Abstract
Background
In brood site pollination mutualisms, pollinators are attracted by odours emitted at anthesis. In Ficus, odours of receptive figs differ among species and the specific pollinators generally only enter figs of their host species ensuring a pre-zygotic barrier to plant interspecific hybridisation. However, field observations recorded that, in Guangdong province in China, Valisia javana hilli, the local pollinator of F. hirta, entered and reproduced successfully in the figs of the closely related F. triloba on a regular basis. We propose that closely related Ficus species produce similar receptive fig odours. Under particular contexts of odours locally present, the receptive fig odours of non-host figs of a Ficus species may become attractive to pollinators of closely related Ficus species. We used the headspace technique to collect in situ receptive fig odours of F. triloba in a series of locations in China. Under controlled conditions, we tested the attraction of fig pollinating wasps from F. hirta and F. triloba to host figs and non-host figs in Y tube experiments.
Results
Receptive fig odours of F. triloba though different from those of F. hirta, were mainly composed of a same set of volatile organic compounds. When given the choice between receptive fig odours and air, the pollinating wasps were only attracted by their host’s odours. However, when given a choice between host and non-host figs the pollinators of F. hirta were equally attracted by the two odours while the pollinators of F. triloba tended to be more attracted by their host’s fig odours.
Conclusions
Receptive fig odours vary geographically within species and the differentiation of receptive fig odours between closely related Ficus species is often incomplete. This allows localised or occasional pollinator sharing following different modalities. Cross stimulation when wasps are exposed simultaneously to odours of host and non-host species may be important. While occasional pollinator sharing may play a marginal role when wasp populations are robust, it may ensure the provisioning of new pollinators from the closest relative of a Ficus species if its pollinators go extinct.
Similar content being viewed by others
Background
Successful speciation involves establishing barriers to gene flow between incipient sister-species. While allopatric speciation is frequent, the distribution of sister species often strongly overlaps [1]. Therefore, reinforcement processes reducing genetic introgression may play a central role in speciation [2]. Sister species with overlapping ranges often occupy different ecological niches [1]. Models show that species coexist more easily if barrier reinforcement relies on habitat preferences rather than on species recognition [3]. In plants, the pre-zygotic barrier often involves change in pollinators [1], and pollinators may be habitat specialists [4].
Within this general context, systems in which plants associate with pollinators that breed in floral structures, i.e. brood pollination mutualisms, may ensure efficient pre-zygotic isolation among plant species. Indeed, the pollinators are often host specialists [5]. Plants typically attract their pollinating insect by releasing particular odours at anthesis that constitute species signatures [6]. Among such systems, figs and fig pollinating wasps provide an extreme case of specialised brood site pollination mutualism in which parallel cladogenesis between plants and insects has been the main form of diversification over the last 70 Ma [7]. They also provide a system where the range of plant species often strongly overlap. Indeed, sympatry is generalised among Ficus species [8]. Do brood site pollination mutualisms and Ficus in particular, follow the general rules associated with pre-zygotic barriers among related species, or do the particularities of these systems allow different diversification processes?
Fig pollinating wasps breed in the enclosed inflorescences (the figs) that characterise genus Ficus. The wasps are the sole pollinators of figs. Generally, a wasp species is associated with a single Ficus species, while a Ficus species is pollinated by a species or a species complex [9,10,11,12] and related Ficus species have related pollinator species [7]. The wasps are attracted to figs by a species-specific odour released when flowers are ready to be pollinated and receive wasp oviposition [13, 14]. Most Ficus species emit distinctive receptive fig odours [6], and wasps are sensitive to the ratio of different volatile organic compounds (VOCs) in the odour [13]. This allows high host-specificity.
The biology of the association suggests a simple, automatically enforced, reproductive isolation mechanism between incipient Ficus species. When the distribution of a Ficus species becomes fragmented (e.g. in glacial refugia during climatic oscillations), allopatric differentiation of pollinator and Ficus host may occur. If there is a local particularity in plant insect communication, i.e. in the odour emitted by receptive figs and in how it is interpreted by the wasps, this may result in a pre-zygotic barrier with respect to other populations surviving in other refugia [15]. In some Ficus species, receptive fig odours vary geographically [10, 16], pollinating wasp species vary geographically [9] and some wasps are attracted by the local odours of their host plant, and not by non-local odours [16]). If geographic receptive fig odour variation has a genetic basis, then a scenario of allopatric speciation in climatic refugia with geographic receptive fig odour differentiation instantly enforcing pre-zygotic isolation is plausible: in case of secondary contact between populations expanding from different refugia, the incipient species will remain distinct.
Receptive fig odours differ markedly between non-sister Ficus species and pollinators are not attracted by the odours of non-sister Ficus species (e.g.[17]). On the other hand, sister Ficus species may present similar odours, because of shared ancestry, and some pollinators are attracted by receptive figs of their host’s sister-species in experimental setups and/or in the field [18,19,20]. With receptive fig odours varying geographically within species and closely related species presenting similar receptive fig odours, we may expect a geographic patchwork of receptive fig odours, where receptive fig odours sometimes locally overlap sufficiently between closely related Ficus species to affect the specificity of wasp attraction.
Ficus hirta and Ficus triloba provide a model system to investigate such a situation. Ficus hirta presents clinal genetic variation across its range [9] and receptive fig odours diverge with increasing distance [16]. Throughout its range, it is pollinated by a set of parapatric wasp species of the Valisia javana species group. Its closest relative, Ficus triloba, occurs throughout most of that range and is pollinated by Valisia esquirolianae [21,22,23], a wasp that is closely related to the Valisia javana species groups [9] but is morphologically distinct [24]. In Guangdong province, South China, notably at Dinghu mountain, both Valisia esquirolianae and Valisia javana hilli, the local species of the V. javana complex [23] develop sucessfully in the figs of Ficus triloba. In samples collected throughout the range of F. hirta, V. esquirolianae was not found in the figs [9], though in the more recent survey it was obtained from some F. hirta figs in two locatities [23].
Here we test the hypothesis that (1) F. hirta and F. triloba receptive fig odours share some main compounds and that (2) in Dinghu mountain, receptive fig odours of F. triloba are attractive to Valisia javana hilli, while V. esquirolianae is not attracted by figs of F. hirta.
Results
Variation in scent profiles
The chemical composition of the odours emitted by receptive F. triloba figs is summarised per location in Table 1. Gas Chromatography-Mass Spectrometer (GC–MS) analysis revealed 46 compounds, with 20 compounds shared by all the locations. Based on their biosynthetic origin [25], the detected compounds fell into three distinct chemical classes: fatty acid derivatives, monoterpenes, and sesquiterpenes. The odours comprised 3 fatty acid derivatives, 8 monoterpenes, and 28 sesquiterpenes, and 7 compounds could not be identified. Ten compounds represented more than 5% of the odours in at least one location, namely α-cubebene, cyclosativene, α-copaene, β-cubebene, (E)-thujopsene, (E)- β-farnesene, (E)- β-caryophyllene, α-muurolene, germacrene D, δ-cadinene and unknown 6. All these compounds were also found at least once in F. hirta receptive fig odours [16].
The non-metric multidimensional scaling (NMDS) (stress = 0.124) applied to the Ficus triloba dataset (i.e., the relative proportion of each VOC in the odour emitted by each studied sample) showed that, while many point overlapped among locations, the odours of receptive figs differed significantly among locations (Fig. 1; Permutational multivariate analysis of variance (PERMANOVA): F(2;31) = 3.6554, P = 0.001). Pairwise comparisons using permutation MANOVAs on the pairwise distance matrix between localities showed differences between all the localities (P < 0.05). The dispersion of the VOC profiles was significantly heterogeneous among the three locations sampled (F2,29 = 4.3772, P = 0.03), but not between Shimen and DHS (F1,26 = 0.7443, P = 0.3962). The results within location at Shimen and even more at DHS presented a large variance.
NMDS representation of the relative proportions of VOCs in the odors emitted by Ficus triloba. The Bray-Curtis dissimilarity Index was used. Each triangle represents an individual. Colour indicates sampling location ( Shimen, Shimen National Forest Park—brown; DHS, Dinghu mountain—orange; XTBG, Xishuangbanna Tropical Botanical Garden—purple.) Odour profiles vary significantly between each of the three study sites (PERMANOVA: F(2;31) = 3.6554, P = 0.001) (stress = 0.124)
Inter-specific variation in the chemical message emitted by receptive figs
All the compounds representing more than 5% of the odour of F. triloba in at least one location were also detected in the odours produced by at least one individual of F. hirta (Table 2). Reciprocally all the compounds representing more than 5% of the odour of F. hirta in at least one location were also detected in at least one individual of F. triloba (Table 2). In contrast, out of these 17 compounds, 12 were not detected in F. hispida odours, while 5 compounds representing each over 5% of receptive fig odours of F. hispida in at least one location where not detected in F. triloba and F. hirta odours. In agreement, in the NMDS plot including the 3 species, there was a large overlap between F. triloba and F. hirta odours, while F. hispida was separated (Fig. 2a). Nevertheless, receptive fig odour differed between F. hirta and F. triloba (PERMANOVA, F (1,79) = 9.65, P = 0.001, Fig. 2b) despite 28 shared compounds (Tables 1, 2; [16]). Geographic variation in receptive fig odours for F. hirta and F. triloba are presented in Fig. 3. The chemical distance between F. hirta samples from different locations increases with log distance (linear regression between chemical distance and log geographic distance, R2 = 0.65 p < 10–10, Fig. 4) while the chemical distance between F. triloba and F. hirta odours was independent of geographic distance between sampling locations (linear regression between chemical distance and log geographic distance, R2 = 0.006, P = 0.70, Fig. 4). The chemical distances beween F. hirta and F. triloba odours within location at XTBG and at DHS (the two values for low geographic distance in Fig. 4) were close to the median value of interspecific comparisons, with 14 higher values in comparisons between F. triloba and F. hirta odours from different locations and 11 lower values. More locations need to be sampled to test for a correlation between geographic distance and chemical distance of receptive fig odours of F. triloba.
Comparison of receptive fig odours among species. 2a: comparison between Ficus triloba, F. hirta and F. hispida. Black, red and green dots represented F. hirta, F. triloba, F. hispida, respectively. 2b comparison between F. triloba and F. hirta. Non-metric multi-dimentional scaling (NMDS) representation of the relative proportions of VOCs in the odors emitted by individual fig plants showing groupings according to location based on Bray–Curtis dissimilarity Index (stress = 0.18 for 2a and stress = 0.204 for 2b)
Geographic variation in receptive fig odour composition for Ficus hirta and Ficus triloba. The solid black circle represents the location of each population. Colour within the pie shows volatile organic compounds representing more than 5% of the odor in at least one location in at least one species. The color within the pie shows the proportional contribution of different volatile compounds. Odors of F. hirta and F.triloba are marked with black and blue letters in the pie chart at the location
Insect behavioral tests
Results of Y-tube olfactometer tests are presented in Fig. 5. When given the choice between the odour of receptive figs against cleaned air, both V. javana hilli and V. esquirolianae were attracted by the receptive fig odour of their host species (two-tailed binomial test, P < 0.005, n = 36 and P < 0.001, n = 41, respectively) and they were not attracted by the receptive fig odours of the other species (two-tailed binomial test, P = 0.253; n = 49, and P = 0.323, n = 37, respectively).
Wasp choices when confronted with different odours in a Y-tube olfactometer. We used binomial tests for statistical comparisons between the number of choices for receptive fig odour versus clean air or choice between receptive fig odours of the two Ficus species. N: number of tested wasps. P: probability, two tailed
However, when Valisia javana hilli were first exposed to a mix of receptive fig odours of the two Ficus species in the first section of the Y tube olfactometer, they became equally attracted by the two branches of the olfactomer although one presented a flow of F. hirta odours and the other one of F. triloba odours (two tailed binomial test, P = 1; n = 42). Exposed to the same conditions, Valisia esquirolianae tended to be more attracted by the odours of F. triloba (two-tailed binomial test, P = 0.066; n = 43).
Discussion
Despite significant differences in receptive fig odours between F. hirta and F. triloba, there was a large overlap in the VOCs constituting these odours. All the compounds present at a concentration above 5% in at least one location in one species were also detected in the other species. This overlap was much more marked than with the VOCs constituting the receptive fig odours of F. hispida suggesting an effect of phylogenetic distance. A similar situation was observed for the species group of Ficus itoana (subgenus Sycomorus) in Papua New-Guinea, with overlap of receptive figs odours of the species group on an NMDS plot and separation from other species of subgenus Sycomorus belonging to other sections [20].
The similarity in the main VOCs constituting the odours suggests that the pollinating wasps of both F. hirta and F. triloba are capable of detecting some of the VOCs composing the receptive fig odour of the other Ficus species. If, by chance, the relative proportions of the VOCs the wasps detect, are locally sufficiently similar between the two species, then the wasp may be attracted to the usually non-host species [13]. On the other hand, the wasps, with their limited repertoire of olfactory genes [26], may not have the olfactory receptors allowing them to detect the VOCs constituting receptive fig odours of F. hispida.
The difference between receptive fig odours in F. hirta increased with geographic distance [16] while the difference between F. hirta and F. triloba receptive fig odours was independent of geographic distance. This suggests lack of interference between the two species in the local evolution of their receptive fig odours. We suggest that independent odour variation, of genotypic or phenotypic origin, in the two species may lead to occasional situations of local overlap of the part of the chemical message detected by one or the other species of wasp.
In the Y tube experiment, when wasps were given the choice between purified-air and receptive fig odours, they were attracted by their host species’ figs and were not attracted by non-host figs.
However, when the wasps were first exposed to a mix of odours of the two species, Valisia javana hilli became attracted by figs of both species. We propose that during the exposition to the mix of odours, F. hirta odours stimulate Valisia javana hilli so that it subsequently responds to the previously non-attractive odour of F. triloba. Such situations could occur under natural conditions, as we have several times observed F. hirta growing under the cover of F. triloba. In a similar process, male European corn borer moths are initially highly discriminative according to VOC relative concentrations in pheromones. However, after initial stimulation, they respond to a broader range of relative concentrations [27].
The attraction of host specialist pollinating wasps by receptive figs of closely related Ficus species has previously been investigated in Y tube olfactometer experiments for three situations. Ficus boninsimae and F. nishimurae are two very closely related species co-occurring in the Ogasawara islands, Japan. Ficus boninsimae is an open habitat species while F. nishimurae is an understory tree. In Y tube experiments, pollinators of F. boninsimae were equally attracted by figs of F. boninsimae and F. nishimurae, while the pollinators of F. nishimurae were more attracted by F. nishimurae fig odours [18]. In Papua New Guinea, the closely related F. microdyctia, F. sp. and F. itoana replace each other along an altitudinal gradient. Their receptive fig odours overlap in an NMDS plot. In Y tube tests against air, the pollinator of Ficus sp. was attracted by fig odours of F. sp and of F. microdyctia, but not those of F. itoana. The pollinator of F. itonana was attracted by receptive fig odours of F. itoana, but not those of the two other species. Finally, the pollinator of F. microdyctia was avoiding the odours of receptive figs of F. sp. and F. itoana [20]. Ficus semicordata semicordata and F. s. montana co-occur from Nepal to Laos through South-China but have distinct habitats [19]. Receptive fig odours of Ficus s. semicordata are mainly constituted by a highly unusual compound, p-methylanisole [14], and this compound was also found in receptive fig odours of F. s. montana individuals. Pollinators of F. s. semicordata were preferentially attracted by their host species when given a choice, but when given no choice, they were attracted by non-host figs. Pollinators of F. s. montana were equally attracted by receptive figs of the two varieties. Finally, the ranges of Ficus auriculata, F. oligodon and F. hainanensis, which form a species complex, overlap throughout continental Asia but they occupy distinct habitats [28]. They share pollinators throughout their regions of co-occurrence and the receptive fig odours of F. auriculata and F. oligodon were not distinguishable [28].
Hence, although all investigated sister Ficus species that occur in sympatry present similarities in their fig odours, they occupy different habitats. Generally, they do not share pollinators, but their pollinators may be attracted by non-host receptive figs in Y tube experiments, following variable modalities and directionality. There is no evidence supporting selection for divergence in olfactive signalling between these closely related Ficus species and there is no evidence supporting selection on the wasps to use several hosts. All the investigated cases involve dioecious Ficus species, in which pollinator dispersal is limited [29]. Hence, for dioecious Ficus species, habitat differentiation between closely related species may constitute the main barrier to gene flow between species. Pollinator specificity is a complementary force, but it is leaky. As such, Ficus follow the general pattern of separation between closely related species in the tree of life [1].
On islands, small population sizes may lead to local extinctions of pollinators. In such situations, because of the limited barriers to wasps detecting receptive figs of close relatives of their usual host species, recolonization of a Ficus species by pollinators of a close relative is expected. This is the case in Taiwan where Blastophaga nipponica pollinates Ficus erecta as elsewhere, but distinct host-races of B. nipponica pollinate the more localised F. formosana and F. tannoensis [30]. In an artificial situation in Hawaii, Ficus rubiginosa was introduced with its pollinator Pleistodontes imperialis. Ficus watkinsiana, a close relative of F. rubiginosa was also introduced. It is now beginning to be pollinated by P. imperialis, while in their native range the two Ficus species co-occur and are pollinated by different wasp species [31]. Hence, the barrier to colonisation of closely related host species by a same wasp species could often be competition with the established populations of pollinating wasp. Reciprocally, when a Ficus species is introduced into a part of the world where no closely related species sustains a population of wasps, it will remain unpollinated as long as its pollinator is not introduced [32]. Within this perspective, specialised pollination in Ficus may limit their invasiveness when introduced into new parts of the world as long as pollinators from their continent of origin are not introduced.
Material and methods
Study system and collection sites
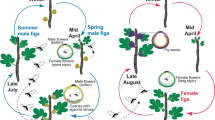
Ficus triloba Buch.-Ham. ex Voigt (= Ficus esquiroliana Léveillé) (subg. Ficus, sect. Eriosycea, subsect. Eriosycea) is a dioecious tree up to 15 m tall while Ficus hirta is a small shrub [32,33,34]. Ficus triloba male trees produce a single main crop releasing pollen loaded wasps in July in time to pollinate the main crop on female trees that ripens in September–October [33]. Ficus hirta, its closest relative [21, 22], produces figs year-round, with seasonal peaks, in June-July, and in October–November [36, 37] thus overlapping with F. triloba phenology. Ficus triloba has large figs, about 30 mm in diameter at receptivity [34], while those of F. hirta are about 10-15 mm [38]. Receptive figs of F. triloba emit a strong floral scent while the smell of receptive figs of F. hirta is hardly detectable by the human nose [22]. Ficus hirta is pollinated by a set of 9 different wasps throughout its distribution [9], while a same pollinator (Valisia esquirolianae) has been collected on F. triloba in Taiwan, in continental China, and in Thailand [24, 39]. The two species are sympatric across most of their distributions that extends from northeast India and subtropical China to the Malay Peninsula [35]. While their habitats differ, the two species may grow side by side in secondary habitats, for instance in abandoned tree plantations or close to each other as in our study sites in Dinghu Mountain (DHS, a National Nature Reserve, established in 1956) and in Shimen (a forest park established in1995) in Guangdong province, China. In these two sites, V. javana hilli was observed to develop in figs of F. triloba [23].
Between November 2017 and June 2019, in wet (May to September) and dry (November to March) season, to explore the diversity of receptive fig odours, we collected receptive fig odours from Ficus triloba at DHS, Shimen and at the Xishuangbanna Tropical Botanical Garden (XTBG) in Yunnan. We collected 15 samples in the region of Dinghu mountain (DHS, 112.54 E, 23.16 N), 13 samples in Shimen National Forest Park (Shimen, 113.45 E, 23.27 N), and 4 samples in the Xishuangbanna Tropical Botanical Garden (XTBG,101.15 E, 21.55 N).
Volatile collections
The chromatoprobe head-space method, which was initially used in Silene, was adopted to collect fig odours in situ [16, 40, 41]. Odour collection was performed outdoors in the shade between 10:00 a.m. and 5:00 p.m. on sunny days, corresponding to the insects’ period of peak activity during our field season. Five-15 receptive figs were enclosed together in a polyethylene terephtalate (Nalophan®, Kalle Nalo GmbH, Wursthüllen, Germany) bag for 30 min. Then, air was pulled out of the bag for 5 min (flow rate: 200 mL/min) through a filter filled with 1.5 mg of Carbotrap 20–40 and 1.5 mg of Tenax 60–80, in which the volatile organic compounds (VOCs) were trapped. In parallel, at every collection, we made a ‘blank’ extraction from a bag that contained no fig, using the same protocol. One microliter of a solution of internal standards (n-Nonane and n-Dodecane, 110 ng/μl of each) was added to each filter before scent extraction, so that we could control for VOC loss during storage and transport. The samples were stored at − 20 °C until VOC analysis.
VOC analysis
The samples were analysed using gas chromatography coupled with mass spectrometry and the compounds were identified as detailed in Deng et al. 2021. We obtained a global dataset, where the composition of the odour extracted from each sample is expressed by the relative proportions of each VOC in the emitted odour (semi-quantitative data). This dataset was complemented by previous data obtained from Ficus hirta [16] to compare the odours of the two species, and from F. hispida (subgenus Sycomorus) (Deng et al. submitted) another sympatric species to provide an outgroup.
Divergence in chemical profiles across locations was estimated with non-metric multidimensional scaling (NMDS) in two dimensions, based on a Bray–Curtis similarity matrix, using the R-package vegan [42]. Pairwise distance between individuals for relative proportions of VOCs was calculated using the Bray–Curtis dissimilarity index, which ranges between 0 and 1. Chemical distance matrices were calculated with the function “vegdist” between locations and between species, using available data for F. hirta [16], Two-dimensional plots were constructed using the “metaMDS” function algorithm after data standardization with “decostand” function in R (v. 3.5.1). A stress value is given, indicating how well the particular configuration represents the distance matrix (stress values < 0.2 are desirable). To test if the variation in chemical composition between locations was significant, we carried out permutational multivariate analysis of variance tests (PERMANOVA) on the distance matrix using the function “adonis” in the vegan package [42]. The model used 999 permutations, and we FDR corrected p-values to control for multiple comparisons. Multivariate homogeneity of group dispersions (variances) was tested using the “betadisper” function. SIMPER (similarity percentage) was used to identify the compounds responsible for dissimilarities between groups.
Insect behavioural tests
In Dinghu Mountain (DHS), Valisia javana hilli was observed to develop in figs of F. triloba along with V. esquirolianane. On the contrary, V. esquirolianae was not observed to develop in the figs of F. hirta at DHS [23].
In order to test if the local populations of Valisia esquirolianae and V. javana hilli are attracted by the odours released by receptive figs of F. triloba and F. hirta, wasp attraction was tested using Y-tube olfactometers in DHS. Bioassays were conducted outside, on a sunny day between 9 and 12 a.m. We tested the response of the wasps when given the choice between floral odours emitted either by F. triloba or by F. hirta and filtered air (i.e. control), and their response to a choice between the floral odours of the two species. Three different series of tests were used: receptive figs of host versus control, receptive figs of non-host versus control and receptive figs of host versus receptive figs of non-host. We used the same size Y-tube olfactometer (stem 8 cm; arms 9 cm; diameter 1.5 cm) as Proffit et al. (2009) to test the attraction of the pollinating wasps of F. hispida. Humidified air was purified with activated charcoal and blown into a glass vial connected to each lateral arm (200 ml/min per arm). The vial connected to one arm contained receptive figs stemming from several trees, and in the other, the vial was either empty (in controls) or it contained receptive figs of the other species. For tests involving Ficus triloba, 2 receptive figs were put into the vial, while for F. hirta, 4 receptive figs were put into the vial. When comparing the attraction by receptive figs of the two species, due to the large difference in size, an equal weight of fresh figs was used. To ensure continuous odour production, we changed the odour source every two hours. Wasps were introduced individually into the central arm of the Y-tube and their movements were recorded for 10 min. To avoid a potential directional bias, the directions of control and odour source were reversed after each trial. To eliminate scent contamination, the Y-tubes were cleaned with pure acetone before each trial, as was the entire network of connecting tubes after each five trials. The observer noted the behavioural choice made by each individually tested fig wasp for 10 min among three modalities: choice for odour, choice for control, or no choice. We considered that wasps made no choice when they stayed motionless for 3 min in the departure section and/or the central arm before the bifurcation of the olfactometer. All the adult female fig wasps were newly emerged from male figs. For each experiment, we used two-tailed binomial tests (with a probability of 0.5) to compare the number of choices for odour versus choices for no odour or other odour (excluding the no-choice response).
Availability of data and materials
All figs sample collection permissions or licenses were obtained. All datasets generated or analyzed during this study are included in this published article.
Abbreviations
- GC–MS:
-
Gas Chromatography-Mass Spectrometer
- NMDS:
-
Non-metric multidimensional scaling
- VOCs:
-
Volatile organic compounds
- PERMANOVA:
-
Permutational multivariate analysis of variance
References
Hernandez-Hernandez T, Miller EC, Roman-Palacios C, Wiens JJ. Speciation across the Tree of Life. Biol Rev Camb Philos Soc. 2021;96(4):1205–42.
Servedio MR, Noor MAF. The role of reinforcement in speciation: theory and data. Annu Rev Ecol Evol S. 2003;34(1):339–64.
Kyogoku D, Kokko H. Species coexist more easily if reinforcement is based on habitat preferences than on species recognition. J Anim Ecol. 2020;89(11):2605–16.
Lami F, Bartomeus I, Nardi D, Beduschi T, Boscutti F, Pantini P, Santoiemma G, Scherber C, Tscharntke T, Marini L. Species-habitat networks elucidate landscape effects on habitat specialisation of natural enemies and pollinators. Ecol Lett. 2021;24(2):288–97.
Brookes DR, Hereward JP, Terry LI, Walter GH. Evolutionary dynamics of a cycad obligate pollination mutualism-Pattern and process in extant Macrozamia cycads and their specialist thrips pollinators. Mol Phylogenet Evol. 2015;93:83–93.
Hossaert-McKey M, Soler C, Schatz B, Proffit M. Floral scents: their roles in nursery pollination mutualisms. Chemoecology. 2010;20(2):75–88.
Cruaud A, Rønsted N, Chantarasuwan B, Chou LS, Clement WL, Couloux A, Cousins B, Genson G, Harrison RD, Hanson PE, et al. An extreme case of plant-insect codiversification: figs and fig-pollinating wasps. Syst Biol. 2012;61(6):1029–47.
Harrison RD. Figs and the diversity of tropical rainforests. AIBS Bull. 2005;55(12):1053–64.
Yu H, Tian E, Zheng L, Deng X, Cheng Y, Chen L, Wu W, Tanming W, Zhang D, Compton SG, et al. Multiple parapatric pollinators have radiated across a continental fig tree displaying clinal genetic variation. Mol Ecol. 2019;28(9):2391–405.
Rodriguez LJ, Bain A, Chou L-S, Conchou L, Cruaud A, Gonzales R, Hossaert-McKey M, Rasplus J-Y, Tzeng H-Y, Kjellberg F. Diversification and spatial structuring in the mutualism between Ficus septica and its pollinating wasps in insular South East Asia. BMC Evol Biol. 2017;17:207.
Darwell CT, Al-Beidh S, Cook JM. Molecular species delimitation of a symbiotic fig-pollinating wasp species complex reveals extreme deviation from reciprocal partner specificity. BMC Evol Biol. 2014;14:189.
Bain A, Borges RM, Chevallier MH, Vignes H, Kobmoo N, Peng YQ, Cruaud A, Rasplus JY, Kjellberg F, Hossaert-Mckey M. Geographic structuring into vicariant species-pairs in a wide-ranging, high-dispersal plant–insect mutualism: the case of Ficus racemosa and its pollinating wasps. Evol Ecol. 2016;30(4):663–84.
Proffit M, Lapeyre B, Buatois B, Deng X, Arnal P, Gouzerh F, Carrasco D, Hossaert-McKey M. Chemical signal is in the blend: bases of plant-pollinator encounter in a highly specialized interaction. Sci Rep. 2020;10:10071.
Chen C, Song Q, Proffit M, Bessière J-M, Li Z, Hossaert-McKey M. Private channel: a single unusual compound assures specific pollinator attraction in Ficus semicordata. Funct Ecol. 2009;23(5):941–50.
Cook JM, Segar ST. Speciation in fig wasps. Ecol Entomol. 2010;35(s1):54–66.
Deng X, Buatois B. Plants are the drivers of geographic variation of floral scents in a highly specialized pollination mutualism: a study of Ficus hirta in China. Res Square. 2021. https://doi.org/10.21203/rs.3.rs-192226/v1.
Okamoto T, Su Z-H. Chemical analysis of floral scents in sympatric Ficus species: highlighting different compositions of floral scents in morphologically and phylogenetically close species. Plant Syst Evol. 2021;307:45.
Yokoyama J. Cospeciation of figs and fig-wasps: a case study of endemic species pairs in the Ogasawara Islands. Popul Ecol. 2003;45(3):249–56.
Wang G, Compton SG, Chen J. The mechanism of pollinator specificity between two sympatric fig varieties: a combination of olfactory signals and contact cues. Ann Bot. 2013;111(2):173–81.
Souto-Vilarós D, Proffit M, Buatois B, Rindos M, Sisol M, Kuyaiva T, Isua B, Michalek J, Darwell CT, Hossaert-McKey M, et al. Pollination along an elevational gradient mediated both by floral scent and pollinator compatibility in the fig and fig-wasp mutualism. J Ecol. 2018;106(6):2256–73.
Berg CC, Corner EJH. Moraceae: Ficeae. Flora Malesiana, Series I. 2005;17(2):1–730.
Hu R, Sun P, Yu H, Cheng Y, Wang R, Chen X, Kjellberg F. Similitudes and differences between two closely related Ficus species in the synthesis by the ostiole of odors attracting their host-specific pollinators: A transcriptomic based investigation. Acta Oecol. 2020;105: 103554.
Yu H, Zhang Z, Liu L, Cheng Y, Deng X, Segar ST. Compton SG Asymmetric sharing of pollinator fig wasps between two sympatric dioecious fig trees: a reflection of supply and demand or differences in the size of their figs? Bot stud. 2022;63(1):1–12.
Chen C-H, Chou L-Y. The Blastophagini of Taiwan (Hymenoptera: Agaonidae: Agaoninae). J-Taiwan Museum. 1997;50:113–54.
Knudsen JT. Diversity and distribution of floral scent. Bot Rev. 2006;72(1):1–120.
Wang R, Yang Y, Jing Y, Segar ST, Zhang Y, Wang G, Chen J, Liu Q-F, Chen S, Chen Y, et al. Molecular mechanisms of mutualistic and antagonistic interactions in a plant-pollinator association. Nat Ecol Evol. 2021;5:974–86.
Kárpáti Z, Tasin M, Cardé RT, Dekker T. Early quality assessment lessens pheromone specificity in a moth. P Natl Acad Sci. 2013;110(18):7377–82.
Wang G, Cannon CH, Chen J. Pollinator sharing and gene flow among closely related sympatric dioecious fig taxa. Proc Royal Soc B. 2016;283:20152963.
Harrison RD, Rasplus J-Y. Dispersal of fig pollinators in Asian tropical rain forests. J Trop Ecol. 2006;22(6):631–9.
Wachi N, Kusumi J, Tzeng HY, Su ZH. Genome-wide sequence data suggest the possibility of pollinator sharing by host shift in dioecious figs (Moraceae, Ficus). Mol Ecol. 2016;25(22):5732–46.
Bernard J, Brock KC, Tonnell V, Walsh SK, Wenger JP, Wolkis D, Weiblen GD. New species assemblages disrupt obligatory mutualisms between figs and their pollinators. Front Ecol Evol. 2020;8: 564653.
Compton SG, Stavrinides M, Kaponas C, Thomas PJ. No escape: most insect colonisers of an introduced fig tree in Cyprus come from the plant’s native range. Biol Invasions. 2019;22(2):211–6.
Lu J, Gui P, Li H-Q, Lu Z-L, Zhang L-F, Tian H-Z, Gilbert MG. Phylogenetic analysis and taxonomic delimitation of the “hairy-fig” complex of Ficus sect Eriosycea (Moraceae) in China. Phytotaxa. 2016;261(2):121–36.
Kuaraksa C, Elliott S, Hossaert-Mckey M. The phenology of dioecious Ficus spp. tree species and its importance for forest restoration projects. Forest Ecol Manag. 2012;265:82–93.
Berg CC. Precursory taxonomic studies on Ficus (Moraceae) for the Flora of Thailand. Thai Forest Bulletin (Botany). 2007;35:4–28.
Yu H, Zhao N, Chen Y, Herre EA. Male and female reproductive success in the dioecious fig, Ficus hirta Vahl in Guangdong Province, China: Implications for the relative stability of dioecy and monoecy. Symbiosis. 2008;45(1):121.
Yu H, Zhao N, Chen Y, Deng Y, Yao J. Phenology and reproductive strategy of a common fig in Guangzhou. Bot Stud. 2006;47(4):435–41.
Yu H, Liang D, Tian E, Zheng L, Kjellberg F. Plant geographic phenotypic variation drives diversification in its associated community of a phytophagous insect and its parasitoids. BMC EvoL Biol. 2018;18(1):134.
Jiang ZF, Huang DW, Zhu CD, Zhen WQ. New insights into the phylogeny of fig pollinators using Bayesian analyses. Mol Phylogenet Evol. 2006;38(2):306–15.
Soler CC, Proffit M, Bessiere JM, Hossaert-McKey M, Schatz B. Evidence for intersexual chemical mimicry in a dioecious plant. Ecol Lett. 2012;15(9):978–85.
Dötterl S, Wolfe LM, Jürgens A. Qualitative and quantitative analyses of flower scent in Silene latifolia. Phytochemistry. 2005;66(2):203–13.
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O'Hara RB, Simpson GL, Solymos P et al. Package ‘vegan’ Community Ecology Package. 2013, 2(9):1–295.
Acknowledgements
We also thank all our guides in the field work and two anonymous reviewers for their thoughtful comments.
Funding
This work was supported by the National Natural Science Foundation of China (3215010364; 31971568) and Province Natural Science Foundation of Guangdong (c20140500001306) and by the International Research Project (IRP)-CNRS-MOST. Xiaoxia Deng was supported by a Grant from the China Scholarship Council No. (2017)3109. Chemical analyses were conducted at the platform PACE which is supported by LabEx CeMEB, an ANR "Investissements d'avenir" program (ANR-10-LABX-04- 01).
Author information
Authors and Affiliations
Contributions
XXD and YFC collected samples, analysed the data and wrote the manuscript. YQP help collecting samples. HY, FK and MP organized the work and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors have no competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Deng, X., Cheng, Y., Peng, YQ. et al. Overlaps in olfactive signalling coupled with geographic variation may result in localised pollinator sharing between closely related Ficus species. BMC Ecol Evo 22, 97 (2022). https://doi.org/10.1186/s12862-022-02055-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12862-022-02055-0