Abstract
Climate change has significantly affected agriculture production, particularly the rice crop that is consumed by almost half of the world’s population and contributes significantly to global food security. Rice is vulnerable to several abiotic and biotic stresses such as drought, heat, salinity, heavy metals, rice blast, and bacterial blight that cause huge yield losses in rice, thus threatening food security worldwide. In this regard, several plant breeding and biotechnological techniques have been used to raise such rice varieties that could tackle climate changes. Nowadays, gene editing (GE) technology has revolutionized crop improvement. Among GE technology, CRISPR/Cas (Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR-associated protein) system has emerged as one of the most convenient, robust, cost-effective, and less labor-intensive system due to which it has got more popularity among plant researchers, especially rice breeders and geneticists. Since 2013 (the year of first application of CRISPR/Cas-based GE system in rice), several trait-specific climate-resilient rice lines have been developed using CRISPR/Cas-based GE tools. Earlier, several reports have been published confirming the successful application of GE tools for rice improvement. However, this review particularly aims to provide an updated and well-synthesized brief discussion based on the recent studies (from 2020 to present) on the applications of GE tools, particularly CRISPR-based systems for developing CRISPR rice to tackle the current alarming situation of climate change, worldwide. Moreover, potential limitations and technical bottlenecks in the development of CRISPR rice, and prospects are also discussed.
Similar content being viewed by others
Introduction
Food security is being challenged by several factors including but not limited to climate change. It is one of the biggest challenges being faced by humanity not only in this era but can also probe the future generations, if not resolved. It is estimated that a huge chunk of world’s population does not have access to enough food for their survival for years, and about two million people are currently facing nutrient deficiency, leading to their stunted growth, as well (Ahmad et al. 2021a, b, c). In such a critical situation, rice (Oryza sativa L.), being one of the major food crops and a staple food of almost half of the world’s population, can play a pivotal role in global food security and can also be a key solution provider. However, about 90% of its cultivation is happening in Asian countries, which seems more vulnerable to changing climatic conditions. The japonica and indica sub-species of rice are dominant in the rice cultivation system, however, indica rice holds more share in rice market (Uyeh et al. 2021). It is estimated that the world’s population can rise to 8.5 billion by 2030, and about 25% increase in rice production would be required to meet the global food by then (Ahmad et al. 2021a, b, c). Whereas climate changes such as severe drought, heat, salinity, cold, deviation in precipitation pattern, increasing diseases and insect pest attacks are posing serious effects on both rice yield and its nutritional quality. For instance, it is projected that the temperature would rise by 8℃, the average drought index will be 129, which is 52.45 now, and the sea level could also rise by 2100 that may cause food havoc (Li et al. 2009). Moreover, every degree increase in temperature could increase 3% and 7% average precipitation and humidity, respectively, that would promote diseases and insect attacks on the crops (Rezvi et al. 2022). Consequently, the world may suffer with impaired food production system, huge yield losses, and ultimately global food insecurity.
Rice is highly sensitive to climate change as it requires optimum irrigation and a suitable temperature to grow normally. It is reported that 1℃ temperature increase could decrease paddy yield by up to 3.44%. While the same increase in precipitation could decrease 0.12% and 0.21% yield and crop harvest, respectively (Alam et al. 2011). So, as a matter of concern, it is reported that the Malaysian granary area’s temperature could increase from 0.3℃ to 0.5℃ along with increased precipitation from 133 to 200 mm (Firdaus et al. 2020). Ancha (2012) has also described that the Cambodian climate could face a 2.5℃ and 8.3% increase in temperature and rainfall, respectively, which would lead to humid environments in rainy seasons and lower humidity in dry seasons. Therefore, swiftly changing climatic scenario has become a central concern for the whole agricultural production system, especially rice, to ensure current and future global food security. Thus, being a major food crop, new climate smart rice cultivars should be developed that could withstand changing climatic conditions to feed the ever-growing human population and to end hunger, globally (Arunrat et al. 2020; Hussain and Ahmad 2022; Riaz et al. 2022).
In this regard, classical plant breeding has not only contributed in the past but also played a crucial role in current rice breeding programs for its improvement (Fiaz et al. 2021; Patel et al. 2020). Whereas the utilization of conventional plant breeding techniques (CPBTs) for crop improvements is limiting with the development of new plant breeding technologies (NPBTs), which are more efficient, robust, cheaper, and less time-consuming to produce promising results as compared to CPBTs. In addition, modern problems like rapid climate change, soil degradation, pollutant accumulation, and altered rainfall pattern also restrict the application of CPBTs due to their low efficiency and slow pace (Ahmad et al. 2021a, b, c). By keeping in view, the recent environmental challenges and high food demand, NPBTs especially gene editing (GE) could be an effective alternative to the CPBTs that can help to end hunger and ensure food security, globally (Ahmad et al. 2020, 2021a, b, c). Among different GE systems CRISPR-Cas (Clustered Regularly Interspaced Palindromic Repeats-CRISPR-associated Protein) system provides a robust, efficient, and economical mode of action that can alter the genetic makeup of crop plants without introducing foreign DNA, a major controversy in the acceptance of genetically modified organisms (GMOs) developed through transgenic breeding method (Ahmad et al. 2021a, b, c; Monsur et al. 2020). Additionally, the current availability of genome sequences and information of vast number of plant species has made it a more suitable tool to manipulate any specific gene/s for the creation of new desirable crop varieties.
Here, to give an updated picture of the application of CRISPR-Cas systems in rice from 2020 to Present (“Present” refers to March 2023), we have explored five different scholarly databases including Google Scholar (http://scholar.google.com), Pubmed (https://pubmed.ncbi.nlm.nih.gov/), Scopus (https://www.scopus.com/home.uri?zone=header&origin=), DOAJ (Directory of Open Access Journals) (https://doaj.org/) and Refseek (https://www.refseek.com/) and collected the relevant literature using web filters and various keywords i.e., rice, Oryza sativa L., gene editing, CRISPR, genome editing in rice, climate change, biotic resistance, and abiotic tolerance. Finally, fully screened, and curated data have been presented and discussed below in this article to highlight the potential of CRISPR-Cas based GE system in rice improvement (Fig. 1).
Although several reviews on GE in rice have been published previously (Fiaz et al. 2019; Khan et al. 2021; Mishra et al. 2018; Tabassum et al. 2021), but an up-to-date progress of rice GE was needed due to its high value and significant contribution to food security. Thus, this spatiotemporal mini review primarily aims to summarize the recent success stories and progress of CRISPR-based GE systems, especially CRISPR-Cas9, over the last three years in the creation of climate-smart rice lines. Moreover, regulatory aspects and future directions are also discussed in detail.
Can CRISPR Help to Breed New Climate-resilient Transgene-free Rice Varieties?
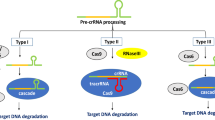
Nowadays, CRISPR-Cas based GE is more valued due to its robustness, accuracy, and applicability in crop plants, especially in rice. Compared to other GE techniques, CRISPR-Cas solely can play a significant role in the development of climate resilient rice lines by performing multiple tasks including but not limited to knock-out, knock-in, epigenetic changes and transcriptional regulation of different genes controlling various traits (Ahmad et al. 2021a, b, c). Further, CRISPR-Cas system based indels, homologous recombination based targeted sequence alteration, and base pair changes that could not be differentiated from natural mutation are spared from GMOs regulation in several countries (Turnbull et al. 2021). Consequently, CRISPR edited foreign DNA free rice lines will be used in rice breeding programs for developing new rice varieties that could be commercialized (Fig. 2). For instance, according to this spatiotemporal study, CRISPR-Cas based GE systems have been used in total 126 research projects since 2020 that have been conducted on the theme of development of climate resilient transgene-free rice lines or validation of the role of gene/s involved in any biotic or abiotic stress tolerance. Out of these published research, 74 and 52 research found related to abiotic and biotic stresses, respectively (Fig. 1). Hence these publications have been further explored and reviewed critically, below in this section, to confirm the promising role of CRISPR technology in developing abiotic (drought, heat, cold, herbicide, salinity, lodging, heavy metal, and others) and biotic stress (bacterial leaf blight, rice blast, sheath blight, and others) tolerant rice varieties.
Illustration of production of publicly acceptable transgene-free climate resilient rice varieties using gene editing tools. (A) Different biotic and abiotic stresses. (B) Diseased or under stress (A/biotic) plants having susceptibility (Su) and sensitivity (Se) genes. (C) Various crop improvement breeding interventions. (D) Gene-edited plant/s (T0) developed using GE tools e.g., CRISPR-Cas-based GE system. At this stage plant/s could have heterozygous or homozygous mutation, hence screening of homozygous plants is required. (E) Final genetically stable homozygous transgene-free desired rice plant/s that can be released commercially as a potential new variety or can be used in rice breeding programs
Re-wiring of Rice Genome via CRISPR for Developing Abiotic Stress Tolerance
Abiotic stresses, particularly drought, heat, cold, salinity, herbicide, and salinity cause substantial yield losses in rice by damaging its growth and developmental processes. It is the need of the hour to develop such rice varieties that could withstand rapidly changing abiotic environmental conditions. CRISPR-Cas systems have been proved as a promising tool in this regard (Table 1). Hence, this section covers its recent applications against abiotic stresses in rice.
Drought
Drought stress is one of the major threats that limits crop productivity and can affect food security significantly (Zhang et al. 2023a, b). It is projected that about half of the global cultivated land would suffer from severe water scarcity by the end of 2050 (Kamanga et al. 2018; Mushtaq et al. 2018). To overcome the possible upcoming consequences, production of drought tolerant crop varieties, especially rice, is undeniable. In this regard, researchers have utilized the CRISPR-Cas based GE systems to knock out the undesirable sensitivity (Se) causing gene/s and have created new drought tolerant rice lines (Table 1).
For instance, 366 bp deletion in drought and salinity tolerant 1 gene through CRISPR-Cas9 enhanced water retention against dehydration stress followed by broader leaves production (Santosh Kumar et al. 2020). The phenotype of CRISPR-edited mutant plants showed tolerance against drought stress. Similarly, a CRISPR-Cas9 based null mutation in pyrabactin resistance-like (OsPYL9) gene resulted in higher cuticular leaf wax accumulation, lower transpiration rate, and increased grain weight along with drought tolerance (Usman et al. 2020). Chung et al. (2020) also identified 66 drought induced microRNAs and of these OsmiR535 and OsmiR818b were found to be drought responsive. The 5 bp- homozygous deletion in drought responsive OsmiR535 induced tolerance against PEG, dehydration, NaCl and abscisic acid (ABA) stresses (Yue et al. 2020). Likewise, the loss of function of OsABA8ox2 gene, an ABA 8,-hydroxylase encoding gene, generated via CRISPR-Cas9 system produced a long and narrow rooting system that is beneficial to acquire water under drought conditions (Zhang et al. 2020a, b). In addition, stomatal density largely affects water transpiration thus playing a critical role in drought stress. For instance, the CRISPR-Cas9 based stomata developmental defect 1 (osrsd1) mutants had decreased stomatal density that prevented water loss under dehydration stress. Consequently, mutant lines also exhibited altered expression of other stomatal related genes i.e., stomatal density and distribution 1 (OsSDD1), and showed tolerance against drought stress (Yu et al. 2020). Lu et al. (2020) identified two drought-sensitive genes (OsAAA-1 and OsAAA-2) through screening of the activation tagging population. Thus, CRISPR-Cas9 mediated disruption introduced pre-mature truncation of protein that resulted in increased grain yield under limited water availability. Moreover, disruption of epidermal patterning factor 10 (EPFL10) through CRISPR-Cas9 system caused lower stomatal density followed by improved water conservation compared to the wild (Karavolias et al. 2021). Similarly, CRISPR-Cas9 generated osmotic stress/ABA–activated protein kinases (sapk3-1 and sapk3-2) mutants displayed lower water loss and normal plant growth under drought conditions (Lou et al. 2023). Cuticular wax synthesis under drought stress inhibits water loss and its production could prevent the plant from severe damages. Under drought stress, both knockdown and knockout mutants of lov kelch repeat protein 2 (Oslkrp2) gene made 10% more cuticular wax along with increased leaf size, which resultant improve the level of tolerance of plants against drought conditions (Shim et al. 2023). In contrast to these studies, scientists have also identified some positive regulators (genes) of drought stress whose disruption has brought drought sensitivity. These genes include nitrate transporter 1/peptide transporter family (OsNPF8.1) (Diyang et al. 2023), and ideal plant architecture 1 (IPA1) genes (Chen et al. 2023a, b). Finally, it could be concluded that CRISPR-Cas mediated over-expression of these genes could contribute to produce drought tolerant rice lines. Similarly, numerous stress-related factors (TFs) have also been found to be positively associated with drought tolerance. For example, CRISPR-Cas9 mediated NAM, ATAF1/2, and CUC2 (OsNAC006) knock-out lines impaired morpho-physiological traits together with decreased antioxidants (catalase, peroxidase dismutase, and peroxidase) growth following drought stress (Wang et al. 2020a, b, c, d). Similarly, CRISPR-Cas 9 oriented disruption of OsWRKY76 (w76-1 and w76-2) TF has brought severe symptoms of wilting, electrolyte leakage, water loss, and chlorosis after treating with 20% PEG (Zhang et al. 2023a, b). Additionally, the rice genome also has 89 basic region/leucine zipper motif (bZIP) TFs that are involved in regulating numerous regulatory and stress related pathways. Two base pairs deletion in the first codon of OsbZIP72 TF led to the premature termination and phenotype of this mutation caused withering of seedlings against drought compared to over-expressed lines demonstrating better morphological appearance against drought (Baoxiang et al. 2021).
Heat
According to temperature stats recently published by FAO in May 2023, 1.4 °C was the average annual temperature change in the world in 2022 (FAO 2023). However, it is predicted that the average global temperature would increase up to 2℃ to 4℃ by the end of 21st century, and this increase in temperature could significantly affect crop productivity (Jagadish et al. 2015). The global mean temperature could rise to 3℃ by the mid twenty-first century (Field et al. 2012). Hence, it is becoming more alarming as it is reported that 1℃ increase in mean temperature could affect 6–7% grain yield of crop plants (Lesk et al. 2016). Considering the adverse effects of prolonged heat stress scientists have made multiple efforts using CRISPR-based GE system to produce heat resilient rice lines (Table 1).
For example, OsNAC006 TF is regulated by indole acetic acid, gibberellin, H2O2, ABA, cold, heat, PEG, and NaCl treatments. CRISPR-Cas9 mediated null mutants of OsNAC006 have displayed thermo-tolerance in rice plants followed by elevated H2O2, O2- levels (Wang et al. 2020a, b, c, d). Similarly, CRISPR-Cas9 oriented disruption of NTL TF at two sites produced ntl3-1 and ntl3-2 mutants. The ntl3-1 produced truncated protein whereas ntl3-2 mutant exhibited a new C-terminus in addition to a truncated protein. Interestingly, regardless of post GE protein structure both mutants showed thermo-tolerance at 45℃ for 5 days with lower survival rate (Liu et al. 2020a, b, c). On the other hand, there are also some genes that are positively associated with heat tolerance whose expression is essential to ensure heat tolerance. For example, leucine rich repeat receptor kinase (LRK1) is positively correlated with leaf dark respiration and CRISPR-Cas9 based single bp mutation at the 508th nucleotide resulted in premature termination of protein by introducing the stop codon at the 180th amino acid sequence. Exposure of lrk1 mutants to 35℃ for 10 days reduced dark respiration along with retarded morphological growth (Qu et al. 2020). Likewise, three mutations consisting of 1-bp, 2-bp, and 4-bp deletion in the first exon of semi rolled leaf 10 (SRL10) gene through CRISPR-Cas9 generated three independent mutant lines that exhibited semi rolled leaf phenotype and compromised thermo-sensitivity (Zhang et al. 2023a, b). Consequently, the application of CRISPR-Cas9 system confirms that constitutive expression of these genes is needed to induce thermotolerance in rice.
Salinity
Salinity stress greatly affects the seedling and reproductive stages of rice plants by introducing osmotic, oxidative, and ion toxicity stresses. Interestingly, salt tolerant rice is a preferred grain crop to utilize the saline-alkali and coastal tidal lands, which has great utilization potential (Han et al. 2022). Numerous studies have been executed to find out and to characterize the salinity associated gene (s) through CRISPR-Cas mediated GE.
For instance, soil surface rooting 1 (OsqSOR1) is a homolog to deep rooting 1 (AtDRO1) gene that governs shallow root growth angle. One bp substitution in the 3rd exon of OsqSOR1 gene caused premature truncation and produced soil surface root which improves salinity tolerance (Kitomi et al. 2020). Likely, double mutants of extra-large GTP-binding protein (osxlg1/osxlg4) genes also increased root length that led to improved salinity tolerance (Biswal et al. 2022). Further, CRISPR-Cas9 oriented two mutations viz., -20 bp in M16 exon and − 1 bp in M18 exon of OsRR22 gave higher root and shoot weight and a greater number of fresh leaves against 0.8% NaCl treatment (Han et al. 2022). Heterotrimeric G proteins are involved in regulating the stress responses in plants. CRISPR-Cas9 based null mutations in G protein encoding genes (gs3, and dep1) induced salinity tolerance (Cui et al. 2020). Similarly, the paraquat tolerance 3 (OsPQT3) gene could switch off the stress mechanism through an off-switch mechanism and disruption of the first and second exons of OsPQT3 gene resulted in higher salt tolerance (150mM NaCl) together with improved germination (Alfatih et al. 2020). For salinity tolerance, balance between Na + and other salts is crucial in salinity tolerance and sensitivity. in this regard, Lian et al. (2020) have observed lower K + concentration and improved morphological growth in open stomata 2 (ossst) mutants against 150mM NaCl. Like genes, TFs could also perform compartmentation of Na + and K + to induce salt tolerance and a single bp deletion in OsbHLH024 TF improved oxidative stress tolerance by maintaining the balanced level of Ca2+, Zn2+, and Mg2 + minerals (Alam et al. 2022a, b, c). Moreover, steroid hormones also have their role in stress tolerance, but they are difficult to use in plant improvement due to their complex role. Dong et al. (2020) have disrupted SERK2, one of the steroid component genes that enhanced salinity tolerance. Similarly, ABA biosynthesis also has some roles in salinity tolerance and CRISPR-Cas9 based disruption of violaxanthin de-epoxidase (OsVDE) gene has brought salinity tolerance followed by increased ABA level higher survival rate, and stomatal closure (Wang et al. 2022a, b, c). Although gene disruption could contribute to stress tolerance, genotypic background largely affects the induction of stress tolerance. In this regard Prodhan et al. (2022) have disrupted the betaine aldehyde dehydrogenase (OsBadh2) gene with CRISPR-Cas9 simultaneously in Jiahua#1 (WT_JH) and Huaidao#5 (WT_HD) cultivars. Surprisingly mutation in OsBadh2 gene induced salinity tolerance in WT_HD lines only, indicating the significant effect of the genetic background to acquire the stress tolerance.
Conversely, positive regulators of salinity tolerance have also been reported whose disruption introduced such characteristics that have produced salt sensitive rice lines. For instance, disruption of Ca2 + sensor, calmodulin (OsCaM1) gene produced lower primary root, and weakened lateral root length with reduced root density confirming its sensitivity to salt stress (Yang et al. 2021). Likewise, CRISPR-Cas9 based mutagenesis in the first exon of glyoxalase (OsGLYi3) gene yielded saline hypersensitivity following elevated methylglyoxal, and glyoxalase I activity (Liu et al. 2022). Balance of Na + and K + in root zones greatly affects salinity tolerance in crop plants. Identically, knock-out lines of mitochondrial phosphate transporters (OsMPT3;1 and OsMPT3;2) genes exhibited compromised morphological growth and reduced Na + efflux and K + and Ca + influx as well (Huang et al. 2020a, b, c). Like genes, TF could also positively regulate the salinity tolerance. CRISPR-Cas9 oriented disruption of a trihelix (OsGTγ-2) TF produced salt hypersensitive phenotypes followed by imbalanced K + and Na + accumulation (Liu et al. 2020a, b, c). Likewise, CRISPR-Cas9 mediated editing of 3rd exon of BEAR1, a bHLH TF, produced salt sensitive phenotypes i.e., reduced plant height compared to control (Teng et al. 2022). Interestingly, knocked out mutants of bHLH044 TF also produced salt sensitive lines. However, this sensitivity was due to the elevated reactive oxygen species levels followed by higher lipid peroxidation and H2O2 levels (Alam et al. 2022a, b, c).
Cold
Rice is vulnerable to chilling stress and induction of chilling tolerance is pivotal to expanding its cultivation to northern areas characterized by much lower annual temperatures (Li et al. 2023). Chilling tolerance is a complex trait and is controlled by several quantitative trait loci such as COLD1, qCTS12/GSTZ2, CTB4a, and Ctb1 (Liu et al. 2018). Therefore, several attempts have been made to induce chilling tolerance or to investigate genes involved in chilling tolerance. Many tolerance causing genes have been reported in the past (Le et al. 2022; Romero and Gatica-Arias 2019), and latest are presented here (Table 1). Such as, CRISPR-Cas9 mediated disruption of a cold tolerance gene (OsMYB30), panicle length gene (OsPIN5b), and grain size gene (GS3) genes yielded cold tolerance along with improved yield. Additionally, two triple mutants comprised of ospin5b/gs3/osmyb30-25 and ospin5b/gs3/osmyb30-4 also exhibited improved cold tolerance together with enhanced grain yield (Zeng et al. 2020a, b). Likewise, exposure of CRISPR-Cas9 based osatp4 and ostcd3 mutants to lower temperature possessed improved cold tolerance coupled with decreased chlorophyll contents (Lin et al. 2020). Moreover, WRKY TFs are also associated with cold tolerance. For instance, CRISPR-Cas9 oriented disruption of OSWRKY63 TF introduced cold tolerance in rice (Li et al. 2021a, b). In contrast, like other stresses, positive regulators of cold stress have also been reported. Like, editing of the fifth exon of annexin (OsAnn5) gene caused cold sensitivity in rice seedlings (Que et al. 2020). Further, CRISPR-Cas9 based editing of OsWRKY76 TF produced cold sensitive rice lines (Zhang et al. 2022a, b). Though editing of positive regulators is not required but the application of CRISPR-Cas9 system helped in elucidating their confirm role in cold stress. Hence it proves that CRISPR-Cas9 system does not only help in developing new cold stress tolerant germplasm but also playing its critical role in functional analysis of the newly identified and cloned gene/s.
Herbicide
Weeds are another big challenge to rice and hinder its production significantly. Several efforts, including but not limited to the utilization of conventional, mechanical, and chemical approaches, have been made to cope with this problem so far. Nevertheless, these strategies have been proven expensive, less efficient, and/or hazardous to health and ecosystem (Chauhan 2013; Kuang et al. 2020; Wu et al. 2023). The development of herbicide tolerant genotypes using CRISPR-Cas systems are the need of the hour and could be one of the popular strategies to overcome the challenge of weeds to rice and to improve its production, globally. For that, extensive studies have been conducted in last three years to investigate the possible roles of several genes towards herbicide tolerance and their utilization in the development of herbicide tolerant rice lines through CRISPR-Cas systems (Table 1).
Acetolectase synthase (ALS) gene, an herbicide resistant gene catalyzes the first step of biosynthesis of branched amino acids that are the first target of Bispyribac sodium (BS) and other herbicides. Recently, conversion of tryptophan to leucine at 548th position of ALS gene improved BS tolerance in basmati rice (Zafar et al. 2023). Likewise, Liying et al. (2022) have also edited OsALS genes earlier and obtained three types of mutants viz., ALSS627N and 1884G-A, ALSS627N, and ALSS627N/G628E that were resistant to imidazole ethylinicotinic acid. Moreover, auxin hormones play a variety of roles in plants including embryogenesis, tissue elongation, and vascular differentiation. Dicot weeds are controlled by auxinic herbicides. CRISPR-Cas9 directed disruption of auxin signaling f-box (OsAFB4) auxin receptor induced tolerance against picloram and 2,4-dichlrophenoxyacetic acid (Guo et al. 2021). Moreover, single bp substitution has proved their crucial role in producing herbicide tolerant rice lines. Xu et al. (2021a, b, c, d) have developed prime-editing-library-mediated saturation mutagenesis to increase the substitution rate at the target site (s) and have identified 16 diverse mutations in OsACC1 corresponding to herbicide resistance. Additionally, paraquat herbicide resistance has also been achieved in CRISPR-Cas9 based triple mutants of OsPUT knock-out lines (Alfatih et al. 2020). Beside BS, glyphosate is a widely used herbicide, but it also affects the main crop. A single bp substitution of 96G to A in glyphosate resistant gene (EPSPS) induced herbicide tolerance (Jiang et al. 2022). Hence, in this case it can also be concluded that CRISPR-based base editors have not only improved the rice resistance against herbicides but also proved that it can generate mutants that could be mimicking natural mutants. Besides, unlike drought, salinity and heat stresses, positive regulators of herbicide tolerance have not been reported in the last three years.
Heavy Metal
Heavy metals are undesirable for plants and humans too as their exposure to the human body could damage organs even at lower concentration. Heavy metals are non-biodegradable, stable, and persistent that made them hazardous for human consumption. Cadmium (Cd) is one of the most threatening heavy metals for living organisms and its pollution in agricultural soil has continuously been increasing (Zhao et al. 2015). Cd is not only affecting rice yield and quality, but it is also a serious threat to human health as well. Cd interacts with metabolic processes and could cause various cancers and bone diseases in the human body (Clemens 2019). Although numerous studies have been conducted to find out the Cd associated genes but unfortunately, till now only a few genes have been found that can take part in Cd metabolism. Such as, null mutants of low cadmium (oslcd) gene accumulated less Cd in shoots under the influence of Cd-contaminated soil (Elkonin et al. 2023). Similarly, the knocked-out mutants of mir535 of brown rice accumulated 35% less Cd compared to the control under controlled stress of 2 µmol/L Cd (Yue et al. 2023). Moreover, Chang et al. (2022) have also got Cd tolerance with limited manganese accumulation by disrupting natural resistance associated macrophage protein (OsNramp5) gene with CRISPR-Cas9. Besides Cd, copper oxide nanoparticles (CuO NPs) are also creating toxication in rice plants and oscerk1 mutants had CuO NPs tolerance followed by the increased accumulation and regulation of H2O2 and antioxidant system respectively (Chen et al. 2023a, b). In addition to heavy metal contamination, pesticides residuals are another source of contaminated cultivated soils. Xu et al. (2023) have identified acetyltransferase (OsACE2) gene that has a primary role in catabolizing the oxyfluorfen pesticide residuals in soil. However, CRISPR-Cas9 based downregulation of this gene resulted in compromised morphological growth and higher oxyfluorfen accumulation suggesting its positive association with oxyfluorfen catabolization.
Others
Besides the prominent stresses, other minor stresses have also been addressed through CRISPR systems. These stresses include Fe homeostasis, lodging, effects of short and long-day span on heading, Pi (phosphorous) starvation, and K+ (potassium ion) uptake. For detail overview please refer to Table 1.
Re-wiring of Rice Genome via CRISPR System for Developing Resistance Against Biotic Stresses
Biotic stresses including diseases and insects cause up to 40% (and sometimes complete) yield losses in rice. Rice is attacked by many pathogens including bacterial, fungal, and viral, which cause several kinds of diseases in rice that eventually hamper or stop the growth and development processes in rice. Hence, developing pathogen and insect resistant rice varieties are quite important to achieve global food security. In this regard, the CRISPR-Cas system is contributing significantly to developing biotic stress resistant rice lines (Table 2). So, the contributions of CRISPR-Cas system are highlighted below in this section.
Rice Blast
Rice blast is a fungal disease caused by Magnaporthe oryzae; a hemi biotrophic fungus. It has been extensively studied due to its lethal damage to rice. Scientists have also developed a Patho-system of rice M. oryzae which has been used as a primary model to study plant-microbe interaction (Li et al. 2019). Using this model, scientists have conducted multiple studies in the last three years to produce rice blast resistant lines through CRISPR-Cas systems.
In this regard, Huang et al. (2020a, b, c) have edited the N terminal sequences of four Ferrona like receptor (FLR) genes that are involved in rice M. oryzae interaction. Double knocked out lines of FLR2 and FLR11 receptors depicted enhanced resistance, while single knocked out lines of FLR1 and FLR13 showed susceptibility indicating their negative and positive association respectively with blast resistance. Similarly, single mutant lines of FLR1 and single knock out lines of FLR2 receptor delayed the pathogen infection respectively. Differences in resistance/susceptibility could be due to the altered expression of other genes. Likewise, MILAzzo et al. (2022) have achieved blast resistance characterized by lower disease symptoms following the disruption of OsDjA2 and ethylene-responsive factor104 (ERF104) genes independently. Salicylic acid (SA) may have a crucial role in the plant immune system and its indirect association with blast resistance has also been reported. Downregulation of UGT74J1, a UDP-glucosyltransferase gene induced several PR-related genes along with improved blast resistance and higher SA accumulation. However, confirmation of this hypothesis needs further investigation whether blast resistance is due to the overexpression of PR-related genes or higher SA accumulation (Tezuka et al. 2021). Further, impairing avirulence activity of pathogen has also been proven to be fruitful to induce blast resistance. For example, CRISPR-Cas9 based ubiquitin-conjugating enzyme26 (osubc26) mutants depicted impaired avirulence activity by impairing proteasome through degradation of AvrPiz-t cells Liu et al. (2021a, b, c). Addition to gene disruption, CRISPR-Cas based gene knock-in approach has also been used to induce blast resistance in rice lines. Xu et al. (2020) have induced blight resistance by introducing exon #2 of Pi-ta gene (blast resistant gene) into pi-ta (susceptible gene) gene resulting in rice blast resistance.
In contrast, there are also such genes and TFs have been reported in the last three years whose expression is required to ensure the blast resistance. For example, CRISPR-Cas9 based null mutations of OsPib (Xie et al. 2022), ent-casbene synthase (Liang et al. 2021), peroxidase3 (OsPerox3) (Liu et al. 2021a, b, c), growth-regulating factor4 (OsGRF4) (Yudong et al. 2021), and partial resistance1 gene (OsPiPR1) (Liu et al. 2020a, b, c) genes have increased blast susceptibility. Similarly, some TFs particularly WRKY TF gene family have been found to be positively associated with blast resistance and disruption of WRKY45, OsWRKY93 and OsWRKY7 TFs caused more susceptibility (Li et al. 2021a, b; Tun et al. 2023). Finally, the expression of specific genes may conclude to ensure blast resistance.
Bacterial Blight
Bacterial blight of rice caused by Xanthomonas oryzae pv. Oryzae (Xoo), is a destructive rice disease that can reduce the rice grain yield by up to 75%. Xoo could activate the host susceptibility gene followed by seizing the host machinery through its endogenous transcription activator-like effectors (TALEs). Moreover, TALEs could bind to effector binding elements (EBE) to cause bacterial diseases that are present in the upstream regions of SWEET genes or other plant susceptible genes. Targeted mutation in the promoter of OsSWEET14 gene characterized with AvrXa7 deletion exhibited improved resistance against Xoo. Consistently Zeng et al. (2020a, b) have targeted the 1st and 3rd exons of Sugars Will Eventually be Exported Transporter14 (OsSWEET14) gene and have obtained resistance against the Asian and African races of Xoo (AXO1947). In addition to gene knock-out, scientists have also engineered other SWEET genes as well and Diana et al. (2022) have introduced indels with CRISPR-Cas9 in the promoter of susceptibility gene (OsSWEET13). However, phenotypic evaluation has yet to be performed. Like SWEET genes, pathogen’s disease inducing genes including Xa13, Xa1, and Xa23 have also been subjected to GE to explore their role towards rice blight resistance. It is worth noticing here that these two genes could contribute to broad spectrum resistance (BSR) as wellWei et al. (2021a, b) have reported BSR to blight by knocking of EBEAvrXa23 elements into the promoter regions of susceptible allele (xa23). Furthermore, altering EBEs of Xa13 gene’s promoters also resulted in blight resistance (Li et al. 2020a, b). Besides EBEs, UPT boxes are also of great importance in acquiring blight resistance. CRISPR-Cas12a mediated site-specific mutations in UPT box of Xa13 gene gave blight resistant phenotypes followed by disturbed TALEs binding sites (Yu et al. 2021). Hence, CRISPR-based GE tools can be some promising tools to combat bacterial blight and reduce yield losses being happening due to it.
Sheath Blight
Sheath blight is caused by Rhizoctonia solani and causes withering and lodging of the entire plant that could reduce grain yield by up to 50%. Identification of susceptibility genes is a prerequisite to induce disease resistance in crop plants. In this regard numerous efforts have been made to identify and to edit the disease responsive genes through CRISPR-Cas system in last three years. In contrast to other studies, most genes are positively associated with sheath blight resistance. For example, employing of CRISPR-Cas9 system on Protein phosphate (OsPP2A1) gene yielded five mutants; pp2a-1-1 had 1 and 2 bp insertions in 1st exon, and pp2a-2 had 1 bp deletion in 11th exon. Moreover, pp2a-3, and pp2a-4, had 1 bp insertion and 2 bp insertion in their 1st and 2nd exon respectively, whereas pp2a-5 contained 1pb deletion in the 1st exon too. Interestingly, all these mutants depicted hypersensitivity to sheath blight disease compared to control (Lin et al. 2021). Similarly, Jung and colleagues have found that NH4+ uptake is positively associated with sheath blight disease as osamt1 lines (an ammonium transporter) exhibited hypersensitivity against R. solani isolates (Jung et al. 2023).
Others
Rice genome editing has also been attempted to induce other biotic stress resilience as well including microbial organisms, brown plant hopper (Zhang et al. 2022a, b), rice tungro virus (Kumam et al. 2022), rice black streaked dwarf virus (Wang et al. 2021a, b, c, d; Wang et al. 2022a; Wang et al. 2022b; Wang et al. 2022c), southern rice black streaked dwarf virus (Lu et al. 2021; Wang et al. 2022a, b, c) and yellow mottle virus (Arra et al. 2023). In addition to these, some genes have also shown resistance to multiple pathogens or diseases (Table 2). Hence, all these applications show that CRISPR-based GE tool has great potential to develop climate-smart rice varieties that can help us to achieve food security, globally.
Challenges, Opportunities, and Future Perspective
Several GE tools, including restriction enzymes, zinc finger nucleases, Transcription activator-like effector nucleases, CRISPR-Cas systems, and transposases have been used for creating a better rice for a long time. However, there is no doubt that among these, CRISPR-Cas based GE systems (especially CRISPR-Cas9 system) have been the most adopted GE tool due to its higher accuracy, robustness, ability to modify the target sequence, and applicability to maximum number of crop plants without any restrictions. Several improvements have been observed in rice DNA and RNA editing using CRISPR-Cas systems since its first application in rice in 2013. The world has witnessed the successful application of different versions of CRISPR-Cas system, prominently CRISPR-Cas9/-Cas12/-Cas13, DNA/RNA base editors, and prime editors, in developing new desirable rice lines (Aqib et al. 2022; Malzahn et al. 2019; Tang et al. 2018; Zhang et al. 2021a, b). Besides its huge potential over others, CRISPR-Cas systems also have some limitations and challenges, including but not limited to off-target effects, bigger size of Cas proteins, limited protospacer adjacent motif (PAM) sites as they are important to determine the target site (e.g., in case of Cas protein “NGG“ is the PAM site), efficiency and accuracy of targeting the desired DNA/RNA fragment, and/or production of transgene free CRISPR-edited mutants (Ahmad et al. 2020). To overcome these limitations, many efforts have been made and many more are needed.
For example, multiple alternative PAM sites (including NAG, NGA, NNGG, NNG, NAA etc.) have been identified to improve the efficiency of the system. The wild-type Cas9 protein can efficiently identify the NGG and NAG PAM sites in rice. Moreover, according to Meng et al. (2023) using NGA alone or in combination with NAG could enhance editing efficiency. In contrast, it is reported that despite of first recognition of NAG PAM by SpyCas9, NGG has shown strong affinity for SpyCas9 protein (Kleinstiver et al. 2015). Hence, the identification of new orthologs of Cas9 nuclease or engineering of available Cas9 are needed that could identify and show strong affinity with new and efficient PAM sites in the genome. Otherwise, the identification of the target/s in the genome for gene disruption using CRISPR-Cas systems could be PAM independent (Collias and Beisel 2021). Moreover, the identification of new variants of Cas9 nuclease can also solve the problem of the transformation of bigger size SpyCas9 nuclease into plants e.g., a smaller Cas9s nuclease has recently been engineered and used effectively in therapeutics as an alternative (Schmidt et al. 2021).
Off-target effects are another growing challenge that should be addressed. Off-targets could be of two types especially in case of base editors: (1) Cas protein dependent, or (2) Cas protein independent. Hence, both types are crucial and need ultimate solution. Nowadays, several in silico, in vitro, in vitro in cellulo, and in cellulo based methods are available for identifying genome-wide CRISPR-Cas off-target sites (Tao et al. 2023). Recent advancements in genomics and next-generation sequencing (NGS) would certainly help to minimize the off-targeting and to identify the CRISPR edited plants without off-target mutations. Recently, Amit et al. (2021) have also developed a model based on NGS data to reduce the off targets. Moreover, the development of base editing, prime editing and CRISPR-Cpf1 type systems have made CRISPR-based GE technology more reliable due to their minimal off-target activities. Collectively, it can be predicted that off-target effects can be further reduced from minimal to even zero level.
Besides off-targets, tissue culture is one of the key steps involved in delivering CRISPR/Cas system reagents into target plant. Optimization of tissue culture for each targeted crop and various economically important tree species (which are difficult to propagate and/or near to their extinction) is a major bottleneck on their way to improvement using CRISPR-Cas based GE technology. In this regard, quite a few successes and advancements have been observed in recent days. Fortunately, researchers have recently combined grafting and mobile CRISPR to avoid the laborious and time-consuming tissue culture technique and delivered the CRISPR/Cas9 reagents as RNA from transgenic roots (rootstock) to distal parts of unmodified grafted scion, where it is translated into proteins to induce heritable mutagenesis at desired loci (Yang et al. 2023). This technique has been critically reviewed by (Zaman et al. 2023), and it is demonstrated that it has tremendous potential to produce transgene-free and genetically stable plants not only in field and vegetable crops, but also fruits and other economical trees. Similarly, Cao et al. (2023) have also developed an extremely simple cut–dip–budding (CDB) tissue culture free delivery system, which uses Agrobacterium rhizogene to inoculate explants and does not need sterile conditions. It has been demonstrated that CDB is a useful tool to achieve the heritable transformation of plant species in multiple plant families. In addition, several in planta transformation attempts have been made to perform tissue culture free GE (Hamada et al. 2018; Liu et al. 2021a, b, c) .
In various countries, genetically modified plants that have traces of any foreign DNA are not legal for commercial cultivation. Similarly, whether CRISPR-edited plants should be considered as GMO and pass through the GMO regulatory process or should not be labeled as GMO and pass-on to the next level of field trials and commercialization straight away, is still a question and part of legislative discussions in many countries including European Union (UN). In this regard, some countries have already finished or near to finish their legislative framework and decided the fate of GE crops. Positively, UK has recently joined the countries (i.e., Argentina, Australia, Brazil, Canada, Chile, China, Colombia, India, Israel, Japan, and USA) that have allowed GE tools for crops’ improvements and exempted the CRISPR-edited plants from GMO’s legislations because they possess mutations or genetic changes as similar as they can happen in conventional breeding or natural populations (Caccamo 2023; Schmidt et al. 2020) (Fig. 3).
Additionally, regarding the biosafety concerns, the Ministry of Agriculture and Rural Affairs of China has also recently approved the safety of GE soybean and issued a certificate to the developers for next five years starting from April 2023 (Mallapaty 2022). However, such biosafety approvals should be granted to rice varieties as well to achieve food security globally. In contrast, the GE crops are regulated as GMO in EU and New Zealand. However, Agriculture Ministers from the European Union (EU) have met for the first time to discuss the EU recent proposal on new genomic techniques (NGT), including GE. According to the reports, several EU countries supported proposed suggestions regarding GE technology, as compared to very few who showed their concerns over potential risks. The draft proposal to deregulate NGT, especially GE, was released in early July. While certain traceability requirements would remain in place for all GE crops, the draft foresees that NGT-based plants, which also means GE-plants, that are indistinguishable from ones obtained by conventional breeding should be treated like their conventional counterparts (Union 2023a, b). Likewise, other countries in the world are either working on the proposal and the legislations i.e., Kingdom of Saudi Arabia, Pakistan etc., or still having discussions about the new genomics and precision breeding technologies.
Unlike GMOs, CRISPR-edited plants do not carry any foreign DNA and manipulate the existing gene/s, and it has been proven by many studies (Gu et al. 2021; Li et al. 2020a, b; Yang et al. 2023; Yu et al. 2021). Particularly, CRISPR-edited transgene-free rice plants have been generated and screened through several approaches including genetic segregation, foreign DNA free delivery method or transient expression of CRISPR-Cas systems. Genetic segregation may not affect the plant’s genetic makeup, but it certainly eliminates the vector backbone/selectable marker gene by crossing with wild plants. However, outcrossing itself is a tedious and time-consuming task that could be substituted in future due to some recent developments in CRISPR systems for developing transgene-free plants. For example, grafting and mobile CRISPR by Yang et al. (2023), CDB by Cao et al. (2023) and CRISPR Combo to knock out and fine tune the gene expression simultaneously by Pan et al., (2022) are the versatile breakthroughs that will potentially pave the way towards their smooth application in rice as well as other crops speed and precision breeding programs.
In conclusion, rice is being consumed as a staple food in half of the world’s countries highlighting the necessity of rice improvement to withstand rapidly changing global climate. Classical plant breeding techniques, along with mutation and transgenic technologies, are struggling hard to meet the global food requirements but cannot be due to their certain limitations and slower pace of action. The rice breeding programs need to be aided through new speed and precision breeding technologies such as CRISPR-Cas based GE systems. Despite of certain limitations, problems and challenges being faced in CRISPR-Cas based GE system, its applications and achievements highlighted in this review show that it has a great potential to revolutionize not only the rice improvement breeding programs but also the future of whole agricultural system to end hunger worldwide. In future, this technology may support the researchers to develop tolerance against multiple abiotic and biotic stresses (both within and between different environmental stresses) simultaneously to save time and resources. In this regard some proof-of-concepts exist in which tolerance or resistance against two or more stresses have been achieved (Tables 1 and 2, and Fig. 4). Here, to conclude our discussion, we hypothesize that development and characterization of CRISPR-edited plants against various stresses at the same time has huge potential that can transform and speed up the future breeding programs (Fig. 4). Additionally, based on the progress and developments being made in CRISPR-Cas systems coupled with rice, we also speculate that they could be one of the key players in bringing the next green revolution.
Illustration of proof-of-concept of development of climate-resilient rice lines by manipulating single, double, or multiple genes at the same time and future possibilities. (A) Network of abiotic stresses, (B) Network of biotic stresses, (C) Combined tolerance between abiotic and biotic stresses, (D) CRISPR-edited rice line possessing tolerance against multiple stresses at the same time. Solid lines represent the proof-of-concepts of development of tolerance or resistance against multiple stresses at the same time, whereas doted lines show future directions and potential areas in rice gene editing to be explored. BLB, bacterial leaf blight; RBSDV, rice black streak dwarf virus; SRBSDV, southern rice black streak dwarf virus
Data Availability
Not applicable.
References
Ahmad S, Wei X, Sheng Z et al (2020) Crispr/cas9 for development of disease resistance in plants: recent progress, limitations and future prospects. Brief Funct Genomics 19(1):26–39
Ahmad S, Shahzad R, Jamil S et al (2021a) Regulatory aspects, risk assessment, and toxicity associated with rnai and crispr methods Crispr and rnai systems, Elsevier: 687–721
Ahmad S, Sheng Z, Jalal RS et al (2021b) Crispr–cas technology towards improvement of abiotic stress tolerance in plants Crispr and rnai systems, Elsevier: 755–772
Ahmad S, Tang L, Shahzad R et al (2021c) Crispr-based crop improvements: a way forward to achieve zero hunger. J Agric Food Chem 69(30):8307–8323
Alam M, Toriman M, Siwar C The impacts of agricultural supports for climate change adaptation: Farm level assessment study on paddy farmers. Alam, MM, Mohd Ekhwan, Siwar T et al (2011) C., Molla, RI, and Talib, B:178–182
Alam M, Kong J, Tao R et al (2022a) Md. Alamin; alotaibi, ss; abdelsalam, nr; xu, j.-h. Crispr/cas9 mediated knockout of the osbhlh024 transcription factor improves salt stress resistance in rice (oryza sativa l.). Plants 2022a, 11, 1184, s Note: MDPI stays neu-tral with regard to jurisdictional claims in …
Alam MS, Kong J, Tao R et al (2022b) Crispr/cas9 mediated knockout of the osbhlh024 transcription factor improves salt stress resistance in rice (oryza sativa l). Plants 11(9):1184
Alam MS, Yang Z-K, Li C et al (2022c) Loss-of-function mutations of osbhlh044 transcription factor lead to salinity sensitivity and a greater chalkiness in rice (oryza sativa l). Plant Physiol Biochem 193:110–123
Alfatih A, Wu J, Jan SU et al (2020) Loss of rice paraquat tolerance 3 confers enhanced resistance to abiotic stresses and increases grain yield in field. Plant Cell Environ 43(11):2743–2754
Amit I, Iancu O, Levy-Jurgenson A et al (2021) Crispector provides accurate estimation of genome editing translocation and off-target activity from comparative ngs data. Nat Commun 12(1):3042
Ancha S (2012) Cambodia: Mainstreaming climate resilience into development planning
Anjala K, Augustine R (2022) Designing of guide rna constructs for crispr/cas9-mediated editing of rice transcription factor osmads26 for enhancing drought tolerance. J Appl Biology Biotechnol 11(1):176–182
Aqib Z, Ahmad S, Tabbasum J et al (2022) Rice grain yield and quality improvement via crispr/cas9 system: an updated review. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 50(3):12388–12388
Arra Y, Auguy F, Stiebner M et al (2023) Rice yellow mottle virus resistance by genome editing of the oryza sativa l. ssp. Japonica nucleoporin gene oscpr5. 1 but not oscpr5. 2. bioRxiv:2023.2001. 2013.523077
Arulganesh T, Kumam Y, Kumar K et al (2021) Genome editing of elite rice cultivar co51 for bacterial leaf blight resistance. Electron J Plant Breed 12(4):1060–1068
Arunrat N, Pumijumnong N, Sereenonchai S et al (2020) Assessment of climate change impact on rice yield and water footprint of large-scale and individual farming in thailand. Sci Total Environ 726:137864
Baoxiang W, Yan L, Yifeng W et al (2021) Osbzip72 is involved in transcriptional gene-regulation pathway of abscisic acid signal transduction by activating rice high-affinity potassium transporter oshkt1; 1. Rice Sci 28(3):257–267
Beyene G, Chauhan RD, Villmer J et al (2022) Crispr/cas9-mediated tetra-allelic mutation of the ‘green revolution’semidwarf-1 (sd-1) gene confers lodging resistance in tef (eragrostis tef). Plant Biotechnol J 20:1716–1729
Biswal AK, Wu T-Y, Urano D et al (2022) Novel mutant alleles reveal a role of the extra-large g protein in rice grain filling, panicle architecture, plant growth, and disease resistance. Front Plant Sci 12:2821
Butt H, Rao GS, Sedeek K et al (2020) Engineering herbicide resistance via prime editing in rice. Plant Biotechnol J 18(12):2370
Caccamo M (2023) New precision-breeding law unlocks gene editing in england. Nat Biotechnol :1–2
Cao X, Xie H, Song M et al (2023) Cut–dip–budding delivery system enables genetic modifications in plants without tissue culture. The Innovation 4(1)
Chang J-D, Gao W, Wang P et al (2022) Osnramp5 is a major transporter for lead uptake in rice. Environ Sci Technol 56(23):17481–17490
Chauhan B (2013) Effect of tillage systems, seeding rates, and herbicides on weed growth and grain yield in dry-seeded rice systems in the philippines. Crop Prot 54:244–250
Chen X, Liu P, Mei L et al (2021) Xa7, a new executor r gene that confers durable and broad-spectrum resistance to bacterial blight disease in rice. Plant Commun 2(3):100143
Chen F, Zhang H, Li H et al (2023a) Ipa1 improves drought tolerance by activating snac1 in rice. BMC Plant Biol 23(1):1–12
Chen Y, Liu Z, Meng S et al (2023b) Oscerk1 contributes to cupric oxide nanoparticles induced phytotoxicity and basal resistance against blast by regulating the anti-oxidant system in rice. J Fungi 9(1):36
Chung PJ, Chung H, Oh N et al (2020) Efficiency of recombinant crispr/rcas9-mediated mirna gene editing in rice. Int J Mol Sci 21(24):9606
Clemens S (2019) Safer food through plant science: reducing toxic element accumulation in crops. J Exp Bot 70(20):5537–5557
Collias D, Beisel CL (2021) Crispr technologies and the search for the pam-free nuclease. Nat Commun 12(1):555
Cui Y, Jiang N, Xu Z et al (2020) Heterotrimeric g protein are involved in the regulation of multiple agronomic traits and stress tolerance in rice. BMC Plant Biol 20:1–13
Diana PA, Shanthinie A, Arulganesh T et al (2022) Targeted editing of ossweet13, a bacterial leaf blight susceptible gene in rice using crispr tool. Electron J Plant Breed 13(3):772–779
Ding Y, Zhang F, Sun F et al (2023) Loss of oshrc function confers blast resistance without yield penalty in rice. Plant biotechnology journal
Diyang Q, Rui H, Ji L et al (2023) Peptide transporter osnpf8. 1 contributes to sustainable growth under salt and drought stresses, and grain yield under nitrogen deficiency in rice. Rice Sci 30(2):113–126
Dong N, Yin W, Liu D et al (2020) Regulation of brassinosteroid signaling and salt resistance by serk2 and potential utilization for crop improvement in rice. Front Plant Sci 11:621859
Duy PN, Lan DT, Pham Thu H et al (2021) Improved bacterial leaf blight disease resistance in the major elite vietnamese rice cultivar tbr225 via editing of the ossweet14 promoter. PLoS ONE 16(9):e0255470
Elkonin LA, Gerashchenkov GA, Borisenko NV et al (2023) Development of sorghum mutants with improved in vitro protein digestibility by crispr/cas9 editing of kafirin genes. The Crop Journal
FAO (2023) Temperature change statistics 1961–2022 – global, regional and country trends. N. FAOSTAT Analytical Brief Series, Rome
Feng P, Guangda W, Peng G et al (2023) Evaluation of new japonica rice lines with low cadmium accumulation and good quality generated by knocking out osnramp5. Chin J Rice Sci 37(1):16
Fiaz S, Ahmad S, Noor MA et al (2019) Applications of the crispr/cas9 system for rice grain quality improvement: perspectives and opportunities. Int J Mol Sci 20(4):888
Fiaz S, Khan SA, Ali Noor M et al (2021) Genome engineering for food security. Genome engineering for crop improvement:380–390
Field CB, Barros V, Stocker TF et al (2012) Managing the risks of extreme events and disasters to advance climate change adaptation: Special report of the intergovernmental panel on climate change, Cambridge University Press
Firdaus RR, Leong Tan M, Rahmat SR et al (2020) Paddy, rice and food security in malaysia: a review of climate change impacts. Cogent Social Sciences 6(1):1818373
Gu X, Liu L, Zhang H (2021) Transgene-free genome editing in plants. Front Genome Editing 3:805317
Guo F, Huang Y, Qi P et al (2021) Functional analysis of auxin receptor ostir1/osafb family members in rice grain yield, tillering, plant height, root system, germination, and auxinic herbicide resistance. New Phytol 229(5):2676–2692
Hamada H, Liu Y, Nagira Y et al (2018) Biolistic-delivery-based transient crispr/cas9 expression enables in planta genome editing in wheat. Sci Rep 8(1):14422
Han X, Chen Z, Li P et al (2022) Development of novel rice germplasm for salt-tolerance at seedling stage using crispr-cas9. Sustainability 14(5):2621
He N, Huang F, Yu M et al (2022) Analysis of a rice blast resistance gene pita-fuhui2663 and development of selection marker. Sci Rep 12(1):14917
Hu X-H, Shen S, Wu J-L et al (2023) A natural allele of proteasome maturation factor improves rice resistance to multiple pathogens. Nat Plants :1–10
Huang S, Xin S, Xie G et al (2020a) Mutagenesis reveals that the rice osmpt3 gene is an important osmotic regulatory factor. Crop J 8(3):465–479
Huang Y-Y, Liu X-X, Xie Y et al (2020b) Identification of feronia-like receptor genes involved in rice-magnaporthe oryzae interaction. Phytopathol Res 2(1):1–10
Huang Y, Han Z, Cheng N et al (2020c) Minor effects of 11 dof family genes contribute to the missing heritability of heading date in rice (oryza sativa l). Front Plant Sci 10:1739
Hussain B, Ahmad S (2022) Crispr/cas9 for rice crop improvement: recent progress, limitations, and prospects. Mod Techniques Rice Crop Prod :701–717
Jagadish S, Murty M, Quick W (2015) Rice responses to rising temperatures–challenges, perspectives and future directions. Plant Cell Environ 38(9):1686–1698
Jiang Y, Chai Y, Qiao D et al (2022) Optimized prime editing efficiently generates glyphosate-resistant rice plants carrying homozygous tap-ivs mutation in epsps. Mol Plant 15(11):1646–1649
Jung JH, Li Z, Chen H et al (2023) Mutation of phytochrome b promotes resistance to sheath blight and saline–alkaline stress via increasing ammonium uptake in rice. Plant J 113(2):277–290
Kamanga RM, Mbega E, Ndakidemi P (2018) Drought tolerance mechanisms in plants: physiological responses associated with water deficit stress in solanum lycopersicum. Adv Crop Sci Technol 6(3):1–8
Karavolias NG, Patel D, Seong K et al (2021) Crispr/cas9 knockout of epfl10 reduces stomatal density while maintaining photosynthesis and enhancing water conservation in rice. bioRxiv:2021.2012. 2021.473329
Khan I, Khan S, Zhang Y et al (2021) Crispr-cas technology based genome editing for modification of salinity stress tolerance responses in rice (oryza sativa l). Mol Biol Rep 48(4):3605–3615
Kim P, Xue CY, Song HD et al (2021) Tissue-specific activation of dof11 promotes rice resistance to sheath blight disease and increases grain weight via activation of sweet14. Plant Biotechnol J 19(3):409
Kim CY, Park JY, Choi G et al (2022) A rice gene encoding glycosyl hydrolase plays contrasting roles in immunity depending on the type of pathogens. Mol Plant Pathol 23(3):400–416
Kitomi Y, Hanzawa E, Kuya N et al (2020) Root angle modifications by the dro1 homolog improve rice yields in saline paddy fields. Proc Natl Acad Sci 117(35):21242–21250
Kleinstiver BP, Prew MS, Tsai SQ et al (2015) Engineered crispr-cas9 nucleases with altered pam specificities. Nature 523(7561):481–485
Komatsu A, Ohtake M, Shimatani Z et al (2020) Production of herbicide-sensitive strain to prevent volunteer rice infestation using a crispr-cas9 cytidine deaminase fusion. Front Plant Sci 11:925
Kuang Y, Li S, Ren B et al (2020) Base-editing-mediated artificial evolution of osals1 in planta to develop novel herbicide-tolerant rice germplasms. Mol Plant 13(4):565–572
Kumam Y, Rajadurai G, Kumar K et al (2022) Genome editing of indica rice asd16 for imparting resistance against rice tungro disease. J Plant Biochem Biotechnol :1–14
Le VT, Kim M-S, Jung Y-J et al (2022) Research trends and challenges of using crispr/cas9 for improving rice productivity. Agronomy 12(1):164
Lesk C, Rowhani P, Ramankutty N (2016) Influence of extreme weather disasters on global crop production. Nature 529(7584):84–87
Li Y, Ye W, Wang M et al (2009) Climate change and drought: a risk assessment of crop-yield impacts. Climate Res 39(1):31–46
Li W, Chern M, Yin J et al (2019) Recent advances in broad-spectrum resistance to the rice blast disease. Curr Opin Plant Biol 50:114–120
Li C, Li W, Zhou Z et al (2020a) A new rice breeding method: Crispr/cas9 system editing of the xa13 promoter to cultivate transgene-free bacterial blight–resistant rice. Plant Biotechnol J 18(2):313
Li S, Zhang Y, Xia L et al (2020b) Crispr-cas12a enables efficient biallelic gene targeting in rice. Plant Biotechnol J 18(6):1351
Li Y, Liao S, Mei P et al (2021a) Oswrky93 dually functions between leaf senescence and in response to biotic stress in rice. Front Plant Sci 12:643011
Li Z, Sun P, Sun P et al (2021b) Osbc1l1 and osbc1l8 function in stomatal development in rice. Biochem Biophys Res Commun 576:40–47
Li C, Zhou L, Wu B et al (2022a) Improvement of bacterial blight resistance in two conventionally cultivated rice varieties by editing the noncoding region. Cells 11(16):2535
Li Z, Huang Q, Lin B et al (2022b) Crispr/cas9-targeted mutagenesis of a representative member of a novel pr10/bet v1-like protein subfamily significantly reduces rice plant height and defense against meloidogyne graminicola. Phytopathol Res 4(1):38
Li Z, Rao MJ, Li J et al (2022c) Crispr/cas9 mutant rice ospmei12 involved in growth, cell wall development, and response to phytohormone and heavy metal stress. Int J Mol Sci 23(24):16082
Li Z, Wang B, Luo W et al (2023) Natural variation of codon repeats in cold11 endows rice with chilling resilience. Sci Adv 9(1):eabq5506
Lian T, Huang Y, Xie X et al (2020) Rice sst variation shapes the rhizosphere bacterial community, conferring tolerance to salt stress through regulating soil metabolites. MSystems 5(6):e00721–e00720
Liang J, Shen Q, Wang L et al (2021) Rice contains a biosynthetic gene cluster associated with production of the casbane-type diterpenoid phytoalexin ent–10–oxodepressin. New Phytol 231(1):85–93
Lin D, Kong R, Chen L et al (2020) Chloroplast development at low temperature requires the pseudouridine synthase gene tcd3 in rice. Sci Rep 10(1):8518
Lin QJ, Chu J, Kumar V et al (2021) Protein phosphatase 2a catalytic subunit pp2a-1 enhances rice resistance to sheath blight disease. Front Genome Editing 3:632136
Lin W, Kuang H, Bai M et al (2023) Multiplex genome editing targeting soybean with ultra-low anti-nutritive oligosaccharides. The Crop Journal
Liu C, Ou S, Mao B et al (2018) Early selection of bzip73 facilitated adaptation of japonica rice to cold climates. Nat Commun 9(1):3302
Liu MH, Kang H, Xu Y et al (2020a) Genome-wide association study identifies an nlr gene that confers partial resistance to magnaporthe oryzae in rice. Plant Biotechnol J 18(6):1376–1383
Liu X, Wu D, Shan T et al (2020b) The trihelix transcription factor osgtγ-2 is involved adaption to salt stress in rice. Plant Mol Biol 103:545–560
Liu XH, Lyu YS, Yang W et al (2020c) A membrane-associated nac transcription factor osntl3 is involved in thermotolerance in rice. Plant Biotechnol J 18(5):1317–1329
Liu H, Dong S, Li M et al (2021a) The class iii peroxidase gene osprx30, transcriptionally modulated by the at-hook protein osath1, mediates rice bacterial blight–induced ros accumulation. J Integr Plant Biol 63(2):393–408
Liu X, Song L, Zhang H et al (2021b) Rice ubiquitin-conjugating enzyme osubc26 is essential for immunity to the blast fungus magnaporthe oryzae. Mol Plant Pathol 22(12):1613–1623
Liu Y, Luo W, Linghu Q et al (2021c) In planta genome editing in commercial wheat varieties. Front Plant Sci 12:648841
Liu S, Liu W, Lai J et al (2022) Osglyi3, a glyoxalase gene expressed in rice seed, contributes to seed longevity and salt stress tolerance. Plant Physiol Biochem 183:85–95
Liying Y, Yuanye Z, Rongtian L et al (2022) Improvement of herbicide resistance in rice by using crispr/cas9 system. Chin J Rice Sci 36(5):459
Lou D, Lu S, Chen Z et al (2023) Molecular characterization reveals that ossapk3 improves drought tolerance and grain yield in rice. BMC Plant Biol 23(1):1–18
Lu G, Wang C, Wang G et al (2020) Knockouts of drought sensitive genes improve rice grain yield under both drought and well-watered field conditions
Lu Q, Luo X, Yang X et al (2021) Crispr/cas9-mediated gene editing of vacuolar atpase subunit d mediates phytohormone biosynthesis and virus resistance in rice
Lu H, Shen Z, Xu Y et al (2023) Immune mechanism of ethylicin-induced resistance to xanthomonas oryzae pv. Oryzae in rice. Journal of Agricultural and Food Chemistry
Lyu Y-S, Cao L-M, Huang W-Q et al (2022) Disruption of three polyamine uptake transporter genes in rice by crispr/cas9 gene editing confers tolerance to herbicide paraquat. Abiotech 3(2):140–145
Mallapaty S (2022) China’s approval of gene-edited crops energizes researchers. Nature 602(7898):559–560
Malzahn AA, Tang X, Lee K et al (2019) Application of crispr-cas12a temperature sensitivity for improved genome editing in rice, maize, and arabidopsis. BMC Biol 17(1):1–14
MILAzzo J, H ADRE DTHARREAU et al (2022) Crispr/cas9-targeted knockout of rice susceptibility genes osdja2 and oserf104 reveals alternative sources of
Mishra R, Joshi RK, Zhao K (2018) Genome editing in rice: recent advances, challenges, and future implications. Front Plant Sci 9:1361
Monsur MB, Shao G, Lv Y et al (2020) Base editing: the ever expanding clustered regularly interspaced short palindromic repeats (crispr) tool kit for precise genome editing in plants. Genes 11(4):466
Mushtaq M, Bhat JA, Mir ZA et al (2018) Crispr/cas approach: a new way of looking at plant-abiotic interactions. J Plant Physiol 224:156–162
Nawaz G, Usman B, Peng H et al (2020) Knockout of pi21 by crispr/cas9 and itraq-based proteomic analysis of mutants revealed new insights into m. Oryzae resistance in elite rice line. Genes 11(7):735
Ni Z, Cao Y, Jin X et al (2021) Engineering resistance to bacterial blight and bacterial leaf streak in rice. Rice 14(1):38
Nithya S, Kumam Y, Varanavasiappan S et al (2020) Targeted mutation in eif4g gene in rice. Electron J Plant Breed 11(04):1194–1199
Ogata T, Ishizaki T, Fujita M et al (2020) Crispr/cas9-targeted mutagenesis of osera1 confers enhanced responses to abscisic acid and drought stress and increased primary root growth under nonstressed conditions in rice. PLoS ONE 15(12):e0243376
Patel SK, Sharma A, Singh GS (2020) Traditional agricultural practices in india: an approach for environmental sustainability and food security. Energy Ecol Environ 5:253–271
Prodhan ZH, Islam SA, Alam MS et al (2022) Impact of osbadh2 mutations on salt stress response in rice. Plants 11(21):2829
Qu M, Essemine J, Li M et al (2020) Genome-wide association study unravels lrk1 as a dark respiration regulator in rice (oryza sativa l). Int J Mol Sci 21(14):4930
Que Z, Lu Q, Liu T et al (2020) The rice annexin gene osann5 is a positive regulator of cold stress tolerance at the seedling stage
Rezvi HUA, Tahjib-Ul–Arif M, Azim MA et al (2022) Rice and food security: Climate change implications and the future prospects for nutritional security. Food and Energy Security:e430
Riaz A, Kanwal F, Ahmad I et al (2022) New hope for genome editing in cultivated grasses: crispr variants and application. Front Genet 13:866121
Romero FM, Gatica-Arias A (2019) Crispr/cas9: development and application in rice breeding. Rice Sci 26(5):265–281
Santosh Kumar V, Verma RK, Yadav SK et al (2020) Crispr-cas9 mediated genome editing of drought and salt tolerance (osdst) gene in indica mega rice cultivar mtu1010. Physiol Mol Biology Plants 26:1099–1110
Schmidt SM, Belisle M, Frommer WB (2020) The evolving landscape around genome editing in agriculture: many countries have exempted or move to exempt forms of genome editing from gmo regulation of crop plants. EMBO Rep 21(6):e50680
Schmidt MJ, Gupta A, Bednarski C et al (2021) Improved crispr genome editing using small highly active and specific engineered rna-guided nucleases. Nat Commun 12(1):4219
Shim Y, Seong G, Choi Y et al (2023) Suppression of cuticular wax biosynthesis mediated by rice lov kelch repeat protein 2 supports a negative role in drought stress tolerance. Plant, Cell & Environment
Tabassum J, Ahmad S, Hussain B et al (2021) Applications and potential of genome-editing systems in rice improvement: current and future perspectives. Agronomy 11(7):1359
Tang X, Liu G, Zhou J et al (2018) A large-scale whole-genome sequencing analysis reveals highly specific genome editing by both cas9 and cpf1 (cas12a) nucleases in rice. Genome Biol 19(1):1–13
Tao H, Shi X, He F et al (2021) Engineering broad-spectrum disease–resistant rice by editing multiple susceptibility genes. J Integr Plant Biol 63(9):1639–1648
Tao J, Bauer DE, Chiarle R (2023) Assessing and advancing the safety of crispr-cas tools: from DNA to rna editing. Nat Commun 14(1):212
Távora FTP (2021) Development of blast resistant rice plants using crispr/cas9 system for genome editing
Távora FT, Meunier AC, Vernet A et al (2022) Crispr/cas9-targeted knockout of rice susceptibility genes osdja2 and oserf104 reveals alternative sources of resistance to pyricularia oryzae. Rice Sci 29(6):535–544
Teng Y, Lv M, Zhang X et al (2022) Bear1, a bhlh transcription factor, controls salt response genes to regulate rice salt response. J Plant Biology 65(3):217–230
Tezuka D, Matsuura H, Saburi W et al (2021) A ubiquitously expressed udp-glucosyltransferase, ugt74j1, controls basal salicylic acid levels in rice. Plants 10(9):1875
Tianshun Z, Dong Y, Ling L et al (2021) Crispr/cas9-mediatedediting of afp1improves rice stress tolerance. Chin J Rice Sci 35(1):11
Tripathy SP, Majhi PK, Patra B et al (2021) Editing the genome for salt tolerance in rice
Tun W, Yoon J, Vo KTX et al (2023) Sucrose preferentially promotes expression of oswrky7 and ospr10a to enhance defense response to blast fungus in rice
Turnbull C, Lillemo M, Hvoslef-Eide TA (2021) Global regulation of genetically modified crops amid the gene edited crop boom–a review. Front Plant Sci 12:630396
Union E (2023a) New techniques in biotechnology
Union E (2023b) Regulation of the european parliament and of the council on plants obtained by certain new genomic techniques and their food and feed, and amending regulation (eu) 2017/625 Brussels
Usman B, Nawaz G, Zhao N et al (2020) Precise editing of the ospyl9 gene by rna-guided cas9 nuclease confers enhanced drought tolerance and grain yield in rice (oryza sativa l.) by regulating circadian rhythm and abiotic stress responsive proteins. Int J Mol Sci 21(21):7854
Uyeh DD, Asem-Hiablie S, Park T et al (2021) Could japonica rice be an alternative variety for increased global food security and climate change mitigation? Foods 10(8):1869
Wang B, Fang R, Chen F et al (2020a) A novel ccch-type zinc finger protein saw1 activates osga20ox3 to regulate gibberellin homeostasis and anther development in rice. J Integr Plant Biol 62(10):1594–1606
Wang B, Zhong Z, Wang X et al (2020b) Knockout of the osnac006 transcription factor causes drought and heat sensitivity in rice. Int J Mol Sci 21(7):2288
Wang F, Itai RN, Nozoye T et al (2020c) The bhlh protein osiro3 is critical for plant survival and iron (fe) homeostasis in rice (oryza sativa l.) under fe-deficient conditions. Soil Sci Plant Nutr 66(4):579–592
Wang K, An W, Liu Y et al (2020d) Disruption of osrhogdi2 by crispr/cas9 technology leads to semi-dwarf in rice. Sheng wu Gong Cheng xue bao = chinese. J Biotechnol 36(4):707–715
Wang F, Xu Y, Li W et al (2021a) Creating a novel herbicide-tolerance osals allele using crispr/cas9-mediated gene editing. Crop J 9(2):305–312
Wang W, Ma S, Hu P et al (2021b) Genome editing of rice eif4g loci confers partial resistance to rice black-streaked dwarf virus. Viruses 13(10):2100
Wang X, Li J, Li F et al (2021c) Rice potassium transporter oshak8 mediates k + uptake and translocation in response to low k + stress. Front Plant Sci 12:730002
Wang Z, Chen D, Sun F et al (2021d) Argonaute 2 increases rice susceptibility to rice black-streaked dwarf virus infection by epigenetically regulating hexokinase 1 expression. Mol Plant Pathol 22(9):1029–1040
Wang C, Feng X, Yuan Q et al (2022a) Upgrading the genome of an elite japonica rice variety kongyu 131 for lodging resistance improvement. Plant Biotechnology Journal
Wang X, Ren P, Ji L et al (2022b) Osvde, a xanthophyll cycle key enzyme, mediates abscisic acid biosynthesis and negatively regulates salinity tolerance in rice. Planta 255:1–15
Wang Z, Zhou L, Lan Y et al (2022c) An aspartic protease 47 causes quantitative recessive resistance to rice black-streaked dwarf virus disease and southern rice black–streaked dwarf virus disease. New Phytol 233(6):2520–2533
Wei H, Wang X, He Y et al (2021a) Clock component osprr73 positively regulates rice salt tolerance by modulating oshkt2; 1-mediated sodium homeostasis. EMBO J 40(3):e105086
Wei Z, Abdelrahman M, Gao Y et al (2021b) Engineering broad-spectrum resistance to bacterial blight by crispr-cas9-mediated precise homology directed repair in rice. Mol Plant 14(8):1215–1218
Wu Y, Xiao N, Cai Y et al (2023) Crispr/cas9-mediated editing of oshppd 3’-utr confers enhanced resistance to hppd-inhibiting herbicide in rice. Plant communications:100605
Xie Y, Wang Y, Yu X et al (2022) Sh3p2, an sh3 domain-containing protein that interacts with both pib and avrpib, suppresses effector-triggered, pib-mediated immunity in rice. Mol Plant 15(12):1931–1946
Xu Y, Wang F, Chen Z et al (2020) Intron-targeted gene insertion in rice using crispr/cas9: a case study of the pi-ta gene. Crop J 8(3):424–431
Xu J, Wang X, Zu H et al (2021a) Molecular dissection of rice phytohormone signaling involved in resistance to a piercing-sucking herbivore. New Phytol 230(4):1639–1652
Xu R, Liu X, Li J et al (2021b) Identification of herbicide resistance osacc1 mutations via in planta prime-editing-library screening in rice. Nat Plants 7(7):888–892
Xu X, Xu Z, Li Z et al (2021c) Increasing resistance to bacterial leaf streak in rice by editing the promoter of susceptibility gene ossulrt3; 6. Plant Biotechnol J 19(6):1101
Xu X, Xu Z, Ma W et al (2021d) Tale-triggered and itale-suppressed xa1 resistance to bacterial blight is independent of ostfiiaγ1 or ostfiiaγ5 in rice. Journal of Experimental Botany
Xu Y, Yan S, Jiang S et al (2023) Identification of a rice leaf width gene narrow leaf 22 (nal22) through genome-wide association study and gene editing technology. Int J Mol Sci 24(4):4073
Yamaguchi K, Yamamoto T, Segami S et al (2020) Gw2 mutation increases grain width and culm thickness in rice (oryza sativa l). Breed Sci 70(4):456–461
Yang J, Ji L, Liu S et al (2021) The cam1-associated ccamk–mkk1/6 cascade positively affects lateral root growth via auxin signaling under salt stress in rice. J Exp Bot 72(18):6611–6627
Yang L, Machin F, Wang S et al (2023) Heritable transgene-free genome editing in plants by grafting of wild-type shoots to transgenic donor rootstocks. Nat Biotechnol :1–10
Yu Q, Chen L, Zhou W et al (2020) Rsd1 is essential for stomatal patterning and files in rice. Front Plant Sci 11:600021
Yu K, Liu Z, Gui H et al (2021) Highly efficient generation of bacterial leaf blight-resistant and transgene-free rice using a genome editing and multiplexed selection system. BMC Plant Biol 21(1):1–10
Yudong C, Xiangyi X, Naizhong Y et al (2021) Auxin regulator osgrf4 simultaneously regulates rice grain shape and blast resistance. Chin J Rice Sci 35(6):629
Yue E, Cao H, Liu B (2020) Osmir535, a potential genetic editing target for drought and salinity stress tolerance in oryza sativa. Plants 9(10):1337
Yue E, Rong F, Liu Z et al (2023) Cadmium induced a non-coding rna microrna535 mediates cd accumulation in rice. J Environ Sci 130:149–162
Zafar K, Khan MZ, Amin I et al (2020) Precise crispr-cas9 mediated genome editing in super basmati rice for resistance against bacterial blight by targeting the major susceptibility gene. Front Plant Sci 11:575
Zafar K, Khan MZ, Amin I et al (2023) Employing template directed crispr-based editing of the osals gene to create herbicide tolerance in basmati rice. AoB PLANTS
Zaman QU, Raza A, Gill RA et al (2023) New possibilities for trait improvement via mobile crispr-rna. Trends in Biotechnology
Zeng X, Luo Y, Vu NTQ et al (2020a) Crispr/cas9-mediated mutation of ossweet14 in rice cv. Zhonghua11 confers resistance to xanthomonas oryzae pv. Oryzae without yield penalty. BMC Plant Biol 20(1):1–11
Zeng Y, Wen J, Zhao W et al (2020b) Rational improvement of rice yield and cold tolerance by editing the three genes ospin5b, gs3, and osmyb30 with the crispr–cas9 system. Front Plant Sci 10:1663
Zhai XY, Chen ZJ, Liu J et al (2022) Expression of cyp76c6 facilitates isoproturon metabolism and detoxification in rice. J Agric Food Chem 70(15):4599–4610
Zhang H, Li L, He Y et al (2020a) Distinct modes of manipulation of rice auxin response factor osarf17 by different plant rna viruses for infection. Proceedings of the National Academy of Sciences 117(16):9112–9121
Zhang Y, Wang X, Luo Y et al (2020b) Osaba8ox2, an aba catabolic gene, suppresses root elongation of rice seedlings and contributes to drought response. Crop J 8(3):480–491
Zhang R, Chen S, Meng X et al (2021a) Generating broad-spectrum tolerance to als-inhibiting herbicides in rice by base editing. Sci China Life Sci 64:1624–1633
Zhang Y, Ren Q, Tang X et al (2021b) Expanding the scope of plant genome engineering with cas12a orthologs and highly multiplexable editing systems. Nat Commun 12(1):1944
Zhang M, Zhao R, Huang K et al (2022a) The oswrky63–oswrky76–osdreb1b module regulates chilling tolerance in rice. Plant J 112(2):383–398
Zhang X, Liu D, Gao D et al (2022b) Cytokinin confers brown planthopper resistance by elevating jasmonic acid pathway in rice. Int J Mol Sci 23(11):5946
Zhang A, He H, Li Y et al (2023a) Mads-box subfamily gene gmap3 from glycine max regulates early flowering and flower development. Int J Mol Sci 24(3):2751
Zhang M, Zhao R, Huang K et al (2023b) Oswrky76 positively regulates drought stress via osbhlh148-mediated jasmonate signaling in rice
Zhao F-J, Ma Y, Zhu Y-G et al (2015) Soil contamination in china: current status and mitigation strategies. Environ Sci Technol 49(2):750–759
Zhao W, Xiao W, Sun J et al (2022) An integration of microrna and transcriptome sequencing analysis reveal regulatory roles of mirnas in response to chilling stress in wild rice. Plants 11(7):977
Zhou Y, Xu S, Jiang N et al (2022) Engineering of rice varieties with enhanced resistances to both blast and bacterial blight diseases via crispr/cas9. Plant Biotechnol J 20(5):876–885
Zhu Z, Yin J, Chern M et al (2020) New insights into bsr-d1–mediated broad–spectrum resistance to rice blast. Mol Plant Pathol 21(7):951–960
Acknowledgements
Not applicable.
Funding
This research was financially supported by the National Natural Science Foundation of China (32188102), Zhejiang Provincial Science and Technology Project (2020R51007), and Intelligent Technology and Platform Development for Rice Breeding (2021PE0AC05).
Author information
Authors and Affiliations
Contributions
Conceptualization, S.A. and G.S.; Methodology, S.A.; Validation, S.A., S.S.A. and G.S.; Resources, S.A. and G.S.; Data Curation, N.S. and S.A.; Writing – Original Draft Preparation, N.S. and S.A.; Writing – Review & Editing, S.S.A., H.M.R., M.A.J., J.T., and G.S.; Visualization & Supervision, S.A.; Funding Acquisition, G.S.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shaheen, N., Ahmad, S., Alghamdi, S.S. et al. CRISPR-Cas System, a Possible “Savior” of Rice Threatened by Climate Change: An Updated Review. Rice 16, 39 (2023). https://doi.org/10.1186/s12284-023-00652-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12284-023-00652-1