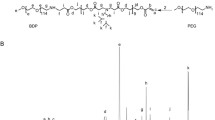
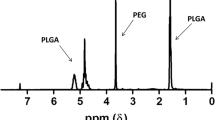
Abstract
PEGylated triacontanol (mPEG2k-b-TRIA) was developed as a dual-functional polymer with remarkable biocompatibility. The polymer could self-assemble to micelles with critical micelle concentration (CMC) 17.62 μg mL−1. Docetaxel-loaded mPEG2k-b-TRIA micelles (DTX PMs) were fabricated to evaluate the feasibility of mPEG2k-b-TRIA as drug delivery system. DTX PMs achieved desirable particle size of 93.7 nm, drug loading of 6.66%, and drug encapsulation efficiency of 89.87%. The drug release was based on first-order kinetics model, thus enabling prolonged release. Meanwhile, pharmacokinetic study also revealed that DTX PMs could improve the exposure level of DTX and prolong its systemic circulation time. Furthermore, DTX PMs demonstrated significantly enhanced cytotoxicity and cellular uptake in vitro compared with DTX solution. The in vivo tumor inhibition study carried out on MCF-7 bearing BALB/c mice model also validated that DTX PMs exhibited stronger anti-tumor activity but low toxicity. Notably, mPEG2k-b-TRIA made some contribution to inhibit the growth of breast cancer cells in vitro and in vivo, indicating the potential as anti-tumor complementary agents. All the results suggested that mPEG2k-b-TRIA polymer as a vehicle in the formulation of anti-cancer drugs may provide an effective way to improve their therapeutic efficacy. Consequently, the mPEG2k-b-TRIA polymers would be another promising carrier for hydrophobic anti-cancer drugs delivery.









Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the curve
- CL:
-
Clearance rate
- CLSM:
-
Confocal laser scanning microscopy
- CMC:
-
Critical micelle concentration
- CMPI:
-
2-Chloro-1-methylpyridinium iodide
- DAPI:
-
4,6-Diamidino-2-phenylindole
- DL:
-
Drug loading
- DMAP:
-
4-Dimethylaminopyridine
- DMF:
-
N,N-Dimethylformamide
- DTX:
-
Docetaxel
- DTX-PMs:
-
Docetaxel-loaded polymeric micelles
- EE:
-
Encapsulation efficiency
- EPR:
-
Enhanced permeability and retention
- FT-IR:
-
Fourier transform infrared
- GPC:
-
Gel permeation chromatography
- HBSS:
-
Hank’s balanced salt solution
- 1H NMR:
-
1H nuclear magnetic resonance
- IC50 :
-
Half maximal inhibitory concentration
- IR:
-
Inhibition rate
- MRT:
-
Mean residence time
- mPEG2k-NH2 :
-
Monomethoxy poly(ethylene glycol)-amine; methoxy poly(ethylene glycol 2000)-triacontanol (mPEG2k-b-TRIA)
- PBS:
-
Phosphate-buffered saline
- PDI:
-
Polydispersity index
- PEG:
-
Polyethylene glycol
- PMs:
-
Polymeric micelles
- PS:
-
Physiological saline
- SA:
-
Succinic anhydride
- t 1/2 :
-
Elimination half-life
- TEM:
-
Transmission electron microscope
- TRIA:
-
Triacontanol
References
DeSantis CE, Fedewa SA, Goding Sauer A, Kramer JL, Smith RA, Jemal A. Breast cancer statistics, 2015: convergence of incidence rates between black and white women. CA Cancer J Clin. 2016;66(1):31–42. https://doi.org/10.3322/caac.21320.
Lu Y, Park K. Polymeric micelles and alternative nanonized delivery vehicles for poorly soluble drugs. Int J Pharm. 2013;453(1):198–214. https://doi.org/10.1016/j.ijpharm.2012.08.042.
Talelli M, Rijcken CJF, Hennink WE, Lammers T. Polymeric micelles for cancer therapy: 3 C’s to enhance efficacy. Curr Opin Solid State Mater Sci. 2012;16(6):302–9. https://doi.org/10.1016/j.cossms.2012.10.003.
Zhang Y, Ren T, Gou J, Zhang L, Tao X, Tian B, et al. Strategies for improving the payload of small molecular drugs in polymeric micelles. J Control Release. 2017;261:352–66. https://doi.org/10.1016/j.jconrel.2017.01.047.
Yu J, Deng H, Xie F, Chen W, Zhu B, Xu Q. The potential of pH-responsive PEG-hyperbranched polyacylhydrazone micelles for cancer therapy. Biomaterials. 2014;35(9):3132–44. https://doi.org/10.1016/j.biomaterials.2013.12.074.
Gong J, Chen M, Zheng Y, Wang S, Wang Y. Polymeric micelles drug delivery system in oncology. J Control Release. 2012;159(3):312–23. https://doi.org/10.1016/j.jconrel.2011.12.012.
Hadinoto K, Sundaresan A, Cheow WS. Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review. Eur J Pharm Biopharm. 2013;85(3):427–43. https://doi.org/10.1016/j.ejpb.2013.07.002.
Omolo CA, Kalhapure RS, Jadhav M, Rambharose S, Mocktar C, Ndesendo VM, et al. PEGylated oleic acid: a promising amphiphilic polymer for nano-antibiotic delivery. Eur J Pharm Biopharm. 2016;112:96–108. https://doi.org/10.1016/j.ejpb.2016.11.022.
Zhang L, Chan JM, Gu FX, Rhee JW, Wang AZ, Radovicmoreno AF, et al. Self-assembled lipid–polymer hybrid nanoparticles: a robust drug delivery platform. ACS Nano. 2008;2(8):1696–702. https://doi.org/10.1021/nn800275r.
Teerawanichpan P, Robertson AJ, Qiu X. A fatty acyl-CoA reductase highly expressed in the head of honey bee (Apis mellifera) involves biosynthesis of a wide range of aliphatic fatty alcohols. Insect Biochem Mol Biol. 2010;40(9):641–9. https://doi.org/10.1016/j.ibmb.2010.06.004.
Du Z, Mao X, Tai X, Wang G, Liu X. Preparation and properties of microemulsion detergent with linear medium chain fatty alcohols as oil phase. J Mol Liq. 2016;223:805–10. https://doi.org/10.1016/j.molliq.2016.09.011.
Eccleston GM, Behan-Martin MK, Jones GR, Towns-Andrews E. Synchrotron X-ray investigations into the lamellar gel phase formed in pharmaceutical creams prepared with cetrimide and fatty alcohols. Int J Pharm. 2000;203(1–2):127–39. https://doi.org/10.1016/S0378-5173(00)00447-6.
Tian L, Gandra N, Singamaneni S. Monitoring controlled release of payload from gold nanocages using surface enhanced Raman scattering. ACS Nano. 2013;7(5):4252–60. https://doi.org/10.1021/nn400728t.
Uner M, Gönüllü U, Yener G, Altinkurt T. A new approach for preparing a controlled release ketoprofen tablets by using beeswax. Farmaco. 2005;60(1):27–31. https://doi.org/10.1016/j.farmac.2004.08.008.
Wong PC, Heng PW, Chan LW. Determination of solid state characteristics of spray-congealed ibuprofen solid lipid microparticles and their impact on sustaining drug release. Mol Pharm. 2015;12(5):1592–604. https://doi.org/10.1021/acs.molpharmaceut.5b00015.
Mi Y, Liu Y, Feng SS. Formulation of docetaxel by folic acid-conjugated-α-tocopheryl polyethylene glycol succinate 2000 (vitamin E TPGS) micelles for targeted and synergistic chemotherapy. Biomaterials. 2011;32(16):4058–66. https://doi.org/10.1016/j.biomaterials.2011.02.022.
Irmak S, Dunford NT, Milligan J. Policosanol contents of beeswax, sugar cane and wheat extracts. Food Chem. 2006;95(2):312–8. https://doi.org/10.1016/j.foodchem.2005.01.009.
Jackson MA, Eller FJ. Isolation of long-chain aliphatic alcohols from beeswax using lipase-catalyzed methanolysis in supercritical carbon dioxide. J Supercrit Fluids. 2006;37(2):173–7. https://doi.org/10.1016/j.supflu.2005.08.008.
Kim SM, Chung HJ, Lim ST. Effect of various heat treatments on rancidity and some bioactive compounds of rice bran. J Cereal Sci. 2014;60(1):243–8. https://doi.org/10.1016/j.jcs.2014.04.001.
Naeem M, Khan MMA, Moinuddin SMH. Triacontanol stimulates nitrogen-fixation, enzyme activities, photosynthesis, crop productivity and quality of hyacinth bean (Lablab purpureus L.). Sci Hortic. 2009;121(4):389–96. https://doi.org/10.1016/j.scienta.2009.02.030.
Evans DA, Hill SW. Dietary supplement composition for blood lipid health. US; 2011.
Gong J, Qin X, Yuan F, Hu M, Chen G, Fang K, et al. Efficacy and safety of sugarcane policosanol on dyslipidemia: a meta-analysis of randomized controlled trials. Mol Nutr Food Res. 2017;62:1700280. https://doi.org/10.1002/mnfr.201700280.
Mcbride PT, Clark L, Krueger G. Evaluation of triacontanol-containing compounds as anti-inflammatory agents using guinea pig models. J Invest Dermatol. 1987;89(4):380–3. https://doi.org/10.1111/1523-1747.ep12471763.
Menéndez R, Más R, AMa A, Pérez Y, RMa G, Fernández J, et al. Antioxidant effects of D002 on the in vitro susceptibility of whole plasma in healthy volunteers. Arch Med Res. 2001;32(5):436–41. https://doi.org/10.1016/S0188-4409(01)00315-0.
Ravelo Y, Molina V, Carbajal D, Fernández L, Fernández JC, Arruzazabala ML, et al. Evaluation of anti-inflammatory and antinociceptive effects of D-002 (beeswax alcohols). J Nat Med. 2011;65(2):330–5. https://doi.org/10.1007/s11418-010-0496-4.
Fan X, Zhang R, Cheng H. Use of triacontanol in preparation of medicaments for treatment of cancers. US; 2011.
Takada Y, Kageyama K, Yamada R, Onoyama Y, Nakajima T, Hosono M, et al. Correlation of DNA synthesis-inhibiting activity and the extent of transmembrane permeation into tumor cells by unsaturated or saturated fatty alcohols of graded chain-length upon hyperthermia. Oncol Rep. 2001;8(3):547–51. https://doi.org/10.3892/or.8.3.547.
Thippeswamy G, Sheela ML, Salimath BP. Octacosanol isolated from Tinospora cordifolia downregulates VEGF gene expression by inhibiting nuclear translocation of NF-<kappa>B and its DNA binding activity. Eur J Pharmacol. 2008;588(2–3):141–50. https://doi.org/10.1016/j.ejphar.2008.04.027.
Zhou Y, Li N, Qiu Z, Lu X, Fang M, Chen X, et al. Superior anti-neoplastic activities of triacontanol-PEG conjugate: synthesis, characterization and biological evaluations. Drug Deliv. 2018;25(1):1546–59. https://doi.org/10.1080/10717544.2018.1477864.
Grudén S, Sandelin M, Rasanen V, Micke P, Hedeland M, Axén N, et al. Antitumoral effect and reduced systemic toxicity in mice after intra-tumoral injection of an in vivo solidifying calcium sulfate formulation with docetaxel. Eur J Pharm Biopharm. 2017;114:186–93. https://doi.org/10.1016/j.ejpb.2017.01.018.
Tang X, Zhou S, Tao X, Wang J, Wang F, Liang Y. Targeted delivery of docetaxel via Pi-Pi stacking stabilized dendritic polymeric micelles for enhanced therapy of liver cancer. Mater Sci Eng C. 2017;75:1042–8. https://doi.org/10.1016/j.msec.2017.02.098.
Zhang H, Wang K, Zhang P, He W, Song A, Luan Y. Redox-sensitive micelles assembled from amphiphilic mPEG-PCL-SS-DTX conjugates for the delivery of docetaxel. Colloids Surf B: Biointerfaces. 2016;142:89–97. https://doi.org/10.1016/j.colsurfb.2016.02.045.
Wang Y, Chen L, Tan L, Zhao Q, Luo F, Wei Y, et al. PEG-PCL based micelle hydrogels as oral docetaxel delivery systems for breast cancer therapy. Biomaterials. 2014;35(25):6972–85. https://doi.org/10.1016/j.biomaterials.2014.04.099.
Palominos MA, Vilches D, Bossel E, Soto-Arriaza MA. Interaction between amphipathic triblock copolymers and L-α-dipalmitoyl phosphatidylcholine large unilamellar vesicles. Colloids Surf B: Biointerfaces. 2016;148:30–40. https://doi.org/10.1016/j.colsurfb.2016.08.038.
Wang Q, Jiang J, Chen W, Jiang H, Zhang Z, Sun X. Targeted delivery of low-dose dexamethasone using PCL-PEG micelles for effective treatment of rheumatoid arthritis. J Control Release. 2016;230:64–72. https://doi.org/10.1016/j.jconrel.2016.03.035.
Zhu Z, Li Y, Yang X, Pan W, Pan H. The reversion of anti-cancer drug antagonism of tamoxifen and docetaxel by the hyaluronic acid-decorated polymeric nanoparticles. Pharmacol Res. 2017;126:84–96. https://doi.org/10.1016/j.phrs.2017.07.011.
Wang W, Zhang L, Le Y, Chen J-F, Wang J, Yun J. Synergistic effect of PEGylated resveratrol on delivery of anticancer drugs. Int J Pharm. 2016;498(1):134–41. https://doi.org/10.1016/j.ijpharm.2015.12.016.
Jain A, Jain SK. In vitro release kinetics model fitting of liposomes: an insight. Chem Phys Lipids. 2016;201:28–40. https://doi.org/10.1016/j.chemphyslip.2016.10.005.
Kim Y-M, Park S-C, Jang M-K. Targeted gene delivery of polyethyleneimine-grafted chitosan with RGD dendrimer peptide in αvβ3 integrin-overexpressing tumor cells. Carbohydr Polym. 2017;174:1059–68. https://doi.org/10.1016/j.carbpol.2017.07.035.
Yang Y, Lu X, Liu Q, Dai Y, Zhu X, Wen Y, et al. Palmitoyl ascorbate and doxorubicin co-encapsulated liposome for synergistic anticancer therapy. Eur J Pharm Sci. 2017;105:219–29. https://doi.org/10.1016/j.ejps.2017.05.038.
Yao H, Wang K, Wang Y, Wang S, Li J, Lou J, et al. Enhanced blood-brain barrier penetration and glioma therapy mediated by a new peptide modified gene delivery system. Biomaterials. 2015;37:345–52. https://doi.org/10.1016/j.biomaterials.2014.10.034.
Rodallec A, Benzekry S, Lacarelle B, Ciccolini J, Fanciullino R. Pharmacokinetics variability: why nanoparticles are not just magic-bullets in oncology. Crit Rev Oncol Hematol. 2018;129:1–12. https://doi.org/10.1016/j.critrevonc.2018.06.008.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81473272, No. 81503148).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Animal studies
All institutional and national guidelines for the care and use of laboratory animals were followed.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Fig. 1
Fitting curve of in vitro release data from DTX PMs for first-order kinetics model (PNG 50 kb)
Supplementary Table 1
(DOCX 25 kb)
Rights and permissions
About this article
Cite this article
Lu, X., Fang, M., Yang, Y. et al. PEG-conjugated triacontanol micelles as docetaxel delivery systems for enhanced anti-cancer efficacy. Drug Deliv. and Transl. Res. 10, 122–135 (2020). https://doi.org/10.1007/s13346-019-00667-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-019-00667-6