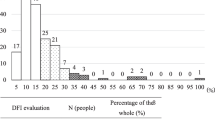
Abstract
Background
Recently, damage to the sperm DNA has been studied as it is associated with reduced fertilization rates, embryo quality, and pregnancy rates, also higher rates of spontaneous miscarriage.
Objective
To develop a diagnostic method in predicting male infertility.
Material and Methods
The design of this study is cross-sectional. Data were retrieved from medical records of Yasmin IVF Clinic Dr. Cipto Mangunkusumo General Hospital and Daya Medika Infertility Clinic from January to December 2015. Subjects were selected by consecutive sampling and divided into two groups: infertile and fertile. Sperm deoxyribonucleic acid fragmentation index (DFI) was determined by sperm chromatin dispersion (SCD) method using Halosperm® Kit.
Results
There were 114 subjects (36 fertile and 78 infertile) selected into this study. We found no significant difference in the age between both of groups. The median value of sperm DFI in infertile group was significantly higher, 29.95 (26.6–34.3)%, compared to 19.90 (15.6–24.4)% of the fertile group, with p < 0.001. Area Under Curve (AUC) of sperm DFI, 0.862 (95% CI 0.783, 0.941), was higher than concentration (AUC 0.744; 95% CI 0.657, 0.831), motility (AUC 0.668; 95% CI 0.572, 0.765), and morphology (AUC 0.718; 95% CI 0.697, 0.864) of the semen analysis. At the cut-off point of 26.1%, the sperm DFI had sensitivity of 80.8% (95% CI; 70.0, 88.5), specificity of 86.1% (95% CI; 69.7, 94.8), positive predictive value (PPV) of 92.6% (95% CI; 83.0, 97.3), negative predictive value (NPV) of 67.4% (95% CI; 51.9, 80.0), positive likelihood ratio (PLR) of 12.6 (95% CI; 5.4, 29.4), and negative likelihood ratio (NLR) of 0.48 (95% CI 0.31, 0.75). Sperm DFI of ≥26.1% had prevalence ratio of 2.84 (95% CI 1.86, 4.33) for the occurrence of male infertility.
Conclusion
There was significant difference between the median value of sperm DFI of infertile men and fertile men. Compared to semen analysis, sperm DFI at cut-off point of 26.1% has a higher diagnostic value (AUC).
Résumé
Objectif
Développer une méthode diagnostique e prédiction de l’infécondité masculine.
Matériel et Méthodes
il s’agit d’une étude transversale. Les données ont été extraites des dossiers médicaux de la Yasmin IVF Clinic Dr Cipto Mangunkusumo General Hospital et de la Daya.
Medika Infertility Clinic, de janvier à décembre 2015. Les sujets ont été sélectionnés par échantillonnage consécutif et divisés en deux groupes : infécond et fécond. L’indice de fragmentation de l’acide désoxyribonucléique (DFI) a été déterminé par la méthode de la dispersion de la chromatine spermatozoïdaire (SCD) en utilisant le kit Halosperm™.
Résultats
Cent quatorze sujets ont été sélectionnés dans cette étude : 36 féconds et 78 inféconds. Aucune différence significative n’a été retrouvée pour l’âge entre les deux groupes. La valeur médiane du DFI spermatozoïdaire dans le groupe infécond était significativement plus élevée, 29.9% (IQ1-IQ3 = 26.6–34.3%), que celle du groupe fécond, 19,90% (15.6–24.4%; P < 0.001). L’aire sous la courbe (AUC) du DFI spermatozoïdaire, 0.862 (95% CI 0.783, 0.941), était plus grande que celle de la concentration (AUC 0.744; 95% CI 0.657, 0.831), mobilité (AUC 0.668; 95% CI 0.572, 0.765), et morphologie (AUC 0.718; 95% CI 0.697, 0.864) des spermatozoïdes retrouvées à l’analyse du sperme. Pour une valeur seuil de 26,1%, le DFI spermatozoïdaire a une sensibilité de 80.8% (95% CI; 70.0, 88.5%), une spécificité de 86.1% (95% CI; 69.7, 94.8%), une valeur prédictive positive (PPV) de 92.6% (95% CI; 83.0, 97.3%), une valeur prédictive négative (NPV) de 67,4% (95% CI; 51.9, 80.0%), un ratio de probabilité positive (PLR) de 12,6 (95% CI; 5.4, 29.4), et un ratio de probabilité négative (NLR) de 0.48 (95% CI 0.31, 0.75). Un DFI spermatozoïdaire ≥ 26,1% a un ratio de prévalence de 2,84 (95% CI 1.86, 4.33) pour la survenue d’une infécondité masculine.
Conclusion
La valeur médiane du DFI spermatozoïdaire diffère significativement entre les féconds et les inféconds. Par comparaison aux paramètres spermatiques de l’analyse du sperme, un DFI spermatozoïdaire à une valeur seuil de 26,1% possède une plus grande valeur diagnostique (AUC).
Similar content being viewed by others
Background
The incidence of infertility ranges from 10 to 20% in the world. This figure is similar with the data in Indonesia, where there are 39.8 million couples of childbearing age with 3.98 million (10–15%) experiencing infertility [1]. The success of a pregnancy is influenced by both men and women. Of all infertility cases, nearly 50% are due to male factor of infertility, either as a single factor or as a combination with female factor [2]. A study conducted by Wiweko, et al., found that male factor had a role in 43.9% of 266 infertile couples in Indonesia [3]. Male infertility is determined by the quality of the spermatozoa, which affects the ability for fertilization. In infertility cases, semen analysis that evaluates sperm concentration, motility, and morphology is done as a standard diagnostic tool to assess sperm quality. Damage to the sperm’s DNA has been studied lately as it is associated with reduced fertilization rates, embryo quality, and pregnancy rates, as well as higher rates of spontaneous miscarriage [4, 5]. A recent study, which studied the relationship between sperm DNA fragmentation in 203 couples underwent In Vitro Fertilization (IVF) and 136 couples underwent Intracytoplasmic Sperm Injection (ICSI), found that following the IVF, couples with <25% sperm DFI had a live-birth rate of 33% while couples with >50% DFI had only 13% birth rate. On the contrary, following the ICSI, there was no significant difference between both groups [6]. Live-birth rate increased significantly in couples with low sperm DFI compared to those with high sperm DFI (RR 1.17, 95% CI 1.07, 1.28; p = 0.0005) in a systematic review and meta-analysis [7]. Therefore, undetected sperm DNA damage could affect the outcome of Assisted Reproductive Technology (ART) and also potentially become a stumbling block in ART making the success rate relatively low, about 20–40% [3]. It also indicates that semen analysis is not optimal to detect male infertility, so that there is an opportunity to use a new predictive factor as a better diagnostic tool. Acridine orange test, Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay, Comet assay, sperm chromatin structure assay (SCSA), and sperm chromatin dispersion (SCD) are some methods commonly used to identify sperm DNA fragmentation. TUNEL assay is considered as the most superior method of all other methods, yet it needs more advanced equipment and high cost [8]. Chohan (2006) concluded that SCD had positive correlation with TUNEL assay results that they had similar predictive values, only SCD was a simpler and a low-cost method [9]. Another study by Zhang (2010) found that SCD was more sensitive than TUNEL assay to detect sperm with DNA fragmentation [10]. This study used Halosperm® Kit, which is a more accurate and efficient form of SCD method [11].
Methods
Patients
This study used a cross-sectional design. The data was retrieved from patient’s medical records of two infertility clinics in Jakarta (Yasmin IVF Clinic of Dr. Cipto Mangunkusumo General Hospital and Daya Medika Infertility Clinic) since January 2015 to December 2015. In this study, there were two groups of male subjects, infertile and fertile. The infertile group was obtained from couples who had undergone infertility workup with abnormal semen analysis (except azoospermia) and no cause of infertility in the female partner, while the fertile group was obtained from men proven to have a living child, taken from secondary infertile couples with abnormalities only in the female factor. Informed consent was obtained before all subjects recruited into this study. Ethical clearance was approved by Ethical Committee Faculty of Medicine Universitas Indonesia. From the sample size calculation, it was determined a minimum sample size of 110 subjects. Potential confounding factors, such as smoking, scrotal heat-exposure jobs, history of varicocele, cancer, genitourinary tract and gland infection, were obtained from the medical history and the examination results cited in the medical records. The last five conditions mentioned above were excluded from this study. Exclusion also applied for azoospermia as sperm DFI determination could not be performed.
Semen analysis
After 7 days of abstinence, a complete sample of neat ejaculate produced by masturbation in a room in the laboratory was collected into a sterile container. In the first 30 min after the collection, sperm concentration was determined by using counting chamber technique, while wet preparation for sperm motility and eosin-stained for vitality determination were prepared. The slides were examined with phase-contrast optics at x400 magnification and only morphologically normal spermatozoa were assessed. An air-dried, fixed, and Giemsa-stained preparation was made for sperm morphology determination by using bright-field optics. The prominent semen analysis parameters in this study were sperm concentration, motility, and morphology by referring to the World Health Organization (WHO) standards 2010 [12]. The sperm DFI was determined by SCD test using Halosperm® Kit. Both determinations, semen analysis and sperm DFI, were carried out by the same expert.
Statistical analysis
Statistical analysis was performed using SPSS version 23. Descriptive method was used to determine the distribution of the demographic profiles and the risk factors. Logistic regression was done to assess the smoking habit as the potential confounding factor. Due to the normality test of Kolmogorov-Smirnov showed abnormal data distribution, the Mann Whitney test was used to determine the association of semen parameters (concentration, motility, or morphology) and sperm DFI with male infertility. Categorization of sperm DFI was based on the cut-off point obtained from the Receiver Operating Characteristic (ROC) curve of sperm DFI.
Results
From the medical records, it was obtained a total of 114 subjects fulfilling the study criteria. Subjects were divided into two groups: 78 of infertile group and 36 of fertile group. The median and range of age were 34 (32–39) years old in infertile group and 37 (35–41) years old in fertile group. Age did no significantly differ between fertile and infertile groups (p = 0.310). Smoking habit was not considered as confounding factor in this study based on logistic regression result (p = 0.401). Infertile group had a significantly higher median value of sperm DFI 29.9 (26.6–34.3)% compared to fertile group 19.9 (15.6–24.4)% with p < 0.001. With the same abstinence/delay in both groups, the values of sperm concentration, total motility, progressive motility, morphology, and total sperm count were significantly lower in the infertile group compared to the fertile group, while semen volume did not differ between these two groups (Table 1).
The ability of sperm DFI and semen analysis (concentration, motility, and morphology) to diagnose male infertility was described by ROC curve (Fig. 1). AUC of sperm DFI was 0.862 (95% CI 0.783, 0.941), which was higher than concentration (AUC of 0.744; 95% CI 0.657, 0.831), motility (AUC of 0.668; 95% CI 0.572, 0.765), and morphology (AUC of 0.718; 95% CI 0.697, 0.864) of semen analysis (Table 2).
The highest sensitivity and specificity for sperm DFI was found at the cut-off point of 26.1%. With this cut-off point, sperm DFI was able to distinguish infertile men from fertile men with sensitivity of 80.8% (95% CI; 70.0, 88.5), specificity of 86.1% (95% CI; 69.7, 94.8), positive predictive value of 92.6% (95% CI; 83.0, 97.3), negative predictive value of 67.4% (95% CI; 51.9, 80.0), positive likelihood ratio of 12.6 (95% CI; 5.4, 29.4), and negative likelihood ratio of 0.48 (95% CI 0.31, 0.75), as contained in Table 3. Logistic regression showed that sperm DFI of ≥26,1% has a prevalence ratio of 2.84 (95% CI; 1.86, 4.33) for infertility.
Discussion
The total number of subjects in the present study was relatively small, yet it fulfilled the minimal number required from the sample size calculation. From the demographic data, there was no significant difference in the age between the two groups. Aging is responsible for a general decrease in the function of tissues and organs, including the reproductive tissues and organs. The effects of aging on male fertility were studied by numerous studies. Robertshaw, et al., found that advanced paternal age has an adverse impact on ART outcomes [13]. Other study also found that the ability of spermatozoa to performed fertilization is inversely correlated with the age [14]. The effects of paternal age on fertility remain controversial. Wu, et al., found that increased paternal age had no impact on fertilization rate, embryo quality, and miscarriage rate after controlling the maternal age [15]. Ferreira, et al., who conducted a study to 1024 couples undergoing ICSI (intracytoplasmic sperm injection), also found no correlation between paternal age and sperm parameters or pregnancy rate [16]. Alfaraj and Yunus conducted a study to 451 couples and also found that advancing paternal age had no significant correlation with the outcomes of semen analysis parameters and IVF in infertile couples [17]. This study found no significant difference between age of the two groups. This might be caused by the subject’s selection method used in this study. The fertile group was taken from men proven to have living children and obtained from secondary infertile couples due to female factors so that the median and the range of the age were similar between the two groups.
We define the fertile men were those who have normal semen parameter (concentration, motility, and morphology), even though the subsequent subfertility condition might have correlated with the male factor that can not be seen in semen analysis result, such as body mass index (BMI). Although the data about male’s BMI were not provided, but it has been under a lot of suspicion as the cause of male infertility. There are emerging facts confirming that obesity negatively affects male reproductive potential not only by reducing sperm quality, but in particular, by altering the physical and molecular structures of germ cells in the testes, and ultimately by affecting the maturation and functions of sperm cells [18]. Anifandis, et al., found that BMI of men did not correlate with sperm parameters, but influenced the quality of the produced embryos which, in turns, influenced the pregnancy rates [19]. Similar result was also found by Petersen, et al., that showed couples with both partners having BMI > 25 kg/m2 had the lowest odds of live birth when compared to couples with both partners having BMI < 25 kg/m2 in IVF [20]. In contrast with those studies, Kupka, et al., retrospectively analyzed data retrieved from the National German IVF Registry, which covered 12 years and included 650,452 cycles, and found that the highest clinical pregnancy rates for both IVF and ICSI were seen in normal weight females with obese male partners (P = 0.0028) [21]. However, because none of those studies were randomized controlled trials, several potential confounders and biases might have influenced the findings.
This study found that sperm concentration, total motility, progressive motility, morphology, and total sperm count values were significantly lower in the infertile group compared to the fertile group, while semen volume did not differ. Although total sperm count, total motility, and progressive motility were significantly lower in infertile group, the median values were considered normal. It was considered that the normal median value of the total sperm count could be the result of the length of abstinence. Both groups have abstinence/delay of 7 days. The recommended abstinence delay for semen analysis are 2–7 days [12]. Many studies found that semen volume and concentration (resulted in total sperm count) increased with the increasing length of abstinence. Carlsen, et al., studied 419 semen samples with abstinence interval of 2–7 days and found that there was increased in semen volume and concentration after 4 days of abstinence and succeeding days, and there was no effect on motility and total motile spermatozoa with increased duration of abstinence [22]. Sunanda, et al., studied 730 men with abstinence interval of 2–7 days and found that semen volume and total count increased with the increasing abstinence period, while sperm motility and vitality declined after 5 days of abstinence [23]. The effects of abstinence length on sperm motility in previous studies were contradicted by a more recent study by Agarwal, et al., who conducted semen analysis by grouping the abstinence interval into three categories: short interval (1 day), the recommended interval of the World Health Organization (WHO) (2–7 days), and long interval (9–11 days). The study found significant increase in volume, total sperm count, total motility, and DNA fragmentation between the short and the recommended abstinence interval (P < .05); and between the recommended and the long abstinence interval (P < .05) [24].
In this study, the median value of sperm DFI was significantly higher in the infertile group compared to the fertile group (Table 1). The values of the three sperm parameters are also significantly lower in the infertile group. These results are consistent with the study of Sergerie, et al., in 2005 that found mean value of sperm DFI in the infertile group was significantly higher than in the fertile group (40.9 ± 14.3% compared to 13.1 ± 7.3%) and the mean sperm concentration in infertile group also significantly lower compared to the fertile group (62.9 ± 33.2 × 106/ml compared to 102.4 ± 66.4 × 106/ml) [25]. Similar result was also reported in a study that assessed the degree of DFI in patients dealing with infertility. Sperm DFI was significantly higher in patients with infertility compared to those of control (22.2 ± 5.6% vs. 16.7 ± 0.7%; p < 0.05) [26]. From these results, it may be concluded that sperm DFI determination could be used to distinguish infertile men from fertile men.
AUC value of sperm DFI was 0.862 (p < 0.001; 95% CI 0.783, 0.941) with sensitivity of 80.8% and specificity of 86.1%. Statistically, AUC value in the range of 80–90% has a good diagnostic strength [27]. This value is 11.8% higher than the highest AUC value of semen analysis that routinely performed in infertility workup (Table 2). This result indicates that clinically, sperm DFI has better diagnostic strength than semen analysis. Sperm DFI also has a stronger predictive value compared to free sperm DNA for the success of pregnancy in IVF and ICSI patients (AUC = 0.7; p < 0.05 compared to AUC = 0.6; p > 0.05) [28]. A meta-analysis by Cui, et al., also supports this result that the sperm DFI may be used to distinguish the sperm of infertile men from fertile men with AUC value of 0.921, and sensitivity of 80% and specificity of 83% [29].
The expected benefit of this study is to increase the outcome of infertility management in infertile couple population. Sperm DFI can well-distinguish infertile men from fertile men with positive predictive value of 92.6% at 26.1% cut-off point. Similar result was reported in a study comparing DNA fragmentation of neat and swim-up spermatozoa to predict pregnancy following ICSI. The cut-off value of the neat spermatozoa that resulted in 80% of pregnancy rate was 26% [30]. There are only a few published papers that specifically used SCD test (or Halosperm test) to assess male infertility and reported the correlation with IVF or ICSI outcomes. Two published papers reported that sperm DFI measured with Halosperm had no impact on the embryo quality and the ongoing pregnancy rates in IVF or ICSI. These studies, however, used different cut-off points from the present study (30 and 35% DFI) [31, 32]. Due to the higher cut-off points, the fact that extremely high DNA damages are associated with total pregnancy failure should not be ruled out. A new study that uses 26% DFI as a cut-off point is needed to establish the impact of sperm DFI measured with Halosperm on male infertility.
In this study, the prevalence ratio for sperm DFI ≥ 26.1% was 2.84 (95% CI, 1.86, 4.33). Thus, it may be concluded that a man with sperm DFI of ≥ 26.1% has 2.84 times greater risk for infertility than men with sperm DFI of < 26.1%. These results are consistent with a cohort study that found the most predictive cut-off point for pregnancy was sperm DFI of > 25.5% with negative predictive value of 72.7% and the odds ratio for sperm DFI < 25.5% was 3.6 (95% CI; 1.66, 7.82) [33].
Conclusion
This study discovered a significant difference between the median value of sperm DFI of infertile and fertile men. In determining male infertility, the diagnostic value (AUC) of sperm DFI was higher than the semen analysis. Sperm DFI of 26,1% was the optimal cut-off point to distinguish infertile men from fertile men with sensitivity of 80.8%, specificity of 86.1%, positive predictive value of 92.6%, and negative predictive value of 67.4%. Thus, the determination of sperm DFI can be considered as an additional diagnostic tool besides the semen analysis before an infertile couple undergoing an ART. In addition, in order to decide whether the cut-off point of 26.1% can be used universally for sperm DFI determination by SCD method using Halosperm® Kit, it is necessary to perform further research in a large scale.
Abbreviations
- AUC:
-
Area under the curve
- CI:
-
Confidence interval
- DFI:
-
DNA fragmentation index
- DNA:
-
Deoxyribonucleic acid
- ICSI:
-
Intra cytoplasmic sperm injection
- IVF:
-
In vitro fertilization
- NLR:
-
Negative likelihood ratio
- NPV:
-
Negative predictive value
- PLR:
-
Positive likelihood ratio
- PPV:
-
Positive predictive value
- ROC:
-
Receiver operator characteristic
- SCD:
-
Sperm chromatin dispersion test
- WHO:
-
World Health Organization
References
World Health Organization. Infecundity, infertility, and childlessness in developing countries, DHS Comparative Reports. Maryland: ORC Macro and the World Health Organization; 2004.
Azis N, Agarwal A. Evaluation of sperm damage: beyond the World Health Organization criteria. Fertil Steril. 2008;90:484–5.
Wiweko B, Hestiantoro A, Sumapraja K, Muharam R, Andriyana H, Febia E, et al. Predictive factors for pregnancy in IVF: an analysis of 348 cycles. Indones J Obstet Gynecol. 2010;34(4):180–4.
Lewis SEM, Aitken RJ, Conner SJ, Luliis GD, Evenson DP, Henkel R. at al. The impact of sperm DNA damage in assisted conception and beyond: recent advances in diagnosis and treatment. Reprod Biomed Online. 2013;27(4):325–7.
Simon L, Murphy K, Shamsi MB, Liu L, Emery B, Aston KI, et al. Paternal influence of sperm DNA integrity on early embryonic development. Hum Reprod. 2014;29(11):2402–12.
Simon L, Proutski I, Stevenson M, Jennings D, McManus J, Lutton D, et al. Sperm DNA damage has a negative association with live-birth rates after IVF. Reprod Biomed Online. 2013;26(1):68–78.
Osman A, Alsomait H, El-toukhy T, Khalaf Y. The effect of sperm DNA fragmentation on live birth rate aftar IVF or ICSI: a systematic review and meta-analysis. RBM Online. 2015;30(2):120–7.
Sharma RK, Said T, Agarwal A. Sperm DNA damage and its clinical relevance in assessing reproductive outcome. Asian J Androl. 2004;6:139–48.
Chohan KR, Jeanine T, Griffin JT, Lafromboise M, De Jonge CJ, Carrel D. Comparison of chromatin assays for DNA fragmentation evaluation in human sperm. J Androl. 2006;27(1):53–9.
Zhang LH, Qiu Y, Wang KH, Wang Q, Tao G, Wang LG. Measurement of sperm DNA fragmentation using bright-field microscopy: comparison between sperm chromatin dispersion test and terminal uridine nick-end labeling assay. Fertil Steril. 2010;94(3):1027–32.
Fernandez JL, Muriel L, Goyanes V, Segrelles E, Gosalvez J, Enciso M, et al. Simple determination of human sperm DNA fragmentation with an improved sperm chromatin dispersion test. Fertil Steril. 2005;84(4):833–42.
Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16:231.
Robertshaw I, Khoury J, Abdallah ME, Warikoo P, Hofmann GE. The effect of paternal age on outcome in assissted reproductive technology using the ovum donation model. Reprod Sci. 2014;21(5):590–3.
Frattarelli JL, Miller KA, Miller BT, Elkind-Hirsch K, Scott Jr RT. Male age negatively impacts embryo development and reproductive outcome in donor oocyte assisted reproductive technology cycles. Fertil Steril. 2008;90:97–103.
Wu Y, Kang X, Zheng H, Liu H, Liu J. Effect of paternal age on reproductive outcomes of fertilization. PLoS One. 2015;10(9):e0135734.
Ferreira R, Braga D, Bonetti C, Pasqualotto F, Iaconelli A, Borges E. Negative influence of paternal age on clinical intracytoplasmic sperm injection cycle outcomes in oligozoospermic patients. Fertil Steril. 2010;93:1870–4.
Alfaraj SS, Yunus F. Advancing paternal age does not affect in-vitro fertilization (IVF) outcomes in a Saudi population. Middle East Fertil Soc J. 2015;20(3):204–8.
Shukla KK, Chambial S, Dwivedi S, Misra S, Sharma P. Recent scenario of obesity and male fertility. J Androl. 2014;2:809–18.
Anifandis G, Dafopoulos K, Messini CI, Polyzos N, Messinis IE. The BMI of men and not sperm parameters impact on embryo quality and the IVF outcome. J Androl. 2013;1:85–9.
Petersen GL, Schmidt L, Pinborg A, Kamper-Jorgensen M. The influence of female and male body mass index on live births after assisted reproductive technology treatment: a nationwide register-based cohort study. Fertil Steril. 2013;99:1654–62.
Kupka MS, Gnoth C, Buehler K, Dahncke W, Kruessel JS. Impact of female and male obesity on IVF/ICSI: results of 700,000 ART-cycles in Germany. Gynecol Endocrinol. 2011;27:144–9.
Carlsen E, Petersen JH, Andersson AM, Skakkebaek NE. Effects of ejaculatory frequency and season on variations in semen quality. Fertil Steril. 2004;82(2):358–66.
Sunanda P, Panda B, Dash C, Padhy RN, Routray P. Effect of age and abstinence on semen quality: a retrospective study in a teaching hospital. Asian Pac J Reprod. 2014;3(2):134–41.
Agarwal A, Gupta S, Du Plessis S, Sharma R, Esteves SC, Cirenza C, et al. Abstinence time and its impact on basic and advanced semen parameters. J Urol. 2016;94:102–10.
Sergerie M, Laforest G, Bujan L, Bissonnette F, Bleau G. Sperm DNA fragmentation: threshold value in male fertility. Hum Reprod. 2005;20(12):3446–51.
Diallo MS, Faye O, Diallo AS, Diallo Y, Diao B. Increased DNA fragmentation in patients with infertility in Dakar (Senegal). Adv Reprod Sci. 2015;3:97–105.
Rao CR, Miller JP, Rao DC. Handbook of statistics vol. 27: epidemiology and medical statistics. Oxford: Elsevier; 2008. p. 726.
Bounartzi T, Dafopoulos K, Anifandis G, Messini CI, Koutsonikou C, Kouris S, et al. Pregnancy prediction by free serm DNA and sperm DNA fragmentation in semen specimens of IVF/ICSI-ET patients. Hum Fertil (Camb). 2016;19(1):56–62.
Cui Z, Zheng D, Chen L, Lin D, Feng-Hua L. Diagnostic accuracies of TUNEL, SCD, and Comet based sperm DNA fragmentation assay for male infertility: A meta-analysis study. Clin Lab. 2015;61(5-6):525–35.
Gonsalves J, Caballero P, Lopez-Fernandez C, Ortega L, Guijarro JA, Fernandez JL, et al. Can DNA fragmentation of neat or swim-up spermatozoa be used to predict pregnancy following ICSI of fertile oocyte donors? Asian J Androl. 2013;15(6):812–8.
Yilmaz S, Zergeroglu AD, Yilmaz E, Sofuoglu K, Delikara N, Kutlu P. Effects of sperm DNA fragmentation on semen parameters and ICSI outcome determines by improved SCD test, Halosperm. Int J Fertil Steril. 2010;4(2):73.
Anifandis G, Bounartzi T, Messini CI, Dafopoulus K, Markandona R, Sotiriou S, et al. Sperm DNA fragmentation measured by Halosperm does not impact on embryo quality and ongoing pregnancy rates in IVF/ICSI treatments. J Androl. 2015;47(3):295–302.
Lopez G, Lafuente R, Checa MA, Cerreras R, Brassesco M. Diagnostic value of sperm DNA fragmentation and sperm high-magnification for predicting outcome of assissted reproduction treatment. Asian J Androl. 2013;15(6):790–4.
Acknowledgement
The authors would like to acknowledge Dr. Sylvi WL, MBioMed, PhD as her expertise of SCD test and all of the team in Indonesian Reproductive Medicine and Training Center (Ina-Repromed) for their support to this study.
Funding
This study has no received research grants.
Availability of data
Data set and SPSS is available in author.
Authors’ contributions
BW conceived of the study, carried out the design study, recruited the subject, drafted and finalized the manuscript; PU participated in recruited subject, data collection and performed statistical analysis. Both authors read and approved the final manuscript.
Authors’ information
Budi Wiweko: Currently he is the research manager of Faculty of Medicine Universitas Indonesia. His research interest is on ART and fertility preservation. He is the President of Indonesian Association for IVF and The President Elect of ASPIRE 2016 – 2018.
Pramety Utami: She has just finished O & G specialist training from Faculty of Medicine Universitas Indonesia in 2016.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Every author has agreed to publish this study.
Ethics approval and consent to participate
The ethical clearance approved by Ethical Committee Faculty of Medicine Universitas Indonesia No. 896/UN2.F1/ETIK/2015. Informed consent was obtained to every subject before recruitment into study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Wiweko, B., Utami, P. Predictive value of sperm deoxyribonucleic acid (DNA) fragmentation index in male infertility. Basic Clin. Androl. 27, 1 (2017). https://doi.org/10.1186/s12610-016-0046-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12610-016-0046-3
Keywords
- Cut-off point
- Male infertility
- Halosperm®
- Semen analysis
- Sperm chromatin dispersion (SCD)
- Sperm DNA fragmentation index