funding
This study was funded by the Japan Racing Association (2–3272) and Strategic International Collaborative Research Project (JPJ008837) Promoted by the Ministry of Agriculture, Forestry and Fisheries, Japan.
Author information
Authors and Affiliations
Contributions
JY, MA, and AO conceived and designed the study. MA, HH and AO conducted sample collection. MA and AO performed the experiments. JY, and TK conducted the literature search, performed data extraction and analysis, and interpreted the results. JY, AO, and TK drafted and wrote the manuscript. MA and HH critically reviewed the manuscript for important intellectual content and revised the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Uniquely gained and lost genes in B. caballi. Table S2. Clustered genes in B. caballi. Table S3A. Estimated function of set of genes in each cluster by delta-blast for functional motif identification. Table S3B. Estimated function of set of genes in each cluster by blastp for homologus gene identification. Table S4. Repeat sequences.
Additional file 2: Fig. S1.
Structural comparison among apicoplast genomes of Babesia species. The alignments among apicoplast genomes of seven Babesia species are shown using dotplots. Fig. S2. Phylogenetic analysis using apicoplast genomes of seven Babesia species. The genomes were aligned, and gaps were trimmed from the alignment. The phylogenetic tree was constructed using the maximum likelihood method. Fig. S3. Phylogenetic tree based on 18S rRNA gene sequences. Three 18S rRNA genes in the B. caballi genome were compared with representative 18S rRNA genes obtained from other B. caballi specimens and different Babesia species. The sequences are shown with their corresponding GenBank IDs. Clade names are classified according to the nomenclature described by Nehra et al [26]. Numbers on branches represent bootstrap values in the analysis.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ochi, A., Kidaka, T., Hakimi, H. et al. Chromosome-level genome assembly of Babesia caballi reveals diversity of multigene families among Babesia species. BMC Genomics 24, 483 (2023). https://doi.org/10.1186/s12864-023-09540-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-023-09540-w