Abstract
Background
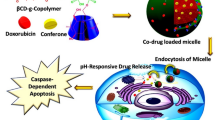
Designing and preparing a co-delivery system based on polymeric micelles have attracted in recent years. Co-delivery of anti-cancer agents within pH-sensitive polymeric micelles could provide superior advantages over the co-administration of free drugs, since it enables simultaneous delivery of drugs to reach an optimum synergistic dose right to the tumor.
Methods
DOX was conjugated to the polymer through a hydrazine linker by Schiff’s base reaction. Then, DTX was encapsulated into the core of the polymer to the resulting DOX-Hyd-PM/DTX micelle with optimum molar ratios of 1:1 and 1:5 (DOX/DTX).
Results
The final formulations showed the desired particle size and increased release of DOX and DTX in acidic media (pH 5.5). The cytotoxicity assay of DOX-Hyd-PM/DTX indicated the highest synergistic effect on both 4T1 and TUBO cell lines over other formulations. Interestingly, in accordance with in vitro results, DOX-Hyd-PM/DTX revealed a promising anti-tumor activity in mice-bearing 4T1 breast cancer tumor with higher tumor accumulation of DOX and DTX after 24 h compared to free drugs combination.
Conclusions
These findings point to the potential use of such smart nanodrug delivery systems in cancer treatment, where the synergistic effect of both drugs may be used to enhance therapeutic response.
Graphical Abstract

Similar content being viewed by others
Introduction
Chemotherapy is associated with a number of challenges, including normal tissue toxicity (Brianna and Lee 2023; Bo et al. 2023; Rose et al. 2023). Furthermore, cancer cells could become less responsive to treatment over time due to the occurrence of resistance, which limits the efficacy of therapy (Schirrmacher 2019). Despite these challenges, chemotherapy is still an important part of cancer treatment, and ongoing research is focusing on improving its efficacy and reducing its side effects. The superior advantages of nanoparticulate drug delivery systems (NDDSs), including desirable size and surface area, allow for enhancing the physico-chemical properties of drug molecules including low aqueous solubility. Furthermore, the functionalization of the carrier could facilitate targeting distinct tumor areas, increase the effectiveness of therapy, and decrease undesirable side effects. Indeed, by prolonging the blood circulation time of the drug moieties, NDDSs could accumulate in the target area by passive targeting known as EPR (enhanced permeability and retention effect) (Alghamdi et al. 2022; Nisha et al. 2022). NDDSs have demonstrated impressive anti-tumor potential, as evidenced by the FDA's approval of Doxil in 1995, which was among the initial successful clinical trials. By reducing toxicity and increasing efficacy, this approach allowed for higher doses of the drug to be administered to patients while minimizing the risk of dose-dependent cardiotoxic effects (Shafei et al. 2017).
Polymers have evolved into smart materials that can adapt to changes in their environment through stimuli-responsive behavior. This behavior is determined by functional groups within the polymer chain. Smart polymers have enabled on-demand drug delivery with customized release profiles and precise dosage control in targeted areas. By designing systems that can recognize and respond dynamically to their microenvironment, similar to biological organisms, drugs can be distributed on demand. (Rao et al. 2018). As there is complexity in tumor tissue and microenvironment, single drug delivery is not enough to reach effective treatment. Hence, dual drug delivery seems to be a promising alternative approach to treating cancer.
Combination therapy has long been used for chemotherapy to assist with tackling dose-dependent side effects, due to the minimum dosage of agents typically used (Rostamizadeh and Torchilin 2020; Vakili-Ghartavol et al. 2022). Furthermore, synergistic therapeutic effect is an added value (Yu et al. 2019b), since multiple drugs could target varying aspects of the cancer cell’s biology or signaling pathways, thus enhancing cancer cell killing (Din et al. 2017). Co-delivery of anti-cancer agents within nanocarriers could provide superior advantages over co-administration of free drugs, since it enables simultaneous delivery of drugs right to the target site (Yu et al. 2019a). A combination of chemotherapeutic agents with precise drug ratio is required for increasing drug payload within the carrier to reach an optimum synergistic dose at the target site (Li et al. 2023; Ghosh et al. 2019). The combination therapy takes advantage of the synergistic effects of various drugs to maximize treatment efficacy. Through precise drug ratio calibration, researchers attempt to target cancer cells via multiple pathways at once, potentially lowering the emergence of drug resistance (Qi et al. 2017). In addition, it is possible to further improve therapeutic efficacy using stimuli-responsive drug delivery system that can minimize nonspecific delivery (Rostamizadeh and Torchilin 2020).
Polymeric micelles are among promising drug delivery carriers that effectively address the bioavailability issues associated with hydrophobic drugs while also enabling simultaneous delivery of hydrophilic drugs (Fathi and Barar 2017). These nanocarriers are made of amphiphilic block copolymer, which self-assemble in an aqueous solution to form a hydrophilic shell that prolongs bloodstream circulation and improves solubility and a hydrophobic core that can encapsulate insoluble drugs (Farhoudi et al. 2022). These micelles possess several key advantages, including small size, and stability due to featured thermodynamic and kinetic properties (Rostamizadeh and Torchilin 2020; Ngoune et al. 2016). This unique structure allows for efficient delivery of a wide variety of therapeutic agents, which lower systemic toxicity while increasing therapeutic efficacy at the target location (Cai et al. 2023). However, the major limiting point is the tendency for burst drug release (Pacheco et al. 2023). Stimuli-responsive systems are the preferred choice for targeted drug delivery due to numerous advantages, including the ability to achieve responsive drug release, thus improving drug availability in the targeted area (Zhou et al. 2022; Long et al. 2022). This innovative approach involves using NDDSs that respond to specific stimuli, such as changes in the pH, temperature, or light, to release their cargo in a controlled manner. Prodrug-based nanoassemblies have also been designed which can release the drug upon exposure to specific stimuli within the cancerous tissue, resulting in an enhanced drug efficacy (Long et al. 2022). Some stimulus-responsive nanomaterials have been developed to ensure that these nanosystems have a longer circulation, increased accumulation in tumor tissues, and stay stable in the circulatory system (Cheng et al. 2019; Chen et al. 2020). Polymeric micelles with pH-responsiveness have been intensively studied as "smart vehicles," and represent a promising strategy in cancer therapy owing to the pH gradient of the tumor and normal tissue (Qin et al. 2021; Xing and Zhao 2018).
Doxorubicin is a type of anthracycline antibiotic that involves by intercalating into DNA and inhibiting DNA synthesis while also generating free radicals that damage cancer cells (Li et al. 2022). DTX belongs to the taxane family and works by inhibiting microtubule disassembly, ultimately leading to cell death (Shokooh et al. 2022). Both drugs are widely used as chemotherapeutic drugs in the treatment of various types of cancer, including breast, lung, and ovarian cancers due to their high efficacy in treating the cancer disease (Mahani et al. 2023; Rarokar et al. 2023). Despite their effectiveness, these drugs can cause significant side effects, including nausea, hair loss, and an increased risk of infections, among others (Shafei et al. 2017). To date, numerous researchers have reported a synergistic effect of DOX and DTX with an optimum ratio in carriers (Li et al. 2019; Sheu et al. 2016) to assess anti-tumor effect in different cancers. What is more, an ideal co-delivery system should have a high drug loading efficiency with an optimum drug ratio into the tumor site. Conjugation of the anti-cancer agent is a promising strategy that avoids the disassembly of the nanocarrier itself or in response to protein plasma upon i.v. administration (Kumbham et al. 2022). This approach guarantees more regulatory and focused drug delivery to tumor cells while also considerably lowering the early release of drugs, thereby minimizing side effects and improving patient outcomes. In this regard, Yakun et al. developed polymeric micelles for the co-delivery of doxorubicin (DOX) and paclitaxel (PTX) with conjugation of DOX to a Pluronic F127-chitosan polymer via an acidic linker to form a pH-sensitive polymeric micelle. At the acidic pH, the cleavage of acid linker was facilitated and released the drug (Ma et al. 2016b). Hence, to address these challenges, it is imperative to develop a novel drug delivery system. We hypothesized that the co-delivery of DOX and DTX within a pH-sensitive carrier could improve drug delivery and release in a sustained and pH-dependent manner, and incorporated DTX could enhance the anti-cancer efficacy of DOX to a considerable level for the treatment of breast cancer.
Thus, for the sake of synergistic efficacy of DOX and DTX, a smart pH-sensitive polymeric micelle that could simultaneously entrap and release DOX and DTX was designed with a precise drug ratio. For this, hydrophilic DOX was first conjugated to poly(N,N-dimethyl acrylamide-co-pentafluorphenyl acrylate) P(DMA-co-PFPA) using a hydrazine linker between drug and polymer backbone to form DOX-Hyd-PM, upon formation of a polymeric micelle, DTX was then incorporated into the core of the micelles through self-assembly to form DOX-Hyd-PM/DTX.
Experiment
Materials
All the materials are listed in supplementary file. (S1–1). The polymer poly(N,N-dimethyl acrylamide-co-pentafluorphenyl acrylate) p(DMA-co-PFPA) was kindly provided by professor Theato’s team (Lin et al. 2017; Gaballa et al. 2018).
Cell line
All cells and their equipment requirements are listed in supplementary file (S1–2).
Docetaxel-loaded polymeric micelles (DTX-PM)
The p(DMA-co-PFPA) polymer was kindly synthesized by prof. Patrick Theato’ team (Li et al. 2021; Lin et al. 2017; Das and Theato 2016). DTX-loaded polymeric micelles (DTX-PM) and blank polymeric micelle (without drug) were prepared using film method. Briefly, equal amounts of DTX and polymer solution at 20 mg/ml were mixed together in a round-bottom flask and the solvent was evaporated under rotary evaporation (Heidolph, Germany). The resulting DTX-PM dry film was hydrated in HEPES-Sucrose buffer (10 mM, pH 7.4) at the 25 ℃ using bath sonicator (Bandelin electronic GmbH & Co. KG, Berlin, Germany) (5 min) to obtain a micelles solution. In the next step, unloaded DTX was removed by centrifugation (11,000 rpm/min, 5 min) and the supernatant was collected. The blank polymeric micelles (without drug) were prepared with the same method without loading DTX. In addition, fluorescence-labeled polymeric micelles were prepared by the same method, except that instead of DTX, DiI dye with a molar ratio of 0.2% of total polymer was incorporated into the polymeric micelles.
Determination of DTX in polymeric micelles (DTX-PM)
Encapsulation efficiency (EE%) was measured using reverse-phase HPLC (Berline, Germany) through by C18 column (3.5µm, 150 × 4.6 mm). In this regard, the 1 mL of each prepared formulation was dissolved in acetonitrile and methanol solution (55:45, v/v) and used for injection in HPLC with UV/Vis detector (Knauer S2600) at 232 nm with a flow rate of 1 mL/min. The drug loaded efficacy was calculated using the following equation:
Determination of critical blank micelles concentration (CMC)
Critical blank micelles concentration (CMC) was determined by the iodine method (Thotakura et al. 2017). Briefly, a standard solution of iodine (16 mg/ml) was prepared and various concentrations (0.125–10 mg/ml) of polymer dissolved in water and 10 µL from iodine solution were added to each dilution. The solution was incubated overnight, and absorbance was measured using UV–Vis spectra (UV-160A Shimadzu) at λmax = 369 nm and CMC was verified.
DOX/DTX optimal ratio
The optimal DOX/DTX ratio in terms of cancer cell killing was determined by incubating the two drugs at different molar ratios with 4T1 and TUBO tumor cells. The cells were placed overnight in 96-well plate at a density of (4000 cells/well). The next day, the cells were treated with different molar ratios of DTX and DOX (1:1, 1:5, 1:10, 5:1, and 10:1, respectively) for three replicates per concentration. The culture medium was replaced with fresh RPMI or DMEM medium (for 4T1 and TUBO tumor cells, respectively) and incubated continued for 48 h at 37 ℃. Following that, MTT solution (5 mg/ml) was added to each well and incubated for 4 h. Finally, the absorption was read at 570 nm with 630 nm references wavelength using microplate reader (Tecan Group Ltd., Mannedorf, Switzerland). The IC50 value was calculated using CalcuSyn Version 2.0 software (BIOSOFT, UK) (Mashreghi et al. 2021). The synergistic effect of both drugs was evaluated using Cho and Talalay's combination index (CI) (Chou 2010) at different molar ratios of DOX/DTX.
Synthesis and characterization of DOX-Hyd-polymer conjugate
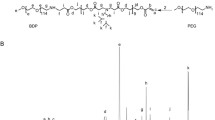
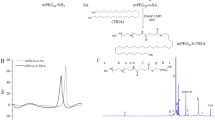
DOX-Hyd-polymer conjugation was prepared according to Van Driessche et al. protocol with modifications (Van Driessche et al. 2018). Briefly, a round-bottom flask was filled with 40 mg of dry pDMA-b-pPFPA and anhydrous DMSO under N2 atmosphere. In addition, 1mL hydrazine (Hyd) and triethylamine (2%, v/v) (TEA) were added to remove the reactive pentafluoro phenyl (PFP) groups in the polymer. The reaction was stirred overnight under N2 atmosphere at 50 ℃. Afterwards, the reaction mixture was dialyzed (MW: 3.5 kDa) against water/methanol mixture (1:1 v/v) for 3 days. The hydrazine-modified polymer) was freeze-dried (taitech, Koshigaya, Japan) and kept at − 20 ℃. In the next step, 10 mg/mL DOX and 25 mg of freeze-dried hydrazine-bearing polymer were dissolved in 2 mL anhydrous methanol and 50 µl acetic acid was added to promote the reaction stirred overnight in the defined condition (out of light and room temperature). At the end of reaction, dialysis was done using dialysis bag (MW: 3.5kDa) against % 0.05 ammonia buffer (pH 9.1). The solution was freeze-dried overnight until a dark red color freeze-dried powder appeared (Fig. 2).
The product was dispersed in HEPES/sucrose buffer (10 mM, pH 7.4) and analyzed by fluorescent spectrophotometer (Perkin-Elmer LS-45) with an emission wavelength of 590 nm and excitation wavelength of 485 nm. The final product (DOX-Hyd-PM) was characterized by 1H and 19F NMR (proton nuclear magnetic resonance) spectroscopy in deuterated (CDCl3) was recorded at 400 MHz (Varian Medical System Inc., Palo Alto, CA, USA). In addition, final reaction was monitored by TLC followed Iodine staining.
Preparation of DTX loaded DOX-Hyd-polymeric micelles
The DTX loaded fabricated polymeric micelles (DOX-Hyd-PM) through film hydration method. DTX solution (20 mg/ml) at optimum ratio of DOX/DTX (1:1 and 1:5) was dissolved in anhydrous methanol and the solvent was removed using rotary evaporation. After that, DOX-Hyd-PM solution was added to DTX and sonicated in a bath sonicator (Bandelin electronics, Germany) at 25 ℃ for 30 min. The obtained mixture was centrifuged (11,000 rpm/min, 10 min) and the contents of DTX in (DOX-Hyd-PM/DTX) was measured using HPLC as mentioned earlier in "Determination of DTX in polymeric micelles (DTX-PM)" section.
Characterization of nano-micelles
Particle diameter, PDI (Polydispersity index), and ζ potential (mV) of different formulations were determined using DLS (dynamic light scattering) method by Zeta Sizer Nano-ZS; Malvern Instruments Ltd., United Kingdom (Nikpoor et al. 2017).
The morphologic examination of polymeric micelles was imaged at a voltage of 120 kV by Leo 912 AB Transmission electron microscope (TEM) (Zeiss, Jena, Germany) with negative stain method as described elsewhere (Zamani et al. 2023). Briefly, 20 µl of diluted polymeric micelles sample (1:50 sample to HEPES/Sucrose buffer) was placed on the copper grid and prior to imagining it was air-dried.
Drug release profile
The release profile of DOX and DTX from all polymeric micelles formulation was evaluated in 50 mL PBS buffer. For this, polymeric micelles were placed in dialysis bags (cutoff: 3.5 kD) and incubated with PBS (pH 7.4 and 5.5). The samples were placed on an incubator, shaken at 100 rpm in a horizontal shake at 37 ℃ for 72 h. Samples from each formulation were collected by withdrawing 2 mL of dialysis solution and then replacing with 2 mL fresh solution buffer. Samples were taken at 0.5. 1, 2, 4, 6, 8, 24, 48 and 72 h (Mashreghi et al. 2022).
Cytotoxicity assay
The cytotoxicity of formulations was studied using MTT assay. For this purpose, 4T1 and TUBO cells were cultured at a density of 4000 cells/well in a 96-well plate for 24 h. After the incubation time, 100 µL of DTX-PM, DOX-Hyd-PM, DOX-Hyd-PM/DTX (1:1molar ratio), Taxotere, free DOX and free DOX/DTX (1:1 molar ratio) at various concentration from (250–0.06 µg/mL) were added to each well in triplicate. The cells incubated for another 48 h at 37 ℃ and the medium was removed and MTT solution was added to each well and incubated for 4 h. The absorbance of solution was measured on a microplate reader at 570 nm as described in "DOX/DTX optimal ratio" section.
Cellular uptake
The cellular uptake was performed using flow cytometry. So, 4T1 and TUBO cells were seeded in 6-well plates (1 \(\times \) 106 cell/well). After an overnight incubation, cells were treated with different DiI-labeled formulations (DiI-PM, DOX-Hyd-PM, and DOX-Hyd-PM/DTX) for 3 h at 37 ℃. Untreated cells were used as control. Afterwards, the cells were washed, detached by trypsinization, and centrifugation (at 1200 rpm for 5 min). Eventually, the cells were suspended in 300 µL PBS before analyzing by flow cytometry. The FlowJo Software version 10 was used for data analysis) (Mirhadi et al. 2022; Vakili-Ghartavol et al. 2022).
Monitoring of cellular uptake using a fluorescence microscope
The uptake capacity of formulations was examined using fluorescence microscopic (Olympus BX-51, Japan) imaging as reported previously (Chacko et al. 2015; Askarizadeh et al. 2023). 4T1 cells were seeded at density of 10 × 105 cells/well on sterile coverslips which were placed into each well of 6-well plates, and incubated overnight at 37 ℃. The next day, cells were treated with FCS free RPMI1640 medium containing DOX-Hyd-PM/DTX (1:1) and free DOX for 3 h, then cells were washed (3 times) with cold PBS and fixed with 4% paraformaldehyde for 10 min. Then, cells were stained with DAPI and mounted on the microscopic slide. The Intrinsic fluorescent of Dox was utilized to estimate drug cell uptake.
Apoptosis assay
For cell apoptosis analysis in vitro, fluorescein isothiocyanate (FITC)-conjugated Annexin-V/Propidium Iodide (PI) was used according to manufacturers’ protocol (BioLegend, CA, USA). Briefly, after seeding 4T1 cells (1 × 105 cells/well) in a 6-well plate and an overnight incubation at 37 ℃ cells were treated with various formulations, and incubated for another 48 h. After that, both control (not treated) and treated cells were collected and washed twice with cold phosphate buffer saline (PBS, pH 7.4), resuspend in 90 μL of diluted Annexin-V binding buffer. Later, 5 μL of the mixture of Annexin-V conjugate and PI solutions was applied and incubated for 15 min in the dark places. In the end, each sample was resuspended with 400 µL Annexin-V binding buffer and analyzed by fluorescence cell sorting (FACS) cytometer (BD Biosciences, San Jose, CA, USA) to evaluate the apoptotic cells.
In vivo study
4–6-week-old female BALB/c mice were purchased from Royan Institute (Tehran, Iran). All animal experiments were approved by Mashhad University of Medical Sciences' Institutional Ethical Committee and Research Advisory Committee (Ethical number: 960881), and all methods and animal experiments were carried out in accordance with the relevant guidelines and regulations approved by the ethical committee and ARRIVE guidelines.
For tumor induction, regarding animal care guidelines, all mice were first anesthetized with 100 mg/kg ketamine/10 mg/kg xylazine via intraperitoneal (i.p.) injection. Then, 3 × 105 viable 4T1 cells suspended in 60 μL PBS buffer were subcutaneously injected into the right hind flank of each mouse (Mirhadi et al. 2022, 2024).
Biodistribution study
Two weeks after the tumor inoculation, when tumor size reached 5 mm3, mice were randomly divided into 7 groups (n = 3). Mice were treated through the tail vein with 5mg/ml of DTX-PM, DOX-Hyd-PM and combination of DOX-Hyd-PM/DTX, DOX/DTX (1:1 ratio). After 24 h, mice were sacrificed and different organs including a portion of the liver, the whole tumor, lung, heart, spleen, and kidney were dissected. The equal amount of tissues was transferred into propylene micro vials containing zirconia beads and 1 mL acidified isopropanol alcohol was added and then homogenized by Mini-Beadbeater-1 (Biospec, UK). To extract all drugs, the homogenized tissues were stored at 4 ℃ overnight (Amin et al. 2013). The next day, samples were centrifuged (14,000 rpm for 10 min) and the supernatant was collected and the amount of DTX was evaluated using HPLC(Berlin, Germany), and DOX concentration was assessed by spectrofluorometer (Perkin-Elmer LS-45) (Ex: 470 nm, Em: 590 nm).
Anti-tumor efficacy
To evaluation the anti-tumor effects of different therapeutics, 8 days after tumor inoculation, the mice with palpable tumors, were randomly divided into 7 groups (n = 5). Mice were treated through the tail vein with PBS (150 µL), free drugs combination DOX/DTX (1:1) (5 mg/ml) mg/kg, free Dox (10 mg/kg), polymeric micelles containing single drug (DTX-PM and DOX-Hyd-PM, 5 mg/kg), polymeric micelles containing both drugs (DOX-Hyd-PM/DTX,) and Taxotere (5 mg/kg). The tumor size and weight of mice were followed for 60 days 3 times a week. These monitoring continued until their tumor volume exceeded 1000 mm3, or during treatment their body weight lost 20% of their initial mass or they were found dead (Amin et al. 2013). The tumor volume was measured according to the Eq. 2:
For each mouse, the time to reach the end-point (TTE) was calculated using the equation of the line derived from the tumor growth curve's exponential regression.
Histochemical staining of tissues
To assess tissue-related toxicity, all mice were sacrificed on day 21 after treatment and the main organs (heart, liver, spleen, lung, and kidney) as well as tumor tissues were isolated. The samples were rinsed with 0.9% NaCl solution before fixing with a 10% (v/v) formalin solution. The samples were then embedded in paraffin. All tissue blocks were sectioned in standard sizes (4–5 m) and imaged microscope after staining with hematoxylin and eosin (H &E) (Askarizadeh et al. 2023).
Statistical analysis
All data were statically analyzed by GraphPad Prism 8.0 (GraphPad Software, Inc., San Diego, USA). One-way ANOVA analysis was used to access the statistical analysis.
Result
Physicochemical characterization of polymeric nanomicelles
Polymer–Dox conjugate was prepared by Schiff’s base reaction between the amine group of DOX and the ester group of Penta fluorophenyl (PFP) of p(DMA-co-PFPA) through pH-sensitive hydrazine moieties (Fig. 1). The conjugation reaction was confirmed by TLC and 1H and 19FNMR (supplementary figure S2–S3).
Different types of nanomicelles comprising of blank polymeric micelles (without drug), single drug polymeric micelles and dual-drug polymeric micelles (DOX/DTX at ratios of 1:1 and 1:5) were prepared. All the micelles structures were characterized by particle diameter, charge, distribution, morphology and drug loading contents. The results are summarized in Table 1.
By encapsulating DTX in the core of polymer micelles, the particle diameter of the blank micelles significantly increased from 15.3 ± 6.9 nm to 38.5 ± 8.2 nm. The particle diameter for a pH-sensitive form of polymeric micelles (DOX-Hyd-PM) increased to 45.6 ± 0.4, while for dual drug-loaded polymeric micelles (DOX-HYD-PM/DTX) the diameter increased from 69.9 ± 6.7 to 122.6 ± 2.5 nm after drug loading. Different forms of polymeric micelles showed negative charge (− 19.2 ± 3.46 mV) and the DOX-Hyd-PM exhibited higher zeta potential compared to DTX-PM. The surface charge of DOX-Hyd-PM/DTX were − 15.67 ± 8.11 and − 16.7 ± 4.41 mV for 1:1 and 1:5 molar ratio, respectively. TEM images confirmed that all nanomicelles were spherical in morphology with ideal dispersity as shown in Fig. 2.
The DOX conjugation was determined to be 23% (w/w). The DTX encapsulation efficiency (EE) in DTX-PM was 81.2 ± 3.1%, however, by loading DTX in the conjugation system, the EE was decreased to 57.9%, which could be explained by the limited encapsulation capacity of the DOX conjugated polymer.
Critical micelles concentration (CMC)
The Iodine method was used to determine the CMC value of nanomicelles. The CMC or critical micelles concentration is defined as the minimum concentration of polymer required to form stable micelles in solution, where the hydrophobic segments of the polymer are sequestered in the core of the micelle and the hydrophilic segments are exposed to the solvent. (Perumal et al. 2022). The CMC value for blank polymeric micelles (without drug) was determined around 5.53 µM. The low CMC of polymeric micelles guaranteed better dilution stability in plasma conditions (Kumar et al. 2017).
In vitro drug release study
To evaluate the effectual of the pH-sensitive hydrazine linker, the release study of DOX was investigated under physiological (pH 7.4) as well as acidic condition to mimic the endosomal compartment of the tumor cells (pH 5.5) Fig. 3. In this regard, we have observed that the release of DOX was completely affected by the environmental acidity. After 72 h, about 60% of DOX was released at pH 5.5 while slight release of DOX was observed in phosphate buffer media (pH 7.4). Moreover, the release profile of DTX in DTX-PM and DOX-Hyd-PM/DTX (1:1) displayed approximately 53% release at pH 5.5 while it remained stable at pH 7.4 during the release study test.
In vitro release profile of DOX and DTX from polymeric micelles formulation. A, C release of DOX in single and combination form of polymeric micelles, (B, D) release of DTX in single and combination form of formulation at pHs of 7.4 and 5.5 at 37 ℃ under sterile condition. The results represented as the mean ± SD (n = 3)
Synergistic cytotoxic effects of DOX and DTX for combination therapy
To reach an ideal synergistic effect, combination index (CI) was calculated. For this purpose, IC50 was used to calculate the CI. Data were evaluated to obtain synergistic additive and antagonistic effects according to Cho and Talalay (Chou 2010) Eq. (3) as follows:
where IC50 ab1 and ab2 shows the drugs in combination, while IC50 d1 and d2 are used for single agents. CI < 1 and CI = 1 demonstrated the synergistic effect and additive effect, respectively, While CI > 1 revealed antagonistic effect.
The IC50 values of free DOX and DTX were 2.5 and 10.76 µM for 4T1, and 3.58 and 17.72 µM for TUBO cells, respectively. The IC50 and CI values of free drugs combination are summarized in Table 2. When compared to the CI values of different DOX/DTX molar ratios, we conclude that DOX/DTX at both (1:1) and (1:5) molar ratios, showed a strong synergistic effect, so we have opted 1:1 ratio for preparation of nanomicellar formulations.
Cytotoxicity assay
The cytotoxicity of DOX/DTX free combination and all PM formulations were evaluated in vitro against both 4T1 and TUBO using MTT assay (Fig. 4). All polymeric nanomicelles formulations exhibited cytotoxicity over control groups. As shown in Fig. 4, all groups including both single and dual drug-loaded polymeric micelles exhibited significant cytotoxicity on 4T1 and TUBO cells lines. The result showed significant cytotoxicity of DTX-PM compared to commercial Taxotere. As regards, DOX-Hyd-PM formulation showed higher cytotoxicity compared to free DOX. Interestingly, DOX-Hyd-PM/DTX 1:1 combination showed more potent anti-cancer activity than drugs free combination (1:1) in both 4T1 and TUBO cells.
Cellular uptake
Cellular uptake of formulations for 4T1 and TUBO cells was measured by flow cytometry analysis (Fig. 5A, B). Based on flow cytometer analysis (FACS, BD Biosciences, San Jose, CA, USA), cell uptake of pH-sensitive polymeric micelles was higher than control group (P < 0.0001), and there was no significant differences among both cell lines. The mean fluorescent intensity (MFI) of 4T1 and TUBO cells incubated with either DOX-PM or DiI-PM increased over time. It should be noted that DOX release after 3 h incubation in uptake study played an inconsiderable effect on the detected MFI over the maximum release rate of polymeric micelles formulations.
Furthermore, we used fluorescence microscopy to assess the intracellular evaluation of DOX and DOX-Hyd-PM/DTX (Fig. 6). As expected, DOX was seen in cells 3 h after incubation, indicating rapid internalization of both free DOX and DOX-Hyd-PM/DTX formulation.
Apoptosis assay
To demonstrate how pH-sensitive polymeric micelles may impact cells through the apoptotic process, 4T1 cells were treated with DTX-PM, DOX-Hyd-PM and DOX-Hyd-PM/DTX (1:1 ratio) and then stained with Annexin V and PI before being examined using flow cytometry. For this, DOX-Hyd-PM/DTX (1:1 ratio) formulation was compared with polymeric micelles that includes single drug or untreated cells. According to our findings, when treated with DOX-Hyd-PM/DTX (1:1 ratio), 36% of the cells were in an apoptotic condition after 48 h while 98.1% of the control cells were alive (Fig. 7).
Biodistribution study
Biodistribution of all nanomicelles, as well as free DOX/DTX (1:1), was evaluated in different organs (liver, spleen, heart, kidney, lung, and tumor) in BALB/c mice-bearing 4T1 tumors after 24 h of single-dose injection. As shown in Fig. 8. DOX-Hyd-PM and DTX-PM revealed significantly higher accumulation compared to free DOX/DTX in tumor (P < 0.01). The concentration of both drugs in the liver was similar to free combination, while concentration of drugs in the heart significantly reduced compared free combination drugs (P < 0.01). Analysis of the kidney indicated a significantly lower levels of DOX accumulation following DOX-Hyd-PM/DTX administration compared to the free drugs combination, while we demonstrated a much higher accumulation of DTX in the lung. DiR dye was chosen to replace DTX for in vivo imaging experiments. An optimally synthesized DOX-Hyd-PM/DiR were injected to intravenously at a dosage of 5 mg/kg and DiR fluorescent was monitored 24 after injection. The strong fluorescent signals were detected in the tumor at 24 h. Fig. 9 shows the in vivo and ex vivo biodistribution pattern in mice 24 h following the DOX-Hyd-PM/DTX injection.
In vivo result of biodistribution of all polymeric micelles formulations and free drugs combination after 24 h in different tissues (tumor, kidney, spleen, liver, heart and lung,). Two weeks after tumor post inoculation, BALB/c mice-bearing 4T1 tumor treated with single dose of 5 mg/kg polymeric micelles (*P < 0.05 and **P < 0.01, respectively)
Anti-tumor efficacy
Anti-tumor efficacy and survival were studied for 55 days. Figure 10A. shows the tumor growth curve during treatment. A single administration of polymeric micelles of either DOX, DTX or the combination showed substantial efficacy in reducing the tumor growth rate. However, treatment with combination polymeric micelles seems to significantly affect the rate of survival compared to free drugs combination or other groups. Moreover, no significant changes in the body weight was observed between groups (Fig. 10B). In addition, the therapeutic efficacy of treatment was evaluated in vivo by monitoring the change in the life prolongation indices (TTE, % TGD, % ILS and MST) over a 55-day period for the treatment groups, as shown in Table 3. According to the results, the DOX-Hyd-PM/DTX (1:1) showed the highest TTE of 49.2 days, followed by DTX-PM and DOX-Hyd-PM, free DOX/DTX (1:1), DOX and Taxotere 48.6, 46.6, 41.2, 34.8, and 35.2 days, respectively. These durations were significantly higher than the TTE value of 33.2 days observed in PBS.
In vivo experiments including (A percentage change of bodyweight in animal and B anti-tumor efficacy and C survival curve) of formulations (PBS, free DOX, Taxotere, free drugs combination DOX:DTX 1:1 and all PM formulation) in female BALB/c mice-bearing 4T1 tumor after post injection of 5:5 mg/kg DOX and DTX (****indicates the P value < 0.0001). All formulation compared with PBS
Histological evaluations
Further studies were carried out to evaluate the safety of formulations using H&E staining of different organs (heart, liver, lung, kidney, and spleen). As shown in Fig. 11, no detectable pathological changes were present in the treated groups with dual or single drug-loaded polymeric micelles compared to the control group. Meanwhile, free drugs combination demonstrated a morphological change in heart relevant to DOX cytotoxicity (Ma et al. 2016a).
Discussion
This study was conducted to scrutinize the synergistic effect and anti-tumor potency of conjugation of doxorubicin through the acid-labile hydrazine as a pH-sensitive linker to form polymeric micelles in combination with docetaxel at an optimum ratio.
Polymers have evolved into smart materials that can adapt to changes in their environment through stimuli-responsive behavior. This behavior is determined by functional groups within the polymer chain. Smart polymers have enabled on-demand drug delivery with customized release profiles and precise dosage control in targeted areas. By designing systems that can recognize and respond dynamically to their microenvironment, similar to biological organisms, drugs can be distributed on demand (Rao et al. 2018). As there is complexity in tumor tissue and microenvironment, single drug delivery is not enough to reach effective treatment. Hence, dual drug delivery seems to be a promising alternative approach to treating cancer.
In the present study, the synthesized polymer (Li et al. 2021; Lin et al. 2017) was modified by reacting the PFP esters through a hydrazine linker for dual delivery of DOX and DTX (Van Driessche et al. 2018). To conjugate DOX to polymer, the active ester group was first converted into hydrazide. After that, DTX was physically encapsulated into the pH-sensitive to form DOX-Hyd-PM/DTX. Assessment of CMC was estimated for the minimum concentration of blank co-polymer for preparation of polymeric micelles was (5.53 µM). The lower CMC of co-polymer would able to maintain structural integrity when once diluted in body fluids (Lukyanov and Torchilin 2004). The hydrodynamic diameter of blank polymeric micelles, DTX-PM, DOX-Hyd-PM, and DOX-Hyd-PM/DTX had small sizes ranging from 15 nm to 122.6 nm (< 200 nm), indicating that they were likely to spread through the vascular space and reach the tumor site through the EPR effect. Hydrophobic drugs can be incorporated physically into the hydrophobic core and stabilized by hydrophobic interaction. Here, we could successfully encapsulate DTX as a water-insoluble agent into the core of micelles. The DTX encapsulation efficiency (EE) in DTX-PM was 81.2 ± 3.1%; however, the lower EE by loading DTX in the conjugation system, which could be explained by the limited encapsulation capacity of the DOX conjugated polymer (Liao et al. 2019). The nanoparticles’ surface charge has a significant effect on protein adsorption, which in turn influences RES activities and blood circulation times. The negative charge of all different polymeric micelles is essential for the stability of micelles owing to the repulsive force inhibiting nanomicelles from aggregation (Finbloom et al. 2020).
Before adding DTX and DOX to the carrier, we thoroughly inquired into the optimal ratio of both drugs for the synergistic effect in different types of breast cancer (4T1 and TUBO). To date, numerous researchers have reported a synergistic effect of DOX and DTX with an optimum ratio in carriers (Li et al. 2019; Sheu et al. 2016) to assess the anti-tumor effect in different cancers. What is more, an ideal co-delivery system should have a high drug loading efficiency with an optimum drug ratio into the tumor site. The molar ratio of DOX/DTX was broadly consistent with that of feed as the polymeric micelles were prepared, which is an obvious synergistic effect with higher cytotoxicity due to effective antineoplastic properties of DTX and activity of DOX with intercalate into DNA in cancer cell (Yu et al. 2020). More importantly, DOX-Hyd-PM/DTX (1:1) exhibited the strongest synergism in compared to DOX/DTX (1:1) against 4T1 and TUBO cells, respectively.
The extracellular pH of tumors is typically lower than that of normal tissue, and polymeric micelles face even more acidic conditions once internalized into tumor cells. To exploit this, acid-liable bonds such as Hydrazine are utilized in polymer–drug conjugation to create pH-sensitive polymeric micelles. Conjugation of the anti-cancer agent represents a noteworthy and beneficial approach in drug delivery. This approach able to improve the stability and control release of an anti-cancer drug, thereby lowering side effects and increasing therapeutic efficacy (Kumbham et al. 2022; Farhoudi et al. 2023).
In this conjugate, DOX-Hyd-PM has been demonstrated to evaluate pH-sensitivity. Hydrazine linker is cleaved at pH 5–6, the pH-sensitive formulation was expected to stay stable in physiologic media and cleave of DOX occurs upon internalization of conjugation in acidic condition (endosomes or lysosome) (Zhao et al. 2015). The amount of DOX released from both single and dual drugs polymeric micelles at pH 7.4 was significantly less while DOX become fast released in acidic media. However, DTX release pattern was not significantly different from DOX during 72 h in acidic media. This phenomenon could be pertinent to faster disassembly of polymeric micelles and result in more release of DTX. This could be relevant physical entrapment of DTX which was easy to release by physical diffusion compared to DOX conjugation (Ma et al. 2016b). It appears that intracellular cleavage and release of both DOX and DTX improved the synergistic cancer cytotoxicity to both 4T1 and TUBO cell lines. Based on the overall cytotoxicity screening results, the DTX-PM expressed a stronger effect in the 4T1 cells compared to the Taxotere.
Cellular uptake of both single and dual nanomicelles was considerably higher compared to non-treated cells. This may be relevant to the acidic condition in the tumor, leading to more electrostatic interaction on the cell membrane which results in expedited cellular uptake (Yue et al. 2011). In addition, cellular uptake of free drugs has been reported by a passive transfer while polymeric micelles tend to uptake to an endocytosis pathway with higher uptake (Xu et al. 2021). Moreover, we found that a higher amount of cell apoptosis is relevant to DOX-Hyd-PM/DTX (1:1) than the free combination, which could be attributed to the high cytotoxicity of the combination polymeric micelles. The above-mentioned results are in agreement with that of (Fan et al. 2020) which indicated that biomimetic nanoparticles comprised of DOX and Gamabufotalin (CS-6) effectively apoptosis in over 89% of MDA-MB-231 cells at an optimum ratio. They suggested that the combination of two drugs in nanoparticles is able to induce cell apoptosis through distinct signaling pathways, resulting in a synergistic effect.
In the present study, the mice receiving single or dual drugs of polymeric micelles showed substantial efficacy in reducing the tumor growth rate. This may be attributed to the polymeric micelles platform’s capacity for passive targeting and tumor accumulation brought on by the EPR effect. However, treatment with combination polymeric micelles DOX-Hyd-PM/DTX (1:1) seems to significantly affect the rate of survival and anti-tumor efficacy which is relevant to the synergistic effect of DOX and DTX with the highest therapeutic response compared to free drugs combination or other groups. There is also evidence indicating that tumors treated with combination therapy exhibited much smaller volume than those treated with free combination drugs (Zhang et al. 2016). These features highlight the synergistic effect of intelligent nanomedicine in treatment of cancer. The tumor growth effect of DTX prodrug and cisplatin which co-loaded in nanoparticle was assessed by Wu et al., and they found a higher accumulation in tumor and lower in the heart (Wu et al. 2020). In this study, a similar phenomenon was observed, with DOX-Hyd-PM/DTX showing the greatest accumulation in the tumor tissue and lower in the heart. It is worth noticing that both drugs showed low accumulation in the heart due to the EPR effect. The higher concentration of DTX in the lung showed no significance compared to the free drug combination, which could be explained by drug absorption by RES (Esmaeili et al. 2010). This result may be beneficial for the treatment of lung cancer and breast cancer metastasis of lung cancer (Jo et al. 2020). It seems that survival factors (TTE and MST) of codelivery improve following treatment. Moreover, monitoring of body weight loss and survival rate of all mice demonstrated body weight alteration in groups receiving free drug combinations. The histological analyses showed that the polymeric micelles had no harmful effect on major organs (Xiao et al. 2023). However, some structural lesions were found in heart tissue after injection of a free combination of DOX/DTX which may be relevant to DOX cytotoxicity (Ma et al. 2016a; Askarizadeh et al. 2023).
Conclusion
In this study, the incorporation of DTX into DOX-conjugated PM through an acid linkage was designed to improve treatment of Breast cancer through enhancing synergistic effect. The blank polymeric micelles with low CMC easily self-assemble into the nanosize range and desirable distribution. In particular, the combination of DOX and DTX in one platform could effectively improve antitumor efficacy, survival, and apoptosis compared to free DOX/DTX in a 1:1 ratio. Overall, this study demonstrated how nano-based drug delivery formulation can be beneficial; and described a potential translational pathway for DOX and DTX delivered in combination via polymeric micelles for the treatment of breast cancer.
Availability of data and materials
Data will be made available on request.
References
Alghamdi MA, Fallica AN, Virzì N, Kesharwani P, Pittalà V, Greish K (2022) The promise of nanotechnology in personalized medicine. J Pers Med 12(5):673
Alka, Nisha R, Singh P, Pal RR, Singh N, Mishra N, Saraf SA (2022) Bridging bio-nanoscience and cancer nanomedicine. Pharmacokinetics and Pharmacodynamics of Nanoparticulate Drug Delivery Systems, 295–313.
Amin M, Badiee A, Jaafari MR (2013) Improvement of pharmacokinetic and antitumor activity of PEGylated liposomal doxorubicin by targeting with N-methylated cyclic RGD peptide in mice bearing C-26 colon carcinomas. Int J Pharm 458(2):324–333
Askarizadeh A, Mashreghi M, Mirhadi E, Mirzavi F, Shargh VH, Badiee A et al (2023) Doxorubicin-loaded liposomes surface engineered with the matrix metalloproteinase-2 cleavable polyethylene glycol conjugate for cancer therapy. J Cancer Nanotechnol 14(1):1–26
Bo L, Wang Y, Li Y, Wurpel JND, Huang Z, Chen ZS (2023) The battlefield of chemotherapy in pediatric cancers. Cancers 15(7):1963. https://doi.org/10.3390/cancers15071963
Brianna L, Lee B (2023) Chemotherapy: how to reduce its adverse effects while maintaining the potency? J Med Oncol 40(3):88
Cai R, Zhang L, Chi H (2023) Recent development of polymer nanomicelles in the treatment of eye diseases. Front Bioeng Biotechnol. 11:1246974
Chacko SM, Nevin KG, Dhanyakrishnan R, Kumar BP (2015) Protective effect of p-coumaric acid against doxorubicin induced toxicity in H9c2 cardiomyoblast cell lines. Toxicol Reports. 2:1213–1221
Chen Y, Du Q, Zou Y, Guo Q, Huang J, Tao L et al (2020) Co-delivery of doxorubicin and epacadostat via heparin coated pH-sensitive liposomes to suppress the lung metastasis of melanoma. Int J Pharm 584:119446
Cheng X, Li D, Sun M, He L, Zheng Y, Wang X et al (2019) Co-delivery of DOX and PDTC by pH-sensitive nanoparticles to overcome multidrug resistance in breast cancer. Colloids Surf, B 181:185–197
Chou T-C (2010) Drug combination studies and their synergy quantification using the Chou-Talalay methodsynergy quantification method. Cancer Res J 70(2):440–446
Das A, Theato PJCR (2016) Activated ester containing polymers: opportunities and challenges for the design of functional macromolecules. Chem Rev 116(3):1434–1495
Din FU, Aman W, Ullah I, Qureshi OS, Mustapha O, Shafique S et al (2017) Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int J Nanomed 12:7291–7309
Esmaeili F, Dinarvand R, Ghahremani MH, Ostad SN, Esmaily H, Atyabi F (2010) Cellular cytotoxicity and in-vivo biodistribution of docetaxel poly (lactide-co-glycolide) nanoparticles. J Anti-Cancer Drugs 21(1):43–52
Fan J, Liu B, Long Y, Wang Z, Tong C, Wang W et al (2020) Sequentially-targeted biomimetic nano drug system for triple-negative breast cancer ablation and lung metastasis inhibition. J Nanobiotechnology 113:554–569
Farhoudi L, Kesharwani P, Majeed M, Johnston TP, Sahebkar A (2022) Polymeric nanomicelles of curcumin: potential applications in cancer. Int J Pharm 617:121622
Farhoudi L, Fobian S-F, Oei AL, Amin M, Jaafari MR, ten Hagen TLJNT (2023) Applications of biomimetic nanoparticles in breast cancer as a blueprint for improved next-generation cervical cancer therapy. Nano Today 53:102032
Fathi M, Barar J (2017) Perspective highlights on biodegradable polymeric nanosystems for targeted therapy of solid tumors. BioImpacts 7(1):49
Finbloom JA, Sousa F, Stevens MM, Desai TAJADDR (2020) Engineering the drug carrier biointerface to overcome biological barriers to drug delivery. Adv Drug Deliv Rev 167:89–108
Gaballa H, Lin S, Shang J, Meier S, Theato P (2018) A synthetic approach toward a pH and sugar-responsive diblock copolymer via post-polymerization modification. Polym Chem 9(24):3355–3358
Ghosh S, Lalani R, Patel V, Bardoliwala D, Maiti K, Banerjee S et al (2019) Combinatorial nanocarriers against drug resistance in hematological cancers: opportunities and emerging strategies. J Controlled Release 296:114–139
Jo MJ, Lee YJ, Park C-W, Chung YB, Kim J-S, Lee MK et al (2020) Evaluation of the physicochemical properties, pharmacokinetics, and in vitro anticancer effects of docetaxel and osthol encapsulated in methoxy poly (ethylene glycol)-b-poly (caprolactone) polymeric micelles. Int J Mol Sci 22(1):231
Kumar R, Kumar P, Singh B, Sharma G, Katare OP, Raza K (2017) In vivo pharmacokinetic studies and intracellular delivery of methotrexate by means of glycine-tethered PLGA-based polymeric micelles. Int J Pharm 519(1–2):138–144
Kumbham S, Ghosh A, Ghosh B, Biswas S (2022) Human serum albumin-poly (Lactide)-conjugated self-assembly NPs for targeted docetaxel delivery and improved therapeutic efficacy in oral cancer. Int J Biol Macromol 222:1287–1303
Li K, Zhan W, Chen Y, Jha RK, Chen X (2019) Docetaxel and doxorubicin codelivery by nanocarriers for synergistic treatment of prostate cancer. Front Pharmacol 10:1436. https://doi.org/10.3389/fphar.2019.01436
Li X, Mutlu H, Fengler C, Wilhelm M, Theato PJPC (2021) Dual-faced borax mediated synthesis of self-healable hydrogels merging dynamic covalent bonding and micellization. Polym Chem 12(3):361–369
Li Z, Liu J, Sun Z, Li Y, Yu B, Zhao F et al (2022) Nanomicelles co-loaded with doxorubicin and salvianolic acid A for breast cancer chemotherapy. Cancer Nanotechnol 13(1):21
Li Y, Matsumoto Y, Chen L, Sugawara Y, Oe E, Fujisawa N et al (2023) Smart nanofiber mesh with locally sustained drug release enabled synergistic combination therapy for glioblastoma. Nanomater 13(3):414
Liao J, Song Y, Liu C, Li D, Zheng H, Lu BJPC (2019) Dual-drug delivery based charge-conversional polymeric micelles for enhanced cellular uptake and combination therapy. Polym Chem 10(43):5879–5893
Lin S, Das A, Theato PJPC (2017) CO 2-Responsive graft copolymers: synthesis and characterization. Polym Chem 8(7):1206–1216
Long H, Tian W, Jiang S, Zhao J, Zhou J, He Q et al (2022) A dual drug delivery platform based on thermo-responsive polymeric micelle capped mesoporous silica nanoparticles for cancer therapy. J Microporous Mesoporous Mater 338:111943
Lukyanov AN, Torchilin VP (2004) Micelles from lipid derivatives of water-soluble polymers as delivery systems for poorly soluble drugs. Adv Drug Deliv Rev 56(9):1273–1289
Ma M, Lei M, Tan X, Tan F, Li N (2016a) Theranostic liposomes containing conjugated polymer dots and doxorubicin for bio-imaging and targeted therapeutic delivery. RSC Adv 6(3):1945–1957
Ma Y, Fan X, Li L (2016b) pH-sensitive polymeric micelles formed by doxorubicin conjugated prodrugs for co-delivery of doxorubicin and paclitaxel. Carbohydr Polym 137:19–29
Mahani M, Bahmanpouri M, Khakbaz F, Divsar F (2023) Doxorubicin-loaded polymeric micelles decorated with nitrogen-doped carbon dots for targeted breast cancer therapy. J Drug Delivery Sci Technol 79:104055
Mashreghi M, Faal Maleki M, Karimi M, Kalalinia F, Badiee A, Jaafari MR (2021) Improving anti-tumour efficacy of PEGylated liposomal doxorubicin by dual targeting of tumour cells and tumour endothelial cells using anti-p32 CGKRK peptide. J Drug Target 29(6):617–630
Mashreghi M, Maleki MF, Askarizadeh A, Farshchi H, Farhoudi L, Nasrollahzadeh MS et al (2022) A novel and easy to prepare azo-based bioreductive linker and its application in hypoxia-sensitive cationic liposomal doxorubicin: synthesis, characterization, in vitro and in vivo studies in mice bearing C26 tumor. Chem Phys Lipids 247:105226
Mirhadi E, Mashreghi M, Askarizadeh A, Mehrabian A, Alavizadeh SH, Arabi L et al (2022) Redox-sensitive doxorubicin liposome: a formulation approach for targeted tumor therapy. Sci Rep 12(1):1–17
Mirhadi E, Askarizadeh A, Farhoudi L, Mashreghi M, Behboodifar S, Alavizadeh SH et al (2024) The impact of phospholipids with high transition temperature to enhance Redox-Sensitive liposomal doxorubicin efficacy in colon carcinoma model. Chem Phys Lipids 261:105396
Ngoune R, Peters A, von Elverfeldt D, Winkler K, Pütz GJJ (2016) Accumulating nanoparticles by EPR: a route of no return. J Controlled Release 238:58–70
Nikpoor AR, Tavakkol-Afshari J, Sadri K, Jalali SA, Jaafari MR (2017) Improved tumor accumulation and therapeutic efficacy of CTLA-4-blocking antibody using liposome-encapsulated antibody: in vitro and in vivo studies. NBM 13(8):2671–2682
Pacheco C, Baião A, Ding T, Cui W, Sarmento B (2023) Recent advances in long-acting drug delivery systems for anticancer drug. Adv Drug Deliv Rev 194:114724. https://doi.org/10.1016/j.addr.2023.114724
Perumal S, Atchudan R, Lee W (2022) A review of polymeric micelles and their applications. Polymers 14(12):2510
Qi S-S, Sun J-H, Yu H-H, Yu S-Q (2017) Co-delivery nanoparticles of anti-cancer drugs for improving chemotherapy efficacy. Drug Deliv 24(1):1909–1926
Qin J, Zhu Y, Zheng D, Zhao Q (2021) pH-sensitive polymeric nanocarriers for antitumor biotherapeutic molecules targeting delivery. Bio-Design Manufactur 4(3):612–626
Rao NV, Ko H, Lee J, Park JH (2018) Recent progress and advances in stimuli-responsive polymers for cancer therapy. Front Bioeng Biotechnol 6:110
Rarokar N, Agrawal R, Yadav S, Khedekar P, Ravikumar C, Telange D et al (2023) Pteroyl-γ-l-glutamate/Pluronic® F68 modified polymeric micelles loaded with docetaxel for targeted delivery and reduced toxicity. J Mol Liq 369:120842
Rose L, Lustberg M, Ruddy KJ, Cathcart-Rake E, Loprinzi C, Dulmage B (2023) Hair loss during and after breast cancer therapy. J Supportive Care in Cancer 31(3):186
Rostamizadeh K, Torchilin VP (2020) Polymeric nanomicelles as versatile tool for multidrug delivery in chemotherapy. Nanopharmaceuticals (pp. 45–72). Elsevier.
Schirrmacher V (2019) From chemotherapy to biological therapy: a review of novel concepts to reduce the side effects of systemic cancer treatment. Int J Oncol 54(2):407–419
Shafei A, El-Bakly W, Sobhy A, Wagdy O, Reda A, Aboelenin O et al (2017) A review on the efficacy and toxicity of different doxorubicin nanoparticles for targeted therapy in metastatic breast cancer. Biomed Pharmacot 95:1209–1218
Sheu MT, Jhan HJ, Su CY, Chen LC, Chang CE, Liu DZ et al (2016) Codelivery of doxorubicin-containing thermosensitive hydrogels incorporated with docetaxel-loaded mixed micelles enhances local cancer therapy. Colloids Surf B Biointerfaces 143:260–270. https://doi.org/10.1016/j.colsurfb.2016.03.054
Shokooh MK, Emami F, Duwa R, Jeong J-H, Yook SJ (2022) Triple-negative breast cancer treatment meets nanoparticles: Current status and future direction. J Drug Deliv Technol 71:103274
Thotakura N, Dadarwal M, Kumar P, Sharma G, Guru SK, Bhushan S et al (2017) Chitosan-stearic acid based polymeric micelles for the effective delivery of tamoxifen: cytotoxic and pharmacokinetic evaluation. AAPS PharmSciTech 18(3):759–768. https://doi.org/10.1208/s12249-016-0563-6
Vakili-Ghartavol R, Mehrabian A, Mirzavi F, Rezayat SM, Mashreghi M, Farhoudi L et al (2022) Docetaxel in combination with metformin enhances antitumour efficacy in metastatic breast carcinoma models: a promising cancer targeting based on PEGylated liposomes. JPP 74(9):1307–1319
Van Driessche A, Kocere A, Everaert H, Nuhn L, Van Herck S, Griffiths G et al (2018) pH-sensitive hydrazone-linked doxorubicin nanogels via polymeric-activated Ester scaffolds: synthesis, assembly, and in vitro and in vivo evaluation in tumor-bearing zebrafish. Chem Mater 30(23):8587–8596
Wu R, Zhang Z, Wang B, Chen G, Zhang Y, Deng H et al (2020) Combination chemotherapy of lung cancer–co-delivery of docetaxel prodrug and cisplatin using aptamer-decorated lipid–polymer hybrid nanoparticles. Drug Des Devel Ther 14:2249–2261
Xiao C, Sun Y, Fan J, Nguyen W, Chen S, Long Y et al (2023) Engineering cannabidiol synergistic carbon monoxide nanocomplexes to enhance cancer therapy via excessive autophagy. Acta Pharm Sin b 13(11):4591–4606
Xing P, Zhao Y (2018) Supramolecular vesicles for stimulus-responsive drug delivery. Small Methods 4:1700364
Xu M, Yao C, Zhang W, Gao S, Zou H, Gao J (2021) Anti-cancer activity based on the high docetaxel loaded poly (2-oxazoline) s micelles. Int J Nanomed 16:2735
Yu G, Ning Q, Mo Z, Tang S (2019a) Intelligent polymeric micelles for multidrug co-delivery and cancer therapy. Artif Cells Nanomed Biotechnol 47(1):1476–1487. https://doi.org/10.1080/21691401.2019.1601104
Yu G, Ning Q, Mo Z, Tang S (2019b) Intelligent polymeric micelles for multidrug co-delivery and cancer therapy. Artific Cells Nanomed Biotechnol 47(1):1476–1487
Yu Z, Li H, Jia Y, Qiao Y, Wang C, Zhou Q et al (2020) Ratiometric co-delivery of doxorubicin and docetaxel by covalently conjugating with mPEG-poly (β-malic acid) for enhanced synergistic breast tumor therapy. Polym Chem 11(46):7330–7339
Yue Z-G, Wei W, Lv P-P, Yue H, Wang L-Y, Su Z-G et al (2011) Surface charge affects cellular uptake and intracellular trafficking of chitosan-based nanoparticles. Biomacromol 12(7):2440–2446
Zamani P, Mashreghi M, Bazaz MR, Zargari S, Alizadeh F, Dorrigiv M et al (2023) Characterization of stability, safety and immunogenicity of the mRNA lipid nanoparticle vaccine Iribovax® against COVID-19 in nonhuman primates. J Contrloed Release 360:316–334
Zhang Y, Yang C, Wang W, Liu J, Liu Q, Huang F et al (2016) Co-delivery of doxorubicin and curcumin by pH-sensitive prodrug nanoparticle for combination therapy of cancer. Sci Rep 6(1):21225
Zhao D, Li B, Han J, Yang Y, Zhang X, Wu G (2015) PH responsive polypeptide based polymeric micelles for anticancer drug delivery. J Biomed Mater Res A 103(9):3045–3053
Zhou M, Wen L, Wang C, Lei Q, Li Y, Yi X et al (2022) Recent advances in stimuli-sensitive amphiphilic polymer-paclitaxel prodrugs. Front Bioeng Biotechnol 10:875034
Acknowledgements
This study was a part of Leila Farhoudi Ph.D. thesis (Grant number: 971980) supported by Nanotechnology Research Center, Mashhad University of Medical Sciences (MUMS), Mashhad, Iran. Prof. Patrick Theato is gratefully acknowledged for his comments during the writing of the manuscript.
Author information
Authors and Affiliations
Contributions
Leila. Farhoudi: Investigation, Conceptualization, Formal analysis, Writing - Original Draft, Visualization Seyedeh Maryam Hoseinikhah: Investigation, Amin Kazemi Beydokhti: Project administration, Investigation Leila Arabi: Project administration, Review & Editing Seyedeh Hoda Alavizadeh: Project administration, Seyedeh Alia Moosavian: Project administration Mahmoud Reza Jaafari: Supervision, Funding acquisition, Conceptualization, Writing - Review & Editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Farhoudi, L., Hosseinikhah, S.M., Kazemi-Beydokhti, A. et al. pH-sensitive polymeric micelles enhance the co-delivery of doxorubicin and docetaxel: an emerging modality for treating breast cancer. Cancer Nano 15, 37 (2024). https://doi.org/10.1186/s12645-024-00275-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12645-024-00275-1