Abstract
This work studied the thermal stability, electrical, and thermoelectrical properties of copper(I) selenide, Cu2Se synthesized by high-energy milling in a planetary ball mill. The phase composition was investigated by X-ray powder diffraction analysis and scanning electron microscopy. The conversion of the precursors during mechanochemical synthesis and the stability of the product was monitored by thermal analysis. The dependence of electrical properties on the product porosity was observed. For the densification of Cu2Se, the method of spark plasma sintering was applied to prepare suitable samples for thermoelectric characterization. High-temperature thermoelectric properties of synthetic Cu2Se were compared to its natural analogue-mineral berzelianite in terms of its potential application in energy conversion. Based on the results a relatively high figure-of-merit, ZT parameter (~ 1.15, T = 770 K) was obtained for undoped Cu2Se, prepared by rapid mechanochemical reaction (5 min). Cyclic voltammetry measurements of Na/NaClO4/Cu2Se cell implied that mechanochemically synthesized Cu2Se could be used as a promising intercalation electrode for sodium-ion batteries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Copper (I) selenide Cu2Se is an interesting p-type semiconductor for its numerous potential applications such as solar cells, thermoelectric converters, photodetectors, superionic materials, optical filters, photovoltaics, and ion batteries [1,2,3,4,5] due to its thermal stability, electrical and thermoelectric properties. Cu2Se exists even at room temperature in different crystallographic modifications, including orthorhombic, monoclinic, and cubic structures, depending on the preparation method. Lévy-Clément and co-workers have prepared cubic Cu2Se by chemical bath deposition on an inert Pt-substrate from a selenosulfite-containing bath at 75 °C. By electrochemical transformation, its orthorhombic phase at room temperature was achieved [6]. A monoclinic structure was reported by Murray and Heyding [7], while Stevels and Jellinek documented an orthorhombic structure of Cu2Se [8]. These copper (I) selenide investigations and references belong to the period of 70–90 years of the last century. Later in 2011–12, Gulay and co-authors reported the monoclinic phase at room temperature [9], and Liu and co-workers cubic anti-fluorite Cu2Se structure at 450 K [10]. The Cu2Se preparation methods as a solvothermal [11], hydrothermal [12, 13], microwave-assisted hydrothermal method [14, 15], electrodeposition [16], chemical bath deposition [17], chemical synthesis reduction [18, 19], thermal evaporation [20], magnetron sputtering [21], solid-state reaction with pulsed laser deposition [22], microwave heating [23], and classical melting, annealing or sintering [24,25,26] were used in the last 10 years. However, most of these methods of Cu2Se preparation needed expensive equipment or a complicated post-treatment process. For the first time in 2012, Yu and co-authors prepared tetragonal α-Cu2Se by subjecting the Cu and Se starting materials to high-energy milling in a Spex mixer mill for between about 10–30 h, and milling speed > 1000 rpm [27, 28]. Later in 2015, Gahtori et al. synthesized nanostructured Cu2Se during 50 h of milling with a speed of 400 rpm [29], and Butt et al. during 5 h of milling with a speed of 450 rpm in planetary ball mills [30]. They used the milling technique to generate diverse nanoparticles from starting materials for subsequent consolidating of the product nanoparticles under pressure and temperature to examine thermoelectric performance. Recently, mechanochemical synthesis for the preparation of nanostructured orthorhombic modification of Cu2Se after 5 min of milling in a planetary ball mill was realized and appealed extensive attention due to the simple, one-pot, solvent-free and very fast synthesis [31]. The electrical properties, thermoelectric performance, and thermal stability of such mechanochemically synthesized copper(I) selenide have not yet been investigated.
In this paper, just the abovementioned properties of mechanochemically synthesized Cu2Se were studied in order to obtain new and unique knowledge about the potential use of this advantageously very fast-prepared semiconductor material in the energy conversion and storage field. The high-temperature thermoelectric performance of natural Cu2Se- mineral berzelianite was also measured and compared to its synthetic analogue. The properties of synthetic Cu2Se were studied by several characterization techniques, evaluated, and compared with reported copper selenides prepared by more laborious and costly methods.
2 Materials and methods
Mechanochemically synthesized Cu2Se was prepared by milling in a laboratory planetary ball mill Pulverisette 6 (Fritsch, Germany) according to the conditions: loading of the mill-50 balls (10 mm in diameter), the material of the milling chamber and balls-tungsten carbide, the volume of the milling chamber-250 ml, the mass of Cu powder-3.08 g, the mass of Se powder-1.92 g, ball-to-powder ratio 73:1, milling atmosphere-Ar, rotation speed-550 rpm, and milling time-5 min [31].
Natural Cu2Se (Chelopech, Bulgaria) contained cubic Cu2Se (berzelianite; ICDD-PDF2 01-072-7490) and traces of CaCO3 (calcite; ICDD-PDF2 01-071-3699) see Fig. 4. For thermoelectric measurements, 5 g of the sample was treated by dry milling for 5 min in the same mill type and conditions as mentioned above.
The X-ray diffraction analysis (XRD) was carried out using a D8 Advance diffractometer (Bruker, Germany) with Bragg–Brentano geometry and CuKα as a radiation source. The Diffracplus Eva tool and the ICDD-PDF 2 database were utilized for phase identification.
Thermoanalyzer STA 449 F3 Jupiter (Netzsch, Germany) was used for differential thermal analysis (DTA) under dynamic conditions in argon (50 cm3 min−1) and a heating rate of 10 °C min−1.
Scanning electron microscopy (SEM) observations were made with a MIRA3 FE-SEM microscope (TESCAN, Czech Republic) with an energy-dispersive X-ray (EDX) detector (Oxford Instrument, UK).
A standard four-point probe method with a test head (Ossila Ltd., UK), and an electrical source were used to study the electrical properties of Cu2Se [32, 33]. The preparation of Cu2Se tablets weighing 0.37 g was carried out by pressing with a laboratory hydraulic tablet press (Specac, USA), at a pressure of 1 and 2 t, without retention time, and at room temperature. The round pressed tablets had a diameter of 7.06 ± 0.01 mm with a density reported in the literature of 6.9–7.0 g.cm3. To obtain reproducible results, the probe tips were fixed in a constant position (the distance between them was 1.27 mm) and loaded with a constant contact force [34].
The thermoelectric properties of natural Cu2Se and its synthetic analogue were studied on 10 mm round pellets with a thickness of 4 mm sintered by the spark plasma sintering (SPS) in graphite matrices at 500 °C, under the pressure of 50 MPa with holding time 10 min. After SPS, the pellets were polished and cut into geometrically suitable pieces for further measurements. The electrical resistivity, ρ and the Seebeck coefficient, S were measured by the four-terminal static direct-current method, using an LSR-3 m (Linseis, Germany) at temperatures from 300 to750 K. For the determination of thermal diffusivity, α in the temperature range from 100 to 700 K the laser flash apparatus LFA 427 (Netzsch, Germany) was used. The specific heat capacity, cp was measured by a comparative method using Inconel-718 alloy as a reference. Subsequently, thermal conductivity, κ was calculated according to the formula \(\kappa =\alpha .{c}_{p}.\varrho\), where ρ is the density of the sample.
Cyclic voltammetry (CV) and galvanostatic cycling with potential limitation (GCLP) experiments were carried out with an MPG-200 potentiostat/galvanostat (BioLogic Science Instruments SAS, France) at room temperature. In an Ar-filled glove box, the Swagelok cells were prepared from a mixture of Cu2Se: polyvinylidene fluoride: carbon in an 8:1:1 ratio cast on etched Al-foil as a working electrode and a Li-sheet as a counter and reference electrodes. As an electrolyte, 1 mol NaClO4 was used in a non-aqueous solution of ethylene carbonate and dimethyl carbonate in a volume ratio of 1:1. The cells were cycled between 1.5 and 2.5 V vs. Na+/Na and for GCLP charge–discharge measurements between 1.5 and 2.4 V vs. Na+/Na at a current density of 10 mA.
3 Results and discussion
3.1 Completeness of mechanochemical synthesis and thermal stability of the Cu2Se
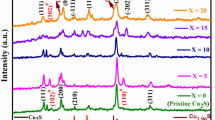
Copper (I) selenide was prepared by mechanochemical synthesis with a milling time of 5 min, which was confirmed by the XRD pattern in Fig. 1. Unreacted elemental precursors Cu and Se were not present in the product Cu2Se with orthorhombic structure (ICDD PDF 47-1448), as shown by their reference patterns. The sample milled for 0.5 min contained, in addition to unreacted precursors, a Cu3Se2 phase (ICDD PDF 65-1656) as an intermediate product of the mechanochemical synthesis of Cu2Se which gradually disappeared with increasing milling time. The kinetics of this mechanochemical reaction, together with the proposed mechanism and refined structure of the Cu2Se product, has recently been described in detail and published by [31].
The unmilled and milled 2Cu/Se mixtures for 0.5 and 5 min were subjected to thermal analysis to monitor the conversion of precursors during mechanochemical synthesis DTA curves of 2Cu/Se mixtures with the various times of milling are shown in Fig. 2.
Only one thermal effect was visible in the DTA curve of the unmilled mixture of the precursors. The endothermic peak at 215 °C corresponds to the melting of hexagonal selenium as described in our earlier research of mechanochemically synthesized bismuth selenides [35]. The reduction of this DTA signal was more pronounced after 0.5 min of milling. The traces of the melting of unreacted selenium can be observed. A slight sign of an exotherm at ~ 127 °C is probably related to the transformation of a very small amount of low-temperature (LT) Cu2Se synthesized by milling alongside the predominant intermediate Cu3Se2 phase (see Fig. 1). According to Gulay and co-authors, the LT modification of Cu2Se is transformed to the high-temperature (HT) modification above ~ 400 K [9]. After 5 min of milling, no peaks and thus no thermal transformations were detected in the DTA curve, which means that HT modification of Cu2Se was prepared by mechanochemical synthesis with complete conversion of precursors to the final product and agreed with the XRD pattern in Fig. 1. The negligible indirectness of the DTA curve was due to the residual moisture in the product. In addition, it was clear from thermal measurements that mechanochemically synthesized Cu2Se was stable up to 300 °C.
3.2 The study of the electrical properties of synthesized Cu2Se
As the representative engineering parameter, the porosity of potential electrode materials is important. The porosity of Cu2Se was varied by changing the pressure of the tablet press from 1 to 2 t (see Table 1). When the pressure of 2 t was applied the porosity decreased by 9%. Therefore, the electrical properties of mechanochemically synthesized Cu2Se product depending on the porosity were also experimentally investigated. Namely, sheet resistance, resistivity, and conductivity were derived from the measurement data population consisting of 200 values for each Cu2Se product with different porosity. The resulting values are in Table 1.
The conductivity of Cu2Se increased 4.4-fold due to a decrease in the porosity of the material by applying a pressure of 2 t in preparation of the tablets. The value was in agreement with the electronic conductivity of the Cu2Se sample conventionally sintered at 973 K for 20 h [36]. Analogously, the resistivity of Cu2Se decreased 4.1 times and the sheet resistance 3.7 times. The reason was the contacts between nanoparticles, which formed better conductive networks [37].
The SEM observations of the Cu2Se products obtained by applying the pressures of 1 and 2 t in Fig. 3 also clearly documented the decrease in porosity of Cu2Se/2 t due to a reduction in pore size and number. Due to the higher pressure, the grain boundaries are reduced, thereby the grain boundary scattering of carriers is also reduced and the conductivity is improved.
3.3 The study of the thermoelectric properties of synthesized and natural Cu2Se
The results of the Rietveld analysis showed that nanostructured synthetic Cu2Se prepared by high-energy ball milling crystallized in an HT orthorhombic phase [31]. After SPS, which was applied for densification of the samples for measurement of thermoelectric properties, the phase composition and crystal structure of natural (mineral berzelianite) and synthetic Cu2Se were unchanged which was confirmed by XRD patterns in Fig. 4.
However, the superionic α–>β phase transition was reflected in the transport and thermolectric properties measurements (Fig. 5a–c) for both the synthetic and the mineral samples. For the synthetic sample was the phase transition well visible from the fluctuations of the electrical resistivity ρ, the Seebeck coefficient S and the thermal conductivity κ at T ~ 400 K. Much less pronounced changes in these properties can be observed for the natural sample, consisting of a mixture of polymorphs and contaminated with calcite mineral (CaCO3), at temperatures around 350 K. The positive values of S hinted that holes represented the major charge carrier for both studied samples. This fact was confirmed also by a positive sign of the Hall coefficient, RH values observed for the synthetic sample. The concentration of the free carriers for this sample around 300 K started at 4.7 × 1020 cm−3 and increased up to 8.2 × 1020 cm−3 at 570 K with a steep downward fluctuation in the area below the phase transition, i.e. between 360 and 410 K (see Fig. 6). Such high free carrier concentration, the linear increase of S and ρ (except the fluctuations in the phase change region) classified both samples as degenerate semiconductors. The slopes of the temperature dependence of Hall mobility µH = f(T) outside of the phase change region indicated in both the pure α-phase (T < 360 K) and the pure β-phase (T > 410 K) showed a dominance of acoustic scattering mechanism. Inside the region, one can observe dramatically decreased mobility with an extremely large temperature dependence, which was according to [38, 39] fully consistent with the ideal characteristic of electron critical scattering.
High-temperature thermoelectric properties of natural (cyan squares) and synthetic (red squares) Cu2Se (5 min ball milling time) as a function of temperature in the range of 200–750 K: a electrical resistivity ρ, b Seebeck coefficient S, c thermal conductivity κ, and d dimensionless figure-of-merit ZT. The results of the published experiments using 50 h ball milling time (black circles) [29] and 5 h ball milling time (violet circles) [30] are presented for comparison. The inset in Fig. 5c represents a comparison of the temperature dependence of specific heat (Cp) values of our synthetic samples (red squares), which was determined by comparative method on LFA 427 (Netzsch, Germany) apparatus, with the values determined by DSC (black circles) [29]
Free carrier concentration p (red circles) and Hall mobility µH (blue circles) as a function of temperature T for the synthetic Cu2Se sample. The red dashed line serves only as a guide to the eye. Blue dashed lines represent Hall mobility slopes for the pure α-phase (T < 360 K) and the pure β-phase (T > 410 K). The violet dashed line represents fit according to a critical power law with Tc = 410 K and the critical exponent r = 0.25
The values of the dimensionless thermoelectric figure-of-merit (ZT) of the synthetic sample surpassed more than three times these for the natural sample (see Fig. 5d). It was due to the almost twice higher values of thermal conductivity of the natural samples, which can be caused by the presence of a quite high concentration of calcite impurity phase in the mineral sample (see Fig. 4) and mainly by a lower Cu stoichiometry of the sample, which is given approximately as Cu1.8Se. Just the difference in the stoichiometries of both samples explained also the observed changes between ρ and S values of both samples. The maximal value of the ZT parameter, i.e. ZT = 1.15 at 770 K, was well comparable with the previously reported ones for Cu2Se composition [27, 29]. One of the highlights of this work was the preparation of high-quality Cu2Se material, synthesized in a very high yield (100%) within a very short reaction time (5 min).
Table 2 compares the ZT value of mechanochemically synthesized Cu2Se with published ZT values and preparation conditions of Cu2Se synthesized by various and combined techniques. It is obvious that the highest ZT values were achieved just by the Cu2Se products synthesized by milling-mechanical alloying, in comparison with techniques that are also multi-step and especially more temperature-demanding. Our mechanochemical synthesis process was the fastest among all the mentioned techniques, one-step, one-pot and performed at room temperature with the ZT value of > 1. In addition, the main advantage of such synthesis is easy scaling up using the industrial vibratory mills, e.g. ESM 324-1 ks (Siebtechnik, Germany), capable of producing up to tons of material per year. Further research and testing is still needed for verification.
3.4 The study of the electrochemical properties of synthesized Cu2Se as a cathode for sodium-ion battery
In Fig. 7a) are presented the cyclic voltammetry (CV) curves of mechanochemically synthesized Cu2Se electrode-cathode at the scan rate of 0.5 mV s−1 from 1.5 to 2.5 V. As can be seen, the electrode showed quasi-reversible sodium ion insertion/extraction ability. During these five reduction processes, two obvious cathodic peaks at about 1.6 and 1.8 V were observed. For the corresponding oxidation processes, two anodic peaks were observed at about 1.8 and 2.1 V. The cathodic peak at about 1.8 V and anodic peak at 2.1 eV were in agreement with the measurement for the Cu2Se electrode fabricated by a single-step postselenized method exposing the surface of the copper grid to selenide vapour in a vacuum chamber at 400 °C according to [44]. A voltage polarization of around 0.3 V between the charge and discharge profiles is suitable for practical application.
The cyclic performance of the Cu2Se electrode together with the reversibility of Na intercalation was tested by GCLP measurements. In Fig. 7b) the first 5, 50th, and 100th charge–discharge voltage profiles of the Na/NaClO4/Cu2Se battery cycled between 1.5 and 2.4 V at a constant current of 10 mA are shown. From these measurements, an even lower polarization value of 0.1 V was confirmed, since the plateau recorded during discharge was around 1.95 V and the charging plateau was around 2.05 V. These plateaus are related to Na-ions intercalation into Cu2Se and its electrochemical reaction. The first results implied that mechanochemically synthesized Cu2Se could be used as a promising intercalation electrode for sodium-ion batteries (SIBs).
Ex-situ XRD analysis of discharged Cu2Se cathode was performed to identify the products of electrochemical reaction with sodium reported by [44]. Figure 8a) showed patterns of mechanochemically prepared Cu2Se cathode (as-prepared) and after discharging to 1.5 V. By comparing these patterns, it was clear that there was a total conversion of Cu2Se and its diffraction peaks disappeared after discharging. Two aluminium peaks come from the Al-foil substrate. However, the peaks of Na2Se and Cu have not appeared as products of the electrochemical reaction between Cu2Se and Na+, probably due to the formation of nanoparticles, which could not be identified using the XRD technique. In addition, unprominent Cu2Se3 (ICDD PDF 65-1656) peaks belonging to the electrochemical reaction by-product appeared, which is known as a low-temperature phase in the Cu-Se system stable at room temperature [45]. This by-product arising during the discharge process can be transformed from the metastable β-Cu2Se through the Cu-ion migration with high mobility in the Se-sublattice [46].
Lower magnification SEM image with element mapping of the discharged Cu2Se cathode in Fig. 8b) demonstrated the presence of Na a Se which probably corresponded to the Na2Se product of the electrochemical reaction. The presence of copper can correspond to elemental Cu but also to Cu2Se3 a by-product of the electrochemical reaction which was also indicated by the XRD pattern of the discharged cathode in Fig. 8a).
4 Conclusion
In this work, the properties of mechanochemically prepared Cu2Se, which have the potential to be applied in practice, were investigated for the first time. The thermal analysis confirmed the complete conversion of the precursors of the mechanochemical reaction Cu and Se to the Cu2Se product after only 5 min of milling and its thermal stability. The reduction of its porosity due to press pressure had a positive impact on the increase in conductivity and the decrease in sheet resistance and resistivity, which is an interesting technological parameter for the fabrication of electrodes from the semiconductor synthesized in this way. Competitive values of the ZT parameter 1.15 at 770 K were achieved by the rapid mechanochemical reaction for the synthetic Cu2Se sample. The first results of electrochemical measurements of the Na/NaClO4/Cu2Se cell indicated that the mechanochemically synthesized Cu2Se has a promising potential for use as an intercalation electrode in SIBs.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper. Should any raw data files be needed in another format they are available from the corresponding author upon reasonable request. Source data are provided in this paper.
References
Okimura H, Matsumae T, Makabe R. Electrical properties of Cu2-xSe thin-films and their application for solar cells. Thin Solid Films. 1980;71:53–9. https://doi.org/10.1016/0040-6090(80)90183-2.
Korzhuev M. Dufour effect in superionic copper selenide. Phys Solid State. 1998;40:217–9. https://doi.org/10.1134/1.1130276.
Pathan HM, Lokhande CD, Amalnerkar DP, Seth T. Modified chemical deposition and physico-chemical properties of copper(I) selenide thin films. App Surf Sci. 2003;211:48–56. https://doi.org/10.1016/s0169-4332(03)00046-1.
Yakuphanoglu F, Viswanathan C. Electrical conductivity and single oscillator model properties of amorphous CuSe semiconductor thin film. J Non-Cryst Solids. 2007;353:2934–7. https://doi.org/10.1016/j.jnoncrysol.2007.06.055.
El Akkad F, Mansour B, Hendeya T. Electrical and thermoelectric properties of Cu2Se and Cu2S. Mater Res Bull. 1981;16:535–9. https://doi.org/10.1016/0025-5408(81)90119-7.
Lévy-Clement C, Neumann Spallart M, Haram SK, Santhanam KSV. Chemical bath deposition of cubic copper (I) selenide and its room temperature transformation to the orthorhombic phase. Thin Solid Films. 1997;302:12–6. https://doi.org/10.1016/s0040-6090(97)00021-7.
Murray RM, Heyding RD. The copper–selenium system at temperatures to 850 K and pressures to 50 Kbar. Can J Chem. 1975;53:878–87. https://doi.org/10.1139/v75-122.
Stevels A, Jellinek F. Phase transitions in copper chalcogenides. 1. Copper-selenium system. Recl Trav Chim Pays-Bas. 1971;90:273–83. https://doi.org/10.1002/recl.19710900307.
Gulay L, Daszkiewicz M, Strok O, Pietraszko A. Crystal structure of Cu2Se. Chem Met Alloy. 2011;4:200–5. https://doi.org/10.30970/cma4.0184.
Liu HL, Shi X, Xu FF, Zhang LL, Zhang WQ, Chen LD, Li Q, Uher C, Day T, Snyder GJ. Copper ion liquid-like thermoelectrics. Nat Mater. 2012;11:422–5. https://doi.org/10.1038/nmat3273.
Li S, Zhang H, Cao Y, Zhang S, Liu Z, Tang S, Yang C, Wen D. Micro-nano Cu2Se as a stable and ultralong cycle life anode material for sodium-ion batteries. Int J Hydrog Energy. 2022;47:28280–8. https://doi.org/10.1016/j.ijhydene.2022.06.157.
Yang B, Yang J, Huang Z, Qin L, Lin H, Li Q. Green fabrication of large-size Cu2Se hexagonal sheets with visible light photocatalytic activity. App Surf Sci. 2021;535: 147712. https://doi.org/10.1016/j.apsusc.2020.147712.
Mascarenhas F, Rodney J, Mishra P, Bhat B. Revolutionizing energy storage: a novel Cu2Se-GO nanocomposite for supercapacitors. Inorg Chem Commun. 2023;156: 111253. https://doi.org/10.1016/j.inoche.2023.111253.
Han X, Liao F, Zhang Y, Yuan Z, Chen H, Xu C. CTAB-assisted hydrothermal synthesis of Cu2Se films composed of nanowire networks. Mater Lett. 2018;210:62–5. https://doi.org/10.1016/j.matlet.2017.08.124.
Khan M, Zhai J, Su W, Mehmood F, Chen T, Feng J, Wang H, Wang C. Microstructure and thermoelectric properties of nanoparticled copper selenide alloys synthesized using a microwave-assisted hydrothermal method. Chem Phys Mater. 2023;2:207–16. https://doi.org/10.1016/j.chphma.2022.08.001.
Shinde S, Ghodake G, Dubal D, Patel R, Saratale R, Kim D, Maile N, Koli R, Dhaygude H, Fulari V. Electrochemical synthesis: monoclinic Cu2Se nano-dendrites with high performance for supercapacitors. J Taiwan Inst Chem Eng. 2017;75:271–9. https://doi.org/10.1016/j.jtice.2017.01.028.
Phokojoe R, Motloung S, Motaung T, Kebede M, Swart H, Koao L. Effect of annealing time on copper selenide thin films prepared by chemical bath deposition. Phys B Condens Matter. 2023;666: 415112. https://doi.org/10.1016/j.physb.2023.415112.
Rapaka S, Anwar S, Singh J. Transport studies of two-step synthesized Cu2Se-Graphene nanocomposites. Phys B Condens Matter. 2023;665: 415044. https://doi.org/10.1016/j.physb.2023.415044.
Liu H, Li D, Liu H, Wang C, Wang Y, Chen Y, Linghu Y, Tian Z, Song H, Zhou J, Guo L. Devisable three-dimensional Cu2Se nanoarrays boosts high rate Na-Ion storage. App Surf Sci. 2023;612: 155725. https://doi.org/10.1016/j.apsusc.2022.155725.
Moger S, Mahesha M. Characterization of thermally evaporated copper selenide thin films for device applications. Mater Today Proc. 2022;55:22–5. https://doi.org/10.1016/j.matpr.2021.11.644.
Fan P, Huang X, Chen T, Li F, Chen Y, Jabar B, Chen S, Ma H, Liang G, Luo J, Zhang X, Zheng Z. α-Cu2Se thermoelectric thin films prepared by copper sputtering into selenium precursor layers. Chem Eng J. 2021;410: 128444. https://doi.org/10.1016/j.cej.2021.128444.
Zhou L, Li Q, Jin M, Zhang R, Zhang Q. Preparation of (111)c-oriented Cu2Se films by pulsed laser deposition method. Thin Solid Films. 2022;741: 139024. https://doi.org/10.1016/j.tsf.2021.139024.
Sakulkalavek A, Rudradawong C, Gobpant J, Harnwunggmoung A, Limsuwan P, Voraud A, Sakdanuphab R, Somdock N. Enhancement of thermoelectric properties in rapidly synthesised β-Cu2Se using optimized Cu content and microwave hybrid heating. Ceram Int. 2024;50:3680–5. https://doi.org/10.1016/j.ceramint.2023.11.119.
Shao L, Wang S, Qi J, Sun Z, Shi X, Shi Y, Lu X. Highly infiltrative micro-sized Cu2Se as advanced material with excellent rate performance and ultralong cycle-life for sodium ion half/full batteries. Mater Today Phys. 2021;19: 100422. https://doi.org/10.1016/j.mtphys.2021.100422.
Tie J, Xu G, Li Y, Fan X, Yang Q, Nan B. The effect of SPS sintering temperatures on the structure, thermoelectric properties, and scattering mechanism of Cu2Se. J Mater Res Technol. 2023;27:3506–16. https://doi.org/10.1016/j.jmrt.2023.10.108.
Nieroda P, Kruszewski M, Leszczynski J, Mars K, Kolezynski A. Influence of DC and AC current in the SPS sintering process on homogeneity of thermoelectric properties of Cu2S and Cu2Se. Ceram Int. 2023;49:9681–90. https://doi.org/10.1016/j.ceramint.2022.11.139.
Yu B, Liu W, Chen S, Wang H, Wang H, Chen G, Ren Z. Thermoelectric properties of copper selenide with ordered selenium layer and disordered copper layer. Nano Energy. 2012;1:472–8. https://doi.org/10.1016/j.nanoen.2012.02.010.
Ren Z, Chen S, Liu WS, Wang H, Wang H, Yu B, Chen G. Methods of synthesizing thermoelectric materials. Massachusetts Institute of Technology, Cambridge, USA, US Patent Appl. 13/788,932, 2016 .
Gahtori B, Bathula S, Tyagi K, Jayasimhadri M, Srivastava A, Singh S, Budhani R, Dhar A. Giant enhancement in thermoelectric performance of copper selenide by incorporation of different nanoscale dimensional defect features. Nano Energy. 2015;13:36–46. https://doi.org/10.1016/j.nanoen.2015.02.008.
Butt S, Xu W, Farooq MU, Ren G, Zhang Q, Zhu Y, Khan SU, Liu L, Yu M, Mohmed F, Lin YH, Nan C. Enhanced thermoelectricity in high-temperature β-phase copper (I) selenides embedded with Cu2Te nanoclusters. ACS Appl Mater Interfaces. 2016;8:15196–204. https://doi.org/10.1021/acsami.6b02086.
Gáborová K, Achimovičová M, Hegedüs M, Girman V, Kaňuchová M, Dutková E. Advantageous mechanochemical synthesis of copper(I) selenide semiconductor, characterization, and properties. Front Chem Sci Eng. 2022;13:433–42. https://doi.org/10.1007/s11705-021-2066-6.
Topsoe H. Geometric correction factors in four point resistivity measurement. Semicond Div. 1968;63:472–513.
Kumar V, Kelekanjeri G, Gerhardt R. A closed-form solution for the computation of geometric correction factors for four-point resistivity measurements on cylindrical specimens. Meas Sci Technol. 2008;19: 025701. https://doi.org/10.1088/0957-0233/19/2/025701.
Achimovičová M, Baláž M, Girman V, Kurimský J, Briančin J, Dutková E, Gáborová K. Comparative study of nanostructured CuSe semiconductor synthesized in a planetary and vibratory mill. Nanomater. 2020;10:2028. https://doi.org/10.3390/nano10102038.
Achimovičová M, Gotor F, Real C, Daneu N. Mechanochemical synthesis and characterization of nanocrystalline BiSe, Bi2Se3 semiconductors. J Mater Sci Mater Electron. 2012;23:1844–50. https://doi.org/10.1007/s10854-012-0672-2.
Shi D, Geng Z, Lam K. Study of conventional sintered Cu2Se thermoelectric material. Energies. 2019;12:401. https://doi.org/10.3390/en12030401.
Orikasa Y, Gogyo Y, Yamashige H, Katayama M, Chen K, Mori T, Yamamoto K, Masese T, Inada Y, Ohta T, Siroma Z, Kato S, Kinoshita H, Arai H, Ogumi Z, Uchimoto Y. Ionic conduction in lithium ion battery composite electrode governs cross-sectional reaction distribution. Sci Rep. 2016;6:26382. https://doi.org/10.1038/srep26382.
Liu H, Yuan X, Lu P, Shi X, Xu F, He Y, Tang Y, Bai S, Zhang W, Chen L, Lin Y, Shi L, Lin H, Gao X, Zhang X, Chi H, Uher C. Ultrahigh thermoelectric performance by electron and phonon critical scattering in Cu2Se1-xIx. Adv Mater. 2013;25:6607–12. https://doi.org/10.1002/adma.201302660.
Brown D, Day T, Borup K, Christensen S, Iversen B, Snyder G. Phase transition enhanced thermoelectric figure-of-merit in copper chalcogenides. APL Mater. 2013;1: 052107. https://doi.org/10.1063/1.4827595.
Huang X, Ao D, Chen T, Chen Y, Li F, Chen S, Liang G, Zhang X, Zheng Z, Fan P. High-performance copper selenide thermoelectric thin films for flexible thermoelectric application. Mater Today Energy. 2021;21: 100743. https://doi.org/10.1016/j.mtener.2021.100743.
Zheng Z, Zhang D, Jabar B, Chen T, Nisar M, Chen Y, Li F, Chen S, Liang G, Zhang X, Fan P, Chen Y. Realizing high thermoelectric performance in highly (0l0)-textured flexible Cu2Se thin film for wearable energy harvesting. Mater Today Phys. 2022;24: 100659. https://doi.org/10.1016/j.mtphys.2022.100659.
Piyasin P, Palaporn D, Kurosaki K, Pinitsoontorn S. High-performance thermoelectric properties of Cu2Se fabricated via cold sintering process. Solid State Sci. 2024;149: 107448. https://doi.org/10.1016/j.solidstatesciences.2024.107448.
Mac T, Ta T, Ngyen H, Van Hoang N, Pham T, Duong V, Pham A, Phan B, Cho S, Duong A. The impact of sintering temperature on the thermoelectric performance of Cu2Se synthesized by solid state reaction method. J Solid State Chem. 2023;322: 123998. https://doi.org/10.1016/j.jssc.2023.123998.
Yue J, Sun Q, Fu Z. Cu2Se with facile synthesis as a cathode material for rechargeable sodium batteries. Chem Commun. 2013;49:5868–70. https://doi.org/10.1039/c3cc41934g.
Glazov V, Pashinkin A, Fedorov V. Phase equilibria in the Cu-Se system. Inorg Mater. 2000;36:641–52. https://doi.org/10.1007/BF02758413.
Tashiro Y, Taniguchi K, Miyasaka H. Copper selenide as a new cathode material based on displacement reaction for rechargeable magnesium batteries. Electrochim Acta. 2016;210:655–61. https://doi.org/10.1016/j.electacta.2016.05.202.
Acknowledgements
This work was realized within the Slovak Research and Development Agency under contract No. APVV-18-0357, and the Slovak Grant Agency VEGA (projects 02/0036/23).
Funding
This work was realized within the Slovak Research and Development Agency under contract No. APVV-18-0357, and the Slovak Grant Agency VEGA (projects 02/0036/23).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study's conception and design. Material preparation, data collection and analysis were performed by Marcela Achimovičová, Katarína Gáborová, Jiří Navrátil, Petr Levinský, Olha Skurikhina, Juraj Kurimský, Jaroslav Briančin, Tomáš Plecháček and Dáša Drenčaková. The first draft of the manuscript was written by Marcela Achimovičová and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflicts of interest/competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Achimovičová, M., Gáborová, K., Navrátil, J. et al. Transport properties of mechanochemically synthesized copper (I) selenide for potential applications in energy conversion and storage. Discover Nano 19, 73 (2024). https://doi.org/10.1186/s11671-024-04025-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11671-024-04025-5