Abstract
Background
Biological age (BA) has been recognized as a more accurate indicator of aging than chronological age (CA). However, the current limitations include: insufficient attention to the incompleteness of medical data for constructing BA; Lack of machine learning-based BA (ML-BA) on the Chinese population; Neglect of the influence of model overfitting degree on the stability of the association results.
Methods and results
Based on the medical examination data of the Chinese population (45–90 years), we first evaluated the most suitable missing interpolation method, then constructed 14 ML-BAs based on biomarkers, and finally explored the associations between ML-BAs and health statuses (healthy risk indicators and disease). We found that round-robin linear regression interpolation performed best, while AutoEncoder showed the highest interpolation stability. We further illustrated the potential overfitting problem in ML-BAs, which affected the stability of ML-Bas’ associations with health statuses. We then proposed a composite ML-BA based on the Stacking method with a simple meta-model (STK-BA), which overcame the overfitting problem, and associated more strongly with CA (r = 0.66, P < 0.001), healthy risk indicators, disease counts, and six types of disease.
Conclusion
We provided an improved aging measurement method for middle-aged and elderly groups in China, which can more stably capture aging characteristics other than CA, supporting the emerging application potential of machine learning in aging research.
Similar content being viewed by others
Introduction
In the context of global aging, exploring the representation methods, evaluation indicators, and influencing factors of aging based on big medical data has become an important social issue and a new research hotspot [1]. Aging is an organismal phenomenon manifested by an increased chance of healthy risk (e.g. the likelihood of disease, death) or decreased function over time [2]. The introduction of biological age (BA) is a critical step in aging research. BA is an ideal indicator to provide evidence on aging independent of chronological age (CA) and measures the rate of human aging associated with the functional decline more accurately than CA [3, 4]. Besides, BA is closely related to health characteristics such as physical function, cognition, morbidity, and mortality by measuring the cumulative level of impairment [5]. Effective BA construction methods and quantitative assessments of the associations between BA with health status will contribute to further understanding of aging and provide effective risk stratification [6, 7].
Current BAs are mainly based on statistical models of a series of biological features [8]. These features include clinical indicators [4, 9, 10], instrumental parameters [11, 12], and molecular genetic measures [13, 14]. The methods commonly used in BA models are based on univariate or multivariate regression methods [7], such as principle component analysis (PCA) [15], multilayer perceptron (MLP) [16], and the Klemera and Doubal method (KDM) [17]. Although these classical methods perform well in predicting adverse aging outcomes, they have limitations in processing multidimensional data, especially when the shape of the distribution is not suited for parametric methods [18], and recognizing the actual interactions between the biomarkers and outcomes [19], as some significant biomarkers were proved to be nonlinear [17]. While recently, new approaches applying machine learning (ML) algorithms have shown considerable accuracy and efficiency in BA prediction [20, 21], causing wide attention [22]. Furthermore, the stacking and bagging algorithm displays better performance in distinguishing significant features [23], revealing the complicated non-linear relationships between biomarkers and the target condition [24], but few applications in ML-BA construction.
The Pearson correlations, MAE, and RMSE between BA and CA are the preferred and most commonly used indicators to compare different BA estimation algorithms [25, 26]. Exploring the associations of ML-BA with epidemiological variables (e.g. health risk indicators, mortality), genetic and environmental factors, and common age-related chronic diseases (e.g. heart disease, kidney disease) can further examine its potential as a biomarker of aging in the general population [6, 27, 28]. Notably, we found in the previous ML-BAs that the correlations between BA and CA attained from the test data used for comparing model performances, and the full data, including both the training data and test data, showed obvious differences. [18, 29]. The reason might be that overfitting makes the model outperform the test set on the training set. And then the model trained on the training set predicted the full dataset’s BA, resulting in a different but better BA performance than the test set. Under such circumstances, whether the degree of overfitting will affect the stability of the association results needs to be further considered.
Valid BA and reliable conclusions are usually based on large population data, but complete large datasets for data mining in public health research are rare, as related medical databases are often lost for various reasons [30, 31], such as sample missing [32], human error [33]. A normal method to solve the problem is ignoring samples with missing values. However, omitting the missing data will greatly limit the downstream analysis performance [18]. Hence, using interpolation methods to estimate incomplete datasets, which will contribute to improving the performance of subsequent analysis [34, 35], becomes a more suitable choice. Some machine learning-based (ML-based) methods have exhibited great application potential in recent years [36,37,38,39]. However, most of the current studies on BA used relatively complete datasets, or deal with missing values only with the most common methods (filled with mean, median, mode, zero or random values) [18]. Insufficient attention has been paid to the complexity and incompleteness of medical data. Therefore, exploring novel and effective interpolation methods will be a constructive and worthy practice in the data preprocessing before building BA models with physical examination data. Besides, to reduce the influence of overfitting on the results, cross-validation methods should be adopted, such as K-fold cross-validation [40], and generalized cross-validation (GCV) [41].
Additionally, most of the current ML-BA studies were from European and American populations [42, 43], and ML-BA based on large Chinese population data (more than 30,000 people) was still very limited [18]. The correlation of ML-BA with CA will vary due to differences in populations and biomarkers [44]. Constructing ML-BA with a large Chinese population from different sources and linking ML-BA with important health statuses will help to further explore the validity and application potential of ML-BA in the Chinese population.
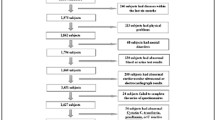
In the research, we used medical examination data (45–90 years) in Zhejiang Province, China, and Fig. 1 illustrated our analysis flow. We focused on four aspects: (1) comparing the applicability of different interpolation methods in medical examination data (e.g. round-robin linear regression, AutoEncoder); (2) constructing ML-BAs based on Chinese large population samples with several machine-learning algorithms; (3) examining associations of ML-BAs with health statuses (e.g. health risk indicators, disease status); and most importantly, (4) exploring the influence of overfitting degree on the stability of the associated results and proposed the optimized ML-BA model.
Results
Comparison of missing value interpolation methods
As shown in Fig. 2A–D, the interpolation results of mean, KNN, AE, RRLR, and MICE for continuous variables on MNAR and MCAR simulation data sets were presented. MSE and R2 compared the accuracy and validity of interpolation respectively. The parameter selection process of KNN and MICE was presented in Additional file 1: Table S1 (MCAR) and Additional file 1: Table S2 (MNAR), and the optimal parameter of both models varied with missing proportions (Additional file 1: Tables S1 and S2). Subsequently, the best results were selected and compared with other models under different missing conditions. AE hyper-parameters considered encoder layers, epochs, activation function, batch size, and learning rate. The optimized parameters of AE and RRLR were presented in Additional file 1: Table S3. Additional file 1: Table S4 recorded the interpolation time consumed by the different models.
The results showed that RRLR outperformed other methods under MCAR and MNAR (Fig. 2A–D). The MSE of MICE and RRLR increased significantly with the increase in missing ratio (Fig. 2A, B), but AE showed more excellent stability, with the missing ratio growing from 5 to 30%, and the R2 only decreased by 4.61%. The lower the missing rate, the greater the advantage of RRLR, while AE was more suitable for cases with a high missing rate. The results in the MNAR simulation dataset (Fig. 2C, D) were similar to those in MCAR (Fig. 2A, B). RRLR reduces MSE by 33.12% compared to MICE, the second-most accurate interpolation method in MNAR. R2 possessed the same trend as MSE, and RRLR interpolation results displayed the best correlation. In addition to interpolation performance, the time spent in interpolation should also be considered (Additional file 1: Table S4). RRLR exhibited a similar time cost to AE and mean, while the time consumed by KNN mainly depended on the missing ratio. MICE needed the most time to complete interpolation. In general, RRLR was used to fill missing values, and the predicted value of the binary variable greater than 0.5 was marked as 1, otherwise, it was 0. BA will be predicted on the new dataset.
Features selection and BA predictor construction
A total of 22 potential biological features were considered for this study. Additional file 1: Fig. S1 showed the optimized lambda and feature selection process in Lasso regression. Urine sugar, urine occult blood, and urine acetone bodies were excluded (Additional file 1: Table S5 and Fig. S1). Figure 2E presented the correlation between variables, with an ‘X’ mark indicating no significant correlation (P > 0.05). Notably, all features were significant (P < 0.05). These two steps yielded 19 features for estimating BA.
Among the machine learning and neural network models explored, ML-BA predicted by Xgboost showed the highest correlation with CA (Pearson’s r = 0.64 in the test set), while Catboost, LGBM, GBDT, and Extra Tress showed similar results (Table 1). Among the five models, R2 ranged from 0.32 to 0.41, and RMSE ranged from 4.49 to 4.89. The parameters of all the above models were detailed in Additional file 1: Table S6. However, the evaluation metrics of these five models were significantly different in training and test set (Table 1), which was attributed to the choice of parameters in the model that greatly affected the model’s fit during training. If over-fitting on the training set was ignored and the model obtained from the training set was used to predict BA of the entire dataset, overfitting will be introduced into the final result, resulting in higher instability of BA. Thus, in addition to determining the optimal model by test set results, the introduction of the prediction results of the overfitting should be avoided in the final prediction.
To this end, we applied the Stacking approach to fusing the model, where the parameters were inherited from a single model. This method could further improve the prediction accuracy besides effectively lowering the interference of the overfitting. Considering the training time, complexity, and fitting effects of the meta-model, the GAM (spline regression) was finally selected to fuse the above five models. The RMSE in the training and test sets were 5.78 and 5.77, respectively, and the R2 was both 0.43. Therefore, we used the fusion model with 19 biological characteristics to get STK-BA. The STK-BA of the entire study population ranged from 44 to 89 years (Table 2), with a mean of 67.8 (SD = 5.0). For females, BA ranged from 43 to 88 years, with a mean of 67.2 (SD = 5.6). For males, BA ranged from 47 to 89 years, with a mean of 68.5 (SD = 4.2). Compared with males, BA in the female population was significantly younger (P < 0.001) and tended to be more normally distributed (Fig. 3A). Table 2 presented that STK-BA was significantly correlated with CA (R = 0.660–0.668, P < 0.001).
To further highlight the advantages of STK-BA and the influences of over-fitting, we constructed two XGB-BAs with similar performance in the test set (the results and parameters were shown in Additional file 1: Table S7). Although XGB-BA2 and XGB-BA1 had similar results on the test set (0.4% MAE difference), XGB-BA2 further improved the fit of the training set, showing a higher correlation with CA (13.1%-increase). Therefore, as shown in Table 2 and Fig. 3, compared with STK-BA, XGB-BAs showed poorer results in the test set, but both improved the correlation with CA in the whole sample (XGB-BA1: 0.738–0.745; XGB-BA2: 0.822–0.827)., the effect of gender on XGB-BAs was similar to that of STK-BAs, but XGB-BAs exhibited a wider BA range (Table 2 and Fig. 3). Taking XGB-BA2 as an example, compared with STK-BA, the BA range was expanded by 42.9%.
The importance of features for the stacking model
Additional file 1: Table S8 recorded the feature importance values of the sub-models in the Stacking model, and Additional file 1: Fig. S2 showed the average feature importance value for the Stacking model. DBP, height, SBP, gender, and platelet content were the top 5 biometric characteristics in the Stacking model. Furthermore, weight, SGPT, waist, and SGOT also showed above-average importance. Conversely, the presence or absence of urinary protein was the least essential marker.
The associations between health risk indicators and STK-BA, XGB-BAs
In this evaluation, we chose ABSI and WHtR as health risk status indicators. Previous studies have pointed out that WHtR was a better measure of an individual’s health than BMI [6, 45]. ABSI based on physical characteristics appeared to be an indicator of premature death in the general population, predicting mortality risk across age, gender, and weight [46]. The three BAs were of the same type and therefore numerically comparable. As shown in Additional file 1: Tables S9, S10, and S11 and Fig. 4, we observed all three ML-BAs exhibited significant positive correlations between ABSI and WHtR (P < 0.001). Results did not change after adjusting for covariates of CA, BMI, and family disease (P < 0.001). And, the correlation strength increased from the first quantile to the fifth quantile, showing a consistent trend. This suggested that the association between ML-BAs and health risk was stable. However, not all ML-BAs showed consistent trends. In an anthropometrically constructed DNN model, the log-rank test for SBSI and WHtR quartiles found that the X2 statistic increased from Q1 to Q2, then decreased from Q2 to Q3, but the overall (Q1–Q4) showed an increasing trend [6]. It was worth noting that from STK-BA to XGB-BA1 and XGB-BA2, the strength and significance of the association of BAs with two health risk indicators continued to decline according to the model coefficients and t-statistics (Fig. 4 and Additional file 1: Tables S8, S9, S10, S11, and S12). Compared with the Q1 group (Model 2) with the lowest ABSI (WHtR) value (Additional file 1: Tables S9, S10, S11, and S12), STK-BA, XGB-BA1, and XGB-BA2 in the Q5 group increased by 2.67 (4.04), 2.31 (3.47), 1.81 (2.73), respectively. Therefore, the increased degree of overfitting of the model reduced the association between BAs and health risk indicators. It could be inferred that when the association strength was small or the degree of overfitting was too high, ML-BA may no longer be correlated with health risk indicators.
Associations of STK-BA and XGB-BAs with health risk indicators (A Body Shape Index (ABSI), Waist-to-height ratio (WHtR)). Health risk indicators as continuous variables (ABSI: A, WHtR: B). Health risk indicators as categorical variables (Model 2, ABSI: C, WHtR: D). Model 1 was a crude model, Model 2 was adjusted for CA, BMI, and family disease status
The associations between disease statuses and STK-BA, XGB-BAs
The increase in STK-BA and XGB-BAs counted for each disease compared to disease-free participants was shown in Fig. 5A, B, Table 3 and Additional file 1: Table S12. Overall, participants with the disease had higher STK-BA and XGB-BAs, and the results remained significant after adjusting for CA and family disease (P < 0.01). In Model 1, XGB-BA2 had the largest BA response to disease count change, while STK-BA had the smallest. Compared with those without the disease, for STK-BA, XGB-BA, and XGB-BA2, those with 1 disease were 0.998, 1.053, and 1.240 years older, and those with 2 or more diseases were 2.422, 2.623, and 3.047 years older. Interestingly, after adjusting for covariates (CA and family disease status), the results were just the opposite. Those with 1 disease were 0.170, 0.100, and 0.069 years older than those without the disease, and were 0.461, 0.372, and 0.284 years older than those with 2 diseases. Also changing was the significance between disease counts and BA (Model 2), the least significant for XGB-BA2 (1:0.024, 2+ : 0.001) and the strongest for STK-BA (1: < 0.001, 2+ : < 0.001).
Poisson regression models were used to examine the associations between BAs and disease counts in the full sample (Table 3). Both STK-BA and XGB-BAs were significantly associated with disease counts (P < 0.001). Consistent results (P < 0.01) were observed after adjusting for CA and family diseases, although the absolute values decreased. Consistent with the trend in the linear regression model, STK-BA showed the strongest association with disease counts (Model 2: Coef = 0.008, SE = 0.001), while XGB-BA2 was the weakest (Model 2: Coef = 0.005, SE = 0.002).
To gain further insights into the relations between the BAs and disease counts, the associations between each disease and STK-BA, XGB-BAs were explored (Fig. 5C and Additional file 1: Table S13). As expected, STK-BA showed a significant positive correlation (P < 0.05) with almost all diseases (except for vascular disease, P = 0.190). XGB-BA1 showed no significant association with vascular disease, kidney disease, and nervous system disease. Notably, in addition to being unrelated to kidney, eye, and nervous system disease, XGB-BA2 was significantly negatively correlated with vascular disease (OR: 0.96, 95% CI: 0.93–0.99) with vascular disease. Furthermore, it was found from the z-score and P values in Additional file 1: Table S13 that compared with XGB-BA1, the associations between XGB-BA2 and diseases (except vascular diseases) were further weakened. This illustrated that overfitting would lead to obvious instability in the results. This also explained why, after adjusting for CA and family disease, XGB-BAs showed weaker associations with disease counts as overfitting degree increased. However, our proposed STK-BA showed fascinating results. After adjusting for covariates, each 1-year increase in STK-BA was associated with a 7% increase in the risk of developing kidney disease (OR: 1.07, 95% CI: 1.00–1.13), 2% for heart disease (OR: 1.02, 95% CI: 1.00–1.05), 5% for cerebrovascular disease (OR: 1.05, 95% CI: 1.03–1.06), 9% for eye disease (OR: 1.09, 95% CI: 1.04–1.17), 8% for nervous system disease (OR: 1.08, 95% CI: 1.02 ~ 1.15) and 1% for other system diseases (OR: 1.01, 95% CI: 1.01–1.01). The results were similar to previous studies. BA has been attested to be a strong indicator and predictor of multiple morbidities, especially chronic diseases [47, 48]. This might be attributed that diseases are closely related to aging. One study showed a stronger association between BA and all-cause morbidity than CA or the traditional biomarkers of age-related diseases (Hazard ratio 1.06 vs. 1.05 and 1.03), including stroke, dementia, Alzheimer’s disease, cancer, coronary heart disease, and diabetes mellitus [49].
Discussion
There is no general missing value interpolation method, but only the most appropriate one. We compared five classical but effective methods for the Chinese physical examination data and found that RRLR performed best under the same missing ratio of the original data. However, the superior performance of the RRLR method was not universal, and it was more suitable for low missing ratios (e.g. less than 30%). This is because the strategy of RRLR is to build regression models to predict and impute the missing features according to other complete samples in an iterative loop [50]. Although this strategy allows RRLR to utilize as many observations as possible during interpolation, regression typically requires many samples with non-missing data to produce stable results [33]. Therefore, under the condition of MCAR, the performance of RRLR interpolation results will decrease significantly with the increase of the missing rate. However, since the overall missing rate in MNAR data is only about 5%, the RRLR model is suitable. Likewise, Yu et al. [51] pointed out that multiple regression imputation was suitable for filling in the missing in the WHO ARI Multicentre Study of clinical signs and etiologic agent dataset. In addition, MICE is widely used for interpolation in medical data, but is usually used in cases assuming missing at random (MAR) [52, 53]. Introduction of missing data through MCAR and MNAR may lead to poor MICE performance. Hegde H pointed out that MICE was suitable for situations with fewer missing variables and fewer missing data [53], which explained why the performance of MICE decreased significantly with an increasing missing rate in MCAR, but was second only to RRLR in MNAR. AE interpolation showed the best stability. As a common artificial neural network in deep learning, deep AE can perform representation learning on the input information, form a higher-level feature map, and then reconstruct the data at the output, reducing sensitivity to higher missing rates [54, 55]. Furthermore, AE has the advantage of capturing more complex or nonlinear relationships between inputs and has a highly robust noise reduction capability [34, 56]. An important problem in data interpolation is dimensionality reduction. The large data vector is reduced to a smaller data vector after interpolation, which shows better results in electronic health record data [57]. AE typically includes the coding layers leading to a central part, followed by the symmetric decoding layers. The symmetrical structure and the central part offer an internal representation of the input data with lower dimensions and thus have the advantages described above [34]. Peralta M also found that when AE was trained on 10–40% missing data, the accuracy index did not change significantly [34]. Furthermore, according to the variance of R2 and MSE, the results are stable and convincing. However, given the uneven distribution of physical examination data, LOOCV or GCV can be introduced when the results are highly biased [58, 59].
More importantly, we found an interesting phenomenon in the previous Chinese population-based ML-BA, which had not been discussed before. When the correlation or R2 between BA and CA was taken as the criterion, the results on the test set were quite different from the final prediction of BA on the full dataset. Taking the previous XGB-BA as an example, the R2 of the model in the test set was 0.27, while the correlation between BA and CA was 0.75 in the final results (BA to CA regression belonged to simple linear regression, so R = cor = 0.75, R2 = 0.56) [18]. The same was also found in the XGB-BA based on the Dongfeng–Tongji cohort [29]. This might be because the model trained on the training set predicted BA on the full dataset, which introduced interference from parameter tuning and training overfitting. However, this still requires further confirmation, as previous studies did not explicitly state how the model was obtained when it finally predicted BA. In any case, the consistency of the test set with the final results is what we would expect.
The correlation between BA and CA was usually regarded as an indispensable index to evaluate BA prediction models. However, after selecting the best model, how to obtain stable correlation analysis results with BA in the whole sample is also of high value. Two generally used health statuses (health risk indicators and disease status) were used as different evaluation aspects to illustrate the influence of different overfitting degrees on correlation strength and significance in ML-BAs. We found that even with similar test set results, as the overfitting degree increased, XGB-BA2 exhibited less obvious associations with health risk indicators (ABSI, WHtR), disease counts, nervous system disease, and eye disease. This finding suggested that the results of association analysis would vary due to parameter selection and other reasons. This can be attributed to the fact that the core purpose of BA is to capture aging features beyond CA, while overfitting causes the model to over-learn the CA feature of the training set. Cao et al. [38] adopted default parameters in the model to overcome this problem, but it did not work fundamentally.
To avoid overfitting affecting the stability of the association results between BA and health outcomes in the entire dataset, we propose three possible solutions. The first is to let the model show basically the same fitting results on the training set and test set, which is the most convenient and least expensive. Secondly, the method of using cross-modeling to predict, such as LOOCV or K-fold, always keeps the final predicted samples from participating in the construction of the model, but this will produce multiple models that are not exactly the same. The prediction accuracy of each model also usually varies due to parameters and different training samples. Therefore, this method presents new challenges for practical application and less time cost. The third is to use only the sample results on the test set for further analysis, but this does not meet the principle of maximizing the use of data and reduces the reliability of the results.
For this case, our proposed STK-BA could improve the correlation between BA and CA while maintaining the consistency of the model results (the correlation of the training set and the test set are the same in three decimal places). What’s more, the positive association of STK-BA with health risk indicators, disease counts, and specific diseases was also more pronounced, suggesting that it better captures the aging-related features behind diseases. This may be attributed to the biological features we considered to represent different physiological functions or dimensions: immune system (e.g. platelet count, white blood cell), cardio-metabolic system (e.g. HDL, DBP), liver function (e.g. SGPT, SGOT), phenotypic dimension (e.g. height, waist), kidney injury (e.g. urine protein). Additionally, the associations we considered included eye disease and kidney disease, which were also not covered in previous Chinese population studies [26].
The Stacking method we adopted is a mechanism to combine the learned types of models into one, consisting of base models and a meta-model [60]. Instead of selecting a model from multiple models for generalization or simple averaging, Stacking uses a meta-model to balance the features (the output of the base model) and predict [33], which is somewhat like a two-layer neural network. Cross-validation of base models and the simple meta-model are the keys to overcoming the overfitting influences. Because the new training and test sets (as input to the second layer) are derived from the predicted values of data sets other than the ones used to build the model, overfitting during training will not be introduced. Meanwhile, the combined data of prediction values from several different models makes the new data sets cover more potential features, which provides support for better prediction performance. Furthermore, a simple meta-model, such as linear regression and generalized additive model, can well reduce the possibility of model overfitting and have good generalization ability, so that the meta-model has similar fitting effects in the training set and test set in the second layer. However, an overly complex meta-model will also lead to overfitting. We observed this when utilizing RF as a meta-model (Table 1). Overall, while outperforming the single base model, Stacking model can overcome the difficulties of overfitting and obtain stable predicted BA on the whole sample for association analysis. More importantly, the Stacking method is equally applicable to the BA based on a single model and can be further generalized.
The correlation between our STK-BA and CA (r = 0.66) on the test set was better than previously published BA (r = 0.52) based on 19 blood biomarkers [18] but weaker than BA (r = 0.74) which considered 44 biomarkers including lung function. This phenomenon is plausible, depending on the population-specific and age-related biosignatures in different datasets [29]. However, it is worth noting that we showed better CA correlations with the same number of biomarkers in the Chinese population. Additionally, Mamoshina et al. [44] found that models trained in a given population declined in correlation when tested across ethnicities (given population: R2 ranged from 0.49 to 0.69; different populations: R2 ranged from 0.24 to 0.34). ML-BA would exhibit different correlations with CA due to differences in population and biometrics [44] Therefore, we constructed ML-BA using Chinese populations from different sources, and this helped to further confirm the applicability of ML-BA in the Chinese population by associating aging measures with important health conditions and outcomes.
DBP, height, SBP, gender, and platelet content were the five most essential variables screened out in the Stacking model, which may play a vital role in assessing BA differences in different populations. In fact, DBP, SBP, and PC have been widely found to be biomarkers closely related to biological aging. Pinto [61] noted that elevated pulse pressure due to decreased DBP and increased SBP was the most potent risk predictor in older adults and was associated with older age. In epidemiological studies, aging populations were more likely to exhibit features of lower PC and higher platelet activity, which are associated with higher rates of cardiovascular disease [62,63,64]. The link between gender or height and aging was also frequently mentioned [65, 66]. In a study of conscripts from Italian inland villages, short people (height less than 161.1 cm) generally had higher survival rates than tall peers [67]. This may be related to caloric restriction, cell replication potential, telomere shortening, and cardiac pumping efficiency [67, 68]. What’s more, the gender-driven characteristics of aging have become the focus of current attention, with gender differences in life expectancy, biological aging, and frailty indices [69]. Of these, women are generally biologically younger than men, consistent with a lower BA assessed by molecular biomarkers [4].
Overall, the BA measurement model we developed integrated multidimensional biosignatures that more systematically reflected human aging. This line of evidence reinforces our findings and suggests that the variable screening results of the Stacking model are biologically interpretable. Besides, although fewer biological features are considered in the model, this facilitates the generalization and practical application of the model and its workflow.
The large sample data of Chinese medical examination data enables us to explore the influence of fitting on the stability of correlation results and develop a new composite BA prediction model after comparing the most suitable interpolation methods. Nonetheless, several limitations need to be discussed. First, although the interpolation methods explored in this study are convenient and practical, more novel missing value imputation methods can be further attempted to be transferred to the medical examination dataset [39], such as the variational AE applied to Genomic data imputation [38]. Second, our data lacked information on outcome variables (e.g., death) to establish a link between BA and survival analysis. We, therefore, associated BA with a health risk indicator that predicted mortality risk instead. Third, the training and test sets of the BA prediction model are both from the same dataset. Testing with external datasets will further evaluate the generalization ability of the ML-BAs [70]. Finally, the biological features used in the study were mostly limited to biochemical indicators, and aging-related indicators that have been discovered, such as mean corpuscular volume, are not included in our data. These may weaken the interpretability of predicted BA and fail to supplement the validation of more existing results [18, 71]. However, with the popularization of big medical data, phenotype information (e.g. cognitive level, gait speed [72, 73]), methylation data (e.g. CpG sites [74, 75]), metabolomic features and pathways (e.g. C-glycosyl tryptophan, α-ketoglutarate and TCA cycle [76,77,78]) will be more convenient, which assists in predicting and explaining the aging process more systematically. Therefore, as more dimensions of individual indicators are taken into account, our composite BA and its construction process will have a broader reference value.
Conclusion
We found RRLR best suited for interpolation on our medical examination dataset, while AE exhibited the highest stability at high missing rates. We pointed out a potential problem of over-fitting affecting the association results in recently proposed ML-BAs. After comparing machine learning methods, we constructed two XGB-BAs with different fitting degrees on the training set (similar performance on the test set) to illustrate the degree of fit by the association between ML-BAs and health statuses that will affect the stability of BA application. For this case, we proposed a composite ML-BA based on the Stacking method with a simple meta-model (STK-BA), which overcame the overfitting problem, and associated more strongly with CA (r = 0.66, P < 0.001), healthy risk indicators, disease counts and six types of disease. Furthermore, we found that DBP, height, SBP, gender, and platelet content were the top five important biological features in STK-BA. However, the influence of the degree of overfitting on the longitudinal association results and the use of external data sets to test the generalization ability of STK-BA are lacking in our study, which deserves further exploration. Overall, our findings supported the application of ML in geriatric research and suggested improvements to existing ML-based BA models. This new aging measurement method captures aging characteristics beyond CA more stably, and provides new possibilities for future work such as the application of BA in risk stratification and aging intervention studies.
Methods
Study population and assessment of physical examination measurements
Based on the electronic health records of residents in Zhejiang Province, China, this study conducted a representative physical examination survey among different age groups. According to the national code for basic public health services, the records were established by substrate medical and health institutions, including township health centers and community health service centers, in 23 cities, and districts of Zhejiang Province.
This study selected potential age-related features missing under 80% and observations with features missing under 20%. Out of the 418,161 participants aged 30–100 years old, we excluded observations those included outliers in comparison with data of the same age and sex (N = 30,935) and those with more than 20% missing data on variates (N = 309,416), leaving the analytic sample of 77,810 adults. Middle age starts around age 45, while the very old are vulnerable to NCDs and socially disadvantaged [18, 79]. Additionally, due to the relatively small size of the oldest-old group and the differences between participants aged 45–90 and others, we excluded participants aged under 45 and over 90 (N = 666). A total of 77,144 participants with 17 biochemical indicators (i.e. systolic blood pressure (SBP), diastolic blood pressure (DBP), hemoglobin, white blood cell, platelets, fasting serum glucose (FSG), serum glutamic pyruvate transaminase (SGPT), serum glutamic oxaloacetic transaminase (SGOT), serum bilirubin, total cholesterol (TC), triglycerides (TG), total bilirubin, low-density lipoprotein (LDL), high-density lipoprotein (HDL), urine protein, urine sugar, urine ketone body, urine occult blood) and 5 physical indicators (i.e. gender, height, weight, waist, body mass index (BMI)) were included in the study. The above indicators were obtained from regular physical examinations. The biological features’ attributions of study populations were shown in Additional file 1: Table S14. The BMI was calculated as weight in kilograms divided by height in meters squared. The data of urine protein, urine sugar, urine ketone body, and urine occult blood were defined as positive and negative levels.
Comparison of interpolation methods for missing values
Interpolating the missing values helps improve the model’s predictive power. Nevertheless, no specific interpolating method is universal. We compared the mean value, k-Nearest Neighbor (KNN), multiple imputations by chained equations (MICE), AutoEncoder (AE), and round-robin linear regression (RRLR) interpolation under the condition of missing completely at random MCAR) and missing not at random (MNAR) to choose the method that best fitted our data. Mean value, KNN, MICE, RRLE and AE respectively represent five typical interpolation methods: simple interpolation, unsupervised learning interpolation, multiple interpolation, regression interpolation, and deep learning network with generative ability methods [33, 38]. The different interpolation principles of these five methods make them applicable to different situations of missing medical data. Thus, it is of great significance to explore a more appropriate interpolation method for the specific missing data.
In real world medical examination data sets, the true values corresponding to the missing locations could not be obtained, nor could the accuracy of the filled value be intuitively evaluated. Therefore, in order to better evaluate the filling performance of different filling methods, we introduced missing values on real world data without missing values to carry out simulation experiments. The process of introducing and interpolating missing values is shown in Additional file 1: Fig. S3. The primary process is as follows:
(1) The missing ratio of each variable (variables with a missing ratio > 2% were considered) and the total missing ratio of all variables in the original data set were calculated, which were used to simulate the missing situation under MNAR and MCAR. (2) The samples without missing values (n = 37,320) were selected to form the simulation data set, of which 80% were used for training and adjusting core parameters of models (such as K in the KNN method), and 20% were used for testing and comparing results. The mean and variance of each variable in the training set were calculated. (3) Based on the results in (1), the missing ratio of different variables (MNAR) or random missing ratio (MCAR, 5%, 10%, 20%, 30%) were introduced into the simulation dataset, and the missing location information was recorded with matrices of the same size at the same time. (4) After interpolation, the imputed value of the test set of each method was compared with the true value by MSE and R2 (with a view to the dimensional difference between different variables, the results in (3) were used to standardize the variables). To reduce the influence of overfitting on the results, we further used tenfold cross-validation as a reliable criterion to evaluate the performance of different methods.
Feature selection and BA calculation
To avoid the redundancy of latent features, lasso regression was used for feature selection first (the data was standardized to avoid dimensional effects). In the second step, Pearson’s correlation was applied to evaluate the correlation of each feature with CA, and features that did not show significant correlations with age (P > 0.05) were excluded.
Similar to that described in previous publications [18], A total of 19 selected biological features were used as independent variables to construct ML-BA. Our work considered machine learning methods (Multiple Linear Regression (MLR), Generalized Additive Models (GAM), Support Vector Machine (SVM), Adaboost, Gradient Boosting Decision Tree (GBDT), Light Gradient Boosting Machine (LGBM), Catboost, Xgboost, Extra Trees) and neural network methods (Deep Neural Networks (DNN), Convolutional Neural Network (CNN)) that can be used for regression analysis. The Pearson correlations, MAE, and RMSE between BA and CA are the indicators used to compare different BA estimation algorithms, which are done in the test set [25, 26].
Finally, stacking model fusion was performed using the top five performing models to calculate the final BA in years (base models). The meta-model considered MLR, GAM (spline regression), SVM, and random forest (RF). Meanwhile, the two xgboost-based BA was calculated, one took the parameters from the Stacking model (XGB-BA1); one amplifies the fit of the training set while keeping the test set results approximately unchanged (XGB-BA2). Both models were trained on the training set to predict the full data set and used to compare the effect of training set overfitting on BA.
A schematic diagram of the Stacking method was presented in Fig. 6. Specifically, the data was divided into the training set (1) and test set (1) with an 8:2 ratio, using CA as the response variable. Each base model was subjected to tenfold cross-validation in the training set (1), a total of 10 times (9 folds as training data for constructing models and a fold as test data per time). The merged result of the predicted values on each test data was the training set (2). The model obtained by each training data also predicted the test set (1), and the mean of the 10 results on the test set (1) was the test set (2). Both training set (2) and test set (2) were provided by the single base model. After repeating these steps for the selected five models, the combined training sets (2) and test sets (2) provided by different models were the training set (3) and test set (3) for the meta-model (The response variable was inherited from the initial training and test set). The training process of the meta-model was the same as that of the single model.
The associations with general health statuses
The way to investigate the performance of estimated BAs in capturing health risk was to consider their possible relationship to known health risk indicators, or how estimated BAs differentiate between subjects with known disease and those without the disease.
Health risk indicators describe the general health state of an individual, such as the A Body Shape Index (ABSI) [46], Surface-based body shape index (SBSI) [80], waist-to-height ratio (WHtR), waist-to-hip ratio (WHR), etc. These indicators are associated with various mortality risks. Considering the biological features covered in the dataset, we used ABSI and WHtR as health risk indicators and further adjusted them for BMI, CA, and family disease. WHtR was obtained from the ratio of waist to height. ABSI was obtained by adjusting waist circumference (WC) for height and weight:
For an effective BA model, when BA increases, the health risk indicator should show a corresponding upward trend. Rahman et al. [6] found a clear separation of BA acceleration by WHtR and SBSI categories (quartiles) in different BA predictive models.
Analyze whether BA will characterize any differences between healthy subjects and subjects with certain known chronic diseases [6, 28]. Individuals with more chronic diseases should have higher mean BA levels than people without any chronic diseases. There are 7 types of diseases diagnosed after physical examination, including cerebrovascular disease, kidney disease, heart disease, vascular disease, eye disease, nervous system disease, and other system diseases. We created a binary variable for each type of disease, with the disease marked as 1 and 0 otherwise. As described above, we added up the disease types of each individual to obtain a disease count variable (ranging from 0 to 7). After accounting for the population distribution, a three-category variable for disease counts was created, no disease, 1 disease, and 2 or more diseases.
Statistical analysis
We trained and optimized BA using training data (80%) and compared the different model results with RMSE, R2, and MAE on test data (20%). All interpolation methods were implemented in Python. The Stacking method with the simple meta-model covering GBDT, LGBM, Catboost, Xgboost, and Extra Trees was selected to calculate the optimal BA (STK-BA) in years. To emphasize the advantages of the Stacking fusion model, the two Xgboost-based BAs (XGB-BAs) with different over-fitting in the training set were also introduced. Furthermore, to assess the importance of features to BA, the feature importance value (FIV) of the five models in the Stacking model was converted to weights and added together [18].
As shown in Fig. 1, we performed two primary analyses, one for health risk indicators, and one for disease counts and specific diseases. To account for confounding effects and to perform further subgroup analyses, we considered the following covariates: chronological age, family disease status, BMI. The details were provided in Additional file 1: Tables S14 and S15.
The associations between ML-BAs and health risk indicators were analyzed by MLR. And the health risk indicators were further classified according to quintiles (Q1–Q5) to compare whether the changes in BA are consistent with the increase of quantiles (Model 1 was a crude model, Model 2 was adjusted for CA, BMI, and family disease status).
To assess the associations between ML-BAs with full-sample disease counts, we first built the MLRs with ML-BAs as the dependent variable. Based on the results of the regression, we estimated BA increments for each disease count category compared with disease-free participants. Subsequently, we used Poisson regression models to examine the associations between ML-BAs and disease counts (the dependent variable). Moreover, the logistic regression model (with or without disease as the dependent variable) was used to assess the association of specific diseases with BAs. We considered two models: Model 1 was a crude model, Model 2 was adjusted for CA and family disease status.
For linear and Poisson regression models, we recorded coefficients, standard errors (SE), z-score, and P-values; for logistic regression models, we recorded odds ratios (ORs), corresponding 95% confidence intervals (95% CI), z-score, and P-values. Statistical analysis and visualization of all data were performed using R Version 4.1.2, Python Version 3.8.8, and Prism 8. Continuous variables were presented as mean ± SD, while categorical variables were presented as numbers (proportions). P < 0.05 (two-tailed) was considered statistically significant.
Availability of data and materials
The data that support the findings of this study are available from the Center for Disease Control of Zhejiang Province, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. However, data are available from the authors upon reasonable request and with permission of the Center for Disease Control of Zhejiang Province.
Abbreviations
- BA:
-
Biological age
- CA:
-
Chronological age
- ML-BA:
-
Machine learning-based BA
- STK-BA:
-
BA based on Stacking method with a simple meta-model
- XGB-BA:
-
Xgboost-based BA
- PCA:
-
Principle component analysis
- MLP:
-
Multilayer perceptron
- KDM:
-
The Klemera and Doubal method
- ML:
-
Machine learning
- SD:
-
Standard deviation
- XGBoost:
-
Extreme gradient boosting
- BMI:
-
Body mass index
- KNN:
-
K-nearest neighbor
- MICE:
-
Multiple imputations by chained equations
- AE:
-
Autoencoder
- RRLR:
-
Round-robin linear regression
- MCAR:
-
Missing completely at random
- MNAR:
-
Missing not at random
- MSE:
-
Mean squared error
- MLR:
-
Multiple linear regression
- GAM:
-
Generalized additive models
- SVM:
-
Support vector machine
- GBDT:
-
Gradient boosting decision tree
- LGBM:
-
Light gradient boosting machine
- DNN:
-
Deep neural networks
- CNN:
-
Convolutional neural network
- RMSE:
-
Root mean squared error
- MAE:
-
Mean absolute error
- DBP:
-
Diastolic blood pressure
- SBP:
-
Systolic blood pressure
- SGPT:
-
Serum glutamic-pyruvic transaminase
- SGOT:
-
Serum glutamic-oxaloacetic transaminase
- CI:
-
Confidence interval
- TC:
-
Total cholesterol
- TG:
-
Triglycerides
- LDL:
-
Low-density lipoprotein
- HDL:
-
High density lipoprotein
- PC:
-
Platelets count
- TCA cycle:
-
Tricarboxylic acid cycle
- ABSI:
-
A Body Shape Index
- SBSI:
-
Surface-based body shape index
- WHtR:
-
Waist-to-height ratio
- WHR:
-
Waist-to-hip ratio
- FIV:
-
Feature importance value
- ORs:
-
Odds ratios
- NCDs:
-
Non-communicable chronic disease
- LOOCV:
-
Leave-one-out cross-validation
- WC:
-
Waist circumference
- GCV:
-
Generalized cross-validation
References
Zhang B, Trapp A, Kerepesi C, Gladyshev VN. Emerging rejuvenation strategies—reducing the biological age. Aging Cell. 2022;21(1):e13538. https://doi.org/10.1111/acel.13538.
Galkin F, Zhang B, Dmitriev SE, Gladyshev VN. Reversibility of irreversible aging. Ageing Res Rev. 2019;49:104–14.
Hannum G, Guinney J, Zhao L, Zhang L, Hughes G, Sadda S, Klotzle B, Bibikova M, Fan J-B, Gao Y, et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell. 2013;49(2):359–67.
Jylhävä J, Pedersen NL, Hägg S. Biological age predictors. EBioMedicine. 2017;21:29–36.
Levine ME. Modeling the rate of senescence: can estimated biological age predict mortality more accurately than chronological age? J Gerontol A Biol Sci Med Sci. 2013;68(6):667–74. https://doi.org/10.1093/gerona/gls233.
Ashiqur Rahman S, Giacobbi P, Pyles L, Mullett C, Doretto G, Adjeroh DA. Deep learning for biological age estimation. Brief Bioinform. 2021;22(2):1767–81. https://doi.org/10.1093/bib/bbaa021.
Gialluisi A, Di Castelnuovo A, Donati MB, de Gaetano G, Iacoviello L, Moli-sani Study I. Machine learning approaches for the estimation of biological aging: the road ahead for population studies. Front Med (Lausanne). 2019;6:146–146. https://doi.org/10.3389/fmed.2019.00146.
Jia L, Zhang W, Jia R, Zhang H, Chen X. Construction formula of biological age using the principal component analysis. BioMed Res Int. 2016;2016:e4697017.
Park J, Cho B, Kwon H, Lee C. Developing a biological age assessment equation using principal component analysis and clinical biomarkers of aging in Korean men. Arch Gerontol Geriatr. 2009;49(1):7–12. https://doi.org/10.1016/j.archger.2008.04.003.
Tzemah-Shahar R, Hochner H, Iktilat K, Agmon M. What can we learn from physical capacity about biological age? A systematic review. Ageing Res Rev. 2022;77:101609. https://doi.org/10.1016/j.arr.2022.101609.
di Giuseppe R, Arcari A, Serafini M, Di Castelnuovo A, Zito F, De Curtis A, Sieri S, Krogh V, Pellegrini N, Schünemann HJ, et al. Total dietary antioxidant capacity and lung function in an Italian population: a favorable role in premenopausal/never smoker women. Eur J Clin Nutr. 2012;66(1):61–8. https://doi.org/10.1038/ejcn.2011.148.
Russoniello CV, Zhirnov YN, Pougatchev VI, Gribkov EN. Heart rate variability and biological age: implications for health and gaming. Cyberpsychol Behav Soc Netw. 2013;16(4):302–8. https://doi.org/10.1089/cyber.2013.1505.
Hannum G, Guinney J, Zhao L, et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell. 2013;49(2):359–67. https://doi.org/10.1016/j.molcel.2012.10.016.
Zhang W-G, Zhu S-Y, Bai X-J, et al. Select aging biomarkers based on telomere length and chronological age to build a biological age equation. Age. 2014;36(3):9639. https://doi.org/10.1007/s11357-014-9639-y.
Nakamura E, Miyao K. A method for identifying biomarkers of aging and constructing an index of biological age in humans. J Gerontol Ser A. 2007;62(10):1096–105. https://doi.org/10.1093/gerona/1062.1010.1096.
Bae C-Y, Kang YG, Kim S, et al. Development of models for predicting biological age (BA) with physical, biochemical, and hormonal parameters. Arch Gerontol Geriatr. 2008;47(2):253–65.
Klemera P, Doubal S. A new approach to the concept and computation of biological age. Mech Ageing Dev. 2006;127(3):240–8.
Cao X, Yang G, Jin X, He L, Li X, Zheng Z, Liu Z, Wu C. A machine learning-based aging measure among middle-aged and older Chinese adults: the China health and retirement longitudinal study. Front Med (Lausanne). 2021;8:698851–698851. https://doi.org/10.3389/fmed.2021.698851.
Jin X, Xiong S, Ju S-Y, Zeng Y, Yan LL, Yao Y. Serum 25-hydroxyvitamin D, albumin, and mortality among Chinese older adults: a population-based longitudinal study. J Clin Endocrinol Metab. 2020;105(8):2762–70. https://doi.org/10.1210/clinem/dgaa349.
Putin E, Mamoshina P, Aliper A, Korzinkin M, Moskalev A, Kolosov A, Ostrovskiy A, Cantor C, Vijg J, Zhavoronkov A. Deep biomarkers of human aging: application of deep neural networks to biomarker development. Aging (Albany NY). 2016;8(5):1021–33. https://doi.org/10.18632/aging.100968.
Bobrov E, Georgievskaya A, Kiselev K, Sevastopolsky A, Zhavoronkov A, Gurov S, Rudakov K, Tobar MDPB, Jaspers S, Clemann S. PhotoAgeClock: deep learning algorithms for development of non-invasive visual biomarkers of aging. Aging (Albany NY). 2018;10(11):3249–59. https://doi.org/10.18632/aging.101629.
Zhavoronkov A, Mamoshina P, Vanhaelen Q, Scheibye-Knudsen M, Moskalev A, Aliper A. Artificial intelligence for aging and longevity research: recent advances and perspectives. Ageing Res Rev. 2019;49:49–66. https://doi.org/10.1016/j.arr.2018.11.003.
Chen L-K. Machine learning improves analysis of multi-omics data in aging research and geroscience. Arch Gerontol Geriatr. 2021;93:104360. https://doi.org/10.1016/j.archger.2021.104360.
Pyrkov TV, Slipensky K, Barg M, Kondrashin A, Zhurov B, Zenin A, Pyatnitskiy M, Menshikov L, Markov S, Fedichev PO. Extracting biological age from biomedical data via deep learning: too much of a good thing? Sci Rep. 2018. https://doi.org/10.1038/s41598-018-23534-9.
Bae C-Y, Im Y, Lee J, et al. Comparison of biological age prediction models using clinical biomarkers commonly measured in clinical practice settings: AI techniques vs. traditional statistical methods. Front Anal Sci. 2021. https://doi.org/10.3389/frans.2021.709589.
Liu Z. Development and validation of 2 composite aging measures using routine clinical biomarkers in the Chinese population: analyses from 2 prospective cohort studies. J Gerontol A Biol Sci Med Sci. 2021;76(9):1627–32. https://doi.org/10.1093/gerona/glaa238.
Finkel D, Sternäng O, Wahlin Å. Genetic and environmental influences on longitudinal trajectories of functional biological age: comparisons across gender. Behav Genet. 2017;47(4):375–82. https://doi.org/10.1007/s10519-017-9851-5.
Rahman SA, Adjeroh DA. Deep learning using convolutional LSTM estimates biological age from physical activity. Sci Rep. 2019;9(1):11425–11425. https://doi.org/10.1038/s41598-019-46850-0.
Wang C, Guan X, Bai Y, et al. A machine learning–based biological aging prediction and its associations with healthy lifestyles: the Dongfeng-Tongji cohort. Ann N Y Acad Sci. 2022;1507(1):108–20. https://doi.org/10.1111/nyas.14685.
Srivastava S, Soman S, Rai A, Srivastava PK: Deep learning for health informatics: recent trends and future directions. In: 2017 International Conference on Advances in Computing, Communications and Informatics (ICACCI): 13–16 Sept. 2017 2017. 1665–1670.
Beaulieu-Jones BK, Lavage DR, Snyder JW, Moore JH, Pendergrass SA, Bauer CR. Characterizing and managing missing structured data in electronic health records: data analysis. JMIR Med Inform. 2018;6(1):e11–e11. https://doi.org/10.2196/medinform.8960.
Zhang X, Yan C, Gao C, Malin BA, Chen Y. Predicting missing values in medical data via XGBoost regression. J Healthc Inf Res. 2020;4(4):383–94. https://doi.org/10.1007/s41666-020-00077-1.
Emmanuel T, Maupong T, Mpoeleng D, Semong T, Mphago B, Tabona O. A survey on missing data in machine learning. J Big Data. 2021;8(1):140. https://doi.org/10.1186/s40537-021-00516-9.
Peralta M, Jannin P, Haegelen C, Baxter JSH. Data imputation and compression for Parkinson’s disease clinical questionnaires. Artif Intell Med. 2021;114:102051. https://doi.org/10.1016/j.artmed.2021.102051.
Das S, Datta S, Chaudhuri BB. Handling data irregularities in classification: foundations, trends, and future challenges. Pattern Recognit. 2018;81:674–93. https://doi.org/10.1016/j.patcog.2018.03.008.
Zahid FM, Heumann C. Multiple imputation with sequential penalized regression. Stat Methods Med Res. 2018;28(5):1311–27. https://doi.org/10.1177/0962280218755574.
Lee JY, Styczynski MP. NS-kNN: a modified k-nearest neighbors approach for imputing metabolomics data. Metabo Off J Metabol Soc. 2018;14(12):153–153. https://doi.org/10.1007/s11306-018-1451-8.
Qiu YL, Zheng H, Gevaert O. Genomic data imputation with variational auto-encoders. GigaScience. 2020. https://doi.org/10.1093/gigascience/giaa082.
Silva HD, Perera AS: Missing data imputation using Evolutionary k- Nearest neighbor algorithm for gene expression data. In: 2016 Sixteenth International Conference on Advances in ICT for Emerging Regions (ICTer): 1–3 Sept 2016. 141–146.
Rose S. Machine learning for prediction in electronic health data. JAMA Netw Open. 2018;1(4):e181404–e181404. https://doi.org/10.1001/jamanetworkopen.2018.1404.
Roozbeh M. Optimal QR-based estimation in partially linear regression models with correlated errors using GCV criterion. Comput Stat Data Anal. 2018;117:45–61. https://doi.org/10.1016/j.csda.2017.08.002.
Lin H, Lunetta KL, Zhao Q, Mandaviya PR, Rong J, Benjamin EJ, Joehanes R, Levy D, van Meurs JBJ, Larson MG, et al. Whole blood gene expression associated with clinical biological age. J Gerontol Ser A. 2019;74(1):81–8. https://doi.org/10.1093/gerona/gly164.
Pyrkov TV, Slipensky K, Barg M, Kondrashin A, Zhurov B, Zenin A, Pyatnitskiy M, Menshikov L, Markov S, Fedichev PO. Extracting biological age from biomedical data via deep learning: too much of a good thing? Sci Rep. 2018;8(1):5210–5210. https://doi.org/10.1038/s41598-018-23534-9.
Mamoshina P, Kochetov K, Putin E, Cortese F, Aliper A, Lee W-S, Ahn S-M, Uhn L, Skjodt N, Kovalchuk O, et al. Population specific Biomarkers of human aging: a big data study using South Korean, Canadian, and Eastern European patient populations. J Gerontol A Biol Sci Med Sci. 2018;73(11):1482–90. https://doi.org/10.1093/gerona/gly005.
Mørkedal B, Romundstad PR, Vatten LJ. Informativeness of indices of blood pressure, obesity and serum lipids in relation to ischaemic heart disease mortality: the HUNT-II study. Eur J Epidemiol. 2011;26(6):457–61. https://doi.org/10.1007/s10654-011-9572-7.
Krakauer NY, Krakauer JC. A new body shape index predicts mortality hazard independently of body mass index. PLoS ONE. 2012;7(7):e39504–e39504. https://doi.org/10.1371/journal.pone.0039504.
Rivero-Segura NA, Bello-Chavolla OY, Barrera-Vázquez OS, Gutierrez-Robledo LM, Gomez-Verjan JC. Promising biomarkers of human aging: In search of a multi-omics panel to understand the aging process from a multidimensional perspective. Ageing Res Rev. 2020;64:101164. https://doi.org/10.1016/j.arr.2020.101164.
Yoo J, Kim Y, Cho ER, Jee SH. Biological age as a useful index to predict seventeen-year survival and mortality in Koreans. BMC Geriatr. 2017;17(1):7–7. https://doi.org/10.1186/s12877-016-0407-y.
Waziry R, Gras L, Sedaghat S, Tiemeier H, Weverling GJ, Ghanbari M, Klap J, de Wolf F, Hofman A, Ikram MA, et al. Quantification of biological age as a determinant of age-related diseases in the Rotterdam study: a structural equation modeling approach. Eur J Epidemiol. 2019;34(8):793–9. https://doi.org/10.1007/s10654-019-00497-3.
Song Q, Shepperd M. Missing data imputation techniques. Int J Bus Intell Data Min. 2007;2(3):261–91. https://doi.org/10.1504/IJBIDM.2007.015485.
Yu L, Liu L, Peace KE. Regression multiple imputation for missing data analysis. Stat Methods Med Res. 2020;29(9):2647–64. https://doi.org/10.1177/0962280220908613.
Mongin D, Lauper K, Turesson C, Hetland ML, Klami Kristianslund E, Kvien TK, Santos MJ, Pavelka K, Iannone F, Finckh A, et al. Imputing missing data of function and disease activity in rheumatoid arthritis registers: what is the best technique? RMD Open. 2019;5(2):e000994. https://doi.org/10.1136/rmdopen-2019-000994.
Hegde H, Shimpi N, Panny A, Glurich I, Christie P, Acharya A. MICE vs PPCA: missing data imputation in healthcare. Inf Med Unlocked. 2019;17:100275. https://doi.org/10.1016/j.imu.2019.100275.
LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521(7553):436–44. https://doi.org/10.1038/nature14539.
Pereira RC, Santos M, Rodrigues P, Henriques Abreu P. Reviewing autoencoders for missing data imputation: technical trends, applications and outcomes. J Artif Intell Res. 2020;69:1255–85. https://doi.org/10.1613/jair.1.12312.
Vincent P, Larochelle H, Lajoie I, Bengio Y, Manzagol PA. Stacked denoising autoencoders: learning useful representations in a deep network with a local denoising criterion. J Mach Learn Res. 2010;11(12):3371–408.
Xiao C, Choi E, Sun J. Opportunities and challenges in developing deep learning models using electronic health records data: a systematic review. J Am Med Inform Assoc. 2018;25(10):1419–28. https://doi.org/10.1093/jamia/ocy068.
Roine A, Veskimäe E, Tuokko A, et al. Detection of prostate cancer by an electronic nose: a proof of principle study. J Urol. 2014;192(1):230–5. https://doi.org/10.1016/j.juro.2014.01.113.
Roozbeh M, Arashi M, Hamzah NA. Generalized cross-validation for simultaneous optimization of tuning parameters in ridge regression. Iran J Sci Technol Trans A Sci. 2020;44(2):473–85. https://doi.org/10.1007/s40995-020-00851-1.
Chen Y, Wong M-L, Li H. Applying Ant Colony Optimization to configuring stacking ensembles for data mining. Expert Syst Appl. 2014;41(6):2688–702. https://doi.org/10.1016/j.eswa.2013.10.063.
Pinto E. Blood pressure and ageing. Postgrad Med J. 2007;83(976):109–14. https://doi.org/10.1136/pgmj.2006.048371.
Le Blanc J, Lordkipanidzé M. Platelet function in aging. Front Cardiovasc Med. 2019. https://doi.org/10.3389/fcvm.2019.00109.
Segal JB, Moliterno AR. Platelet Counts differ by sex, ethnicity, and age in the United States. Ann Epidemiol. 2006;16(2):123–30. https://doi.org/10.1016/j.annepidem.2005.06.052.
Troussard X, Vol S, Cornet E, Bardet V, Couaillac J-P, Fossat C, Luce J-C, Maldonado E, Siguret V, Tichet J, et al. Full blood count normal reference values for adults in France. J Clin Pathol. 2014;67(4):341. https://doi.org/10.1136/jclinpath-2013-201687.
Krakauer JC, Franklin B, Kleerekoper M, Karlsson M, Levine JA. Body composition profiles derived from dual-energy X-ray absorptiometry, total body scan, and mortality. Preven Cardiol. 2004;7(3):109–15. https://doi.org/10.1111/j.1520-037X.2004.3326.x.
Samaras TT. Should we be concerned over increasing body height and weight? Exp Gerontol. 2009;44(1):83–92. https://doi.org/10.1016/j.exger.2008.02.002.
Salaris L, Poulain M, Samaras TT. Height and survival at older ages among men born in an inland village in Sardinia (Italy), 1866–2006. Biodemography Soc Biol. 2012;58(1):1–13. https://doi.org/10.1080/19485565.2012.666118.
Maier AB, van Heemst D, Westendorp RGJ. Relation between body height and replicative capacity of human fibroblasts in nonagenarians. J Gerontol Ser A. 2008;63(1):43–5. https://doi.org/10.1093/gerona/63.1.43.
Hägg S, Jylhävä J. Sex differences in biological aging with a focus on human studies. Elife. 2021;10:e63425. https://doi.org/10.7554/eLife.63425.
Li J, Guasch-Ferré M, Chung W, Ruiz-Canela M, Toledo E, Corella D, Bhupathiraju SN, Tobias DK, Tabung FK, Hu J, et al. The Mediterranean diet, plasma metabolome, and cardiovascular disease risk. Eur Heart J. 2020;41(28):2645–56. https://doi.org/10.1093/eurheartj/ehaa209.
Lam AP, Gundabolu K, Sridharan A, Jain R, Msaouel P, Chrysofakis G, Yu Y, Friedman E, Price E, Schrier S, et al. Multiplicative interaction between mean corpuscular volume and red cell distribution width in predicting mortality of elderly patients with and without anemia. Am J Hematol. 2013;88(11):E245–9. https://doi.org/10.1002/ajh.23529.
Passarino G, Montesanto A, De Rango F, Garasto S, Berardelli M, Domma F, Mari V, Feraco E, Franceschi C, De Benedictis G. A cluster analysis to define human aging phenotypes. Biogerontology. 2007;8(3):283–90. https://doi.org/10.1007/s10522-006-9071-5.
Guida JL, Ahles TA, Belsky D, et al. Measuring aging and identifying aging phenotypes in cancer survivors. JNCI J Natl Cancer Inst. 2019;111(12):1245–54. https://doi.org/10.1093/jnci/djz136.
Bell CG, Lowe R, Adams PD, Baccarelli AA, Beck S, Bell JT, Christensen BC, Gladyshev VN, Heijmans BT, Horvath S, et al. DNA methylation aging clocks: challenges and recommendations. Genome Biol. 2019;20(1):249. https://doi.org/10.1186/s13059-019-1824-y.
Salameh Y, Bejaoui Y, El Hajj N. DNA methylation biomarkers in aging and age-related diseases. Front Genet. 2020. https://doi.org/10.3389/fgene.2020.00171.
Menni C, Kastenmüller G, Petersen AK, Bell JT, Psatha M, Tsai P-C, Gieger C, Schulz H, Erte I, John S, et al. Metabolomic markers reveal novel pathways of ageing and early development in human populations. Int J Epidemiol. 2013;42(4):1111–9. https://doi.org/10.1093/ije/dyt094.
Srivastava S. Emerging insights into the metabolic alterations in aging using metabolomics. Metabolites. 2019;9(12):301. https://doi.org/10.3390/metabo9120301.
Shahmirzadi AA, Edgar D, Liao C-Y, et al. Alpha-ketoglutarate, an endogenous metabolite, extends lifespan and compresses morbidity in aging mice. bioRxiv. 2019. https://doi.org/10.1101/779157.
Prineas RJ, Le A, Soliman EZ, Zhang Z-M, Howard VJ, Ostchega Y, Howard G. United States national prevalence of electrocardiographic abnormalities in black and white middle-age (45- to 64-year) and older (≥65-year) adults (from the reasons for geographic and racial differences in stroke study). Am J Cardiol. 2012;109(8):1223–8. https://doi.org/10.1016/j.amjcard.2011.11.061.
Rahman SA, Adjeroh D. Surface-based body shape index and its relationship with all-cause mortality. PLoS ONE. 2015;10(12):e0144639–e0144639. https://doi.org/10.1371/journal.pone.0144639.
Acknowledgements
We gratefully acknowledge all the people who helped in the establishment of the medical examination data set.
Funding
This work was supported by the 151 Talent Project of Zhejiang Province (first level); the National Natural Sciences Foundation of China (32070677); Health technology Plan of Zhejiang Province (2021PY004); Jiangsu Collaborative Innovation Center for Modern Crop Production and Collaborative Innovation Center for Modern Crop Production cosponsored by the province and ministry.
Author information
Authors and Affiliations
Contributions
Resources, project administration, Funding acquisition: QY, JL, YZ, WW, TL, HP, MC; investigation, conceptualization, methodology, data analysis, formal analysis: SG, KL; writing and visualization: SG, KL; writing-review & editing, supervision: SG, KL, QY, ZW, YC, MC. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This paper has passed the ethics review of the Ethics Committee of Zhejiang Provincial Center for Disease Control and Prevention (Approval No.: 2022-033-01). All the data and methods involved in this paper are in line with the relevant regulations of ethical review. The Ethics Committee of Zhejiang Provincial Center for Disease Control and Prevention makes the decision that the project and the papers produced by the project can be exempted from signing the informed consent. The ethics committee conducted an ethical review of the project and held that: 1. The project data is derived from existing medical data, which will not cause any damage to any individual, and will not bring any harm and inconvenience to any individual’s daily life and work. 2. The data does not contain personal information such as the residents’ names, telephone numbers, addresses, etc., and the project researchers have been unable to get in touch with the residents, and objectively cannot give informed consent to the relevant individuals. 3. After the working system developed in this project is technically mature, it will be provided to grass-roots community hospitals throughout the province for free, and will not involve commercial interests. According to the relevant provisions of the “Measures for Ethical Review of Biomedical Research Involving Humans”, the ethics committee makes the decision that the project and the papers produced by the project can be exempted from signing the informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1. Fig. S1
. Optimized lambda selection (A) and feature selection (B) in Lasso regression. Fig. S2. Feature importance values in the Stacking model. Fig. S3. Schematic diagrams of the process for introducing missing values and interpolation in MCAR (A) and MNAR (B). Table S1. Parameter optimization results of KNN and MICE in MCAR. Table S2. Parameter optimization results of KNN and MICE in MNAR. Table S3. The optimized parameters of AE and RRLR. Table S4. The interpolation time consumed by the different models. Table S5. Coefficients of biological features in Lasso regression (lambda = 0.00072). Table S6. Parameter optimization results of GAM (splines), AdaBoost, CNN, DNN, Extra Trees, GBDT, LGBM, CatBoost, XGBoosts. Table S7. The results and parameters of two XGBoost models. Table S8. The variable importance values of the sub-models in the Stacking model. Table S9. Associations of STK-BA and XGB-BAs with health risk indicators. Table S10. Associations of STK-BA and XGB-BAs with health risk indicators (Quintile, ABSI). Table S11. Associations of STK-BA and XGB-BAs with health risk indicators (Quintile, WHtR). Table S12. Predicted increase in STK-BA and XGB-BAs for each disease count. Table S13. The associations between each disease and STK-BA, XGB-BAs. Table S14. The 19 biological features’ attributes of study participants (n=77,144). Table S15. The disease status of study population (n=77, 144).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, Q., Gao, S., Lin, J. et al. A machine learning-based data mining in medical examination data: a biological features-based biological age prediction model. BMC Bioinformatics 23, 411 (2022). https://doi.org/10.1186/s12859-022-04966-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12859-022-04966-7