Abstract
Background
Recent evidence suggests that certain fetal anomalies detected upon prenatal ultrasound screenings are associated with autism spectrum disorder (ASD). In this cross-sectional study, we aimed to identify genetic variants associated with fetal ultrasound anomalies (UFAs) in children with ASD.
Methods
The study included all children with ASD who are registered in the database of the Azrieli National Center of Autism and Neurodevelopment and for whom both prenatal ultrasound and whole exome sequencing (WES) data were available. We applied our in-house integrative bioinformatics pipeline, AutScore, to these WES data to prioritize rare, gene-disrupting variants (GDVs) probably contributing to ASD susceptibily. Univariate statistics and multivariable regression were used to assess the associations between UFAs and GDVs identified in these children.
Results
The study sample comprised 126 children, of whom 43 (34.1%) had at least one UFA detected in the prenatal ultrasound scan. A total of 87 candidate ASD genetic variants were detected in 60 children, with 24 (40%) children carrying multiple variants. Children with UFAs were more likely to have loss-of-function (LoF) mutations (aOR = 2.55, 95%CI: 1.13–5.80). This association was particularly noticeable when children with structural anomalies or children with UFAs in their head and brain scans were compared to children without UFAs (any mutation: aOR = 8.28, 95%CI: 2.29–30.01; LoF: aOR = 5.72, 95%CI: 2.08–15.71 and any mutation: aOR = 6.39, 95%CI: 1.34–30.47; LoF: aOR = 4.50, 95%CI: 1.32–15.35, respectively). GDVs associated with UFAs were enriched in genes highly expressed across all tissues (aOR = 2.76, 95%CI: 1.14–6.68). There was a weak, but significant, correlation between the number of mutations and the number of abnormalities detected in the same children (r = 0.21, P = 0.016).
Conclusions
The results provide valuable insights into the potential genetic basis of prenatal organogenesis abnormalities associated with ASD and shed light on the complex interplay between genetic factors and fetal development.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Autism spectrum disorder (ASD) is a multifactorial neurodevelopmental disorder with a remarkably heterogeneous clinical presentation and a wide range of associated clinical symptoms [1,2,3]. At present, ASD diagnosis is determined after birth [4], but a growing body of evidence suggests that in some cases initial signs of ASD can be detected in utero [5,6,7,8,9,10,11,12]. Indeed, several studies have reported that certain fetal abnormalities detected in standard prenatal ultrasound screening are associated with ASD [5, 7,8,9,10,11, 13,14,15,16].
Our group recently completed two studies that explored abnormalities in fetal development associated with ASD. In the first, using prenatal ultrasound biometric data from the second and third trimesters of gestation, we showed that children later diagnosed with ASD had abnormal fetal head growth compared with other fetuses [5]. In the second study, which focused on prenatal ultrasound data from the late anatomy survey conducted during mid-gestation, we showed that children later diagnosed with ASD had higher rates of fetal structural anomalies and fetal soft markers than their non-ASD counterparts [11, 17]. This ASD-specific excess of ultrasonography fetal anomalies (UFAs) was mainly seen in the head and brain, the heart, and the urinary system. Taken together, these findings suggest genetic or other gestational predisposition (i.e. maternal viral exposure) [18] to abnormal fetal development associated with ASD.
It is widely accepted that genetic factors play a significant role in ASD susceptibility [19,20,21,22,23]. In the past two decades, advances in next-generation sequencing (NGS) technologies have facilitated the identification of hundreds of rare genetic variants implicated in ASD susceptibility [24,25,26,27,28]. Importantly, some of the genetic variants associated with ASD have also been associated with various congenital diseases and malformations. For example, facial and head abnormalities usually occur in ASD-associated genetic syndromes that involve the CHD8 [29, 30] and ADNP [31, 32] genes and genes in the 22q11.2 region [33]. Furthermore, comorbidity of ASD and congenital renal abnormalities may be found in people with Phelan-McDermid syndrome [34, 35], 17q12 microdeletion syndrome [13, 14, 36], or 16q24.2 deletions [16], while comorbidity of ASD and congenital heart defects is associated with 22q11.2 deletion syndrome [33] and with a set of genes involved in chromatin organization [37]. Taken together, these findings suggest shared molecular mechanisms underlying both ASD predisposition and abnormal embryonic organogenesis of various body parts.
Despite the emerging literature suggesting a range of fetal anomalies associated with ASD, the specific mechanisms underlying this association are still vague. In this study, we investigated whether genetic variations associated with ASD are also associated with fetal anomalies in these children. In addition, we looked for the genetic variations associated with the specific fetal anomalies associated with ASD (i.e., those found in the heart, urinary system, and head&brain) [11].
Materials and methods
Study design
We conducted a cross-sectional study comprising all children diagnosed with ASD who are registered in the database of the Azrieli National Center of Autism and Neurodevelopment (ANCAN) [38], who are members of Clalit Health Services (CHS; the largest health services provider in Israel), and for whom both prenatal ultrasound and whole exome sequencing (WES) data are available (Fig. 1). Enrolment to the ANCAN database is conducted at the time ASD diagnosis is given to children in one of the associated clinics of ANCAN [38]. Then, participation in additional studies conducted at ANCAN is offered to the children’s families. Approximately, one-third of these families consent to participate in the genetic study that involves WES analysis of the child and his/her parents.
ASD diagnosis
All ASD patients registered in the ANCAN database undergo the same multidisciplinary diagnosis process, which entails a comprehensive intake interview (sociodemographic and clinical factors), a behavioral evaluation with ADOS-2 [39], and a full neurocognitive assessment using either the Bayley Scales of Infant and Toddler Development - third edition (Bayley-III) [40] or the Wechsler Preschool and Primary Scale of Intelligence - version three (WPPSI-III) [41], all translated into Hebrew as described previously [38, 42]. In addition, the level of language development is assessed using the Preschool Language Scale - fifth edition (PLS-5) [43]. The final diagnosis of ASD is made by a pediatric psychiatrist or neurologist, according to DSM-5 criteria [44].
Fetal ultrasound and birth data
The study is based on prenatal ultrasound data from the fetal anatomy survey that is routinely conducted in Israel during gestational weeks 20–24. The fetal ultrasound data were obtained from all the prenatal ultrasound clinics of the CHS in southern Israel (the intake area for ANCAN). In these clinics, fetal anatomy surveys are performed by experienced physicians, who record fetal anomalies and biometric measures according to standard clinical guidelines [45, 46]. The ultrasound screening includes the examination of different anatomical landmarks according to the various body systems, including the head, brain, thorax, abdomen, spine, limbs, and umbilical cord. Any abnormalities in each examined organ are classified as structural anomalies or soft markers [45,46,47]. It should be noted that soft markers are not equivalent to structural anomalies; they may represent normal variations or transient conditions and are frequently not included in clinical reports or utilized in clinical decision-making. The list of abnormalities detected can be seen in Supplementary Table S1. Additional measurements of the fetal head and brain include head circumference, biparietal diameter, size of the cisterna magna, and lateral ventricle width [45, 48]. For the current study, the gestational age of each fetus was calculated from the last menstrual period (LMP) and confirmed by the crown-rump length (CRL) in the ultrasound scan obtained in the first trimester. If the LMP date was not known, gestational age was calculated based on CRL.
Birth characteristics of the children included in the study were obtained from the database of the Division of Obstetrics and Gynecology, Soroka University Medical Center (SUMC). The birth characteristics of children born outside of SUMC were obtained from the “Ofek” database, which includes medical data from most hospitals in Israel.
Identification of candidate ASD genetic variants
WES was performed on DNA purified from saliva samples collected from the affected children and their parents with Genotek OG-500 and OG-575 saliva collection kits, as described previously [49]. An in-house integrative bioinformatics pipeline, AutScore [50], was then applied to the WES data to prioritize rare, gene-disrupting variants (GDVs) probably contributing to ASD susceptibily. AutScore anlyzes each candidate GDV through a variety of bioinformatics tools and databases and subsequently assigns a summary score to each GDV, based on its predicted clinical pathogenicity, its inheritence characteristics, and the relevance of the affected gene to ASD or other neurodevelopmental disorders. Genetic variants with AutScore ≥ 11 were included in this study as candidate ASD genetic variants.
Storage of the genetic data, data processing, and the detection of GDVs were conducted on a high-performing computer cluster in a Linux environment using Python version 3.5 and R Studio version 1.1.456.
Statistical analysis
We divided the study sample into children with (Cases) and without (Controls) any UFAs in their ultrasonography anatomical surveys as described in their medical records, and then further divided the UFA group into those with soft markers and those with structural anomalies (Fig. 1). Differences in sociodemographic and clinical characteristics between the study groups were tested using appropriate univariate statistics while adjusting for multiple testing using the Bonferroni correction. Spearman’s correlation was used to assess the correlation between the number of mutations and the number of UFAs in the sample. Logistic regression was used to assess the association between the identified GDVs and the existence of any UFA as well as the existence of different types of UFAs while adjusting for the sex of the children.
We used data from the Genotype-Tissue Expression (GTEx) Multi Gene Query in the GTEx Portal [51] to assess the level of transcripts per million (TPM) of genes with identified variants in different tissues. Then, we applied the GTEx Multi Gene Query genetic clustering [51, 52] to identify four clusters of gene expression patterns. Finally, we assessed the association of different types of UFAs with these clusters using logistic regression, as described above.
All statistical analyses were conducted using SPSS Statistics V. 25 and R software. A two-sided test significance level of 0.05 was used throughout the entire study.
RESULTS
Sample characteristics
The study sample included 126 children with ASD (6 sibling pairs and 114 singletons) for whom both WES and ultrasound data were available (Fig. 1). The children in the study sample had a relatively lower age at diagnosis and a higher ADOS score compared to other children with ASD in the ANCAN database (Supplementary Table S2). Of the 126 children, 43 (34.1%) had at least one reported UFA in their anatomical ultrasonography surveys; of these 43 children, in turn, 17 (13.5%) had UFAs defined as structural anomalies, 23 (18.3%) had UFAs defined as soft markers, and 3 children (2.4%) had both structural anomalies and soft markers. In the remaining 83 children (65.9%), no UFAs were detected in the anatomical ultrasonography survey.
Table 1 presents the sociodemographic and clinical characteristics of the study sample, including a comparison of these characteristics between children with and without UFAs. The study sample comprised 77.8% males and 73.8% children of Jewish ethnicity, without significant differences between cases and controls. There were also no significant differences between children with and without UFAs in terms pregnancy, birth, and ultrasound details, and clinical severity characteristics. Of note, children in the soft marker group had slightly higher, although not statistically significant, cognitive and language abilities compared to children with structural markers and children without UFAs (82.4 ± 19.1 vs. 74.8 ± 13.3 and 74.8 ± 16.4, respectively, for IQ score, and 82.2 ± 25.3 vs. 57.7 ± 11.5 and 61.7 ± 20.6, respectively, for PLS score).
Genetic findings
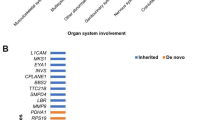
Overall, 87 GDVs with AutScore ≥ 11 were identified in 60 children in the study sample (children with both ultrasound and WES data). Of these, 23 children (38.3%) carried more than one GDV. A list of these GDVs, their genetic characteristics, and their associated UFAs is given in Supplementary Table S3. Seventy-two of the detected GDVs were inherited (69 dominant, and 3 recessive), while 15 GDVs were de-novo variants. In terms of functional consequences, 43 variants were classified as loss-of-function (LoF) mutations (i.e., frameshift, stop gain/loss, and splice acceptor/donor variants), while the remaining 44 variants were missense mutations. The genetic characteristics of GDVs with AutScore ≥ 11 did not vary significantly between children with and without ultrasound data (Supplementary Table S4).
Association between UFAs and candidate ASD genetic variants
UFAs in children with ASD were associated with different characteristics of detected GDVs (Table 2). Specifically, children with UFAs were 2.5 times more likely to carry LoF mutations than children without UFAs (aOR = 2.55, 95%CI: 1.13–5.80). This association was particularly noticeable when comparing children with structural anomalies or children with head and brain UFAs to children without UFAs (any mutation: aOR = 8.28, 95%CI: 2.29–30.01; LoF: aOR = 5.72, 95%CI: 2.08–15.71 and any mutation: aOR = 6.39, 95%CI: 1.34–30.47; LoF: aOR = 4.50, 95%CI: 1.32–15.35, respectively). Of note, given the significant overlap between structural anomalies and head and brain UFAs (Kappa = 0.38, P value < 0.001), the significant associations of genetic variants with these types of UFA are not mutually exclusive. In addition, there was a weak, but statistically significant, correlation between the number of detected GDVs and the number of detected UFAs in each child (r = 0.21, P = 0.016).
Association between UFAs and gene expression patterns
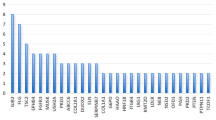
Figure 2 shows the tissue expression patterns of genes affected by the detected GDVs according to the GTEx Portal [51]. We selected the largest four clusters of co-expressed genes (≥ 10 genes per cluster) and examined their association with the detected UFAs in the study sample (Table 3). The genes of cluster 4 that are highly expressed across all tissues were significantly more prevalent in children with any UFA (aOR = 2.76, 95%CI: 1.14–6.68), and specifically in children with structural anomalies (aOR = 4.07, 95%CI: 1.47–11.30) and UFAs in the head and brain (aOR = 4.50, 95%CI: 1.31–15.42). Genes in cluster 3, which were characterized by moderate expression across all tissues, were more likely to be mutated in children with UFAs, specifically soft markers, in the urinary system, but these associations were not statistically significant (aOR = 2.73 95%CI: 0.86–8.62; and aOR = 3.31, 95%CI: 0.89–12.38, respectively).
Discussion
In this study, we examined the association between UFAs and GDVs in children with ASD. Approximately one-third of the study sample had UFAs in their fetal anatomy survey, a similar rate to the rate found in children with ASD in our previous study [11]. Furthermore, mutations in candidate ASD genes were mostly associated with structural anomalies in the head and brain, a finding that is consistent with the results of multiple genetic studies reporting a probable genetic cause of different head and brain congenital anomalies in ASD children. For example, three children with fetal macrocephaly (a structural anomaly) in our study sample had genetic mutations in genes previously linked to this phenotype. One of these genes is CEP290, a gene linked to Joubert Syndrome, which has been associated with multiple fetal anomalies, including macrocephaly, increased biparietal diameter, and ventriculomegaly, on fetal MRI and ultrasound [53]. In addition, two children in our sample, one with a mutation in PTPN11, and the other with mutations in TSC2 and SRCAP, also exhibited macrocephaly (a structural anomaly), as reported previously [54,55,56,57]. Furthermore, one child in our sample with a de-novo mutation in MTOR, which is a key member in the mTOR pathway that regulates cell growth and proliferation [58, 59], also exhibited fetal microcephaly (a structural anomaly). It has been reported for animal models that alterations in the mTOR pathway lead to microcephaly during prenatal life as a result of reduced cell number and cell size [58]. Moreover, a recent study on ASD children found an increased risk of microcephaly in mTOR variant carriers as compared to carriers of mutations in non-mTOR genes [59]. Finally, three children with ventriculomegaly (a structural anomaly) in our sample had genetic mutations in DPYD, NAA15, and SEZL6. Consistent with these findings, ventriculomegaly has previously been reported in a child with a DPYD mutation [60] as well as in children with a 4q31.1 deletion and 16p11.2 duplications, which include the NAA15 and SEZL6 genes, respectively [61, 62].
Our sample included children from several multiplex ASD families. For two siblings with ASD with the same missense/dominant genetic variant in ANK2, there were UFAs in their urinary systems. The scan of the older child, who also had a LoF mutation in MMP9, exhibited pyelectasis (a soft marker), while that of the younger one showed a polycystic kidney (a structural anomaly). ANK2 mutations are associated with an increased number of excitatory synapses with augmented axonal branching, which supports the presence of altered connectivity and penetrant behavioral impairments in humans carrying the ASD-related ANK2 mutation [63]. In addition, ANK2 is located on the 4q deletion, previously linked to congenital anomalies affecting different body systems, including developmental delay, cardiac involvement and polycystic kidney or isolated kidney [64]. Importantly, MMP9, which was disrupted in one of these siblings, is known to be involved in prenatal kidney organogenesis and thus may contribute to the upper urinary tract dilatation [65, 66]. In another multiplex family, the fetal ultrasound scan for a child with missense/dominant mutations in both CHD7 and CHD8 genes showed a single kidney (a structural anomaly). For this child, two older siblings with fetal ultrasonography hydronephrosis and dilated bowels died shortly after birth. CHD8 and its paralog CHD7 have been previously linked to the CHARGE syndrome in which renal anomalies have been described [12, 67]. In addition, in studies on both animals and humans, mutations in CHD8 were characterized by macrocephaly due to expansion of the forebrain/midbrain and a number of distinct facial characteristics [29], as well as genitourinary abnormalities [68].
We showed that the association between UFAs and GDVs is particularly relevant to genes that are broadly expressed in all body tissues (cluster 4 in our analysis). Previous studies indicated that most early prenatal ASD regulatory genes are broadly expressed in multiple organs in addition to the developing prenatal brain, suggesting that broadly expressed regulatory genes might be a major driver of prenatal neural maldevelopment in ASD and might also affect the development and function of other organs and tissues [6, 69,70,71]. Fifteen of the twenty genes comprising cluster 4 in our analysis (75%) were also reported in ASD genetic studies to be broadly expressed during prenatal life in the fetal body and the fetal brain, causing disruptions of cell proliferation, neurogenesis, migration, and cell fate, and consequently affecting prenatal brain development [6, 71] These findings suggest that mutations affecting normal organ development are predominantly expressed during prenatal life and lead to abnormal neurodevelopment and organogenesis, which can be detected on prenatal ultrasound.
We also noticed a weak, but statistically significant, correlation between the number of UFAs and number of GDVs in our sample. Such a correlation may be indicative of a cumulative effect of genetic variations on fetal development. Multiple genetic mutations may lead to more pronounced disruptions of organogenesis, resulting in multiple UFAs. This conclusion highlights the complexity of genetic factors involved in prenatal organ development and suggests that the risk of UFAs in ASD may be influenced by a combination of genetic factors rather than individual mutations alone. Indeed, previous studies found that ASD children with multiple mutations in known ASD genes demonstrate more severe ASD phenotypes (e.g., worse cognitive ability, symptom impairment, and more seizures), suggesting that multiple gene-disruptive events may co-occur in probands and act synergistically or additively to lead to a more severe phenotype [57, 72, 73]. In addition, a study that explored prenatal-stage regulatory ASD risk genes showed that many of them are upstream regulators of key signaling pathways (e.g., RAS/ERK, PI3K/AKT, WNT/β-catenin) and that the degree of dysregulation in these signaling pathways is associated with the severity of ASD symptoms [6, 74]. Thus, higher dysregulation in the signaling pathways due to multiple genetic mutations may cause more severe ASD alongside higher rates of abnormal organ development.
Limitations
This study has several limitations. First, most (~ 80%) of the GDVs included in our analyses were inherited from one parent (dominant inheritance). Although the contribution of heritable factors to ASD susceptibility is well established [75, 76], evidence for specific dominantly inherited mutations that are robustly associated with ASD is limited. Nonetheless, we decided to include inherited dominant mutations in our analyses with the aim of increasing the number of GDVs and, subsequently, the statistical power of this study. Of note, the associations found between dominantly inherited variants and UFAs in this study were also seen for de-novo variants and UFAs, but the latter did not reach statistical significance due to their small numbers. Although there is some evidence of the role of inherited dominant mutations in ASD susceptibility, further studies are needed to confirm the clinical relevance of these variants to ASD. Also, as our study focused on rare, GDVs, our findings apply to a smaller subset of the ASD population harboring rare variants and may not generalize to all children with ASD, since the vast majority of genetic susceptibility is predicted to arise from inherited common variants of low effect size. In addition, the study relied on ultrasound data collected retrospectively from the CHS database. Although these data are based on ultrasound anatomy scans conducted by experienced physicians according to strict clinical guidelines, variation between physicians can still occur. Since we didn’t have information about the physician conducting each ultrasound examination, we could not account for such potential bias, in our analyses. Also, since the study is based on the anatomy survey conducted during gestational weeks 20–24, UFAs that evolve after this gestational period would not be seen at the time of this ultrasound and thus, were not included in this study. It should also be noted that while soft markers are examined for and documented by physicians as part of the anatomy survey, they may also be considered as normal variants or transient. Thus, care should be taken in the association between soft markers and ASD. Also, the current study lacked sufficient statistical power to draw conclusions about specific UFAs. Finally, our study is focused on a sample of the Israeli population, which may limit the generalizability of the findings to other populations.
Conclusions
Our findings suggest a potential genetic basis for some fetal anomalies associated with a later diagnosis of ASD, thus shedding light on the complex interplay between rare, genetic variants and fetal development. Notably, since other non-genetic factors (i.e., in-utero viral infection) could also underlie such associations, future studies are needed to address the pathogenetic pathways from the particular causes through their intermediate fetal presentation to the subsequent neurodevelopmental outcomes.
Data availability
WES data were generated as part of the ASC and are available in dbGaP with study accession: phs000298.v4.p3. The fetal ultrasound results are available upon reasonable request to the corresponding author Prof. Idan Menashe (idanmen@bgu.ac.il).
Abbreviations
- ANCAN:
-
Azrieli National Center of Autism and Neurodevelopment
- ASD:
-
Autism spectrum disorder
- CHS:
-
Clalit Health Services
- CRL:
-
Crown-rump length
- GDV:
-
Gene-disrupting variants
- GTEx:
-
Genotype-Tissue Expression
- LMP:
-
Last menstrual period
- LoF:
-
Loss of function
- NGS:
-
Next-generation sequencing
- PLS:
-
Preschool Language Scale
- SA:
-
Structural anomaly
- SM:
-
Soft marker
- SUMC:
-
Soroka University Medical Center
- UFA:
-
Ultrasonography fetal anomalies
- WES:
-
Whole exome sequencing
References
Bauman ML. Medical comorbidities in autism: challenges to diagnosis and treatment. Neurotherapeutics. 2010;7:320–7.
Doshi-Velez F, Ge Y, Kohane I. Comorbidity clusters in Autism Spectrum disorders: an Electronic Health Record Time-Series Analysis. Pediatrics. 2014;133:e54–63.
Khachadourian V, Mahjani B, Sandin S, Kolevzon A, Buxbaum JD, Reichenberg A, et al. Comorbidities in autism spectrum disorder and their etiologies. Transl Psychiatry. 2023;13. https://doi.org/10.1038/s41398-023-02374-w.
Tanner A, Dounavi K. The emergence of autism symptoms prior to 18 months of age: a systematic literature review. J Autism Dev Disord. 2021;51:973–93.
Regev O, Cohen G, Hadar A, Schuster J, Flusser H, Michaelovski A, et al. Association between abnormal fetal Head Growth and Autism Spectrum Disorder. J Am Acad Child Adolesc Psychiatry. 2021;60:986–97.
Courchesne E, Gazestani VH, Lewis NE. Prenatal origins of ASD: the when, what, and how of ASD Development. Trends Neurosci. 2020;43:326–42.
Fulceri F, Guzzetta A, Athanasiadou A, Iaconianni L, Scattoni ML. Antenatal ultrasound value in risk calculation for Autism Spectrum disorder: a systematic review to support future research. Neurosci Biobehav Rev. 2018;92:83–92.
Gamliel M, Ebstein R, Yirmiya N, Mankuta D. Minor fetal sonographic findings in Autism Spectrum Disorder. Obstet Gynecol Surv. 2012;67:176–86.
Bonnet-Brilhault F, Rajerison TA, Paillet C, Guimard-Brunault M, Saby A, Ponson L, et al. Autism is a prenatal disorder: evidence from late gestation brain overgrowth. Autism Res. 2018;11:1635–42.
Blanken LME, Dass A, Alvares G, van der Ende J, Schoemaker NK, El Marroun H, et al. A prospective study of fetal head growth, autistic traits and autism spectrum disorder. Autism Res. 2018;11:602–12.
Regev O, Hadar A, Meiri G, Flusser H, Michaelovski A, Dinstein I, et al. Association between ultrasonography foetal anomalies and autism spectrum disorder. Brain. 2022;145:4519–30.
Miller MT, Strömland K, Ventura L, Johansson M, Bandim JM, Gillberg C. Autism associated with conditions characterized by developmental errors in early embryogenesis: a mini review. Int J Dev Neurosci. 2005;23:201–19.
Jing X-Y, Huang L-Y, Zhen L, Han J, Li D-Z. Prenatal diagnosis of 17q12 deletion syndrome: a retrospective case series. J Obstet Gynaecol (Lahore). 2019;39:323–7.
Gilboa Y, Perlman S, Pode-Shakked N, Pode-Shakked B, Shrim A, Azaria-Lahav E, et al. Prenatal diagnosis of 17q12 deletion syndrome: from fetal hyperechogenic kidneys to high risk for autism. Prenat Diagn. 2016;36:1027–32.
Jiang YL, Qi QW, Zhou XY, Geng FF, Bai JJ, Hao N, et al. [Prenatal diagnosis of 17q12 microdeletion syndrome in fetal renal abnormalities]. Zhonghua Fu Chan Ke Za Zhi. 2017;52:662–8.
Handrigan GR, Chitayat D, Lionel AC, Pinsk M, Vaags AK, Marshall CR, et al. Deletions in 16q24.2 are associated with autism spectrum disorder, intellectual disability and congenital renal malformation. J Med Genet. 2013;50:163–73.
Menashe I, Regev O, Hadar A, Meiri G, Michaelovski A, Dinstein I, et al. Reply: methodological drawbacks in the alleged association between foetal sonographic anomalies and autism. Brain. 2022;145:e92–4.
Neu N, Duchon J, Zachariah P. TORCH infections. Clin Perinatol. 2015;42:77–103.
De Rubeis S, Buxbaum JD. Recent advances in the Genetics of Autism Spectrum Disorder. Curr Neurol Neurosci Rep. 2015;15:36.
Geschwind DH. Genetics of autism spectrum disorders. Trends Cogn Sci. 2011;15:409–16.
Goldstein J, Ross DA, Moreno, De Luca D. Found in Translation: Autism Genetics and the Quest for Its Rosetta Stone. Biol Psychiatry 2019; 85: e29–e30.
Hussein Y, Tripathi U, Choudhary A, Nayak R, Peles D, Rosh I, et al. Early maturation and hyperexcitability is a shared phenotype of cortical neurons derived from different ASD-associated mutations. Transl Psychiatry. 2023;13:246.
Ploeger A, Raijmakers MEJ, van der Maas HLJ, Galis F. The Association between Autism and errors in early embryogenesis: what is the causal mechanism? Biol Psychiatry. 2010;67:602–7.
Sanders SJ, Murtha MT, Gupta AR, Murdoch JD, Raubeson MJ, Willsey AJ, et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature. 2012;485:237–41.
Satterstrom FK, Kosmicki JA, Wang J, Breen MS, De Rubeis S, An J-Y, et al. Large-scale exome sequencing study implicates both developmental and functional changes in the Neurobiology of Autism. Cell. 2020;180:568–e58423.
Fu JM, Satterstrom FK, Peng M, Brand H, Collins RL, Dong S, et al. Rare coding variation provides insight into the genetic architecture and phenotypic context of autism. Nat Genet. 2022;54:1320–31.
Michaelson JJ, Shi Y, Gujral M, Zheng H, Malhotra D, Jin X, et al. Whole-genome sequencing in Autism identifies hot spots for De Novo Germline Mutation. Cell. 2012;151:1431–42.
Yuen RKC, Thiruvahindrapuram B, Merico D, Walker S, Tammimies K, Hoang N, et al. Whole-genome sequencing of quartet families with autism spectrum disorder. Nat Med. 2015;21:185–91.
Bernier R, Golzio C, Xiong B, Stessman HA, Coe BP, Penn O, et al. Disruptive CHD8 mutations define a subtype of Autism Early in Development. Cell. 2014;158:263–76.
Cotney J, Muhle RA, Sanders SJ, Liu L, Willsey AJ, Niu W, et al. The autism-associated chromatin modifier CHD8 regulates other autism risk genes during human neurodevelopment. Nat Commun. 2015;6:6404.
Helsmoortel C, Vulto-van Silfhout AT, Coe BP, Vandeweyer G, Rooms L, van den Ende J, et al. A SWI/SNF-related autism syndrome caused by de novo mutations in ADNP. Nat Genet. 2014;46:380–4.
Van Dijck A, Vulto-van Silfhout AT, Cappuyns E, van der Werf IM, Mancini GM, Tzschach A, et al. Clinical presentation of a Complex Neurodevelopmental Disorder caused by mutations in ADNP. Biol Psychiatry. 2019;85:287–97.
Mlynarski EE, Xie M, Taylor D, Sheridan MB, Guo T, Racedo SE, et al. Rare copy number variants and congenital heart defects in the 22q11.2 deletion syndrome. Hum Genet. 2016;135:273–85.
Droogmans G, Swillen A, Van Buggenhout G. Deep phenotyping of Development, Communication and Behaviour in Phelan-McDermid syndrome. Mol Syndromol. 2019;10:294–305.
Oberman LM, Boccuto L, Cascio L, Sarasua S, Kaufmann WE. Autism spectrum disorder in Phelan-McDermid syndrome: initial characterization and genotype-phenotype correlations. Orphanet J Rare Dis. 2015;10:105.
Clissold RL, Shaw-Smith C, Turnpenny P, Bunce B, Bockenhauer D, Kerecuk L, et al. Chromosome 17q12 microdeletions but not intragenic HNF1B mutations link developmental kidney disease and psychiatric disorder. Kidney Int. 2016;90:203–11.
Jin SC, Homsy J, Zaidi S, Lu Q, Morton S, DePalma SR, et al. Contribution of rare inherited and de novo variants in 2,871 congenital heart disease probands. Nat Genet. 2017;49:1593–601.
Dinstein I, Arazi A, Golan HM, Koller J, Elliott E, Gozes I, et al. The National Autism Database of Israel: a resource for studying autism risk factors, biomarkers, outcome measures, and treatment efficacy. J Mol Neurosci. 2020;70:1303–12.
Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–23.
Albers CA, Grieve AJ. Test Review: Bayley, N. (2006). Bayley Scales of Infant and Toddler Development– Third Edition. San Antonio, TX: Harcourt Assessment. J Psychoeduc Assess 2007; 25: 180–190.
Warschausky, S., Raiford, S.E. (2018). Wechsler Preschool and Primary Scale of Intelligence. In: Kreutzer, J.S., DeLuca, J., Caplan, B. (eds) Encyclopedia of Clinical Neuropsychology. Springer, Cham. https://doi.org/10.1007/978-3-319-57111-9_1606
Meiri G, Dinstein I, Michaelowski A, Flusser H, Ilan M, Faroy M, et al. Brief report: the Negev Hospital-University-based (HUB) autism database. J Autism Dev Disord. 2017;47:2918–26.
Zimmerman IL, Steiner VG, Pond RE. Preschool language scale- fifth edition Spanish screening test. 2011 doi:https://doi.org/10.1037/t15141-000
Battle DE. Diagnostic and statistical Manual of Mental disorders (DSM). CoDAS. 2013;25:191–2.
Cargill Y, Morin L, Morin L, Bly S, Butt K, Cargill Y, et al. Content of a complete routine second trimester Obstetrical Ultrasound Examination and Report. J Obstet Gynaecol Can. 2009;31:272–5.
Edwards L, Hui L. First and second trimester screening for fetal structural anomalies. Semin Fetal Neonatal Med. 2018;23:102–11.
Van den Hof MC, Wilson RD, Bly S, Gagnon R, Lewthwaite MB, Lim K, et al. RETIRED: fetal soft markers in Obstetric Ultrasound. J Obstet Gynaecol Can. 2005;27:592–612.
Seeds JW. The routine or Screening Obstetrical Ultrasound Examination. Clin Obstet Gynecol. 1996;39:814–30.
Tal-Ben Ishay R, Shil A, Solomon S, Sadigurschi N, Abu-Kaf H, Meiri G, et al. Diagnostic Yield and Economic implications of whole-exome sequencing for ASD diagnosis in Israel. Genes (Basel). 2021;13:36.
Shil A, Arava N, Levi N, Levine L, Golan H, Meiri G et al. AutScore – an integrative scoring approach for prioritization of ultra-rare autism spectrum disorder candidate variants from whole exome sequencing data. medRxiv 2024; doi: https://doi.org/10.1101/2024.01.24.24301544
GTEx Consortium. The genotype-tissue expression (GTEx) project. Nat Genet. 2013;45:580–5.
Aguet F, Brown AA, Castel SE, Davis JR, He Y, Jo B, et al. Genetic effects on gene expression across human tissues. Nature. 2017;550:204–13.
Chen C-P, Su Y-N, Huang J-K, Liu Y-P, Tsai F-J, Yang C-K, et al. Fetal magnetic resonance imaging demonstration of Central Nervous System abnormalities and Polydactyly Associated with Joubert Syndrome. Taiwan J Obstet Gynecol. 2010;49:243–6.
Fahrner JA, Frazier A, Bachir S, Walsh MF, Applegate CD, Thompson R, et al. A rasopathy phenotype with severe congenital hypertrophic obstructive cardiomyopathy associated with a PTPN11 mutation and a novel variant in SOS1. Am J Med Genet Part A. 2012;158A:1414–21.
Bourgeron T. A synaptic trek to autism. Curr Opin Neurobiol. 2009;19:231–4.
Qiu Y, Arbogast T, Lorenzo SM, Li H, Tang SC, Richardson E, et al. Oligogenic effects of 16p11.2 Copy-number variation on Craniofacial Development. Cell Rep. 2019;28:3320–e33284.
Girirajan S, Rosenfeld JA, Cooper GM, Antonacci F, Siswara P, Itsara A, et al. A recurrent 16p12.1 microdeletion supports a two-hit model for severe developmental delay. Nat Genet. 2010;42. https://doi.org/10.1038/ng.534.
Cloëtta D, Thomanetz V, Baranek C, Lustenberger RM, Lin S, Oliveri F, et al. Inactivation of mTORC1 in the developing brain causes Microcephaly and affects gliogenesis. J Neurosci. 2013;33:7799–810.
Arenella M, Mota NR, Teunissen MWA, Brunner HG, Bralten J. Autism spectrum disorder and brain volume link through a set of < scp > mTOR -related genes. J Child Psychol Psychiatry. 2023;64:1007–14.
Chen BC, Mohd Rawi R, Meinsma R, Meijer J, Hennekam RCM, van Kuilenburg ABP. Dihydropyrimidine Dehydrogenase Deficiency in two Malaysian siblings with abnormal MRI findings. Mol Syndromol. 2014;5:299–303.
Li D, Strong A, Hou C, Downes H, Pritchard AB, Mazzeo P, et al. Interstitial deletion 4p15.32p16.1 and complex chromoplexy in a female proband with severe neurodevelopmental delay, growth failure and dysmorphism. Mol Cytogenet. 2022;15:33.
Filges I, Sparagana S, Sargent M, Selby K, Schlade-Bartusiak K, Lueder GT, et al. Brain MRI abnormalities and spectrum of neurological and clinical findings in three patients with proximal 16p11.2 microduplication. Am J Med Genet Part A. 2014;164:2003–12.
Hashem S, Nisar S, Bhat AA, Yadav SK, Azeem MW, Bagga P, et al. Genetics of structural and functional brain changes in autism spectrum disorder. Transl Psychiatry. 2020;10:229.
Strehle E-M, Yu L, Rosenfeld JA, Donkervoort S, Zhou Y, Chen T-J, et al. Genotype-phenotype analysis of 4q deletion syndrome: proposal of a critical region. Am J Med Genet Part A. 2012;158A:2139–51.
Mure P-Y, Mouriquand P. Upper urinary tract dilatation: prenatal diagnosis, management and outcome. Semin Fetal Neonatal Med. 2008;13:152–63.
Lelongt B, Trugnan G, Murphy G, Ronco PM. Matrix metalloproteinases MMP2 and MMP9 are produced in early stages of kidney morphogenesis but only MMP9 is required for renal Organogenesis in Vitro. J Cell Biol. 1997;136:1363–73.
Batsukh T, Pieper L, Koszucka AM, von Velsen N, Hoyer-Fender S, Elbracht M, et al. CHD8 interacts with CHD7, a protein which is mutated in CHARGE syndrome. Hum Mol Genet. 2010;19:2858–66.
Dingemans AJM, Truijen KMG, van de Ven S, Bernier R, Bongers EMHF, Bouman A, et al. The phenotypic spectrum and genotype-phenotype correlations in 106 patients with variants in major autism gene CHD8. Transl Psychiatry. 2022;12:421.
Kasah S, Oddy C, Basson MA. Autism-linked CHD gene expression patterns during development predict multi-organ disease phenotypes. J Anat. 2018;233:755–69.
Gamsiz ED, Sciarra LN, Maguire AM, Pescosolido MF, van Dyck LI, Morrow EM. Discovery of rare mutations in Autism: elucidating neurodevelopmental mechanisms. Neurotherapeutics. 2015;12:553–71.
Courchesne E, Pramparo T, Gazestani VH, Lombardo MV, Pierce K, Lewis NE. The ASD living Biology: from cell proliferation to clinical phenotype. Mol Psychiatry. 2019;24:88–107.
Guo H, Wang T, Wu H, Long M, Coe BP, Li H, et al. Inherited and multiple de novo mutations in autism/developmental delay risk genes suggest a multifactorial model. Mol Autism. 2018;9:64.
Guo H, Duyzend MH, Coe BP, Baker C, Hoekzema K, Gerdts J, et al. Genome sequencing identifies multiple deleterious variants in autism patients with more severe phenotypes. Genet Med. 2019;21:1611–20.
Gazestani VH, Pramparo T, Nalabolu S, Kellman BP, Murray S, Lopez L, et al. A perturbed gene network containing PI3K–AKT, RAS–ERK and WNT–β-catenin pathways in leukocytes is linked to ASD genetics and symptom severity. Nat Neurosci. 2019;22:1624–34.
Sandin S, Lichtenstein P, Kuja-Halkola R, Larsson H, Hultman CM, Reichenberg A. The familial risk of Autism. JAMA. 2014;311:1770.
Colvert E, Tick B, McEwen F, Stewart C, Curran SR, Woodhouse E, et al. Heritability of Autism Spectrum Disorder in a UK Population-based twin sample. JAMA Psychiatry. 2015;72:415.
Acknowledgements
We thank Mrs. Inez Mureinik for critical reviewing and editing of the manuscript.
This study was conducted in partial fulfillment of the requirements for the MD, PhD degree in medicine awarded by the Joyce & Irving Goldman Medical School, Faculty of Health Sciences, Ben-Gurion University of the Negev. The article has been previously posted in MedRxiv preprint server.
Funding
This study was supported by a grant from the Israel Science Foundation (1092/21).
Author information
Authors and Affiliations
Contributions
Conceptualization: MI, RO. Data curation: RO, SA, BT. Formal analysis: RO, SA, MA, MG, DZ. Funding acquisition: MI. Investigation: RO, SA, MA, BT, HA, MI. Methodology: RO, HR, MI. Project administration: HA, HR, MI. Resources: HA, MA, MG. Supervision: HR, MI. Validation: RO, HA, BT, HR, MI. Visualization: RO, BT, SA, HA, DZ. Writing – original draft: RO. Writing – review and editing: MI.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the SUMC Ethics Committee per the Declaration of Helsinki SOR 295 − 18.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Regev, O., Shil, A., Bronshtein, T. et al. Association between rare, genetic variants linked to autism and ultrasonography fetal anomalies in children with autism spectrum disorder. J Neurodevelop Disord 16, 55 (2024). https://doi.org/10.1186/s11689-024-09573-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11689-024-09573-6