Abstract
Background
The interactions between rhizosphere microbial community and ephemeral desert plants strongly affect the ecological protection, restoration and reconstruction in deserts. Ephemeral desert plants as the pioneer plants in the succession are widely distributed in deserts. However, how the ephemeral desert plants assemble their rhizosphere microbiota to adapt to arid and semi-arid environments has been little explored. Here, we used high-throughput sequencing techniques to compare rhizosphere bacterial communities and functions with different ephemeral desert plants composition from Gurbantünggüt Desert in western China.
Results
These plants had the same dominant bacterial phyla, which approximately counted 98% of the total bacterial communities. But the bacterial communities had significant differences (P < 0.05) in the composition, structure, diversity and functions of all groups. When comparing every two groups, similarities appeared in the composition, structure and functions of rhizosphere microbiota, and within each group, it is more likely that the rhizosphere bacterial communities of closely related ephemeral desert plants tend to be consistent. Additionally, the rhizosphere bacterial taxa had more similarities in predicted functions. And the predicted functions were correlated with the dominant bacterial phyla.
Conclusions
Overall, to adapt to the arid and semi-arid environments, the rhizosphere bacterial communities of ephemeral desert plants appear similarities based on having differences. This finding will help to understand the importance of how plants and soil microorganisms cooperate in the process of adaption to arid and semi-arid environments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
Ephemeral desert plants have the ability to survive in arid and semi-arid regions, especially in Central Asia, Junggar Basin, Mediterranean Coast and West Asia [61]. These ephemeral desert plants play a non-negligible role in the process of ecological protection [64], restoration and reconstruction in arid areas. Previous studies have demonstrated that arid regions have complex and a large number of microbial communities [8], and the maintenance of plant communities is assisted by rhizosphere microorganisms. Many researchers are concentrated on the community and diversity of the rhizosphere bacterial communities of desert plants under different desert depths [67] and different environmental gradients [31]. Some also focused on the diversity and structure of microbiota in rhizocompartments of plants [71]. Rhizosphere microbial communities can strongly affect the survival and recovery of ephemeral desert plants, because rhizosphere microbial communities can be regarded as the second genome to provide plants with a highly evolved external functional environment [4, 24, 66], but few have revealed whether the rhizosphere microbes of different plants are consistent. Thus, it is worth exploring whether the rhizosphere microorganisms of ephemeral desert plants converge to adapt to the arid environment.
Rhizosphere microorganisms can strongly affect plant health, nutrient absorption, yield and stress resistance [19, 26]. Additionally, rhizosphere microorganisms are a hotspot to study the interactions between plants and soil microorganisms [62]. The structure and composition of rhizosphere bacterial communities may be attributed to “rhizosphere effect”, which indicates that plants can select their specific rhizosphere microorganisms through the release of root exudates [18]. Therefore, many researchers have focused on the rhizosphere communities of different plant species in the same environments [14] or the same species under different soil type [59]. Previous study have demonstrated that different pioneer plant species also had similar rhizosphere microbial communities [62]. While under periglacial conditions, the rhizosphere effect of pioneer plants was different [30]. And in a high mountain environment, different pioneer plants would select specific rhizosphere bacteria [9]. Ephemeral desert plants as the pioneer plants in deserts, it is worth investigating whether their rhizosphere microorganisms are biased towards similarities or differences to adapt to the arid and semi-arid environment.
In Gurbantünggüt Desert, widely distributed ephemeral desert plants are key factors in effectively stabilizing sand dune surfaces and reducing the mobile intensity of the sand surface [68]. Under a constantly fluctuating environment, rhizosphere microorganisms will undergo changes to some extent, representing similarities and differences are common in the rhizosphere zone of different ephemeral desert plants. Therefore, high-throughput sequencing technology was used to evaluate the rhizosphere bacterial communities of eighteen ephemeral desert plants grown in Gurbantünggüt Desert. The aims of this study were to examine (1) how the rhizosphere bacterial communities of ephemeral desert plants with different affinities vary and (2) what is the direction of variation in the composition, structure, diversity and functions of rhizosphere bacterial communities in the extreme environment.
Materials and methods
Study sites
The study sites are located in the southern of Gurbantünggüt Desert (44°11′-46°20′N, 84°31′-90°00′E), which has an area of 4.88 × 104 km2. It is a fixed and semi-fixed desert where soils are consisted of 73–83% sand with average altitude 677 m, and the altitude of the southwest desert is lower than 400 m [46]. The climate of this region is the typical continental arid and semi-arid desert climate with mean annual precipitation 120 mm and annual mean temperatures ranging from 4 to 7 ℃ [70]. As the second largest desert in China, there are many ephemeral desert plants which account for 37.1% of the total plant species growing in this desert [53].
Soil sample collection and processing
The ephemeral desert plants have relatively short growth cycle, concentrating flowering and fruiting in May [53]. And the inconsistent seed germination of different plants and the difficulty of identifying them in the early stages of growth. Thus, in May, plants with 70% of their layers in flower were selected for the collection of rhizosphere microorganisms, which could better represent the adaption of plants to their environments [36]. In May, 2020, rhizosphere soil was collected from Eighteen different plant species, including Eremurus chinensis (A),Erodium stephanianum (B); Nepeta micrantha (C); Eremopyrum orientale (D); Scorzonera austriaca (F); Alyssum simplex (G); Descurainia Sophia (H); Trigonella arcuata (I); Koelpinia linearis (J); Echinops sphaerocephalus (K); Alyssum linifolium (M); Malcolmia scorpioides (N); Lappula semiglabra (O); Nonea caspica (P); Arnebia guttata (Q); Euphorbia turczaninowii (R); Lactuca sativa (S) and Hypecoum parviflorum (U) in Gurbantünggüt Desert in Xinjiang Uygur Autonomous Region. In this study, the ephemeral desert plants were taken as research objects and selected randomly. Pierce the soil with a shovel to a depth of 30 cm to cut any of the lateral roots holding the plant in the soil. Excavate the plant roots by leveraging the shovel and place the root ball in the labeled bucket with plant sample details. And then, shake the roots and carefully removed the loose soil at the roots. Next, following the previous methods, the roots with closely adhered soil were placed into phosphate buffer saline with sterilized forceps to obtain rhizosphere soil [13]. Rhizosphere soils from randomly selected ephemeral desert plant species in Gurbantünggüt Desert were mixed to form composite samples to reduce geographical differences. To avoid type II errors, three replicates per rhizosphere soil of 18 plant species were performed [51] and 54 soil samples were collected. These samples were placed in a tank of liquid nitrogen, refrigerated, transported back to the laboratory immediately, and then stored at – 80 ℃ [63], until further processed.
DNA extraction and quantitative PCR analysis
Genomic DNA was extracted by CTAB method, followed by agarose gel electrophoresis to check the purity and concentration of DNA. An appropriate amount of DNA was taken and diluted to 1 ng/μL in sterile water. Using diluted genomic DNA as a template, the V4 region of the bacterial 16S rRNA gene was selected and PCR amplification was performed using 515F 5ʹ- GTGYCAGCMGCCGCGGTAA-3ʹ [44] and 806R 5ʹ- GGACTACNNGGGTATCTAAT-3ʹ [49] as primers to identify bacterial diversity. To ensure amplification efficiency and accuracy, Phusion® High-Fidelity PCR Master Mix (New England Biolabs) with GC Buffer were performed. Then the PCR products were detected by electrophoresis using 2% agarose gels and target DNA bands were purified by GeneJET Gel Recovery Kit (Thermo Scientific).
Sequencing of DNA
The sequencing library was constructed using the Ion Plus Fragment Library Kit 48 rxns (Thermo Scientific). After the constructed library was qualified by Qubit quantification and library testing, they were sequenced using IonS5TMXL. Raw reads were obtained by preliminary quality control, and chimeric sequences were removed [39]. Based on the IonS5TMXL sequencing platform, a single-end sequencing method was used to construct a small fragment library for single-end sequencing. The sequences were clustered into Operational Taxonomic Units (OTUs) with 97% identity using UPARSE, and chimeric sequences were identified and removed using UCHIME [12], and then species annotation was performed on the OTUs sequences and the Silva 132 database (https://www.arb-sliva.de).
Statistical analyses
The relative abundance of dominant bacterial communities and alpha diversity were shown using the ggplot2 of R software (version 4.1.3). Additionally, the heatmap of the dominant bacterial communities was also used the same R software (version 4.1.3). Based on the Bray–Curtis distance, PCoA was drawn by vegan package to find the similarities and dissimilarities of bacterial taxa in plant species and cluster analysis based on the Bray–Curtis dissimilarity matrix. One-way analysis of variance (ANOVA) with LSD test was used to identify differences in the dominant bacterial taxa, alpha diversity [including richness (Chao1 index, Richness), diversity (Shannon index, Simpson index), and evenness (Pielou index)] and predicted functions among different treatments in microbial communities. T-test was used to compare the bacterial community composition of the different plants within each group and find differences. Spearman correlations were conducted to determine the relationships between dominant bacterial phyla and predicted functions. Rarefaction analysis showed that the number of samples and the sequencing depth were sufficient (Additional file 1: Fig. S1).
Results
A total of 4,393,802 raw reads were obtained from IonS5TMXLA high-throughput sequencing. After using a series of processes: screening, filtering, and chimera remove, 4,100,809 clean reads were obtained in total. What’s more, a total of 223,128 OTUs were obtained by OTU clustering with 97% concordance from 54 samples collected by high-throughput sequencing.
Composition of the bacterial communities in different ephemeral plant species
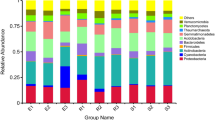
The rhizosphere microbial community was dominated by Proteobacteria with 52.54% average relative abundance, followed by Actinobacteria, Bacteroidetes, Cyanobacteria, Chloroflexi, Acidobacteria, Planctomycetes, Gemmatimonadetes, Verrucomicrobia and Firmicutes, approximately counted 45.98% of the total bacterial communities. And at the genus and species level, the composition of the main dominant genera and dominant species of the rhizobacteria of these ephemeral desert plants are the same. The main dominant genera and species are mainly the Gamma-proteobacteria and Alpha-proteobacteria, both of which belong to the phylum Proteobacteria (Additional file 1: Fig. S2). Next according to the DNA of the eighteen plant species, these species were divided into five groups: N. micrantha(C), S. austriaca(F), K. linearis(J), E. sphaerocephalus(K) and L. sativa(S) as G1; L. semiglabra(O), N. caspica(P) and A. guttata(Q) as G2; E. stephanianum(B) and T. arcuata(I) as G3; A. simplex(G), D. Sophia(H), A. linifolium(M), M. scorpioides(N) and E. turczaninowii(R) as G4; and finally E. chinensis(A), E. orientale(D) and H. parviflorum(U) as G5 (Fig. 1a). There were significant differences (P < 0.05, Fig. 1b) among the five groups, which suggested that the rhizosphere microbiota were reproducibly different in these plants surviving in the same desert environment. Then we compared each phyla, 80% similarities were found in Cyanobacteria and Chloroflexi, respectively, among the five groups (P > 0.05). Between G2 and G3, the similarities of top 10 bacterial phyla accounted 70%. And 50% similarities were observed between G4 and G5 (P > 0.05).
The composition (phylum level) of bacterial communities in different groups. a The composition and relative abundance of major bacterial phyla in rhizosphere soil of eighteen ephemeral plant species and five groups. Each bar represents the average value of three replicates in each sample. Capital letters represent plant species: A, E. chinensis; B, E. stephanianum; C, N. micrantha; D, E. orientale; F, S.austriaca; G, A. simplex; H, D. Sophia; I, T. arcuata; J, K. linearis; K, E. sphaerocephalus; M, A. linifolium; N, M. scorpioides; O, L. semiglabra; P, N. caspica; Q, A. guttata; R, E. turczaninowii; S, L. sativa; U, H. parviflorum. b The community compositions of bacteria with the dominant phyla. Different lowercase letters above the boxes represent significant differences between different group plant species based on one-way ANOVA with LSD multiple comparisons (P < 0.05)
A two-by-two comparison of the bacterial community composition of the different plants within each group revealed that there were also significant differences in the dominant microbial composition of the different plants within the group, particularly in G1, G4 and G5 (Fig. 2 and Additional file 1: Fig. S3, P < 0.05). While in G2, there were no significant variations in the dominant bacterial community composition of P and Q (Fig. 2cd P > 0.05). In G3, except Proteobacteria, Actinobacteria and Chloroflexi, no significant differences were found in the other dominant bacteria phyla (Fig. 2ef P > 0.05).
The community composition of dominant bacterial phyla within each group. Horizontal axis is the plant; vertical axis is the relative abundance of the corresponding plant. The community composition of dominant bacterial phyla within G1 (a and b). The community composition of dominant bacterial phyla within G2 (c and d). The community composition of dominant bacterial phyla within G3 (e and f). T-test was used to compare the bacterial community composition of the different plants within each group. “*”, “**”, “***” and “****”indicate significant differences at 0.05, 0.01, 0.001 and 0.0001, respectively
Bacterial community structure of different plant species
Based on the Bray–curtis distance, the principal coordinate analysis (PCoA) was used to analyze the differences in the rhizosphere bacterial community structures. The overall bacterial community was significantly divided by the five groups (ANOSIM, P = 0.001). This result indicated that the rhizosphere microorganisms of different ephemeral desert plants had distinct structures. Along the second principal coordinate axis, the plants could be divided into two parts, which could be corresponded to Fig. 3b. Additionally, the clustering of different plant species was somewhat different from the previous clustering based on the DNA (Fig. 1a), indicating that high similarity of rhizosphere bacterial communities brings different ephemeral desert plants together. Furthermore, we compared the structure between every two groups. No significant differences were observed between G1 and G3 (ANOSIM, P = 0.326; Table 1). Similarly, G3 was also not different from G4 and G5, respectively (ANOSIM, P = 0.060 and P = 0.294, respectively; Table 1).These results indicated that the rhizosphere bacterial communities of G3 were more similar to those of G1, G4 and G5, respectively, and the ratio of similarities and dissimilarities was 3:7 (Table 1).
The dissimilarity of bacterial communities among different groups. a Principal coordinated analysis (PCoA) derived from dissimilarity matrix of Bray–curtis distance of five groups. An analysis of similarities (ANOSIM) was calculated at the OTU level based on Bray–Curtis distance, indicating community similarity across sampling groups. b The Bray–curtis based cluster analysis of bacterial community composition among plant species
Bacterial community diversity of different plant species
There were significant differences in the alpha diversity among the five groups (ANOVA, P < 0.05; Fig. 4). Then, we compared the within-sample diversity in five groups. Shannon index values ranging from 4.24 to 5.54 and 4.93 to 5.78 were observed within G2 and G3, respectively, but there were no significant differences in the Shannon diversity (ANOVA, P = 0.066 and P = 0.1 respectively; Fig. 5). Similarly, higher Chao 1 richness was observed in the rhizosphere samples of L. semiglabra(O) when compared with those of N. caspica(P) and A. guttata(Q), but no significant differences within G2 were observed (ANOVA, P = 0.066; Fig. 5). And within G3 and G5, respectively, still no significant differences were observed in Chao 1 richness (ANOVA, P = 0.1 and P = 0.061; Fig. 5). These results indicated that the capacity of recruiting rhizosphere microbial bacteria was similar among these plant species with close affinities. Compared to the mentioned groups, the Shannon diversity and Chao 1 richness had significant variations within the rest groups (ANOVA, P < 0.05; Fig. 5). And the ratios of similarities and dissimilarities in Shannon diversity and Chao 1 richness were 2:3 and 3:2, respectively.
Rhizosphere bacterial predicted functional profiles and relations with bacteria
Using Tax4Fun based on species annotations from the SILVA database, we annotated the functions related to 43 pathways (Additional file 1: Fig. S4). The predicted bacterial functions indicated that chemoheterotrophy and aerobic chemoheterotrophy were the two most important predicted metabolic functions for bacteria, which dominated both OTU richness (27.8% and 22.2%, respectively) across all groups (Fig. 6). Moreover, chemoheterotrophy and aerobic chemoheterotrophy were significantly abundant in G1 and G4, but no differences were found (ANOVA, P > 0.05; Fig. 6b). Phototrophy, photoautotrophy and oxygenic_photoautotrophy did not differentiate from G2 to G5 (ANOVA, P > 0.05; Fig. 6b). No significant variations were found in methylotrophy and methanol_oxidation in G1, G3 and G5 (ANOVA, P > 0.05; Fig. 6b). Additionally, chemoheterotrophy and aerobic chemoheterotrophy were the two most important predicted metabolic functions within each group (Additional file 1: Fig. S5).
Predicted functional profiles. a The relative abundance of the dominant predicted functions among five groups. b Bubble chart of the dominant predicted functional profiles of bacterial communities across five groups of plant species. The functional groups of bacteria were inferred using Tax4Fun. Dot size and color indicate the absolute abundance of predicted functions. Different lowercase letters above the boxes represent significant differences between different plant species based on one-way ANOVA with LSD multiple comparisons (P < 0.05)
Chemoheterotrophy was significantly related to four dominant bacterial phyla, while aerobic_chemoheterotrophy was significantly related to five dominant bacterial phyla (Fig. 7). Phototrophy, photoautotrophy and oxygenic_photoautotrophy showed stronger relationships with Cyanobacteria, followed by Proteobacteria. Thus, the three functions were highly abundant in G1 because of the higher relative abundance of Cyanobacteria (Figs. 1b and 6). Meanwhile, methylotrophy and methanol_oxidation were highly abundant in G4 (higher relative abundance of Actinobacteria), which can be due to that these two functions were significantly related to Proteobacteria and Actinobacteria (Figs. 1b and 7).
Discussion
In this study, the rhizosphere bacterial communities in ephemeral desert plants have a tendency to be consistent, because multitrophic interactions may have co-evolved in the rhizosphere of plants grown in the native habitats [37]. Additionally, plants can transmit specific bacteria from one generation to another by recruiting a core rhizosphere microbiota from seeds, which has an important role in the co-evolution of the rhizosphere in natural ecosystems [33]. However, the structures and diversity of rhizosphere bacterial communities in all five groups were significantly different. Plants can modulate their rhizosphere microbiome in a host-dependent way [32]. And it has been shown that differences in the composition of rhizosphere microbial assemblages appear to increase with increasing phylogenetic distance between plant species [7, 34]. The composition, structures, diversity, and predicted functions either between groups or within each group all existed differentiation. These are consistent with previous studies that demonstrated the rhizosphere microorganisms will appear different even if plant species grow on the same soil [10, 27]. Thus, it is normal to find that the rhizosphere microbial communities of ephemeral desert plants from Gurbantünggüt Desert had differences.
In all plant species, Proteobacteria, Actinobacteria, Cyanobacteria, Bacteroidetes, etc. were the dominant bacterial phylum (Fig. 1a), which is similar to the results of previous studies in rhizosphere soils of woody plants [38] and perennial shrub plant species [31]. And the most abundant bacterial groups from the bacterial genera Variovorax, unidentified_Cyanobacteria, Methylobacillus, Nocardiodies, Massilia, Pseudoxanthomonas, Microvirga, Chitinophaga, Streptomyces, Ensifer, Phenylobacterium, Pedobacter, Sphingomonas, Ramlibacter and Lysobacter (Additional file 1: Fig. S2ab). Most of the dominant bacterial genera belong to the class of Gemma-proteobacteria and Alpha-proteobacteria. The results demonstrated that plants grown in the same soil environment have similar microbial communities, which is similar to previous study [25]. In arid desert environments, Proteobacteria had the highest relative abundance, which is consistent with the findings of previous studies [50, 58]. As fast-growing r-strategists, Proteobacteria can use root-derived carbon substrates [33] and play a critical role in the nutrient cycle [43]. The relative abundance of Proteobacteria in G2 and G3 was significantly higher than the rest (Fig. 1), which may be due to the increase in organic matter. Under arid conditions, Actinobacteria are the dominant metabolic group in desert ecosystems due to their wide range of metabolic pathways and high metabolic capacity, complete UV-radiation repair mechanisms [54] and promotion of plant growth [28]. In this study, Cyanobacteria were a prevalent bacterial phylum. Since carbon and nitrogen are usually limited in arid environments, Cyanobacteria increase the fixation of soil carbon and nitrogen by producing organic matter which contains carbon and nitrogen [35].
Interactions between species are a critical determinant of soil bacterial community composition, which may lead to significant differences in soil bacterial community composition in homogeneous environments [69]. The rhizosphere microorganisms of the ephemeral desert plants within each group had more significant differences (Fig. 2 and Additional file 1: Fig. S3), which may be related to that the soil bacterial community competes for limited nutrients (especially soil moisture). This result is consistent with previous studies that species with close affinities are more likely to have a stronger competition for resources [16, 41]. Additionally, plant species may affect the composition and activity of the rhizosphere bacterial communities [6, 22]]. Thus the composition and structure of rhizosphere bacterial communities in ephemeral desert plants show differentiation. In contrast, there were still similarities between each group and within each group (Figs. 1b, 2 and Additional file 1: Fig. S3), which may be due to the homogeneous selection. Recent studies have reported that abundant microbes were controlled more by homogeneous selection in the community assembly processes [29], while abundant subcommunity was governed by dispersal limitation. Meanwhile, rare subcommunity of Panax notoginseng was dominated by homogeneous selection of the rhizosphere bacterial communities [65].
In the present study, there was a distinct rhizosphere microbial structure based on the analysis of PCoA (Fig. 3a). This phenomenon may be attributed to the fact that plant species can influence indigenous microbial population in soil, and each plant species have the ability to choose their own rhizosphere microbes by root exudates [5]. Additionally, the distinct rhizosphere bacterial communities could be related to that bacteria were more sensitive to disturbance [60]. Bacteria with small size and short life cycle can respond rapidly to weak changes in a fluctuating environment [62]. However, no significant differences (accounted for 30%) were observed in the comparison between every two groups (Table 1). Because previous study found that the interactions between plants and microbes are very important to the convergence of bacterial structures [20]. The new clustering of different plant species (Fig. 3b) may be related to root architectural and morphological traits, which can change the interactions of plants and microorganisms [17] and plant phylogeny [57].
The diversity of plant species differed significantly (Figs. 4 and 5), probably because environmental fluctuations can lead to heterogeneity, and the diversity of desert microorganisms may quickly reflect the heterogeneity of desert environments [40]. A recent study indicated that the diversity of microorganisms can be altered by the species richness of neighboring plants [3]. More diverse plant communities can lead to higher diversity because of more diverse composition of plant residues and rhizodeposits [33]. Similarly, the diversity of plant community strongly influences the rhizosphere bacterial communities in natural, which supported by the diversity of rhizosphere microbiota showed significant variations (Fig. 4). In contrast, the diversity of the rhizosphere bacterial communities within some groups (such as G2, G3 and G6 respectively) was similar (Fig. 5). In other words, besides divergence of the rhizosphere microbes, convergence also exists because some plant species can create similar communities [56]. Furthermore, though the amount and composition of rhizodeposits vary between different plant species, they can also vary as time goes by, especially in single plant species [33]. Thus it is not surprising to find that the diversity appeared similar, because the rhizosphere bacterial communities have the ability to change [42].
In the predicted functions of bacteria, chemoheterotrophy and aerobic chemoheterotrophy were the most abundant and important functions of ephemeral desert plants (Fig. 6 and Additional file 1: Fig. S5). These two functions can drive microbial bacteria to metabolize organic materials in soil [55]. And these two functions were extremely significantly related to Proteobacteria and Actinobacteria (Fig. 7), which are sensitive to organic materials. The study of plant genome-wide association study (GWAS) revealed that the leaf microbiome GWAS is important for understanding the processes in rhizosphere [21], because the microbiomes of leaves and roots overlap [1]. Proteobacteria are enriched in rhizosphere and phyllospheric [11], so among leaf microorganisms, some species of Proteobacteria, such as Pesudomonas, Ensifer, Pantoea and etc., have the photosynthetic capacity required for chlorophyll synthesis, providing a pathway for nutrient transformation in arid environments [47]. Compared to rhizosphere environmental conditions, the phyllosphere is a short-lived environment with limited access to nutrients [11, 45]. The carbon fixed by plant photosynthesis is partially transferred to the root zone and released as root secretions [2]. In rhizosphere, the release of various carbohydrates, amino acids, organic acids and other compounds provides a source of nutrients for root-associated bacteria [52]. Proteobacteria in rhizosphere have the ability to provide organic carbon for soil uptake, so that chemoheterotrophy and aerobic chemoheterotrophy functions dominate [15]. Phototrophy, photoautotrophy and oxygenic_photoautotrophy showed extremelly relationships with Cyanobacteria (Fig. 7), which can be responsible for fixed carbon [35]. The predicted functions showed significant different differences, this may be due to the harsh conditions in the desert, and thus the rhizosphere bacterial communities maintain basic functional groups [23]. Compared to the composition of rhizosphere bacterial communities in all groups, the differences in predicted functions of microorganisms were not more significant (Figs. 1b and 6b). This phenomenon is normal because the previous study showed that the community function is more stable than community composition which is in accordance with the hypothesis of functional redundancy [62]. In other words, the same functional taxa may have different composition of microbial communities [48].
Conclusion
Rhizosphere microorganisms of different ephemeral desert plants evolve or succumb in the same direction in response to extreme environments. No significant variation in bacterial community composition, structure and predicted function was observed when comparing between every two groups. Furthermore, these plant groups had distinct bacterial structures but the different species in all groups also clearly clustered together, indicating that rhizosphere microbes of ephemeral plants seem to converge in the same soil environment as time goes by. Compared to the composition of rhizosphere bacterial communities in ephemeral desert plants, predicted functions were more stable and have more tendency to convergence. In summary, under the same arid conditions, the rhizosphere microorganisms of ephemeral plants shape their specific microbial communities to keep the differences between each other, but they can recruit similarly functioning microorganisms to adapt to the desert environment. Adequate understanding of the differences and similarities in rhizosphere microbial communities of plants in the same soil environment could help further study to clarity how ephemeral desert plants respond to drought stress through these microbial communities from a genetic perspective and how they affect the physicochemical properties of the soil.
Availability of data and materials
The datasets analyzed during the current study are not publicly available due to that the data will be used to finish the Master’s thesis, but are available from the corresponding author on reasonable request.
References
Yang B, Müller DB, Srinivas G, Garrido-Oter R, Potthoff E, Rott M, Dombrowski N, Münch PC, Spaepen S, Remus-Emsermann M, Hüttel B, McHardy AC, Vorholt JA, Schulze-Lefert P (2015) Functional overlap of the Arabidopsis leaf and root microbiota. Nature. https://doi.org/10.1038/nature16192
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol. https://doi.org/10.1146/annurev.arplant.57.032905.105159
Bakker MG, Bradeen JM, Kinkel LL (2012) Effects of plant host species and plant community richness on streptomycete community structure. FEMS Microbiol Ecol. https://doi.org/10.1111/1574-6941.12017
Berendsen RL, Pieterse CMJ, Bakker PAHM (2012) The rhizosphere microbiome and plant health. Trends Plant Sci. https://doi.org/10.1016/j.tplants.2012.04.001
Gabriele B, Smalla K (2009) Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol Ecol. https://doi.org/10.1111/j.1574-6941.2009.00654.x
Maria B-V, Prins ME, Raaijmakers JM (2005) Influence of plant species on population dynamics, genotypic diversity and antibiotic production in the rhizosphere by indigenous Pseudomonas spp. FEMS Microbiol Ecol. https://doi.org/10.1016/j.femsec.2004.10.007
Marie-Lara B, Poirier M-A, Muller D, Moënne-Loccoz Y (2014) Root microbiome relates to plant host evolution in maize and other Poaceae. Environ Microbiol. https://doi.org/10.1111/1462-2920.12442
Bull AT, Asenjo JA (2013) Microbiology of hyper-arid environments: recent insights from the Atacama Desert, Chile. Antonie Van Leeuwenhoek. https://doi.org/10.1007/s10482-013-9911-7
Sonia C, Esposito A, Rolli E, Zerbe S, Daffonchio D, Brusetti L (2014) Different pioneer plant species select specific rhizosphere bacterial communities in a high mountain environment. Springerplus. https://doi.org/10.1186/2193-1801-3-391
Díaz-Cruz GA, Cassone BJ (2022) Changes in the phyllosphere and rhizosphere microbial communities of soybean in the presence of pathogens. FEMS Microbiol Ecol. https://doi.org/10.1093/femsec/fiac022
Chun-Juan D, Wang L-L, Li Q, Shang Q-M (2019) Bacterial communities in the rhizosphere, phyllosphere and endosphere of tomato plants. PLoS ONE. https://doi.org/10.1371/journal.pone.0223847
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. https://doi.org/10.1093/bioinformatics/btr381
Joseph E, Johnson C, Santos-Medellín C, Lurie E, Podishetty NK, Bhatnagar S, Eisen JA, Sundaresan V (2015) Structure, variation, and assembly of the root-associated microbiomes of rice. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.1414592112
Xiaoxue F, Wang H, Zhao L, Wang M, Sun M (2022) Diversity and structure of the rhizosphere microbial communities of wild and cultivated ginseng. BMC Microbiol. https://doi.org/10.1186/s12866-021-02421-w
Noah F, Bradford MA, Jackson RB (2007) Toward an ecological classification of soil bacteria. Ecology. https://doi.org/10.1890/05-1839
Foster KR, Bell T (2012) Competition, not cooperation, dominates interactions among culturable microbial species. Curr Biol. https://doi.org/10.1016/j.cub.2012.08.005
Horn HC, Hennessy RC, Bak F, Dresbøll DB, Nicolaisen MH (2022) Back to our roots: exploring the role of root morphology as a mediator of beneficial plant–microbe interactions. Environ Microbiol. https://doi.org/10.1111/1462-2920.15926
Xing-Feng H, Chaparro JM, Reardon KF, Zhang R, Shen Q, Vivanco JM (2014) Rhizosphere interactions: root exudates, microbes, and microbial communities1. Botany. https://doi.org/10.1139/cjb-2013-0225
Xinqi H, Zhou X, Zhang J, Cai Z (2019) Highly connected taxa located in the microbial network are prevalent in the rhizosphere soil of healthy plant. Biol Fertil Soils. https://doi.org/10.1007/s00374-019-01350-1
Igwe AN, Quasem B, Liu N, Vannette RL (2021) Plant phenology influences rhizosphere microbial community and is accelerated by serpentine microorganisms in Plantago erecta. FEMS Microbiol Ecol. https://doi.org/10.1093/femsec/fiab085
Richard J, Peukert M, Succurro A, Koprivova A, Kopriva S (2017) The role of soil microorganisms in plant mineral nutrition-current knowledge and future directions. Front Plant Sci. https://doi.org/10.3389/fpls.2017.01617
Kowalchuk GA, Buma DS, de Boer W, Klinkhamer PGL, van Veen JA (2002) Effects of above-ground plant species composition and diversity on the diversity of soil-borne microorganisms. Antonie Van Leeuwenhoek. https://doi.org/10.1023/a:1020565523615
Carla K, Segura AM, Costa LS, Lacerot G, Kosten S, Peeters ETHM, Huszar VLM, Mazzeo N, Scheffer M (2016) Functional redundancy increases towards the tropics in lake phytoplankton. J Plankton Res. https://doi.org/10.1093/plankt/fbw083
Venkatachalam L, Selvaraj G, Bais HP (2014) Functional soil microbiome: belowground solutions to an aboveground problem. Plant Physiol. https://doi.org/10.1104/pp.114.245811
Shaonan L, Xiaohong Xu, Cheng Z, Xiong J, Ma R, Zhang L, Yang X, Zhu Y, Zhang B, Tian B (2018) Analysis of the community composition and bacterial diversity of the rhizosphere microbiome across different plant taxa. MicrobiologyOpen. https://doi.org/10.1002/mbo3.762
Ning L, Wang T, Kuzyakov Y (2022) Rhizosphere bacteriome structure and functions. Nat Commun. https://doi.org/10.1038/s41467-022-28448-9
Liangliang L, Huang X, Zhang J, Cai Z, Jiang K, Chang Y (2020) Deciphering the relative importance of soil and plant traits on the development of rhizosphere microbial communities. Soil Biol Biochem. https://doi.org/10.1016/j.soilbio.2020.107909
Qi L, Zhao X, Liu Y, Xie S, Xing Y, Dao J, Wei B, Peng Y, Duan W, Wang Z (2021) Response of sugarcane rhizosphere bacterial community to drought stress. Front Microbiol. https://doi.org/10.3389/fmicb.2021.716196
Jiaming Ma, Ma K, Liu J, Chen N (2022) Rhizosphere soil microbial community under ice in a high-latitude wetland: different community assembly processes shape patterns of rare and abundant microbes. Front Microbiol. https://doi.org/10.3389/fmicb.2022.783371
Massaccesi L, Benucci GMN, Gigliotti G, Cocco S, Corti G, Agnelli A (2015) Rhizosphere effect of three plant species of environment under periglacial conditions (Majella Massif, central Italy). Soil Biol Biochem. https://doi.org/10.1016/j.soilbio.2015.07.010
Xiaofan Na, Tingting Xu, Li M, Zhou Z, Ma S, Wang J, He J, Jiao B, Ma F (2018) Variations of bacterial community diversity within the rhizosphere of three phylogenetically related perennial shrub plant species across environmental gradients. Front Microbiol. https://doi.org/10.3389/fmicb.2018.00709
Pérez-Jaramillo JE, Mendes R, Raaijmakers JM (2015) Impact of plant domestication on rhizosphere microbiome assembly and functions. Plant Mol Biol. https://doi.org/10.1007/s11103-015-0337-7
Laurent P, Raaijmakers JM, Lemanceau P, van der Putten WH (2013) Going back to the roots: the microbial ecology of the rhizosphere. Nat Rev Microbiol. https://doi.org/10.1038/nrmicro3109
Neelawan P, Nimnoi P, Lumyong S (2011) Genotypic diversity among rhizospheric bacteria of three legumes assessed by cultivation-dependent and cultivation-independent techniques. World J Microbiol Biotechnol. https://doi.org/10.1007/s11274-011-0855-7
Puxty RJ, Evans DJ, Millard AD, Scanlan DJ (2018) Energy limitation of cyanophage development: implications for marine carbon cycling. ISME J. https://doi.org/10.1038/s41396-017-0043-3
Qian YB, Wu ZN, Zhang LY, Zhao RF, Wang XY, Li YM (2007) Spatial patterns of ephemeral plants in Gurbantünggüt Desert. Sci Bull. https://doi.org/10.1007/s11434-007-0465-9
Reinhart KO, Tytgat T, Van der Putten WH, Clay K (2010) Virulence of soil-borne pathogens and invasion by Prunus serotina. New Phytol. https://doi.org/10.1111/j.1469-8137.2009.03159.x
Frank R, Verboom W, Pate J, Chittleborough D (2019) Collaborative involvement of woody plant roots and rhizosphere microorganisms in the formation of pedogenetic clays. Ann Bot. https://doi.org/10.1093/aob/mcz065
Torbjørn R, Flouri T, Nichols B, Quince C, Mahé F (2016) VSEARCH: a versatile open source tool for metagenomics. PeerJ. https://doi.org/10.7717/peerj.2584
Romina R, Giuffré L, Costantini A, Nannipieri P (2011) Assessment of soil microbial diversity measurements as indicators of soil functioning in organic and conventional horticulture systems. Ecol Ind. https://doi.org/10.1016/j.ecolind.2011.02.008
Jakob R, Røder HL, Madsen JS, Burmølle M, Sørensen SJ (2017) Antagonism correlates with metabolic similarity in diverse bacteria. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.1706016114
Schlemper TR, Leite MFA, Lucheta AR, Shimels M, Bouwmeester HJ, van Veen JA, Kuramae EE (2017) Rhizobacterial community structure differences among sorghum cultivars in different growth stages and soils. FEMS Microbiol Ecol. https://doi.org/10.1093/femsec/fix096
Achim S, Kertesz MA (2007) Desulfurization of aromatic sulfonates by rhizosphere bacteria: high diversity of the asfA gene. Environ Microbiol. https://doi.org/10.1111/j.1462-2920.2006.01172.x
Anne S, Jacquiod S, Vestergaard G, Schulz S, Schloter M (2017) Analysis of soil microbial communities based on amplicon sequencing of marker genes. Biol Fertil Soils. https://doi.org/10.1007/s00374-017-1205-1
Mateen S, Khalid A, Mahmood T, Siddique MT, Han J-I, Habteselassie MY (2017) Evaluation of bacteria isolated from textile wastewater and rhizosphere to simultaneously degrade azo dyes and promote plant growth. J Chem Technol Biotechnol. https://doi.org/10.1002/jctb.5357
Lan S, David TA, Bethany RJ, Garcia-Pichel Ferran Wu, Li, and Hu Chunxiang, (2021) Cyanobacterial community composition and their functional shifts associated with biocrust succession in the Gurbantunggut Desert. Environ Microbiol Rep. https://doi.org/10.1111/1758-2229.13011
Spain AM, Krumholz LR, Elshahed MS (2009) Abundance, composition, diversity and novelty of soil Proteobacteria. ISME J. https://doi.org/10.1038/ismej.2009.43
Strickland MS, Lauber C, Fierer N, Bradford MA (2009) Testing the functional significance of microbial community composition. Ecology. https://doi.org/10.1890/08-0296.1
Abid U, Akbar A, Luo Q, Khan AH, Manghwar H, Shaban M, Yang X (2018) Microbiome diversity in cotton rhizosphere under normal and drought conditions. Microb Ecol. https://doi.org/10.1007/s00248-018-1260-7
Van Goethem Marc W, Makhalanyane TP, Cowan DA, Valverde A (2017) Cyanobacteria and Alphaproteobacteria may facilitate cooperative interactions in niche communities. Front Microbiol. https://doi.org/10.3389/fmicb.2017.02099
Gisle V, Schulz S, Schöler A, Schloter M (2017) Making big data smart—how to use metagenomics to understand soil quality. Biol Fertil Soils. https://doi.org/10.1007/s00374-017-1191-3
Walker TS, Bais HP, Grotewold E, Vivanco JM (2003) Root exudation and rhizosphere biology. Plant Physiol. https://doi.org/10.1104/pp.102.019661
Wang X, Jin J, Yuanchao W, Weilin L, Chunwu S, Junjie C (2006) Responses of ephemeral plant germination and growth to water and heat conditions in the southern part of Gurbantunggut Desert. Sci Bull. https://doi.org/10.1007/s11434-006-8214-z
Yang W, Luo X, Zhang L (2017) Draft genome sequence of glycomyces fuscus TRM 49117, isolated from a hypersaline soil sample. Genome Announc. https://doi.org/10.1128/genomea.01258-17
Guangshan W, Li M, Shi W, Tian R, Chang C, Wang Z, Wang N, Zhao G, Gao Z (2020) Similar drivers but different effects lead to distinct ecological patterns of soil bacterial and archaeal communities. Soil Biol Biochem. https://doi.org/10.1016/j.soilbio.2020.107759
Gabriele W, Neumann R, Backhaus H (2001) Variation of microbial communities in soil, rhizosphere, and rhizoplane in response to crop species, soil type, and crop development. Appl Environ Microbiol. https://doi.org/10.1128/aem.67.12.5849-5854.2001
Alex W, Langridge H, Straathof AL, Muhamadali H, Hollywood KA, Goodacre R, Vries FT (2021) Root functional traits explain root exudation rate and composition across a range of grassland species. J Ecol. https://doi.org/10.1111/1365-2745.13630
Ming-Hui Wu, Li T, Zhang G-S, Fa-Si Wu, Chen T, Zhang B-L, Xiu-Kun Wu, Liu G-X, Zhang K-C, Zhang W (2022) Seasonal variation of hypolithic microbiomes in the Gobi Desert. Microb Ecol. https://doi.org/10.1007/s00248-022-02043-3
Yan Xu, Ge Yi, Song J, Rensing C (2019) Assembly of root-associated microbial community of typical rice cultivars in different soil types. Biol Fertil Soils. https://doi.org/10.1007/s00374-019-01406-2
Juejie Y, Li G, Sheng Y, Zhang F (2022) Response and contribution of bacterial and archaeal communities to eutrophication in urban river sediments. Environ Pollut. https://doi.org/10.1016/j.envpol.2022.119397
Shanlin Y, Shi X, Wang S, Liu J, Meng F, Pang W (2019) Is bi-seasonal germination an optimal choice for an ephemeral plant living in a cold desert? J Arid Land. https://doi.org/10.1007/s40333-019-0001-1
Fei Ye, Xiaoxiao Wang Yu, Wang SW, Jiapeng Wu, Hong Y (2021) Different pioneer plant species have similar rhizosphere microbial communities. Plant Soil. https://doi.org/10.1007/s11104-021-04952-7
Xiangquan Yu, Zhang Y, Shen M, Dong S, Zhang F, Gao Q, He P, Shen G, Yang J, Wang Z, Bo G (2022) Soil conditioner affects tobacco rhizosphere soil microecology. Microb Ecol. https://doi.org/10.1007/s00248-022-02030-8
Yong Z, Liu T, Zhou X-B, Sun Q-M, Han Z-Q, Liu K (2016) Effects of climate change on plant composition and diversity in the Gurbantünggüt Desert of northwestern China. Ecol Res. https://doi.org/10.1007/s11284-016-1352-0
Guozhuang Z, Wei G, Wei F, Chen Z, He M, Jiao S, Wang Y, Yuqi Yu, Dong L, Chen S (2021) Homogeneous selection shapes rare biosphere in rhizosphere of medicinal plant. Ecol Ind. https://doi.org/10.1016/j.ecolind.2021.107981
Tao Z, Wang Z, Lv X, Dang H, Zhuang Li (2020) Variation of rhizosphere bacterial community diversity in the desert ephemeral plant Ferula sinkiangensis across environmental gradients. Sci Rep. https://doi.org/10.1038/s41598-020-75591-8
Tao Z, Wang Z, Lv X, Li Y, Zhuang Li (2019) High-throughput sequencing reveals the diversity and community structure of rhizosphere fungi of Ferula Sinkiangensis at different soil depths. Sci Rep. https://doi.org/10.1038/s41598-019-43110-z
Chunxiang Z, Zheng R, Shi X, Wang L (2021) Soil microbes and seed mucilage promote growth of the desert ephemeral plant Nepeta micrantha under different water conditions. Flora. https://doi.org/10.1016/j.flora.2021.151845
Xin-Feng Z, Hao Y-Q, Zhang D-Y, Zhang Q-G (2019) Local biotic interactions drive species-specific divergence in soil bacterial communities. ISME J. https://doi.org/10.1038/s41396-019-0477-x
Zhang Z, Aimin L, Zhibao D, Zhaohui Z (2022) Sand provenance in the Gurbantunggut Desert, northern China. CATENA. https://doi.org/10.1016/j.catena.2022.106242
Ziyuan Z, Minghan Yu, Ding G, Gao G, He Y (2020) Diversity and structural differences of bacterial microbial communities in rhizocompartments of desert leguminous plants. PLoS ONE. https://doi.org/10.1371/journal.pone.0241057
Acknowledgements
This work was supported by the Key Laboratory Project, Xinjiang (Grant No. 2021D04006) and the National Natural Science Foundation of China (Grant No. 32160281).
Funding
This work was supported by the Key Laboratory Project, Xinjiang (Grant No. 2021D04006) and the National Natural Science Foundation of China (Grant No. 32160281). All these funding was used in the design of the study and collection, analysis, and interpretation of data.
Author information
Authors and Affiliations
Contributions
YY (Yang Yang) and WL designed the study, conducted the field works; YY (Yang Yang), TH, and YY (Yaru Yang) conducted data curation; YY (Yang Yang) and YY (Yaru Yang) visualized and interpreted the results; YY (Yang Yang) wrote the first draft of this manuscript, and WL edited the manuscript; WL supported funding acquisition. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: Fig. S1. Species rarefaction curve of plants. Fig. S2 The composition (genus level and species level) of bacterial communities. The composition of bacterial communities in different plants (a) and in different groups at genus level (b). The composition of bacterial communities in different plants (c) and in different groups at species level (d). Fig. S3 The community composition of dominant bacterial phyla within each group. Horizontal axis is the plant; vertical axis is the relative abundance of the corresponding plant. The community composition of dominant bacterial phyla within G4 (a and b). The community composition of dominant bacterial phyla within G5 (c and d). T-test was used to compare the bacterial community composition of the different plants within each group. “*”, “**”, “***” and “****”indicate significant differences at 0.05, 0.01, 0.001 and 0.0001 respectively. Fig. S4 KEGG Function Pathway. Fig. S5 Predicted functional profiles. (a-e) Bubble chart of the dominant predicted functional profiles of bacterial communities of plant species within five groups. The functional groups of bacteria were inferred using Tax4Fun. Dot size and color indicate the relative abundance of predicted functions.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, Y., Liu, W., Huang, T. et al. Similarities and differences in the rhizosphere biota among different ephemeral desert plants in Gurbantünggüt Desert. Environ Sci Eur 35, 18 (2023). https://doi.org/10.1186/s12302-023-00724-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12302-023-00724-5