Abstract
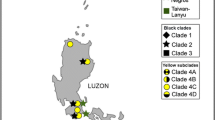
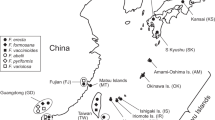
Ficus and their mutualistic pollinating wasps provide a unique model to investigate joint diversification in a high dispersal system. We investigate genetic structuring in an extremely wide-ranging Ficus species, Ficus racemosa, and its pollinating wasp throughout their range, which extends from India to Australia. Our samples were structured into four large, vicariant populations of figs and wasps which may correspond to distinct (sub)species, located in India, China-Thailand, Borneo, and Australia. However, the genetically most divergent group was the Indian population for the figs and the China-Thailand population for the wasps, suggesting different evolutionary histories of populations. Molecular dating for the wasps shows that diversification of the pollinator clade is surprisingly old, beginning about 13.6 Ma. Data on both the host fig species and its pollinating wasps suggest that strong genetic flow within biogeographic groups over several hundreds of kilometers has limited genetic and morphological differentiation and, potentially, local adaptation. This is probably due to long-distance dispersal of pollinating wasps. The genetic clustering into large geographic units observed in F. racemosa and its pollinators is reminiscent of what can be observed in some other high-dispersal organisms characterized by morphology that varies little over huge distances. The implications of strong gene flow for diversification processes and adaptation to different ecological conditions in Ficus and their pollinating wasps are just beginning to emerge.




Similar content being viewed by others
References
Ahmed S, Dawson DA, Compton SG et al (2007) Characterization of microsatellite loci in the African fig Ficus sycomorus L. (Moraceae). Mol Ecol Notes 7:1175–1177
Ahmed S, Compton SG, Butlin RK et al (2009) Wind-borne insects mediate directional pollen transfer between desert fig trees 160 kilometers apart. Proc Natl Acad Sci USA 106:20342–20347
Akaike H (1973) Information theory and an extension of the maximum likelihood principle. In: Petrov PN, Csaki F (eds) Second international symposium on information theory. Akad, Kiado, pp 267–281
Alvarez N, McKey D, Kjellberg F et al (2010) Phylogeography and historical biogeography of obligate specific mutualisms. In: Morand S, Krasnov BR (eds) The biogeography of host-parasite interactions. Oxford University Press, Oxford, p 277
Ashley MV (2010) Plant parentage, pollination, and dispersal: how DNA microsatellites have altered the landscape. Crit Rev Plant Sci 29:148–161
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B Methodol 57:289–300
Berg CC, Corner EJH (2005) Moraceae (Ficus). In: Nooteboom HP (ed) Flora Malesiana. National Herbarium Nederland, Leiden
Borges RM (2015) How to be a fig wasp parasite on the fig–fig wasp mutualism. Curr Opin Insect Sci 8:34–40
Buschbom J, Yanbaev Y, Degen B (2011) Efficient long-distance gene flow into an isolated relict oak stand. J Hered 22:484–493
Chen Y, Compton SG, Liu M et al (2012) Fig trees at the northern limit of their range: the distributions of cryptic pollinators indicate multiple glacial refugia. Mol Ecol 21:1687–1701
Conchou L, Cabioch L, Rodriguez LJV et al (2014) Daily rhythm of mutualistic pollinator activity and scent emission in Ficus septica: ecological differentiation between co-occurring pollinators and potential consequences for chemical communication and facilitation of host speciation. PLoS ONE 9:e103581
Cornille A, Underhill JG, Cruaud A et al (2012) Floral volatiles, pollinator sharing and diversification in the fig–wasp mutualism: insights from Ficus natalensis, and its two wasp pollinators (South Africa). Proc R Soc B 279:1731–1739
Cruaud A, Jabbour-Zahab R, Genson G et al (2010) Laying the foundations for a new classification of Agaonidae (Hymenoptera: Chalcidoidea), a multilocus phylogenetic approach. Cladistics 26:359–387
Cruaud A, Jabbour-Zahab R, Genson G et al (2011) Phylogeny and evolution of life-history strategies in the Sycophaginae non-pollinating fig wasps (Hymenoptera, Chalcidoidea). BMC Evol Biol 11:178–192
Cruaud A, Rønsted N, Chantarasuwan B et al (2012) An extreme case of plant-insect co-diversification: figs and fig-pollinating wasps. Syst Biol 61:1029–1047
Cunha RL, Blanc F, Bonhomme F et al (2011) Evolutionary patterns in pearl oysters of the genus Pinctada (Bivalvia: Pteriidae). Mar Biotechnol 13:181–192
Darwell CT, Al-Beidh S, Cook JM (2014) Molecular species delimitation of a symbiotic fig-pollinating wasp species complex reveals extreme deviation from reciprocal partner specificity. BMC Evol Biol 14:189
Dev SA, Kjellberg F, Hossaert-McKey M et al (2011) Fine-scale population genetic structure of two dioecious Indian keystone species, Ficus hispida and Ficus exasperata (Moraceae). Biotropica 43:309–316
Dick CW, Lewis SL, Maslin M et al (2013) Neogene origins and implied warmth tolerance of Amazon tree species. Ecol Evol 3:162–169
Drummond AJ, Rambaut A (2007) BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol 7:214–221
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14:2611–2620
Gu X (1995) Maximum likelihood estimation of the heterogeneity of substitution rate among nucleotide sites. Mol Biol Evol 12:546–557
Hardy OJ, Vekemans X (2002) SPAGeDi: a versatile computer program to analyse spatial genetic structure at the individual or population levels. Mol Ecol Notes 2:618–620
Hardy OJ, Charbonnel N, Fréville H et al (2003) Microsatellite allele sizes: a simple test to assess their significance on genetic differentiation. Genetics 163:1467–1482
Harrison RD (2005) Figs and the diversity of tropical rainforests. Bioscience 55:1053–1064
Harrison RD, Rasplus J-Y (2006) Dispersal of fig pollinators in Asian tropical rain forests. J Trop Ecol 22:631–639
Heer K, Kalko EKV, Albrecht L, Garcia-Villacorta R, Staeps FC, Herre EA, Dick CW (2015) Spatial scales of genetic structure in free-standing and strangler figs (Ficus, Moraceae) inhabiting Neotropical forests. PLoS ONE 10:e0133581
Heinrichs J, Hentschel J, Feldberg K et al (2009) Phylogenetic biogeography and taxonomy of disjunctly distributed bryophytes. J Syst Evol 47:497–508
Heinze B (2007) A database of PCR primers for the chloroplast genomes of higher plants. Plant Methods 3:4
Hopkins MJ (2011) How species longevity, intraspecific morphological variation, and geographic range size are related: a comparison using late Cambrian trilobites. Evolution 65:3253–3273
Hossaert-McKey M, Soler C, Schatz B et al (2010) Floral scents: their roles in nursery pollination mutualisms. Chemoecology 20:75–88
Jablonski D, Roy K (2003) Geographical range and speciation in fossil and living mollusks. Proc R Soc B 270:401–406
Jevanandam N, Goh AGR, Corlett RT (2013) Climate warming and the potential 16 extinction of fig wasps, the obligate pollinators of figs. Biol Lett 9:20130041
Katoh K, Kuma K, Toh H et al (2005) MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Res 33:511–518
Khadari B, Hochu I, Santoni S et al (2001) Identification and characterization of microsatellite loci in the common fig (Ficus carica L.) and representative species of the genus Ficus. Mol Ecol Notes 1:191–193
Kinland BP, Gaines SD (2003) Propagule dispersal in marine and terrestrial environments: a community perspective. Ecology 84:2007–2020
Kjellberg F, Proffit M (2016) Tracking the elusive history of diversification in plant-herbivorous insect-parasitoid food webs: insights from figs and fig-wasps. Mol Ecol 25:843–845
Kjellberg F, Jousselin E, Hossaert-McKey M et al (2005) Biology, ecology, and evolution of fig-pollinating wasps (Chalcidoidea, Agaonidae). In: Raman A, Schaefer W, Withers TM (eds) Biology, ecology and evolution of gall-inducing arthropods. Science Publishers Inc, Enfield (NH) USA, Plymouth, pp 539–572
Kobmoo N, Hossaert-McKey M, Rasplus J-Y et al (2010) Ficus racemosa is pollinated by a single population of a single agaonid wasp species in continental South-East Asia. Mol Ecol 19:2700–2712
Kremer A, Ronce O, Robledo-Arnuncio JJ et al (2012) Long-distance gene flow and adaptation of forest trees to rapid climate change. Ecol Lett 15:378–392
Krishnan A, Ghara M, Kasinathan S et al (2015) Plant reproductive traits mediate tritrophic feedback effects within an obligate brood-site pollination mutualism. Oecologia 179:797–809
Léotard G, Debout G, Dalecky A et al (2009) Range expansion drives dispersal evolution in an equatorial three-species symbiosis. PLoS ONE 4:e5377
Lewis LR, Rozzi R, Goffinet B (2014) Direct long-distance dispersal shapes a New World amphitropical disjunction in the dispersal-limited dung moss Tetraplodon (Bryopsida: Splachnaceae). J Biogeogr 41:2385–2395
Lin S-Y, Chou L-S, Di Giusto B et al (2015) Sexual specialization in phenology in dioecious Ficus benguetensis and its consequences for the mutualism. Bot stud 56:32
Liu M, Zhao R, Chen Y et al (2014) Competitive exclusion among fig wasps achieved via entrainment of host plant flowering phenology. PLoS ONE 9:e97783
Liu M, Compton SG, Peng F-E et al (2015) Movements of genes between populations: are pollinators more effective at transferring their own or plant genetic markers? Proc R Soc B 282:20150290
Loiselle BA, Sork VL, Nason J et al (1995) Spatial genetic structure of a tropical understory shrub, Psychotria officinalis (Rubiaceae). Am J Bot 82:1420–1425
Moe AM, Weiblen GD (2010) Molecular divergence in allopatric Ceratosolen (Agaonidae) pollinators of geographically widespread Ficus (Moraceae) species. Ann Entomol Soc Am 103:1025–1037
Moe AM, Weiblen GD (2011) Development and characterization of microsatellite loci in dioecious figs (Ficus, Moraceae). Am J Bot Notes Prot 98:e25–e27
Nunes FLD, Norris RD, Knowlton N (2011) Long distance dispersal and connectivity in amphi-atlantic corals at regional and basin scales. PLoS ONE 6:e22298
Nylander JAA (2004) MrAIC.pl. In: Nylander JAA (ed) Evolutionary Biology Centre. Uppsala University, Uppsala
Oleksy R, Racey PA, Jones G (2015) High-resolution GPS tracking reveals habitat selection and the potential for long-distance seed dispersal by Madagascan flying foxes Pteropus rufus. Glob Ecol Conserv 3:678–692
Palumbi SR (1994) Genetic divergence, reproductive isolation, and marine speciation. Ann Rev Ecol Syst 25:547–572
Peakall R, Smouse PE (2006) GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 6:288–295
Perrier X, Jacquemoud-Collet JP (2006) DARwin software. http://darwin.cirad.fr/darwin
Pokorny L, Oliván G, Shaw AJ (2011) Phylogeographic patterns in two Southern Hemisphere species of Calyptrochaeta (Daltoniaceae, Bryophyta). Syst Bot 36:542–553
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Rambaut A (2006) FigTree. http://tree.bio.ed.ac.uk/software/figtree/
Raymond M, Rousset F (1995) GENEPOP (version 1.2): population genetics software for exact tests and ecumenicism. J Hered 86:248–249
Renoult JP, Kjellberg F, Grout C et al (2009) Cyto-nuclear discordance in the phylogeny of Ficus section Galoglychia and host shifts in plant-pollinator associations. BMC Evol Biol 9:248
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Shilton LA, Altringham JD, Compton SG et al (1999) Old World fruit bats can be long-distance seed dispersers through extended retention of viable seeds in the gut. Proc R Soc B 266:219–223
Smith CI, Tank S, Godsoe W et al (2011) Comparative phylogeography of a coevolved community: concerted population expansions in Joshua trees and four yucca moths. PLoS ONE 6:e25628
Soler C, Hossaert-McKey M, Buatois B et al (2011) Geographic variation of floral scent in a highly specialized pollination mutualism. Phytochemistry 72:74–81
Sutton TL, Riegler M, Cook JM (2016) One step ahead: a parasitoid disperses farther and forms a wider geographic population than its fig wasp host. Mol Ecol 25:882–894
Tamura K, Dudley J, Nei M et al (2007) MEGA4: molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Thompson JN (2005) The geographic mosaic of coevolution. University of Chicago Press, Chicago
Thompson AR, Thacker CE, Shaw EY (2005) Phylogeography of marine mutualists: parallel patterns of genetic structure between obligate goby and shrimp partners. Mol Ecol 14:3557–3572
Tian E, Nason JD, Machado CA et al (2015) Lack of genetic isolation by distance, similar genetic structuring but different demographic histories in a fig-pollinating wasp mutualism. Mol Ecol 24:5976–5991
Vignes H, Hossaert-McKey M, Beaune D et al (2006) Development and characterization of microsatellite markers for a monoecious Ficus species, Ficus insipida, and cross-species amplification among different sections of Ficus. Mol Ecol Notes 6:792–795
Voris HK (2000) Maps of Pleistocene sea levels in Southeast Asia: shorelines, river systems and time durations. J Biogeogr 27:1153–1167
Wang G, Cannon CH, Chen J (2016) Pollinator sharing and gene flow among closely related sympatric dioecious fig taxa. Proc R Soc B 283:20152963
Ware AB, Compton SG (1994) Dispersal of adult female fig wasps. 2. Movements between trees. Entomol Exp Appl 73:231–238
Yang L-Y, Machado CA, Dang X-D et al (2015) The incidence and pattern of copollinator diversification in dioecious and monoecious figs. Evolution 69:294–304
Yu H, Nason JD (2013) Nuclear and chloroplast DNA phylogeography of Ficus hirta: obligate pollination mutualism and constraints on range expansion in response to climate change. New Phytol 197:276–289
Zhang G, Song Q, Yang D (2006) Phenology of Ficus racemosa in Xishuangbanna, Southwest China. Biotropica 38:334–341
Acknowledgments
This study was funded by the by the ANR Projects “nicefigs” (ANR-09-BLAN-0392-CSD 7; NSC 99-2923-B-002-001-MY3) and “biofigs” (ANR BDIV-006-001)/National Science Council, Taiwan, R. O. C. Led by M.H.M. We thank Marc Ancrenaz, James Cook, and Edmond Dounias for their help in collecting samples, and Doyle McKey for his useful comments on our paper. We are also grateful to the staff of the Center for Ecological Sciences, Bangalore and of Xishuangbanna Tropical Botanical Garden (XTBG, China) for their field assistance. Laboratory manipulations were done at CEFE (SMGE), CBGP and at the LABEX CEMEB facilities.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bain, A., Borges, R.M., Chevallier, M.H. et al. Geographic structuring into vicariant species-pairs in a wide-ranging, high-dispersal plant–insect mutualism: the case of Ficus racemosa and its pollinating wasps. Evol Ecol 30, 663–684 (2016). https://doi.org/10.1007/s10682-016-9836-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-016-9836-5