Abstract
Background
The protein tyrosine phosphatase 1B (PTP1B) plays a crucial role in the development of insulin resistance. Aerobic training (AT) and vitamin D (Vit D) supplementation have been shown to individually improve glucose tolerance and diabetes-related factors. However, the impact of their combined effect on PTP1B gene expression and serum irisin in the visceral adipose tissue remains unknown. This study aims to investigate whether 8 weeks of combined AT with Vit D supplementation can improve the expression of PTP1B in adipose tissue and serum irisin in obese rats with type 2 diabetes (T2D).
Methods
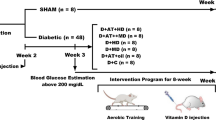
Fifty male Wistar rats were divided into two groups: diabetic (n = 40) and non-diabetic (ND; n = 10). The diabetic rats were further divided into four groups: aerobic training with vitamin D supplementation (D + AT + Vit D; n = 10), aerobic training only (D + AT; n = 10), vitamin D supplementation only (D + Vit D; n = 10), and control (D + C; n = 10). The D + Vit D and D + AT + Vit D groups received 5000 IU of vitamin D via injection once a week, while the D + AT and D + C groups received sesame oil. Diabetes was induced in all groups except the nondiabetic group by intraperitoneal (IP) injection of streptozotocin. At the end of the intervention, blood and adipose tissue samples were collected, and RNA was extracted from adipose tissue for real-time PCR analysis of PPTP1B gene expression.
Results
There was an increase in serum Vit D and irisin levels and a decrease in HOMA-IR and PTP1B gene expression in the diabetic rat model treated with D + AT and injected with 50,000 IU/kg/week of Vit D. Comparatively, when treated with D + AT + Vit D, the downregulation of PTP1B was significantly higher (p = 0.049; p = 0.004), and there was a significant increase in irisin (p = 0.010; p = 0.001).
Conclusion
The present study shows that the combined AT and Vit D supplementation positively impacts the expression of PTP1B in adipose tissue and serum irisin in rats with T2D. These findings suggest that combining AT with Vit D supplementation can provide a new and effective strategy to improve glucose tolerance and diabetes-related factors in individuals with T2D by regulating the expression of PTP1B in adipose tissue and promoting the synthesis of beneficial irisin protein.
Similar content being viewed by others
Introduction
Type 2 diabetes (T2D) is a metabolic condition characterized by hyperglycemia resulting from a combination of insufficient insulin secretion and resistance to insulin action [1]. The Iranian adult population has a prevalence of almost 8% of T2D [2], and approximately 258 million cases were diagnosed with T2D in 2010 worldwide [3]. Genetic mutations and heredity, as well as obesity and obesity-related abnormalities, are among the factors that contribute to the pathogenesis of T2D [4]. Although the disease has multifactorial origins, obesity is one of the primary risk factors that can lead to insulin resistance and the development of T2D. This correlation has been supported by previous research studies [4, 5].
Current research emphasizes identifying genetic factors that contribute to obesity and T2D. Recently, several genetic factors that contribute to the prevalence and severity of both type 1 and type 2 diabetes have been recognized. Impaired expression or polymorphisms of these genes can result in damaged insulin secretion and function [6]. Protein tyrosine phosphatase 1B (PTP1B), a key member of the PTP family, has been identified as a negative regulator of insulin signaling [7] and a potential therapeutic target for T2D [8]. PTP1B has been shown to cause interference with insulin receptor substrate-1 (IRS-1), thereby impairing glucose uptake by degradation of insulin signaling pathways [7]. On the other hand, elevated serum levels of irisin, an important regulator of insulin signaling, have been associated with increased ER (endoplasmic reticulum) stress leading to insulin resistance. This connection has been established through several studies [9, 10]. Irisin is a myokine that is triggered by exercise and is believed to increase energy expenditure by brown adipose tissue [11]. Studies suggest that the browning process could be in response to physical activity and a cold environment, with the upregulation of peroxisome proliferator-activated receptor-gamma (PPARγ) coactivator 1 alpha (PGC-1α) leading to increased expression of the fibronectin type 3 domain containing 5 (FNDC5) gene, which encodes the precursor protein for irisin production [12, 13]. Studies suggest that inhibition of PTP1B and increased serum levels of irisin can lead to improvement in insulin resistance, as well as insulin and blood glucose levels [8, 10]. Several studies have investigated the effects of aerobic training (AT), either alone or in combination with diet management, on hormonal and genetic factors that impact insulin function in nondiabetic and diabetic subjects [14, 15]. Additionally, while some studies have shown that AT can have a significant effect on the expression or protein levels of genes that impact insulin secretion and function, such as transcription factor 7-like 2 (TCF7L2) [4], forkhead box O1 (FOXO1) [16], and PPARγ [17], the direct effect of AT on PTP1B expression in adipose tissue and serum irisin levels in diabetic patients remains understudied. Along with various suggested strategies for metabolic control of diabetes [14, 15], the use of antioxidants like vitamins E, A, and C and coenzyme Q10 have also been proposed [18]. The use of vitamin D (Vit D) to decrease metabolic profiles [19], inflammatory factors [20], and biomarkers of oxidative stress has recently gained attention [21, 22]. Vit D appears to improve insulin sensitivity, which in turn influences metabolic profiles [22]. Limited research is available on the effects of Vit D on the metabolic profiles of diabetic patients. In rats, a diet supplemented with Vit D has been shown to significantly delay the progression of glucose intolerance, hyperglycemia, hyperinsulinemia, dyslipidemia, and oxidative stress [17]. Previous studies have mainly investigated the individual effects of AT and Vit D on glucose tolerance and diabetes-related factors in skeletal muscle. To our knowledge, limited reports exist regarding the effects of combined AT and Vit D on PTP1B expression in adipose tissue and serum irisin levels. While previous research has established the benefits of separate AT and Vit D supplementation, the long-term effects of their combination on PTP1B gene expression in visceral adipose tissue and serum irisin remain unclear. Therefore, the purpose of this study was to investigate the effect of an 8-week combined AT and Vit D supplementation on PTP1B gene expression and serum irisin levels in the visceral adipose tissue of obese rats with T2D.
Methods
Ethical approval
This study followed the scientific protocol, being randomized, single blinded, and placebo controlled. Registration details of the study can be found on the Iranian Registry of clinical trials at http://www.irct.ir with the identifier number IRCT20220510054809N1. The study design and conduction were under the approval and supervision of the Research Ethics Committee of Razi University (no. IR.RAZI.REC.1401.013), Iran. The care, maintenance, and sacrifice of animals were conducted in adherence with the Danish “Animal Welfare Act” (LBK 1343 of 04/12/2007).
Animals
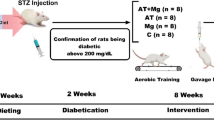
In this experimental research, a total of 50 Wistar rats were used. These rats were adult males, aged 4–5 weeks, and weighed approximately 180 ± 10 g. They were procured from laboratory animals, Kermanshah, Iran. The rats were categorized into two main groups, comprising 40 diabetic rats and 10 nondiabetic (ND) rats. The diabetic rats were induced with diabetes using intraperitoneal streptozotocin (STZ) and nicotinamide along with a high-fat diet. The 40 diabetic rats were further split into four subgroups: AT with Vit D supplementation (D + AT + Vit D; n = 10), AT only (D + AT; n = 10), Vit D supplementation only (D + Vit D; n = 10), and control (D + C; n = 10). The rats in the D + Vit D and D + AT + Vit D groups were given 5000 IU of Vit D by injection once a week, while rats in the D + AT and D + C groups were given sesame oil instead. Additionally, the D + AT and D + AT + Vit D groups underwent an 8-week AT routine, 5 days a week. All rats were kept in transparent polycarbonate cages. The cages were 30 cm in length and 15 cm in width and height. The animals were maintained under a 12-h light/dark cycle, with a temperature of 25 ± 2 °C and humidity of 45–55% [23, 24]. They had free access to food and water in 500-ml bottles, and ethical principles of working with laboratory animals were followed throughout the study.
Obesity induction and diabetes induction method
The male Wister rats used in the study were acclimatized to a new environment for 2 weeks before beginning the intervention program. These rats were given a high-fat diet in the form of pellets, which contained standard mouse food powder (365 mg/kg), sheep fat (310 mg/kg), mixed vitamins and minerals (60 mg/kg), DL methionine (3 mg/kg), yeast powder (1 mg/kg), and chloride sodium (1 mg/kg) to increase their weight. Once their weight exceeded 300 g after 2 weeks, the rats were induced with diabetes by injecting 60 mg/kg BW of streptozotocin dissolved in 0.1-M citrate buffer with pH = 4.5, followed by 110 mg/kg BW of nicotinamide after 15 min intraperitoneally. The induction of T2D was confirmed by measuring blood sugar using a glucometer 2 weeks later. Blood samples were collected from the lateral tail vein after immersing it in 42 °C water for 40–50 s, and blood sugar above 200 mg/l was considered an indicator of diabetes [25]. No insulin treatment was provided to the animals throughout the study.
Intervention programs
Vitamin D supplementation
The study included two groups, D + AT + Vit D and D + Vit D, which received 5000 international units (IU) of Vit D once a week through a single-dose injection. In contrast, the D + AT and D + C groups were injected with sesame oil instead of Vit D. To measure Vit D serum concentration, a Vit D enzyme-linked immunosorbent assay (ELISA) rat kit from Immune Diagnostics System Ltd. in Boldon, UK, was used, which had an intraassay coefficient of variation of 1.63% and method sensitivity of 1.33 mg/dL [17].
Exercise protocol
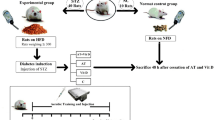
Following diabetes induction, the rats were separated into 4 groups of 10 each, randomized based on body weight. A week before the main AT sessions, rats were subjected to a familiarization AT session on a zero-incline treadmill. The session involved running for 5 min at a speed of 8–10 m/min 5 days a week. The actual AT program spanned over 8 weeks on a zero-incline treadmill with a speed range of 15–25 m/min, for 30–60 min each day, 5 days a week. In the first week, rats ran on the treadmill for 30 min at a speed of 15 m/min, with the AT intensity and duration gradually increasing to reach 25 m/min and 60 min, respectively, by the end of the 8th week. The training intensity equaled 50–60% of VO2max [26]. For both pre- and post-AT, rats were warmed up for 10 min at 5 m/min and cooled down for 10 min. Rats that had stopped running were stimulated to continue running via a combination of sound (from striking the rotating tape wall) and low-voltage electric stimuli. The use of the latter was discontinued after the first week in compliance with laboratory animal ethical guidelines [23, 24] (Fig. 1).
Diagram of the progress through the phases of the parallel randomized trial of four groups (enrolment, intervention allocation, follow-up, and data analysis). D + AT + Vit D, diabetic + aerobic training + vitamin D supplement; D + AT, diabetic + aerobic training; D + Vit D, diabetic + vitamin D supplement; D + C, diabetic + control; NC, nondiabetic control
Measurement variables
Food intake, body weight, and body mass index
At weekly intervals between 9:00 and 11:30 a.m., all animals were weighed using a scale. The nose-to-anus length was measured to calculate the body mass index (BMI). To measure food intake (FI), the weight of uneaten food was subtracted from the total amount (20 g/day) of food given in each cage.
Blood sampling and RNA extraction/real-time PCR
To eliminate the immediate effects of training and to control for uncontrollable variables caused by the training program, animals were anesthetized 48 h after the last training session (46, 15) through an intraperitoneal injection of ketamine (70 mg/kg BW) and xylazine (3–5 mg/kg BW) while considering ethical principles. Blood samples were taken from the vena cava, and plasma concentrations of glucose and insulin were measured via the Hitachi Auto Analyzer (type 7170; Hitachi Electronics, Hitachi, Japan). The homeostatic model assessment for insulin resistance (HOMA-IR) score was calculated by using glucose and insulin concentrations, as follows:
The concentration of irisin was quantified through the use of an ELISA kit manufactured in China, possessing a sensitivity of 0.15 mg/l. Additionally, an ELISA kit was employed to determine the serum concentration of serum 25-hydroxyvitamin D. Furthermore, the visceral adipose tissue was rapidly separated and weighed, washed in saline to prevent RNA degradation, transferred to tubes containing RNA Later, placed in liquid nitrogen, and then preserved in a refrigerator maintained at − 80 °C for subsequent assessments. The RNA present in 20 mg of adipose tissue was isolated using a combined method involving TRI Reagent® (MRC Inc., USA) and miRNeasy techniques (Viragene, Iran). Initially, the frozen tissue was homogenized with a gentleMACS™ Octo Dissociator system and M tubes (Viragene, Iran) using the RNA_02 program along with 2 ml of TRI Reagent® buffer, following the manufacturer’s guidelines. The samples were then allowed to incubate at room temperature for 5 min, followed by centrifugation at 12,000 g at 4 °C for 10 min. After, the desired layer was pipetted into a 1.5-ml tube and mixed with 400 μl of chloroform, followed by a further centrifugation step at 12,000 g at 4 °C for 30 min and keeping the sample at room temperature for 3 min. Next, the upper layer was thoroughly mixed with ethanol by inverting the tube and loaded onto a miRNeasy spin column (Viragene, Iran) according to the manufacturer’s protocol. Finally, RNA was eluted in 30 μl of RNAse-free water. A PrimeScript RT reagent kit (Viragene, Iran) was used for reverse transcription into cDNA using 1 μg of total RNA. TB Green Premix Ex-Taq II (TaKaRa, Dalian, China) was used for quantitative RT-PCR. The expression levels of target genes were calculated using the comparative 2 − ΔΔct method. The Viragene kit from Iran was used to measure the expression of the PTP1B gene using the protein tyrosine phosphatase non-receptor type 1 (NM_002827.4) as a reference gene, and it possessed a sensitivity of 0.034. The sequence of primers utilized for PTP1B can be found in Table 1. All animal testing methods were conducted following the fundamental principles of the 2008 Helsinki Declaration.
Statistical analysis
The normal distribution of the data was examined using the Shapiro–Wilk test. The data was then analyzed utilizing the SPSS 26.0 program (SPSS, Inc., Chicago, IL, USA) through paired sample t-test and one-way ANOVA along with a Tukey post hoc test. Values of p < 0.05 were considered statistically significant.
Results
Table 2 presents the average body weight, FI, and BMI. Following an 8-week intervention, there was a noticeable difference between groups for the average BW, FI, and BMI. The D + AT + Vit D, D + AT, and D + Vit D groups indicated statistically significant reductions in BW, FI, and BMI when compared to the baseline values (p < 0.01). In contrast, the D + C and ND groups reported significant increases in the mean BW, FI, and BMI towards the end of the study in comparison to the commencement of the study.
Figure 2 (A, B, C, and D) presented statistically significant differences in insulin, glucose, HOMA-IR, and visceral fat among the groups, with the lowest levels observed in the ND group and the highest in the D + C group. Additionally, D + AT + Vit D, D + AT, and D + Vit D resulted in lower levels of insulin, glucose, HOMA-IR, and visceral fat compared to the D + C group. The results showed that D + AT + Vit D showed significantly lower insulin (p = 0.003), HOMA-IR (p = 0.012), and visceral fat (p = 0.001) when compared to D + AT. There were no significant differences in blood glucose levels between D + AT + Vit D and D + AT (p = 0.091); however, a significant difference was noted in insulin (p = 0.014), glucose (p = 0.008), HOMA-IR (p = 0.001), and visceral fat (p = 0.001) when comparing the D + AT + Vit D group to D + Vit D (p < 0.05 for all three variables). Moreover, D + AT induced a more significant decrease in insulin (p = 0.026), glucose (p = 0.006), HOMA-IR (p = 0.001), and visceral fat (p = 0.007) than D + Vit D. The ND group showed significant differences in insulin, glucose, HOMA-IR, and visceral fat when compared to the other groups (p < 0.05 for all three variables).
Comparison of mean ± SD of insulin, glucose, HOMA-IR, and visceral fat after the intervention among the groups. D + AT + Vit D, diabetic + aerobic training + vitamin D supplement; D + AT, diabetic + aerobic training; D + Vit D, diabetic + vitamin D supplement; D + C, diabetic + control; NC, nondiabetic control. αSignificantly different compared to AT. ¥Significantly different compared to Vit D. €Significantly different compared to C. £Significantly different compared to NC
The results in Fig. 3 indicate a statistically significant difference in serum 25-hydroxyvitamin D and serum irisin levels among the groups, with the highest levels observed in the ND and D + AT + Vit D groups and the lowest level in the D + C group. D + AT + Vit D, D + AT, and D + Vit D led to increased levels of serum 25-hydroxyvitamin D and serum irisin compared to the D + C group. The results showed that D + AT + Vit D significantly increased serum 25-hydroxyvitamin D (p = 0.006; p = 0.001) compared to D + AT and D + C, respectively. No significant difference was observed in the serum 25-hydroxyvitamin D levels between D + AT + Vit D and D + Vit D (p = 0.286). Additionally, D + AT + Vit D significantly upregulated serum irisin (p = 0.002; p = 0.001, p = 0.001 compared to D + AT, D + Vit D, and C, respectively. Furthermore, D + Vit D induced a more significant increase in the serum 25-hydroxyvitamin D (p = 0.001) than D + AT, while D + AT significantly upregulated serum irisin (p = 0.011) compared to D + Vit D. There was a significant difference in the levels of serum 25-hydroxyvitamin D and serum irisin observed between the ND group and the other groups (p < 0.05 for all three variables).
Comparison of mean ± SD of serum 25-hydroxyvitamin D and irisin after the intervention among the groups. D + AT + Vit D, diabetic + aerobic training + vitamin D supplement; D + AT, diabetic + aerobic training; D + Vit D, diabetic + vitamin D supplement; D + C, diabetic + control; NC, nondiabetic control. Values were calculated using a one-way analysis of variance followed by post hoc Tukey’s test. αSignificantly different compared to AT. ¥Significantly different compared to Vit D. €Significantly different compared to C. £Significantly different compared to NC
Figure 4 displays the results of PTP1B gene expression. A significant difference in PTP1B gene expression was observed among all groups, as determined by one-way ANOVA. Furthermore, D + AT + Vit D, D + AT, and D + Vit D resulted in decreased levels of PTP1B compared to D + C. Based on the results, D + AT + Vit D significantly downregulated PTP1B when compared to D + AT and D + Vit D (p = 0.049; p = 0.004), respectively. Also, D + AT induced a more significant decrease in PTP1B (p = 0.001) compared to D + Vit D. Additionally, there was a significant difference in PTP1B gene expression observed between the ND group and the other groups (p < 0.05 for all three variables).
Comparison between mean ± SD of PTP1B gene expression between groups. D + AT + Vit D, diabetic + aerobic training + vitamin D supplement; D + AT, diabetic + aerobic training; D + Vit D, diabetic + vitamin D supplement; D + C, diabetic + control; NC, nondiabetic control. Value was calculated using a one-way analysis of variance followed by post hoc Tukey’s test. αSignificantly different compared to AT. ¥Significantly different compared to Vit D. €Significantly different compared to C. £Significantly different compared to NC
Discussion
Our study demonstrated that in rats with T2D, combining AT with Vit D for 8 weeks resulted in significant improvements in measures including body weight (BW), body mass index (BMI), visceral fat, insulin, fasting blood glucose, and HOMA-IR. In comparison, separate AT and Vit D treatments showed less improvement. The D + C group showed a significant increase in several measures including BW, BMI, visceral fat, insulin, fasting blood glucose, and HOMA-IR when compared to other groups. Diabetic patients often exhibit insulin resistance, dyslipidemia, and increased systemic inflammation and oxidative stress, which can result in various complications such as diabetic retinopathy, cardiovascular disease (CVD), hypertension, neuropathy, and nephropathy [27, 28]. Previous studies have examined the impact of AT and Vit D on insulin, fasting blood glucose, and HOMA-IR in human subjects with T2D [29] and elderly women with nonalcoholic fatty liver disease (NAFLD) [30]. Similar investigations have also been conducted in animal models, and the outcomes of this study were consistent with other studies [17, 31]. For instance, a study by Hoseini et al. demonstrated that administering AT and Vit D to obese rats improved BW, BMI, and insulin resistance after 8 weeks [32]. Similar results have been reported in multiple experimental studies [33, 34]. The mechanisms by which AT may result in these improvements include increased insulin receptor signaling, increased glucose transport protein-4 (GLUT-4), increased muscular capillaries and mitochondria, and glucose uptake, resulting in increased glycogen synthase and hexokinase enzyme activity [35]. AT also improves free fatty acid homeostasis [36] in serum and muscle tissue, uses it as a metabolic substrate, and reduces body weight and visceral fat [37]. Vit D has been shown to boost insulin production in the pancreas by triggering Vit D receptors (VDR) that are present in the pancreas and skeletal muscle cells [38]. It also enhances the amount of GLUT 4 by working on the skeletal muscle cells [39]. Additionally, Vit D reduces the concentration of inflammatory factors that lead to insulin resistance by suppressing the nuclear factor kappa-light-chain-enhancer of activated b cells (NF-Κb) gene expression [40, 41]. The study found significantly higher levels of serum irisin in the experimental groups compared to the control group after 8 weeks, with the AT + Vit D group experiencing a significantly higher increase than the AT and Vit D alone. The overexpression of irisin in obese mice has been linked to improved insulin sensitivity, enhanced energy expenditure, and reduced hyperlipidemia, hyperglycemia, and hypertension [42]. Irisin treatment of muscle cells stimulates glucose and fatty acid uptake, resembling the metabolic impact of insulin. It also increases the expression of genes such as GLUT4 and PPARalpha, which are involved in glycogenolysis and gluconeogenesis, respectively. In obese individuals, the production of irisin and FNDC5 is increased, enhancing glucose absorption by muscle cells and preventing high blood sugar levels [10, 43]. However, in individuals with diabetes, the amount of FNDC5 in muscle cells decreases by approximately 15%. Studies have confirmed that individuals with prediabetes or T2D have lower levels of irisin [44, 45]. The inflammatory response has been identified as a possible contributing factor to decreased levels of irisin. The precise biological mechanism underlying the higher levels of irisin in obesity and reduced irisin secretion in diabetes remains unclear.
Several previous studies have shown that both adipose and muscle tissues contribute significantly to the elevation of serum irisin levels after exercise training [46, 47]. Irisin levels increase briefly following an exercise session and can reduce both the weight of obese individuals and the insulin resistance of diabetic patients [48]. High levels of serum irisin have been associated with a lower risk of high BMI and coronary atherosclerosis in different population studies [49, 50]. Short-term exercise-induced irisin elevation leads to various positive metabolic changes [51, 52]. Lipid tissue is responsible for the baseline level of irisin in the blood of obese individuals [53]. Recent studies have highlighted the presence of the Vit D receptor in β cells of the pancreatic tissue, with variations in genes associated with Vit D metabolism and VDR expression linked to the pathogenesis of T1DM and T2D [53, 54]. Vit D-deficient mice have impaired insulin secretion to glucose stimulation, which can be improved upon administration of Vit D3. Moreover, Vit D has been observed to have a beneficial effect on glycemic control [54].
According to the findings of our study, the levels of irisin in the serum were increased with Vit D supplementation [55]. This is supported by previous studies which showed that 12 months of Vit D supplementation led to a significant increase in serum irisin levels in albino Vit D-deficient rats and Vit D-deficient subjects, respectively [56]. However, a previous intervention involving healthy subjects found that a single dose of 100,000 IU of Vit D did not result in significant changes in serum irisin levels following 28 days of intervention [57]. The precise mechanism underlying the effect of Vit D on the secretion of irisin and expression of FNDC5 remains to be fully elucidated. However, a recent study has shown that Vit D supplementation and exercise can increase irisin levels in diabetic rats, indicating the potential therapeutic applications of Vit D supplementation, irisin administration, and exercise in the treatment of diabetes. This study also found that PTP1B expression was lower in the Vit D group. This is consistent with recent evidence suggesting that Vit D can act as a stimulator to decrease PTP1B activity and gene expression [58, 59]. The PTP1B gene is of particular significance in the context of insulin function and the treatment of T2D [60]. It has been identified as a regulator of insulin function in mammals and a pharmacological target for the treatment of T2D. The results of the current study demonstrate that Vit D in combination with exercise improves insulin resistance and reduces levels of blood glucose in T2D rats. These beneficial effects are associated with a decrease in the expression of PTP1B, an enzyme that plays a key role in insulin signaling by catalyzing the dephosphorylation of the insulin receptor (IR) [61]. The expression of PTP1B can be augmented by alterations in protein tyrosine kinases (PTKs) activity [62], which leads to impaired binding of insulin receptors to target tissues such as those observed in T2D, obesity, insulin resistance, and leptin deficiency [63]. Previous studies have also demonstrated that knocking out the PTP1B gene in rats is associated with reduced fatty tissue, increased energy consumption, and greater insulin sensitivity [64, 65]. Therefore, noninvasive interventions such as exercise or dietary modifications that lower PTP1B expression could help reduce insulin resistance levels, enhance insulin function, and improve glycemic control. To this end, the study found that the combination of AT and Vit D resulted in a greater reduction in PTP1B expression compared to AT or Vit D alone, potentially offering a promising intervention strategy.
The findings of the study suggest that decreasing the expression of PTP1B in adipose tissue through AT and Vit D intake can potentially result in lower insulin resistance and improved glycemic control. Prior studies have also shown that a reduction in PTP1B expression can affect insulin signaling pathways and improve insulin sensitivity. Specifically, PTP1B-deficient rats exhibit decreased TNF-α-dependent insulin resistance and increased insulin sensitivity in adipose tissue and skeletal muscles. Additionally, deleting PTP1B in the muscles of rats that consume a high-fat diet can improve glucose uptake and insulin signaling in skeletal muscles [66]. Previous research suggests that high levels of PTP1B induced by endoplasmic reticulum (ER) stress via ROS-NF-κB activation can increase proteins that stimulate insulin resistance in obesity. The AT and Vit D combination may reduce PTP1B levels and increase irisin levels in the serum, potentially leading to improved insulin function in target tissues probably by inhibiting ER stress induced by high-fat diets and free radicals. Therefore, decreased PTP1B expression may offer a promising intervention strategy for managing insulin resistance and glycemic control in T2D.
Strengths and limitations of the study
Our research employed a randomized, placebo-controlled trial in an animal model of diabetes to investigate gene expression changes and had several strengths including no dropouts. However, it is too early to make conclusive statements. One of the limitations of our study was the absence of data on the expression of the FND5 gene in skeletal muscle and fat, which could have helped to determine the source of circulating irisin. Another limitation was the lack of evaluation of other significant transcriptional and posttranscriptional factors.
Conclusion
Our study found that 8 weeks of AT with Vit D supplementation resulted in a reduction in HOMA-IR in rats with T2D. This decline was linked to decreases in body weight, BMI, and visceral fat, as well as an increase in serum irisin levels and improvement in PTP1B-dependent insulin function in adipose tissue in response to AT and Vit D. Nevertheless, more research is necessary to elucidate the genetic mechanisms underlying insulin function in response to AT with Vit D.
Availability of data and materials
The datasets generated and analyzed during the current study are not publicly available due to ongoing data analysis but are available from the corresponding author upon reasonable request.
Abbreviations
- AT:
-
Aerobic training
- BMI:
-
Body mass index
- ELISA:
-
Enzyme-linked immunosorbent assay
- ER:
-
Endoplasmic reticulum
- FI:
-
Food intake
- FNDC5:
-
Fibronectin type 3 domain containing 5
- FOXO1:
-
Forkhead box O1
- HOMA-IR:
-
Homeostatic model assessment for insulin resistance
- PGC-1α:
-
Peroxisome proliferator-activated receptor-gamma coactivator-1 alpha
- PPARγ:
-
Peroxisome proliferator-activated receptor-gamma
- PTKs:
-
Protein tyrosine kinases
- PTP1B:
-
Protein tyrosine phosphatase 1B
- T2D:
-
Type 2 diabetes
- TCF7L2:
-
Transcription factor 7-like 2
- Vit D:
-
Vitamin D
References
Li Y, Guo H. Subcutaneous implanted system for the treatment of type 2 diabetes. Zhongguo yi xue ke xue yuan xue bao Acta Academiae Medicinae Sinicae. 2011;33(4):473–7.
Moradi Y, Baradaran HR, Djalalinia S, Chinekesh A, Khamseh ME, Dastoorpoor M, et al. Complications of type 2 diabetes in Iranian population: an updated systematic review and meta-analysis. Diabetes Metab Syndr. 2019;13(3):2300–12.
Neumann A, Schwarz P, Lindholm L. Estimating the cost-effectiveness of lifestyle intervention programmes to prevent diabetes based on an example from Germany: Markov modelling. Cost Eff Resour Alloc. 2011;9(1):1–13.
Eizadi M, Ravasi AA, Soory R, Baesi K, Choobineh S. The effect of three months of resistance training on TCF7L2 expression in pancreas tissues of type 2 diabetic rats. Avicenna J Med Biochem. 2016;4(1):12–34014.
Lazar MA. How obesity causes diabetes: not a tall tale. Science. 2005;307(5708):373–5.
Ruchat S-M, Rankinen T, Weisnagel S, Rice T, Rao D, Bergman R, et al. Improvements in glucose homeostasis in response to regular exercise are influenced by the PPARG Pro12Ala variant: results from the HERITAGE Family Study. Diabetologia. 2010;53:679–89.
Panzhinskiy E, Ren J, Nair S. Pharmacological inhibition of protein tyrosine phosphatase 1B: a promising strategy for the treatment of obesity and type 2 diabetes mellitus. Curr Med Chem. 2013;20(21):2609–25.
Qin Z, Pandey NR, Zhou X, Stewart CA, Hari A, Huang H, et al. Functional properties of claramine: a novel PTP1B inhibitor and insulin-mimetic compound. Biochem Biophys Res Commun. 2015;458(1):21–7.
Lin J, Liu X, Zhou Y, Zhu B, Wang Y, Cui W, et al. Molecular basis of irisin regulating the effects of exercise on insulin resistance. Appl Sci. 2022;12(12):5837.
Yano N, Zhang L, Wei D, Dubielecka PM, Wei L, Zhuang S, et al. Irisin counteracts high glucose and fatty acid-induced cytotoxicity by preserving the AMPK-insulin receptor signaling axis in C2C12 myoblasts. Am J Physiol Endocrinol Metab. 2020;318(5):E791–805.
Huh JY, Siopi A, Mougios V, Park KH, Mantzoros CS. Irisin in response to exercise in humans with and without metabolic syndrome. J Clin Endocrinol Metab. 2015;100(3):E453–7.
Norheim F, Langleite TM, Hjorth M, Holen T, Kielland A, Stadheim HK, et al. The effects of acute and chronic exercise on PGC-1α, irisin and browning of subcutaneous adipose tissue in humans. FEBS J. 2014;281(3):739–49.
Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463–8. https://doi.org/10.1038/nature10777.
Jacobsen E, Boyers D, Manson P, Avenell A. A systematic review of the evidence for non-surgical weight management for adults with severe obesity: what is cost effective and what are the implications for the design of health services?. Curr Obes Rep. 2022;11(4):356–85.
Zanuso S, Jimenez A, Pugliese G, Corigliano G, Balducci S. Exercise for the management of type 2 diabetes: a review of the evidence. Acta Diabetol. 2010;47:15–22.
Soheily S, Eizadi M. Over expression of FOXO1 in subcutaneous fatty tissue and its response to resistance training in high fat diet and type 2 diabetic rat. Iran J Diabetes Obes. 2022;14(2):110–6.
Hoseini R, Damirchi A, Babaei P. Vitamin D increases PPARγ expression and promotes beneficial effects of physical activity in metabolic syndrome. Nutrition. 2017;36:54–9.
Huynh K, Kiriazis H, Du X-J, Love J, Jandeleit-Dahm K, Forbes J, et al. Coenzyme Q 10 attenuates diastolic dysfunction, cardiomyocyte hypertrophy and cardiac fibrosis in the db/db mouse model of type 2 diabetes. Diabetologia. 2012;55:1544–53.
Tang W, Chen L, Ma W, Chen D, Wang C, Gao Y, et al. Association between vitamin D status and diabetic foot in patients with type 2 diabetes mellitus. J Diabetes Investig. 2022;13(7):1213–21.
Gu JC, Wu YG, Huang WG, Fan XJ, Chen XH, Zhou B, et al. Effect of vitamin D on oxidative stress and serum inflammatory factors in the patients with type 2 diabetes. J Clin Lab Anal. 2022;36(5):e24430.
Jamilian M, Mirhosseini N, Eslahi M, Bahmani F, Shokrpour M, Chamani M, et al. The effects of magnesium-zinc-calcium-vitamin D co-supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in gestational diabetes. BMC Pregnancy Childbirth. 2019;19(1):1–8.
Hoseini R, Rahim HA, Ahmed JK. Decreased inflammatory gene expression accompanies the improvement of liver enzyme and lipid profile following aerobic training and vitamin D supplementation in T2DM patients. BMC Endocr Disord. 2022;22(1):1–10.
Mostafavian M, Abdi A, Mehrabani J, Barari A. Effect of eight weeks of aerobic progressive training with capsaicin on changes in PGC-1α and UPC-1 expression in visceral adipose tissue of obese rats with diet. J Complement Med. 2020;10(2):106–17.
Mohammadi K, Khajehlandi A. The effect of a period of swimming training with different temperatures on the expression of β3-AR and ERK2 genes in the visceral adipose tissue of streptozotocin-induced diabetic rats. Feyz, Journal of Kashan University of Medical Sciences. 2022;25(6):1303–12.
Khalili A, Nekooeian AA, Khosravi MB. Oleuropein improves glucose tolerance and lipid profile in rats with simultaneous renovascular hypertension and type 2 diabetes. J Asian Nat Prod Res. 2017;19(10):1011–21.
Rocha-Rodrigues S, Rodríguez A, Gouveia AM, Gonçalves IO, Becerril S, Ramírez B, et al. Effects of physical exercise on myokines expression and brown adipose-like phenotype modulation in rats fed a high-fat diet. Life Sci. 2016;165:100–8.
Adderley NJ, Subramanian A, Toulis K, Gokhale K, Taverner T, Hanif W, et al. Obstructive sleep apnea, a risk factor for cardiovascular and microvascular disease in patients with type 2 diabetes: findings from a population-based cohort study. Diabetes Care. 2020;43(8):1868–77.
Climie RE, van Sloten TT, Bruno R-M, Taddei S, Empana J-P, Stehouwer CD, et al. Macrovasculature and microvasculature at the crossroads between type 2 diabetes mellitus and hypertension. Hypertension. 2019;73(6):1138–49.
Hoseini R, Rahim HA, Ahmed JK. Concurrent alteration in inflammatory biomarker gene expression and oxidative stress: how aerobic training and vitamin D improve T2DM. BMC Complement Med Ther. 2022;22(1):1–13.
Hoseini Z, Behpour N, Hoseini R. Co-treatment with vitamin D supplementation and aerobic training in elderly women with Vit D deficiency and NAFLD: a single-blind controlled trial. Hepat Mon. 2020;20(2):1–11.
da Costa RO, Gadelha-Filho CVJ, de Aquino PEA, Lima LAR, de Lucena JD, Ribeiro WLC, et al. Vitamin D (VD3) intensifies the effects of exercise and prevents alterations of behavior, brain oxidative stress, and neuroinflammation, in hemiparkinsonian rats. Neurochem Res. 2023;48(1):142–60.
Babaei P, Damirchi A, Hoseini R. The interaction effects of aerobic exercise training and vitamin D supplementation on plasma lipid profiles and insulin resistance in ovariectomized rats. J Exerc Nutrition Biochem. 2015;19(3):173–82. https://doi.org/10.5717/jenb.2015.15070703.
Babaei P, Damirchi A, Hoseini R. The interaction effects of aerobic exercise training and vitamin D supplementation on plasma lipid profiles and insulin resistance in ovariectomized rats. J Exerc Nutr Biochem. 2015;19(3):173.
Mehdipoor M, Damirchi A, Razavi Tousi SMT, Babaei P. Concurrent vitamin D supplementation and exercise training improve cardiac fibrosis via TGF-β/Smad signaling in myocardial infarction model of rats. J Physiol Biochem. 2021;77:75–84.
Bruce CR, Hawley JA. Improvements in insulin resistance with aerobic exercise training: a lipocentric approach. Med Sci Sports Exerc. 2004;36(7):1196–201.
Smart N, King N, McFarlane J, Graham P, Dieberg G. Effect of exercise training on liver function in adults who are overweight or exhibit fatty liver disease: a systematic review and meta-analysis. Br J Sports Med. 2018;52(13):834–43.
Dyck DJ. Adipokines as regulators of muscle metabolism and insulin sensitivity. Appl Physiol Nutr Metab. 2009;34(3):396–402.
Wang Y, Zhu J, DeLuca HF. Where is the vitamin D receptor? Arch Biochem Biophys. 2012;523(1):123–33.
Maestro B, Dávila N, Carranza MC, Calle C. Identification of a vitamin D response element in the human insulin receptor gene promoter. J Steroid Biochem Mol Biol. 2003;84(2–3):223–30.
Sung C-C, Liao M-T, Lu K-C, Wu C-C. Role of vitamin D in insulin resistance. BioMed Res Int. 2012;2012:1–11.
Foroughi M, Maghsoudi Z, Khayyatzadeh S, Ghiasvand R, Askari G, Iraj B. Relationship between non-alcoholic fatty liver disease and inflammation in patients with non-alcoholic fatty liver. Adv Biomed Res. 2016;5:28.
Polyzos SA, Anastasilakis AD, Efstathiadou ZA, Makras P, Perakakis N, Kountouras J, et al. Irisin in metabolic diseases. Endocrine. 2018;59:260–74.
Dianatinasab A, Koroni R, Bahramian M, Bagheri-Hosseinabadi Z, Vaismoradi M, Fararouei M, et al. The effects of aerobic, resistance, and combined exercises on the plasma irisin levels, HOMA-IR, and lipid profiles in women with metabolic syndrome: a randomized controlled trial. J Exerc Sci Fit. 2020;18(3):168–76.
Wang R, Liu H. Association between serum irisin and diabetic nephropathy in patients with type 2 diabetes mellitus: a meta-analysis. Horm Metab Res. 2021;53(05):293–300.
Kurdiova T, Balaz M, Vician M, Maderova D, Vlcek M, Valkovic L, et al. Effects of obesity, diabetes and exercise on Fndc5 gene expression and irisin release in human skeletal muscle and adipose tissue: in vivo and in vitro studies. J Physiol. 2014;592(5):1091–107.
Shirvani H, Rahmati-Ahmadabad S. Irisin interaction with adipose tissue secretions by exercise training and flaxseed oil supplement. Lipids Health Dis. 2019;18(1):1–9.
Uysal N, Yuksel O, Kizildag S, Yuce Z, Gumus H, Karakilic A, et al. Regular aerobic exercise correlates with reduced anxiety and incresed levels of irisin in brain and white adipose tissue. Neurosci Lett. 2018;676:92–7.
Fox J, Rioux B, Goulet E, Johanssen N, Swift D, Bouchard D, et al. Effect of an acute exercise bout on immediate post-exercise irisin concentration in adults: a meta-analysis. Scand J Med Sci Sports. 2018;28(1):16–28.
Liu R, Shi L, Peng N, Zhang Q, Li H. Higher baseline serum Irisin decreases risk for body mass index increment in Chinese populations: a 3.2-year cohort study. Diabetes Therapy. 2019;10:713–23.
Hisamatsu T, Miura K, Arima H, Fujiyoshi A, Kadota A, Kadowaki S, et al. Relationship of serum irisin levels to prevalence and progression of coronary artery calcification: a prospective, population-based study. Int J Cardiol. 2018;267:177–82.
Wang Z, Chen K, Han Y, Zhu H, Zhou X, Tan T, et al. Irisin protects heart against ischemia-reperfusion injury through a SOD2-dependent mitochondria mechanism. J Cardiovasc Pharmacol. 2018;72(6):259.
Löffler D, Müller U, Scheuermann K, Friebe D, Gesing J, Bielitz J, et al. Serum irisin levels are regulated by acute strenuous exercise. J Clin Endocrinol Metab. 2015;100(4):1289–99.
Giri D, Pintus D, Burnside G, Ghatak A, Mehta F, Paul P, et al. Treating vitamin D deficiency in children with type I diabetes could improve their glycaemic control. BMC Res Notes. 2017;10(1):1–5.
Felício KM, de Souza ACCB, Neto JF, de Melo FTC, Carvalho CT, Arbage TP, et al. Glycemic variability and insulin needs in patients with type 1 diabetes mellitus supplemented with vitamin D: a pilot study using continuous glucose monitoring system. Curr Diabetes Rev. 2018;14(4):395–403.
Ahmad Raafat N, Mustafa Ali Abulmeaty M. Effect of vitamin D3 administration on irisin levels in vitamin D deficient rat model. Al-Azhar Med J. 2017;46(1):237–50.
Al-Daghri NM, Rahman S, Sabico S, Amer OE, Wani K, Al-Attas OS, et al. Impact of vitamin D correction on circulating irisin: a 12 month interventional study. Int J Clin Exp Med. 2016;9(7):13086–92.
Cavalier É, Mismetti V, Souberbielle J-C, editors. Evaluation of circulating irisin levels in healthy young individuals after a single 100,000 IU vitamin D dose. Annales d'endocrinologie; 2014: Elsevier.
Rocha S, Corvo ML, Fernandes E, Freitas M. The emerging target protein tyrosine phosphatase 1B (PTP1B) for type 2 diabetes mellitus management. J Diabetes Clin Res. 2021;3(4):99–105.
Manna P, Achari AE, Jain SK. Vitamin D supplementation inhibits oxidative stress and upregulate SIRT1/AMPK/GLUT4 cascade in high glucose-treated 3T3L1 adipocytes and in adipose tissue of high fat diet-fed diabetic mice. Arch Biochem Biophys. 2017;615:22–34.
González-Rodríguez Á, Gutierrez JAM, Sanz-González S, Ros M, Burks DJ, Valverde AM. Inhibition of PTP1B restores IRS1-mediated hepatic insulin signaling in IRS2-deficient mice. Diabetes. 2010;59(3):588–99.
Venable CL, Frevert EU, Kim Y-B, Fischer BM, Kamatkar S, Neel BG, et al. Overexpression of protein-tyrosine phosphatase-1B in adipocytes inhibits insulin-stimulated phosphoinositide 3-kinase activity without altering glucose transport or Akt/protein kinase B activation. J Biol Chem. 2000;275(24):18318–26.
He R-j, Yu Z-h, Zhang R-y, Zhang Z-y. Protein tyrosine phosphatases as potential therapeutic targets. Acta Pharmacol Sin. 2014;35(10):1227–46.
Barr AJ. Protein tyrosine phosphatases as drug targets: strategies and challenges of inhibitor development. Future Med Chem. 2010;2(10):1563–76.
Teimouri M, Hosseini H, ArabSadeghabadi Z, Babaei-Khorzoughi R, Gorgani-Firuzjaee S, Meshkani R. The role of protein tyrosine phosphatase 1B (PTP1B) in the pathogenesis of type 2 diabetes mellitus and its complications. J Physiol Biochem. 2022;11(4):356–85.
Yoon S-Y, Kang HJ, Ahn D, Hwang JY, Kwon SJ, Chung SJ. Identification of chebulinic acid as a dual targeting inhibitor of protein tyrosine phosphatases relevant to insulin resistance. Bioorg Chem. 2019;90:103087.
Delibegovic M, Bence KK, Mody N, Hong E-G, Ko HJ, Kim JK, et al. Improved glucose homeostasis in mice with muscle-specific deletion of protein-tyrosine phosphatase 1B. Mol Cell Biol. 2007;27(21):7727–34.
Acknowledgements
The authors would like to thank the participants for their cooperation.
Funding
The authors declared that the research did not receive any financial grants.
Author information
Authors and Affiliations
Contributions
The study design was created by RH, while KHH and AGH were responsible for conducting the experiments. RH analyzed the resulting data and composed the manuscript, which was then reviewed and edited by KHH and AGH. All three authors contributed to interpreting the data and finally endorsed the manuscript after reading and approving it.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Iranian Clinical Trial Registration Center assigned the code IRCT20220510054809N1 to this study on 27 June 2022. Furthermore, the Ethics Committee of Razi University in Kermanshah approved the study under the code IR.RAZI.REC.1401.013.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khaledi, K., Hoseini, R. & Gharzi, A. The impact of vitamin D on type 2 diabetes management: boosting PTP1B gene expression and physical activity benefits in rats. Genes Nutr 19, 4 (2024). https://doi.org/10.1186/s12263-023-00736-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12263-023-00736-z