Abstract
Preharvest sprouting (PHS) is a serious problem in rice production as it leads to reductions in grain yield and quality. However, the underlying mechanism of PHS in rice remains unclear. In this study, we identified and characterized a preharvest sprouting and seedling lethal (phssl) mutant. The heterozygous phssl/+ mutant exhibited normal plant development, but severe PHS in paddy fields. However, the homozygous phssl mutant was seedling lethal. Gene cloning and genetic analysis revealed that a point mutation in OsABA3 was responsible for the mutant phenotypes. OsABA3 encodes a molybdenum cofactor (Moco) sulfurase. The activities of the sulfureted Moco-dependent enzymes such as aldehyde oxidase (AO) and xanthine dehydrogenase (XDH) were barely detectable in the phssl mutant. As the final step of abscisic acid (ABA) de novo biosynthesis is catalyzed by AO, it indicated that ABA biosynthesis was interrupted in the phssl mutant. Exogenous application of ABA almost recovered seed dormancy of the phssl mutant. The knock-out (ko) mutants of OsABA3 generated by CRISPR-Cas9 assay, were also seedling lethal, and the heterozygous mutants were similar to the phssl/+ mutant showing reduced seed dormancy and severe PHS in paddy fields. In contrast, the OsABA3 overexpressing (OE) plants displayed a significant increase in seed dormancy and enhanced plant resistance to PHS. The AO and XDH activities were abolished in the ko mutants, whereas they were increased in the OE plants. Notably, the Moco-dependent enzymes including nitrate reductase (NR) and sulfite oxidase (SO) showed reduced activities in the OE plants. Moreover, the OE plants exhibited enhanced resistances to osmotic stress and bacterial blight, and flowered earlier without any reduction in grain yield. Taken together, this study uncovered the crucial functions of OsABA3 in Moco sulfuration, plant development, and stress resistance, and suggested that OsABA3 is a promising target gene for rice breeding.
Similar content being viewed by others
Background
When cereal crops mature during a season of high temperature and high humidity, grains may germinate within the mother plant before harvest. This phenomenon is termed as Preharvest sprouting (PHS) (Li et al. 2004; Tai et al. 2021). PHS can cause a severe reduction in grain yield and quality, resulting in substantial economic losses (Bewley et al. 2006).
Abscisic acid (ABA) has been proposed as the primary regulator of PHS by maintaining seed dormancy (Gubler et al. 2005). In higher plants, ABA is derived from carotenoid precursors. Therefore, mutants of the critical carotenoid biosynthetic genes, such as phytoene desaturase, ξ-carotene desaturase, carotenoid isomerase and lycopene β-cyclase, all showed reduced ABA level, and much more severe PHS phenotypes (Fang et al. 2008; Hable et al. 1998; Singh et al. 2003). In the plastid, β-carotene undergoes conversion to 9-cisviolaxanthin and 9-cis-neoxanthin through several steps including hydroxylation, epoxidation and isomerization. These steps are catalyzed by enzymes such as β-carotene hydroxylases, zeaxanthin epoxidase (ZEP), and neoxanthin synthase (Finkelstein 2013; North et al. 2007; Rock and Zeevaart 1991; Sun et al. 1996). The resulting 9-cisviolaxanthin and 9-cis-neoxanthin are cleaved by 9-cis-epoxycarotenoid dioxygenase (NCED) to release xanthoxin (Schwartz et al. 1997b). This is the final plastid-localized step in ABA biosynthesis, and is rate limiting (Finkelstein 2013). In the cytosol, xanthoxin is first converted to abscisic aldehyde by xanthoxin dehydrogenase (XanDH) (Gonzalez-Guzman et al. 2002). Subsequently, abscisic aldehyde is oxidized to ABA catalyzed by abscisic aldehyde oxidase (AAO), a molybdoenzyme (Seo et al. 2000). Loss of function of ZEP, NCED, XanDH or AAO resulted in severe PHS phenotypes in various crops (Agrawal et al. 2001; Gonzalez-Guzman et al. 2002; Liu et al. 2019; Schwartz et al. 1997b, 2003).
There are five types of molybdoenzymes in plants, including sulfite oxidase (SO), nitrate reductase (NR), mitochondrial amidoxime reducing component (mARC), aldehyde oxidase (AO), and xanthine dehydrogenase (XDH) (Kaufholdt et al. 2017). SO, NR and mARC require molybdenum cofactor (Moco) for their activity, while AO and XDH require sulfurized Moco (Kaufholdt et al. 2017). The Moco biosynthetic pathway is conserved across eukaryotic kingdoms. In plants, the de novo synthesis of Moco begins with the cyclization of GTP to form a cyclopyranoperin monophosphate (cPMP). This process is catalyzed by Cofactor for Nitrate reductase and Xanthine dehydrogenase (CNX) 2 and CNX3 (Hoff et al. 1995). The resulting cPMP is then sulfurized to form molybdopterin (MPT) by CNX5, CNX6 and CNX7 (Matthies et al. 2004; Suzuki et al. 2006). Finally, the MPT is modified by inserting a molybdenum molecule to form Moco (Stallmeyer et al. 1995). Moco can be further sulfurized by Moco sulfurase (Bittner et al. 2001). The resulting sulfurized Moco is necessary for ABA biosynthesis. Therefore, defects in Moco biosynthesis reduce ABA level, resulting in reduced seed dormancy and severe PHS phenotypes in crops. For instance, rice mutants of OsCNX6, OsCNX1 as well as maize mutant ZmCNX1, ZmCNX7 exhibited obvious PHS (Liu et al. 2019; Porch et al. 2006; Suzuki et al. 2006, 2015).
In Arabidopsis, Moco sulfurase is encoded by abscisic acid insensitive 3 (AtABA3) (Bittner et al. 2001). The ataba3 mutants were impaired in converting ABA-aldehyde to ABA, and exhibited typical ABA-deficiency phenotypes including reduced seed dormancy and excessive water loss (Leon-Kloosterziel et al. 1996; Schwartz et al. 1997a). In addition, the ataba3 mutants were found to be compromised in plant tolerance to freezing, salt, and drought stress (Llorente et al. 2000; Xiong et al. 2001). Heterologous expression of AtABA3 in crops such as tobacco, cotton, soybean, maize, and rice, have been shown to be significantly improve plant drought tolerance (Li et al. 2013; Lu et al. 2013; Yue et al. 2011, 2012). In response to drought stress, OsABA3 expression is activated in rice roots (Huang et al. 2009). However, the functions of OsABA3 in rice remain unclear.
In the present study, we revealed that the null mutants of OsABA3 exhibited severe PHS, impaired Moco sulfuration and seedling lethality. However, overexpression of OsABA3 in rice significantly increased the activities of sulfurated Moco-dependent enzymes and, improved plant resistance to PHS, osmotic stress, and bacterial blight. In addition, plants overexpressing OsABA3 exhibited an earlier flowering time without any penalty in grain yield. Our findings uncovered the underlying mechanism of OsABA3 in PHS resistance, and highlighted the functions of OsABA3 in plant development and stress resistance.
Results
Identification and Characterization of the phssl Mutant
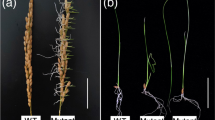
To investigate the underlying mechanism of rice PHS, we screened for severe PHS mutants from an EMS mutant library in paddy fields, and identified nine PHS mutants (Hao-ling et al. 2020). One of the mutants later named as preharvest sprouting and seedling lethal (phssl/+), exhibited normal vegetative and reproductive growth (Fig. 1A), but a severe PHS phenotype (23.60% PHS rate of the mutant plants versus 2.5% of that of the wild type) in paddy fields under high humidity and temperature conditions (Fig. 1B, E). To assess whether seed dormancy is impaired in the phssl/+ mutant, mature panicles were freshly collected from the phssl/+ mutant and the wild-type Huanghuazhan (HHZ), and placed on wet filter paper at 30℃ for 2 days. The rate of germinated seeds per panicle of phssl/+ was 23.58%, which was much higher than that of HHZ (0%) (Fig. 1C, F). Intriguingly, the quickly germinated progeny of phssl/+ showed twisted and slender leaves, and died at the seedling stage of four to five leaves (Fig. 1D). The statistical analysis of a representative population of phssl/+ mutant progeny showed that the segregation ratio of dead seedlings to normal seedlings was consistent with the expected ratio of 1:3 for a single recessive mutation (109:334, χ23:1 = 0.037 < χ20.05,1 = 3.84), indicating that the phenotypes of phssl were caused by a single recessive gene.
Phenotypes of the phssl/+ mutant. (A) The wild-type HHZ and phssl/+ plants. (B) Preharvest sprouting (PHS) phenotypes of the phssl/+ mutant in paddy fields. The magnified views of panicles from the HHZ and phssl/+ mutant are shown on the top right of the graphs, and germinated seeds are marked with red arrow. (C) Germination phenotypes of seeds in freshly collected mature panicles of the HHZ and phssl/+ mutant after 4 days imbibition in water. (D) Phenotypes of the progeny of phssl/+. (E-F) PHS rates of the HHZ and phssl/+ mutant in (B) and (C), respectively. Data represent means ± SD (n > 11), student t-Test, ** represents p < 0.01. Scale bars, 10 cm (A), and 4 cm (C-D)
Molecular Cloning of the Responsible Gene for phssl Mutant
To identify the candidate gene which was responsible for the phenotypes of phssl/+, genomic DNA was extracted from 30 quickly germinated seedlings of phssl/+ progeny, and bulk-sequencing was performed. The resulting sequence data was analyzed by the simultaneous identification of multiple causal mutations (SIMM) method (Yan et al. 2016). Five Single Nucleotide Polymorphisms (SNPs) were identified as candidate mutation sites. Genetic linkage analysis showed that all plants carrying the homozygous candidate site V were lethal (Table S1), suggesting that the site V may be the causal mutation. Site V was located in the 14th exon of LOC_Os06g45860, and caused a missense mutation from TCC (Ser482) to CCC (Pro482) (Fig. 2A). LOC_Os06g45860 is a homolog of Arabidopsis AtABA3, which encodes a molybdenum cofactor sulfurase, and was therefore previously named OsABA3 (Zhang et al. 2019). To confirm that the phenotypes of phssl were resulted from the mutation of OsABA3, mutants of OsABA3 were generated by CRISPR-Cas9 assay with two independent targets in japonica variety Wuyungeng 7 (WYG) (Fig. 2A). A total of 34 independent T0 mutant lines with various genotypes were obtained. Interestingly, the homozygous OsABA3 null mutants exhibited phenotypes similar to the homozygous phssl mutant, with twisted and slender leaves, and early lethality (Fig. 2B-E). Two transgenic-free heterozygous mutant lines, ko1/+ and ko2/+ (Fig. 2D-E), were identified and used for further studies. The homozygous progeny of ko mutants also exhibited a seedling lethal phenotype (Fig. S1). In addition, a construct harboring the wild-type CDS of OsABA3 driven by a ubiquitin promoter was introduced into the ko1/+ mutant, and the homozygous transgenic plants carrying the homozygous ko1 allele were identified and named COM/ko1. The resulting COM/ko1 transgenic plants showed normal plant growth and enhanced PHS resistance, effectively complementing the ko1 mutant phenotypes (Fig. 2F, Fig. S2). Taken together, these data reveled that the mutation of OsABA3 was responsible for the phenotypes of phssl.
Characterization of the knock out (ko) mutants of OsABA3, and the transgenic complementation of the null mutant ko1. (A) The gene structure of OsABA3 is displayed with the mutation site of phssl and target sites of CRISPR-Cas9. (B-C) The seeding phenotypes of T0 mutants of OsABA3 edited by CRISPR-Cas9 at target 1 site (B) and target 2 site (C). The genotypes of mutants in (B) and (C) are displayed in (D) and (E), respectively. (F) Transgenic complementation of ko1 with OsABA3 or OsABA3S482A driven by Ubiquitin promoter. (G) The expression of OsABA3 and OsABA3S482A in the corresponding plants analyzed by western blot. Scale bars, 4 cm (B-C), and 10 cm (F)
Protein Sequence and Phylogenetic Analysis of OsABA3
A typical ABA3 protein contains two conserved domains including the N-terminal NifS-like domain and the ABA3 C-terminal (ABA3-CT) domain (Bittner et al. 2001; Xiong et al. 2001). The NifS-like domain catalyzes the decomposition of L-cysteine to elemental sulfur to form a protein-bound persulfide intermediate (Heidenreich et al. 2005). The ABA3-CT domain may act as a scaffold, facilitating the sulfuration of prebound Moco and mediating the interaction between ABA3 and the target enzymes (Wollers et al. 2008). A homology search of OsABA3 in the public genomic databases Phytozome and NCBI revealed that ABA3 is present in almost all plant species, except for some unicellular algae such as Micromonas pusilla, Chromochloris zofingiensis, Ostreococcus lucimarinus and so on. Phylogenetic analysis showed that ABA3 proteins can be classified into three groups: spermatophytes, chlorophytes, and the group comprising bryophytes and pteridophytes (Fig. S3A). As the plant hormone ABA is critical for drought resistance in land plants, and ABA3 is necessary for ABA de novo biosynthesis (Finkelstein 2013), ABA3 may have played an important role in the plant evolutionary transition from aquatic to terrestrial life. Multiple sequence analysis of ABA3 homologs from 20 represented plant species revealed that ABA3 proteins are highly conserved, especially in higher plants (Fig. S3B). Several amino acid residues of AtABA3 were reported to be essential for its function. The Cys430 residue of AtABA3 was critical for substrate binding and persulfide formation (Heidenreich et al. 2005; Lehrke et al. 2012), and the Arg732 residue might be involved in the binding of sulfurated Moco (Wollers et al. 2008). Both of them were highly conserved in ABA3 proteins. Additionally, heterologous expression of AtABA3 fully rescued the seedling lethal phenotype of ko1 mutant (Fig. S5), implying that AtABA3 could mimic the function of OsABA3 in rice.
Intriguingly, the Ser482 residue of OsABA3, which was mutated to proline residue in the phssl mutant, is absolutely conserved in ABA3 proteins across species (Fig. S3B). Proline is a peculiar cyclic imino acid. The pyrrolidine ring of proline makes the C-N bond more rigid and less prone to rotation, thus reducing the flexibility of the peptide backbone. Given that the S482P mutation of OsABA3 is lethal for rice plants, it is possible that the S482P mutation of OsABA3 reduces the affinity of OsABA3 for its potential interactors. It has been proposed that Alanine, the simplest chiral amino acid that retains the beta carbon but no other side-chain chemistry, provides flexibility to the peptide backbone (Yu et al. 2012). To test the hypothesis, we introduced a CDS of OsABA3 with the codon change for S482A mutation driven by the maize Ubiquitin promoter into the ko1/+ mutant. The homozygous transgenic plants carrying homozygous ko1 allele were identified and named COM-S482A/ko1. The COM-S482A/ko1 plants grew as well as the wild-type plants (Fig. 2F), and exhibited comparable PHS resistance to COM/ko1 plants (Fig. S2), indicating that OsABA3S482A retains most, if not all, of its normal biological function. These data suggested that appropriate flexibility at residue 482 is required for OsABA3 function.
Gene Expression and Protein Subcellular Localization Analyses of OsABA3
To examine the expression pattern of OsABA3 in rice, total RNA was extracted from seedling shoots, seedling roots, mature plant roots, culm leaf sheaths, leaf blades, young panicles and seeds at different developmental stages, and RT-qPCR assay was performed. The results showed that OsABA3 was expressed in all tested tissues (Fig. S4A). ABA3 proteins were presumably localized in cytosol (Selles et al. 2022). Consistently, transient expression of OsABA3-GFP in rice protoplasts showed the GFP fluorescence signal in the cytosol (Fig. S4B), indicating that OsABA3 is a cytosolic protein.
The Role of OsABA3 in Rice PHS Resistance
Following pollination, the embryo undergoes development, maturation and dormancy. In rice, the embryo enters into dormancy approximately 21 days after pollination (Itoh et al. 2005). A weak seed dormancy is a major cause of PHS. To precisely analyze the seed dormancy in phssl/+ mutant, the panicles from HHZ and phssl/+ were harvested at the committed DAP. Seed germination rates were then examined under high humidity conditions for 3 days. The PHS rate of phssl/+ was much higher than that of HHZ (Fig. 3A, C-F), indicating an impaired seed dormancy in phssl/+.
Analysis of seed dormancy of the phssl/+ mutant. (A, C-F) Panicles from HHZ and phssl/+ mutant were collected at 21, 22, 25 and 30 DAP, and were imbibed in water for 3 days. The PHS phenotypes and rates are shown in (A) and (C-F), respectively. Data represent means ± SD (n = 4), student t-Test, ** represents p < 0.01. (B, G) The 22 DAP panicles from HHZ and phssl/+ mutant were treated with or without 10 µM ABA in water for 6 days. (B) The germination phenotypes after 3 days treatment. (G) Time course of germination rate for (B). (H) Comparison of the aldehyde oxidase (AO) and xanthine dehydrogenase (XDH) activities in HHZ and phssl mutant. The quantitative activity was calculated relative to the corresponding enzyme activity in wild-type plants (I-J). The large subunit of rubisco protein stained by Coomassie brilliant blue was used as a loading control. Scale bars = 3 cm in (A-B)
Previous reports showed that AtABA3 encodes a Moco sulfurase enzyme that is required for the activation of AO and XDH in Arabidopsis (Bittner et al. 2001; Schwartz et al. 1997a). OsABA3 shares 60% similarity with AtABA3 in protein sequence. Furthermore, the exogenous expression of AtABA3 in the ko1 mutant effectively restored its phenotypes (Fig. S5), suggesting a conserved function between OsABA3 and AtABA3. Consistent with this hypothesis, we observed that the activities of AO and XDH were almost abolished in the phssl mutant, whereas they were clearly present in WT plants (Fig. 3H-J). Since the AO activity is required for ABA biosynthesis, we tested whether exogenous application of ABA could restore the phenotype of phssl/+ mutant. The panicles from HHZ and phssl/+ at 22 DAP were collected and imbibed with or without 10 µM ABA. The results showed that the application of ABA obviously inhibited the seed germination of phssl/+ mutant (Fig. 3B, G).
During the rice harvest season in June, Guangzhou experiences hot and humid weather, which often leads to occurrences of rice PHS. In the paddy field, the OsABA3 overexpressing plants OE-1 and OE-2 exhibited more resistance to PHS (Fig. 4A, C), while the heterozygous mutants ko1/+ and ko2/+ showed more severe PHS phenotypes (Fig. 4A, C). To assess the impact of OsABA3 on seed dormancy, seeds on panicles were freshly collected from WYG, ko1/+, ko2/+, OE-1 and OE-2 at 31–35 DAP, and a germination experiment was carried out. The seeds of ko mutants germinated more quickly than that of WYG (Fig. 4B, D), whereas OE-1 and OE-2 exhibited much lower PHS rates than WYG (Fig. 4B, D). These results suggested that OsABA3 plays a critical in rice seed dormancy and resistance to PHS.
PHS phenotypes of ko mutants and OE plants. (A) The wild-type WYG and ko1/+, ko2/+, OE-1 and OE-2 plants. Panicles with PHS phenotypes are shown in the enlarged views on the top right of the images. (B) Germination phenotypes of seeds collected at 31–35 DAP from WYG and ko1/+, ko2/+, OE-1 and OE-2 plants after 3 days imbibition on the wet filter papers. (C) PHS rates for (A). Data represent means ± SD (n > 8), and significant differences (p < 0.05) are indicated by different letters (one-way ANOVA with Bonferroni–Holm post hoc test). (D) Time course of seed germination rates for (B). Scale bars = 10 cm in (A)
OsABA3 is Involved in Regulating the Activities of Moco-Dependent Enzymes
To further analyze the function of OsABA3 in Moco biosynthesis, we examined the activities of Moco-dependent enzymes in the ko and OE plants. AO and XDH require sulfurated Moco for their activity. We observed that the activities of AO and XDH were almost abolished in the ko1 and ko2 plants (Fig. 5A-C), while in the OsABA3/ko1 and AtABA3/ko1 plants, these activities were fully recovered (Fig. S6). Moreover, both OE-1 and OE-2 plants exhibited increased activities of AO and XDH (Fig. 5D-F). The results indicated that OsABA3 is essential in regulating the level of sulfurated Moco in rice. Unlike AO and XDH, NR and SO require Moco for their activities. Surprisingly, the activities of NR and SO were significantly reduced in the OE-1 and OE-2 plants (Fig. 5G-H), implying a decrease in Moco levels in these plants. Collectively, these results suggested that OsABA3 is vital for maintaining Moco homeostasis, thereby affecting the activities of Moco-dependent enzymes.
The activities of Moco-dependent enzymes in the ko and OE plants. (A-F) For analyzes of the AO and XDH activities, native PAGE assays were carried out. The quantitative activity was calculated relative to the corresponding enzyme activity in wild-type plants. The large subunit of rubisco protein stained by Coomassie brilliant blue was used as a loading control. (A-C) The activities of AO and XDH in the wild-type WYG and ko mutants. (D-F) The activities of AO and XDH in the WYG and OE plants. (G-H) The activities of nitrate reductase (NR) and sulfite oxidase (SO) in the seedlings of WYG and OE plants, respectively. In (E, F, G), Data represent means ± SD (n = 3), and significant differences (p < 0.05) are indicated by different letters (one-way ANOVA with Bonferroni–Holm post hoc test)
Overexpression of OsABA3 Improves Plant Resistance to Drought and Bacterial Blight
Previous reports have demonstrated that overexpression of AtABA3 in rice, tobacco, cotton, maize and soybean significantly enhanced plant drought tolerance (Li et al. 2013; Lu et al. 2013; Xiao et al. 2009; Yue et al. 2011, 2012). To assess the role of rice OsABA3 in drought resistance, we first analyzed the performance of the OsABA3 OE plants under osmotic stress by polyethylene glycol (PEG) treatment. We observed that the survival rates of OsABA3-OE plants were > 55%, much higher than 22% of the wild-type plants after 7 days of recovery (Fig. 6A-B). We next tested the tolerance of OsABA3 OE plants under drought stress. The seedlings of WT, OE-1 and OE-2 were planted in the same spots, and subjected to drought treatment. After 7 days of water cutoff and 7 days recovery, the seedlings survival rates were calculated. More than 65% seedlings of OE-1 and OE-2 were alive, whereas only 39% WT seedlings remained alive (Fig. 6C-D), indicating that overexpression of OsABA3 in rice improves plant drought tolerance.
The phenotypes of OE plants under osmotic and biotic stresses. (A) Phenotypes of WYG and OE plants after 20% PEG6000 treatment for 7 days plus 7 days recovery. The seedling survival rates are displayed in (B). (C) Phenotypes of WYG and OE plants after drought treatment for 9 days plus 9 days recovery. The seedling survival rates are shown in (D). (E-F) The lesion mimic phenotype of old leaves of OE plants at heading stage. (E) The representative leaf sections of OE plants with brown lesions. (F) Dead cells in leaves stained by trypan blue. (G) H2O2 accumulation in leaves detected by DAB staining. (H) Phenotypes of WYG and OE plants after inoculation with Xoo for 15 days. (I) Statistic analysis of the lesion length. FL: the flag leaf; SL: the second leaf. (J) The expression of PR genes in the leaves from WYG and OE plants at tillering stage. Gene expression of PR1a, PR1b and PR10 analyzed by RT-qPCR. OsUbq5 was used as an internal control. Data represent means ± SD (B and D, N = 3; I, n = 15; J, n = 4), and significant differences (p < 0.05) are indicated by different letters (one-way ANOVA with Bonferroni–Holm post hoc test). Scale bars, 3 cm (A, C), and 1 cm (E-G)
The OE-1 and OE-2 plants appeared normal during vegetative growth. However, during reproductive stage, dark brown lesions were observed in the apical part of older leaves of OE-1 and OE-2 plants (Fig. 6E). Cytological analyzes revealed a reactive oxygen species (ROS) burst and a large number of dead cells were observed in the old leaves of OE plants (Fig. 6F-G). As spontaneous cell death might be associated with plant biotic resistance, we examined the resistance of OE plants to Xanthomonas oryzae pv. oryzae (Xoo) (Fig. 6H-I). The OE plants exhibited much shorter lesions compared to that of wild-type WYG plants. Pathogenesis-related (PR) proteins are very important for plant resistance to pathogen infection. The representative PR genes PR1a, PR1b and PR10 were pronouncedly autoactivated in OE plants (Fig. 6J). These results suggested that overexpression of OsABA3 enhances plant resistance to Xoo.
Overexpression of OsABA3 Promotes Early Flowering But Does Not Impact Grain Yield Under Normal Conditions
To investigate the effects of OsABA3 overexpression on grain yield in rice, the OE plants and the wild-type WYG were grown in the paddy fields in Guangzhou (23.13 N, 113.26E) under regular care. The agronomic traits were examined. The OE plants showed similar appearances to the wild-type in terms of plant height, number of panicles per plant, grain number per panicle, seed setting rate, grain length, grain width, grain weight and grain yield per plant (Fig. 7A-I), indicating that overexpression of OsABA3 did not affect grain yield under normal conditions. Interestingly, the OE-1 and OE-2 plants flowered 87.6 ± 3.1 and 85.4 ± 1.6 day after sowing (DAS) respectively, which were more than 7 days earlier than WYG (94.9 ± 3.2) under natural short-day conditions (Fig. 7J). We also test the agronomic performances of OE plants in the paddy fields in Shenzhen (22.73 N, 113.95E), and similar results were observed (Fig. S7). Collectively, these finding demonstrated that the OE plants exhibited earlier flowering without any reduction in grain yield.
The performances of OE plants in paddy fields. The wild-type plants (WYG) and OE plants were grown in paddy fields in Guangzhou (23.13 N, 113.26E), China. (A) Representative panicles of WYG and OE plants. (B) Comparison of the grain size between WYG and OE plants. (C-I) Comparison of the agronomic traits corresponding to grain yield between WYG and OE plants. (C) Grain length. (D) Grain width. (E) 1000-grain weight. (F) Grain number per panicle. (G) Seed setting rate. (H) Number of panicles per plant. (I) Grain yield per plant. (J) Heading date. Data represent means ± SD (C-I, n = 20; J, n = 48), and significant differences (p < 0.05) are indicated by different letters (one-way ANOVA with Bonferroni–Holm post hoc test). Scale bars, 2 cm (A) and 5 mm (B)
Discussion
Appropriate seed dormancy is essential for seed crop production. A weak seed dormancy may increase the risk of PHS, especially in the regions with high temperature and humidity (Tai et al. 2021). ABA is considered as the core regulator of seed dormancy. The mutants with impaired ABA biosynthesis exhibited significantly reduced seed dormancy in various plant species (Ali et al. 2022). The final step in the de novo ABA biosynthesis pathway is catalyzed by AAO, which requires sulfurated Moco for its activity. The molybdenum cofactor sulfurases are encoded by ABA3 family genes in plants (Finkelstein 2013). The ataba3 mutants were deficient in ABA biosynthesis, displayed pleiotropic phenotypes including loss of seed dormancy, withering under low relative humidity conditions, and susceptibility to cold stress in Arabidopsis (Leon-Kloosterziel et al. 1996; Llorente et al. 2000; Sagi et al. 2002; Xiong et al. 2001). The present study showed that the heterozygous mutants of OsABA3 exhibited more severe PHS in paddy fields (Figs. 1B and 4A). Biochemical analyzes revealed that the AO activities were barely detected in the homozygous mutants (Figs. 3H and 5A), indicating that ABA biosynthesis was interrupted. Furthermore, OsABA3 overexpression enhanced plant resistance to PHS (Fig. 4A). These findings suggest that OsABA3 is essential for seed dormancy and PHS resistance in rice.
The ABA present in developing seeds originates from both maternal and zygotic tissues. Maternal ABA and zygote-derived ABA are both critical for seed development (Frey et al. 2004; Karssen et al. 1983; Qin et al. 2021). However, reciprocal crosses of ABA-deficient mutants and wild-type plants have shown that zygote-derived ABA is predominant for the onset of seed dormancy in Arabidopsis (Kanno et al. 2010; Karssen et al. 1983). The more severe PHS phenotype observed in the heterozygous mutants of OsABA3 indicated that the zygote-derived ABA is also necessary for seed dormancy in rice.
The ABA-deficient mutants typically exhibited severely stunted growth, reduced seed dormancy, and a wilted phenotype in Arabidopsis (Finkelstein 2013). ABA2 encodes an enzyme that catalyze the production of abscisic aldehyde. The ataba2 mutants produced seeds with reduced dormancy and much smaller adult plants with a wilty phenotype under desiccating conditions in Arabidopsis (Leon-Kloosterziel et al. 1996; Nambara et al. 1998). Consistently, the osaba2 mutants showed loss of seed dormancy and sever PHS under wet and high temperature conditions in rice. However, they also displayed species-specific phenotypes including enhanced growth of leaves and stems, and spontaneous cell death on mature leaves (Liao et al. 2018). The null mutants of AtABA3 were viable in Arabidopsis (Leon-Kloosterziel et al. 1996; Xiong et al. 2001). However, our present work showed that the null mutants of OsABA3 were lethal at the seedling stage in rice (Fig. 1D, Fig. S1), indicating ABA3 orthologs may evolved species-specific functions in Arabidopsis and rice. Recently, it was reported that AtABA3 coupled with the sulfur transferase 18 (STR18), represents a new pathway of cytosol sulfur trafficking in Arabidopsis (Selles et al. 2022). Therefore, OsABA3 may have other uncharacterized functions in rice that are essential for rice plant survival.
The activities of molybdoenzymes are coordinated with the levels of Moco or sulfurated Moco in cells. Disruption of the de novo biosynthesis of Moco led a significant decrease in molybdoenzymes activities, including AO, XDH, NR and SO (Liu et al. 2019). ABA3 encodes a Moco sulfurase, which is required for the formation of sulfurated Moco. In our present work, we found that OsABA3 is critical for maintaining Moco homeostasis in rice. We observed that the activities of AO and XDH were barely detected in the null mutants of OsABA3 (Figs. 3H and 5A), but were significantly increased in the OsABA3 overexpressing plants (Fig. 5D). These findings suggest that OsABA3 is indispensable for Moco sulfuration. Interestingly, the NR and SO activities were both decreased in the OsABA3 overexpressing plants (Fig. 5G-H). Moreover, OsABA3 overexpressing plants exhibited enhanced tolerance to osmotic stress (Fig. 6a-d). It was reported that OsABA3 expression was significantly upregulated in response to drought stress (Huang et al. 2009; Kim et al. 2021). In contrast, the NR activity was significantly decreased in rice seedlings subjected to osmotic stress with a rich nitrate supply (Han et al. 2022). These results take the hypothesis that upregulated OsABA3 may enhance Moco sulfuration and thus result in an increase of sulfurated Moco and a decrease of Moco content. This alteration may directly affect the activities of Moco-dependent enzymes including AO and NR, and contribute to plant adaptation to drought stress by activating ABA biosynthesis and inhibiting nitrogen assimilation, respectively. However, further experimental evidence is required to test this hypothesis.
ABA is a positive regulator in plant response to drought stress (Wilkinson and Davies 2002). Drought stress dramatically induces ABA biosynthesis that leads to accumulation of ABA, and then promotes stomatal closure to reduce water loss and activates the expression of drought tolerant genes. Overexpression of ABA biosynthesis genes has been shown to effectively improve plant drought tolerance. As ABA3 is a vital enzyme for ABA biosynthesis, lots of studies have been conducted to test the potential use of ABA3 in improving plant drought tolerance in rice, tobacco, cotton, maize and bean (Li et al. 2013; Lu et al. 2013; Xiao et al. 2009; Yue et al. 2011, 2012). All of these studies showed that overexpression of AtABA3 significantly increased plant fitness under drought stress. It is worth to note that the AtABA3 overexpressing plants appeared similar to wild-type plants under well-watered conditions. Overexpression of OsABA3 in rice enhanced plant resistance to bacterial blight (Liu et al. 2022). Here, we showed that the OsABA3 overexpressing plants displayed enhanced resistance to PHS and bacterial blight (Fig. 6H-I), and early flowering (Fig. 7J). Flowering time, also known as heading date, is critical for rice adaptation and production (Zhou et al. 2020). Earlier flowering leads to a shorter rice growth duration, which facilitates rice production and maximizes the use of field resources. However, early flowering time often results in a reduction in grain yield (Fang et al. 2019). The OsABA3 overexpressing plants flowered earlier but exhibited normal grain yield under well-watered conditions (Fig. 7, Fig. S7). Collectively, these findings suggested that overexpression of OsABA3 may be an effective strategy for simultaneously improving multiple agronomic traits in rice. Recently, the CRISPR-Cas9 genome editing assay was successfully employed to knock-up gene expression in rice by targeting cis elements in the promoter or inducing genomic inversion or duplication (Lu et al. 2021; Tan et al. 2023). Therefore, it is plausible to generate the transgene-free OsABA3 overexpressing plants by genome editing for rice breeding in the future.
In summary, this study demonstrated that OsABA3 is critical for Moco biosynthesis and homeostasis, and essential for plant survival and seed dormancy in rice. Notably, overexpression of OsABA3 significantly improved plant resistance to PHS, osmotic stress and bacterial blight, and promoted plant flowering without any reduction in grain yield, suggesting that OsABA3 is a promising candidate gene for rice breeding.
Materials and methods
Plant Materials
The phssl mutant was identified from an ethyl methanesulfonate (EMS) mutant library of the indica cv. Huanghuazhan (HHZ). The wild-type japonica cv. Wuyungeng 7 (WYG) was used for transgenic analysis. All plants were grown in paddy fields with regular care.
Molecular Cloning of the Mutant Gene of phssl
Genomic DNA was extracted from 30 seedlings of the progeny of phssl/+ plants that had germinated quickly. The resulting DNA was used for bulk-sequencing. The sequence data was analyzed using the simultaneous identification of multiple causal mutations (SIMM) method (Yan et al. 2016). The high resolution melt (HRM) method was employed to examine the mutation sites in the progeny of phssl/+ plant by PCR with specific primers shown in Table S2 (Lochlainn et al. 2011).
Plasmid Construction and Rice Transformation
The null mutants of OsABA3 in the WYG background were generated by using the CRISPR/Cas9 method as previously described (Ma et al. 2015), Briefly, two guide RNAs with different target sequences on OsABA3 were designed and cloned into pYLCRISPR/Cas9. The resulting constructs were introduced to WYG by Agrobacterium-mediated rice transformation (Toki et al. 2006).
To generate OsABA3 overexpressing plants, the OsABA3 coding sequence driven by a ubiquitin promoter was cloned into pCAMBIA1300 using the InFusion HD cloning kit (TaKara, Dalian, China). The final construct was introduced into the Agrobacterium tumefaciens AG10 strain for rice transformation.
To generate the COM/ko1 and COM-S482A/ko1 plants, the pUBQ: OsABA3 and pUBQ: OsABA3-S482A expression cassettes were introduced into the progeny of the heterozygous mutant ko1/+ by Agrobacterium-mediated rice transformation, respectively. The T2 homozygous transgenic plants carrying the homozygous ko1 allele were identified by PCR and HRM genotyping and used for further studies.
Phylogenetic Analysis
The homologs of OsABA3 were identified from the genome database Phytozome (https://phytozome-next.jgi.doe.gov/) and NCBI (https://www.ncbi.nlm.nih.gov/) by using the BlastP method (Goodstein et al. 2012). Sixteen ABA3 proteins from the representative plant species were selected for further analysis. The ABA3 protein sequences from Volvox carteri (XP_002950063.1), Coccomyxa subellipsoidea (19,457), Physcomitrium patens (Pp3c5_23340V3.1), Sphagnum fallax (Sphfalx14G015300.1), Ceratopteris richardii (Ceric.02G066600.3), Diphasiastrum complanatum (Dicom.12G016300.1), Selaginella moellendorffii (173,762), Manihot esculenta (Manes.06G173000.1), Citrus clementina (Ciclev10004324m), Gossypium raimondii (Gorai.002G036800.1), Arabidopsis thaliana (AT1G16540.1), Medicago truncatula (Medtr4g030930.1), Solanum lycopersicum (Solyc07T002813.2), Ananas comosus (Aco008369.1), Oryza sativa (LOC_Os06g45860.1), Triticum aestivum (Traes_7DL_162505318.2), Brachypodium distachyon (Bradi1g32350.5), Panicum hallii (Pahal.D00546.2), Zea mays (Zm00001d036635_P001), and Sorghum bicolor (Sobic.010G224000.3) were aligned by using ClustalX with default parameters. The phylogenetic tree was constructed using the Neighbor-Joining algorithm (1,000 replicates) in MEGA11.
RNA Extraction and RT-qPCR Assay
Total RNA was extracted from various rice tissues using the Plant RNA Kit R6827 (Omega Bio-Tek, Santa Clara, CA, USA), and reverse-transcribed using a PrimeScript RT reagent kit (TaKaRa, Dalian, China). RT-qPCR was performed using SYBR Premix Ex Taq II (TaKaRa, Dalian, China) on a Lightcycler instrument (Roche, Germany).
Subcellular Protein Localization
The OsABA3 coding sequence was amplified from cDNA of WYG using the specific primers and was cloned into pAN580. The resulting plasmid pAN580-OsABA3, carrying the expression cassette 35 S: OsABA3-EGFP was introduced into rice protoplasts using a polyethylene glycol (PEG)-mediated transformation assay. The fluorescence signal was examined and photographed by a confocal laser scanning microscope (LSM 800, Zeiss, Germany).
Assays of XDH and AO Activities
The activities of AO and XDH were analyzed as previously described (Liu et al. 2019). Briefly, 0.2 g of fresh seedling shoots or plant leaves per sample were collected and ground to a powder in liquid nitrogen. The powder was thawed in 1 ml of the extraction buffer (0.1 M potassium phosphate pH 7.5, 1 mM EDTA, 10 mM 2-hydroxy-1-ethanethiol, 1% Triton X-100), and centrifuged at 16,000 g for 30 min at 4 °C. 15 µL of the resulting supernatant was loaded onto 6.5% polyacrylamide gel and subjected to native polyacrylamide gel electrophoresis at 4 °C for 4 h (50 V, 20 mA). Following electrophoresis, the gel was equilibrated in 0.1 M sodium pyrophosphate (pH 8.0) and subsequently stained in AO buffer (0.1 M Tris-HCl pH 8.5, 1 mM indole-3-aldehyde, 1 mM thiazolyl blue tetrazolium bromide, 0.1 mM phenazine methosulfate) for 30 min at room temperature in the dark. For XDH assay, the gel was stained in XDH buffer (0.1 M sodium pyrophosphate, pH 8.0, 2 mM hypoxanthine, 1 mM MTT, 0.1 mM phenazine methosulfate) for 30 min (at room temperature in the dark). After destaining with water, the gel was imaged and analyzed using ImageJ software (http://imagej.nih.gov/ij/).
Assays of NR and SO Activities
The NR activity in rice was analyzed following previously described method (Liu et al. 2019). Briefly, 0.2 g of homogenized sample powder was thawed in 2 ml of NR extraction buffer (25 mM phosphate buffer pH 7.5; 5 mM cysteine; 5 mM EDTA) and centrifuged at 16,000 g for 10 min at 4 °C. Subsequently, 20 µL of the resulting supernatant was mixed with 80 µL of assay buffer (60 µL of 0.2 M KNO3 phosphate buffer; 20 µL of 2.0 mg/ml NADH) and incubated at 30 °C for 30 min. The mixture was subjected to color development by adding 50 µL of stop solution (1% 4-aminobenzene sulfonic acid) and 50 µL of reaction buffer (0.2% 1-naphthylamine) at 30 °C for 30 min in the dark. The absorbance of the sample was immediately measured at 540 nm using a spectrophotometer (Enspire, PerkinElmer). NR activity was determined as the conversion of NO2− per gram of fresh weight per hour.
For the analysis of SO activity, a homogenized sample of 0.2 g was thawed in 1 ml of SO extraction buffer (0.1 M HEPES, 1 mM EDTA, 5% glycerol, 1 mM Na2MoO4, 1 mM phenylmethylsulfonylfluoride, pH 7.3) and centrifuged at 16,000 g at 4 °C for 30 min. 20 µL of the supernatant was mixed with 180 µL of assay buffer (20 mM Tris–acetic acid pH 8.0, 0.1 mM EDTA, 395 µM potassium ferricyanide, 400 µM sodium sulphite). The sample’s absorbance was then measured at 420 nm using a spectrophotometer (Enspire, PerkinElmer). The reduction of 2 mol ferricyanide was assumed to be caused by 1 mol sulfite. The rate of sulfite conversion was used to measure SO activity (Liu et al. 2019).
Germination Assays
More than three fresh panicles from each genotype were harvested at 31–35 DAP. The panicles were then immersed in plastic boxes with distilled water and incubated at 30 °C in a growth chamber under a 14 h/10 h light/dark regime for 2 days. The germination rate per panicle was calculated.
To analyze the seed dormancy more precisely, homogeneous seeds from each genotype were freshly harvested at 31–35 DAP. The seeds were placed on wet filter paper in Petri dishes with at least three repeats. The number of germinated seeds was counted daily, and germination rates were calculated.
Histochemical Analysis
Leaves exhibiting lesion mimic spots from the OsABA3 overexpressing plants, and leaves from the same growth stage of the WYG were collected for histochemical analysis. To examine cell death, trypan blue staining was conducted following established procedures (Liao et al. 2018). In brief, the samples were immersed in a lactic acid-phenol-trypan blue solution (2.5 mg/ml trypan blue, 25% (w/v) lactic acid, 23% water-saturated phenol, and 25% glycerol in H2O), vacuumed for 30 min, and boiled for 2 min, followed by destaining with 30% (w/v) chloral hydrate for several times.
For hydrogen peroxide assay, detached leaves were immersed in the staining solution (0.5 mg/ml 3,3’-diaminobenzidine (DAB)) and vacuumed for 30 min. Subsequently, the leaves were incubated at room temperature overnight, followed by decolorization in 95% ethanol at 80 °C in a water bath.
Data Availability
No datasets were generated or analysed during the current study.
References
Agrawal GK, Yamazaki M, Kobayashi M, Hirochika R, Miyao A, Hirochika H (2001) Screening of the rice viviparous mutants generated by endogenous retrotransposon Tos17 insertion. Tagging of a zeaxanthin epoxidase gene and a novel ostatc gene. Plant Physiol 125:1248–1257
Ali F, Qanmber G, Li F, Wang Z (2022) Updated role of ABA in seed maturation, dormancy, and germination. J Adv Res 35:199–214
Bewley JD, Black M, Halmer P (2006) The encyclopedia of seeds: science, technology and uses. CABI Publishing, 528–530.
Bittner F, Oreb M, Mendel RR (2001) ABA3 is a molybdenum cofactor sulfurase required for activation of aldehyde oxidase and xanthine dehydrogenase in Arabidopsis thaliana. J Biol Chem 276:40381–40384
Fang J, Chai C, Qian Q, Li C, Tang J, Sun L, Huang Z, Guo X, Sun C, Liu M, Zhang Y, Lu Q, Wang Y, Lu C, Han B, Chen F, Cheng Z, Chu C (2008) Mutations of genes in synthesis of the carotenoid precursors of ABA lead to pre-harvest sprouting and photo-oxidation in rice. Plant J 54:177–189
Fang J, Zhang F, Wang H, Wang W, Zhao F, Li Z, Sun C, Chen F, Xu F, Chang S, Wu L, Bu Q, Wang P, Xie J, Chen F, Huang X, Zhang Y, Zhu X, Han B, Deng X, Chu C (2019) Ef-cd locus shortens rice maturity duration without yield penalty. Proc Natl Acad Sci U S A 116:18717–18722
Finkelstein R (2013) Abscisic acid synthesis and response. Arabidopsis Book 11:e0166
Frey A, Godin B, Bonnet M, Sotta B, Marion-Poll A (2004) Maternal synthesis of abscisic acid controls seed development and yield in Nicotiana plumbaginifolia. Planta 218:958–964
Gonzalez-Guzman M, Apostolova N, Belles JM, Barrero JM, Piqueras P, Ponce MR, Micol JL, Serrano R, Rodriguez PL (2002) The short-chain alcohol dehydrogenase ABA2 catalyzes the conversion of xanthoxin to abscisic aldehyde. Plant Cell 14:1833–1846
Goodstein DM, Shu S, Howson R, Neupane R, Hayes RD, Fazo J, Mitros T, Dirks W, Hellsten U, Putnam N, Rokhsar DS (2012) Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res 40:D1178–1186
Gubler F, Millar AA, Jacobsen JV (2005) Dormancy release, ABA and pre-harvest sprouting. Curr Opin Plant Biol 8:183–187
Hable WE, Oishi KK, Schumaker KS (1998) Viviparous-5 encodes phytoene desaturase, an enzyme essential for abscisic acid (ABA) accumulation and seed development in maize. Mol Gen Genet 257:167–176
Han ML, Lv QY, Zhang J, Wang T, Zhang CX, Tan RJ, Wang YL, Zhong LY, Gao YQ, Chao ZF, Li QQ, Chen GY, Shi Z, Lin HX, Chao DY (2022) Decreasing nitrogen assimilation under drought stress by suppressing DST-mediated activation of Nitrate reductase 1.2 in rice. Mol Plant 15:167–178
Hao-ling NI, Wen-shi WU, Yan-min YAN, Yi-yuan F, Jia-yin W, Bi-hu C, Zhi-yi LI, Xiao-yan T, Jian-xin WU (2020) Screening and gene mapping of pre-harvest sprouting mutants in Rice. J Plant Genetic Resour 21:1214–1220
Heidenreich T, Wollers S, Mendel RR, Bittner F (2005) Characterization of the NifS-like domain of ABA3 from Arabidopsis thaliana provides insight into the mechanism of molybdenum cofactor sulfuration. J Biol Chem 280:4213–4218
Hoff T, Schnorr KM, Meyer C, Caboche M (1995) Isolation of two Arabidopsis cDNAs involved in early steps of molybdenum cofactor biosynthesis by functional complementation of Escherichia coli mutants. J Biol Chem 270:6100–6107
Huang P-M, Chen J-Y, Wang S-J (2009) Tissue-specific regulation of rice molybdenum cofactor sulfurase gene in response to salt stress and ABA. Acta Physiol Plant 31:545–551
Itoh J, Nonomura K, Ikeda K, Yamaki S, Inukai Y, Yamagishi H, Kitano H, Nagato Y (2005) Rice plant development: from zygote to spikelet. Plant Cell Physiol 46:23–47
Kanno Y, Jikumaru Y, Hanada A, Nambara E, Abrams SR, Kamiya Y, Seo M (2010) Comprehensive hormone profiling in developing Arabidopsis seeds: examination of the site of ABA biosynthesis, ABA transport and hormone interactions. Plant Cell Physiol 51:1988–2001
Karssen CM, Brinkhorst-van der Swan DL, Breekland AE, Koornneef M (1983) Induction of dormancy during seed development by endogenous abscisic acid: studies on abscisic acid deficient genotypes of Arabidopsis thaliana (L.) Heynh. Planta 157:158–165
Kaufholdt D, Baillie CK, Meinen R, Mendel RR, Hansch R (2017) The Molybdenum Cofactor Biosynthesis Network: in vivo protein-protein interactions of an actin Associated Multi-protein Complex. Front Plant Sci 8:1946
Kim S, Park SI, Kwon H, Cho MH, Kim BG, Chung JH, Nam MH, Song JS, Kim KH, Yoon IS (2021) The Rice Abscisic Acid-Responsive RING finger E3 ligase OsRF1 targets OsPP2C09 for degradation and confers Drought and Salinity Tolerance in Rice. Front Plant Sci 12:797940
Lehrke M, Rump S, Heidenreich T, Wissing J, Mendel RR, Bittner F (2012) Identification of persulfide-binding and disulfide-forming cysteine residues in the NifS-like domain of the molybdenum cofactor sulfurase ABA3 by cysteine-scanning mutagenesis. Biochem J 441:823–832
Leon-Kloosterziel KM, Gil MA, Ruijs GJ, Jacobsen SE, Olszewski NE, Schwartz SH, Zeevaart JA, Koornneef M (1996) Isolation and characterization of abscisic acid-deficient Arabidopsis mutants at two new loci. Plant J 10:655–661
Li C, Ni P, Francki M, Hunter A, Zhang Y, Schibeci D, Li H, Tarr A, Wang J, Cakir M, Yu J, Bellgard M, Lance R, Appels R (2004) Genes controlling seed dormancy and pre-harvest sprouting in a rice-wheat-barley comparison. Funct Integr Genomics 4:84–93
Li Y, Zhang J, Zhang J, Hao L, Hua J, Duan L, Zhang M, Li Z (2013) Expression of an Arabidopsis molybdenum cofactor sulphurase gene in soybean enhances drought tolerance and increases yield under field conditions. Plant Biotechnol J 11:747–758
Liao Y, Bai Q, Xu P, Wu T, Guo D, Peng Y, Zhang H, Deng X, Chen X, Luo M, Ali A, Wang W, Wu X (2018) Mutation in Rice Abscisic Acid2 results in cell death, enhanced Disease-Resistance, altered seed dormancy and development. Front Plant Sci 9:405
Liu X, Wang J, Yu Y, Kong L, Liu Y, Liu Z, Li H, Wei P, Liu M, Zhou H, Bu Q, Fang J (2019) Identification and characterization of the rice pre-harvest sprouting mutants involved in molybdenum cofactor biosynthesis. New Phytol 222:275–285
Liu H, Lu C, Li Y, Wu T, Zhang B, Liu B, Feng W, Xu Q, Dong H, He S, Chu Z, Ding X (2022) The bacterial effector AvrRxo1 inhibits vitamin B6 biosynthesis to promote infection in rice. Plant Commun 3:100324
Llorente F, Oliveros JC, Martinez-Zapater JM, Salinas J (2000) A freezing-sensitive mutant of Arabidopsis, frs1, is a new aba3 allele. Planta 211:648–655
Lochlainn SO, Amoah S, Graham NS, Alamer K, Rios JJ, Kurup S, Stoute A, Hammond JP, Ostergaard L, King GJ, White PJ, Broadley MR (2011) High Resolution Melt (HRM) analysis is an efficient tool to genotype EMS mutants in complex crop genomes. Plant Methods 7:43
Lu Y, Li Y, Zhang J, Xiao Y, Yue Y, Duan L, Zhang M, Li Z (2013) Overexpression of Arabidopsis molybdenum cofactor sulfurase gene confers drought tolerance in maize (Zea mays L). PLoS ONE 8:e52126
Lu Y, Wang J, Chen B, Mo S, Lian L, Luo Y, Ding D, Ding Y, Cao Q, Li Y, Li Y, Liu G, Hou Q, Cheng T, Wei J, Zhang Y, Chen G, Song C, Hu Q, Sun S, Fan G, Wang Y, Liu Z, Song B, Zhu JK, Li H, Jiang L (2021) A donor-DNA-free CRISPR/Cas-based approach to gene knock-up in rice. Nat Plants 7:1445–1452
Ma X, Zhang Q, Zhu Q, Liu W, Chen Y, Qiu R, Wang B, Yang Z, Li H, Lin Y, Xie Y, Shen R, Chen S, Wang Z, Chen Y, Guo J, Chen L, Zhao X, Dong Z, Liu YG (2015) A robust CRISPR/Cas9 system for Convenient, High-Efficiency Multiplex Genome Editing in Monocot and Dicot plants. Mol Plant 8:1274–1284
Matthies A, Rajagopalan KV, Mendel RR, Leimkuhler S (2004) Evidence for the physiological role of a rhodanese-like protein for the biosynthesis of the molybdenum cofactor in humans. Proc Natl Acad Sci U S A 101:5946–5951
Nambara E, Kawaide H, Kamiya Y, Naito S (1998) Characterization of an Arabidopsis thaliana mutant that has a defect in ABA accumulation: ABA-dependent and ABA-independent accumulation of free amino acids during dehydration. Plant Cell Physiol 39:853–858
North HM, De Almeida A, Boutin JP, Frey A, To A, Botran L, Sotta B, Marion-Poll A (2007) The Arabidopsis ABA-deficient mutant aba4 demonstrates that the major route for stress-induced ABA accumulation is via neoxanthin isomers. Plant J 50:810–824
Porch TG, Tseung CW, Schmelz EA, Settles AM (2006) The maize Viviparous10/Viviparous13 locus encodes the Cnx1 gene required for molybdenum cofactor biosynthesis. Plant J 45:250–263
Qin P, Zhang G, Hu B, Wu J, Chen W, Ren Z, Liu Y, Xie J, Yuan H, Tu B, Ma B, Wang Y, Ye L, Li L, Xiang C, Li S (2021) Leaf-derived ABA regulates rice seed development via a transporter-mediated and temperature-sensitive mechanism. Sci Adv 7:eabc8873
Rock CD, Zeevaart JA (1991) The aba mutant of Arabidopsis thaliana is impaired in epoxy-carotenoid biosynthesis. Proc Natl Acad Sci U S A 88:7496–7499
Sagi M, Scazzocchio C, Fluhr R (2002) The absence of molybdenum cofactor sulfuration is the primary cause of the flacca phenotype in tomato plants. Plant J 31:305–317
Schwartz SH, Leon-Kloosterziel KM, Koornneef M, Zeevaart JA (1997a) Biochemical characterization of the aba2 and aba3 mutants in Arabidopsis thaliana. Plant Physiol 114:161–166
Schwartz SH, Tan BC, Gage DA, Zeevaart JA, McCarty DR (1997b) Specific oxidative cleavage of carotenoids by VP14 of maize. Science 276:1872–1874
Schwartz SH, Qin X, Zeevaart JA (2003) Elucidation of the indirect pathway of abscisic acid biosynthesis by mutants, genes, and enzymes. Plant Physiol 131:1591–1601
Selles B, Moseler A, Caubriere D, Sun SK, Ziesel M, Dhalleine T, Heriche M, Wirtz M, Rouhier N, Couturier J (2022) The cytosolic Arabidopsis thaliana cysteine desulfurase ABA3 delivers sulfur to the sulfurtransferase STR18. J Biol Chem 298:101749
Seo M, Peeters AJ, Koiwai H, Oritani T, Marion-Poll A, Zeevaart JA, Koornneef M, Kamiya Y, Koshiba T (2000) The Arabidopsis aldehyde oxidase 3 (AAO3) gene product catalyzes the final step in abscisic acid biosynthesis in leaves. Proc Natl Acad Sci U S A 97:12908–12913
Singh M, Lewis PE, Hardeman K, Bai L, Rose JK, Mazourek M, Chomet P, Brutnell TP (2003) Activator mutagenesis of the pink scutellum1/viviparous7 locus of maize. Plant Cell 15:874–884
Stallmeyer B, Nerlich A, Schiemann J, Brinkmann H, Mendel RR (1995) Molybdenum co-factor biosynthesis: the Arabidopsis thaliana cDNA cnx1 encodes a multifunctional two-domain protein homologous to a mammalian neuroprotein, the insect protein cinnamon and three Escherichia coli proteins. Plant J 8:751–762
Sun Z, Gantt E, Cunningham FX Jr. (1996) Cloning and functional analysis of the beta-carotene hydroxylase of Arabidopsis thaliana. J Biol Chem 271:24349–24352
Suzuki M, Settles AM, Tseung CW, Li QB, Latshaw S, Wu S, Porch TG, Schmelz EA, James MG, McCarty DR (2006) The maize viviparous15 locus encodes the molybdopterin synthase small subunit. Plant J 45:264–274
Suzuki Y, Miura K, Shigemune A, Sasahara H, Ohta H, Uehara Y, Ishikawa T, Hamada S, Shirasawa K (2015) Marker-assisted breeding of a LOX-3-null rice line with improved storability and resistance to preharvest sprouting. Theor Appl Genet 128:1421–1430
Tai L, Wang HJ, Xu XJ, Sun WH, Ju L, Liu WT, Li WQ, Sun J, Chen KM (2021) Pre-harvest sprouting in cereals: genetic and biochemical mechanisms. J Exp Bot 72:2857–2876
Tan W, Miao J, Xu B, Zhou C, Wang Y, Gu X, Liang S, Wang B, Chen C, Zhu J, Zuo S, Yang Z, Gong Z, You A, Wu S, Liang G, Zhou Y (2023) Rapid production of novel beneficial alleles for improving rice appearance quality by targeting a regulatory element of SLG7. Plant Biotechnol J 21:1305–1307
Toki S, Hara N, Ono K, Onodera H, Tagiri A, Oka S, Tanaka H (2006) Early infection of scutellum tissue with Agrobacterium allows high-speed transformation of rice. Plant J 47:969–976
Wilkinson S, Davies WJ (2002) ABA-based chemical signalling: the co-ordination of responses to stress in plants. Plant Cell Environ 25:195–210
Wollers S, Heidenreich T, Zarepour M, Zachmann D, Kraft C, Zhao Y, Mendel RR, Bittner F (2008) Binding of sulfurated molybdenum cofactor to the C-terminal domain of ABA3 from Arabidopsis thaliana provides insight into the mechanism of molybdenum cofactor sulfuration. J Biol Chem 283:9642–9650
Xiao BZ, Chen X, Xiang CB, Tang N, Zhang QF, Xiong LZ (2009) Evaluation of seven function-known candidate genes for their effects on improving drought resistance of transgenic rice under field conditions. Mol Plant 2:73–83
Xiong L, Ishitani M, Lee H, Zhu JK (2001) The Arabidopsis LOS5/ABA3 locus encodes a molybdenum cofactor sulfurase and modulates cold stress- and osmotic stress-responsive gene expression. Plant Cell 13:2063–2083
Yan W, Chen Z, Lu J, Xu C, Xie G, Li Y, Deng XW, He H, Tang X (2016) Simultaneous identification of multiple causal mutations in Rice. Front Plant Sci 7:2055
Yu J, Becker ML, Carri GA (2012) The influence of amino acid sequence and functionality on the binding process of peptides onto gold surfaces. Langmuir 28:1408–1417
Yue Y, Zhang M, Zhang J, Duan L, Li Z (2011) Arabidopsis LOS5/ABA3 overexpression in transgenic tobacco (Nicotiana tabacum Cv. Xanthi-nc) results in enhanced drought tolerance. Plant Sci 181:405–411
Yue Y, Zhang M, Zhang J, Tian X, Duan L, Li Z (2012) Overexpression of the AtLOS5 gene increased abscisic acid level and drought tolerance in transgenic cotton. J Exp Bot 63:3741–3748
Zhang D, Tian C, Yin K, Wang W, Qiu JL (2019) Postinvasive Bacterial Resistance conferred by Open Stomata in Rice. Mol Plant Microbe Interact 32:255–266
Zhou S, Zhu S, Cui S, Hou H, Wu H, Hao B, Cai L, Xu Z, Liu L, Jiang L, Wang H, Wan J (2020) Transcriptional and post-transcriptional regulation of heading date in rice. New Phytol 230:943–956.
Acknowledgements
We thank Dr. Yao-Guang Liu for providing the CRISPR/Cas9 system.
Funding
This work was supported by the Major Program of Guangdong Basic and Applied Research (2019B030302006), the Natural Science Foundation of Guangdong Province (2022A1515012353), and Guangdong Laboratory for Lingnan Modern Agriculture Shenzhen Branch Project (AGIS-ZDXM202202).
Author information
Authors and Affiliations
Contributions
HN and JW conceived this study. HN, WW, YY, YF, CW, JC, SC and KW performed the experiments. HN and JW analyzed the data and wrote the manuscript. XT revised the manuscript. JW. CX and XT supervised the study. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it.The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ni, H., Wu, W., Yan, Y. et al. OsABA3 is Crucial for Plant Survival and Resistance to Multiple Stresses in Rice. Rice 17, 46 (2024). https://doi.org/10.1186/s12284-024-00724-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12284-024-00724-w