Abstract
In this study, systematic development of a portable sensor for the rapid detection of Escherichia coli (E. coli) and Exiguobacterium aurantiacum (E. aurantiacum) was reported. A conductive glass was utilized as a substrate and developed the electrode patterns on it. Trisodium citrate (TSC) and chitosan-stabilized gold nanoparticles (AuNPs) (CHI-AuNP-TSC) and chitosan-stabilized AuNPs (CHI-AuNP) were synthesized and utilized as a sensing interface. The morphology, crystallinity, optical properties, chemical structures, and surface properties of immobilized AuNPs on the sensing electrodes were investigated. The sensing performance of the fabricated sensor was evaluated by using an electrochemical method to observe the current changes in cyclic voltammetric responses. The CHI-AuNP-TSC electrode has higher sensitivity toward E. coli than CHI-AuNP with a limit of detection (LOD) of 1.07 CFU/mL. TSC in the AuNPs synthesis process played a vital role in the particle size, the interparticle spacing, the sensor’s effective surface area, and the presence of CHI around AuNPs, thus enhancing the sensing performance. Moreover, post-analysis of the fabricated sensor surface exhibited the sensor stability and the interaction between bacteria and the sensor surface. The sensing results showed a promising potential for rapid detection using a portable sensor for various water and food-borne pathogenic diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the last decade, Au nanostructures have been utilized in various sensing applications such as the detection of water and food-borne pathogens, heavy metal ions, harmful gas molecules, and pesticides. [1,2,3,4,5]. Au nanostructures have unique properties, such as a large surface–to-volume ratio, high specific surface area, and excellent electrical and optical properties [6]. Colloidal-based synthesis is one of the most simple and cost-effective methods for synthesizing nanoparticles. The synthesis of metal ion precursors with various capping agents in organic and aqueous solutions has developed to achieve multiple sizes and shapes of Au nanostructures [7,8,9]. Owing to their bio-inertness and high physical and chemical stability, diverse applications such as colorimetric sensing, drug delivery, biological imaging, catalysis, and surface-enhanced Raman scattering probing used the colloidal form of Au nanoparticles (AuNPs) [10,11,12,13,14]. Substrate-bound Au nanostructures are more suitable for solid-state device fabrication owing to their stability and reusability. Au nanostructures can be immobilized on various substrates, including glass, conducting glass, fiber optics, silicon, and glassy carbon, and their properties differ from those of the colloidal form. Other alternative techniques, namely electrodeposition, electron beam evaporation, inert gas condensation, pulsed laser deposition, and ion-beam irradiation, have also been applied to fabricate Au nanostructures on desired substrates [15,16,17,18,19].
Although various techniques are used for AuNPs fabrication, colloidal-based synthesis is most commonly used because of its low cost, simplicity, ease of preparation, and unsophisticated surface functionalization. Different methods have been introduced to fabricate substrate-bound AuNPs, namely the self-assembly of colloidal AuNPs on functionalized substrates through electrostatic interaction [20, 21], Au precursor was loaded into diblock copolymer micelles to form ordered AuNPs arrays on the substrate [22], and layer-by-layer deposition of colloidal AuNPs on the substrate [23]. However, the immobilization of colloidal AuNPs on the desired substrate results in poor adhesion between the NPs and the substrate, which can cause the detachment of AuNPs from the substrate in different chemical environments. Thus, several techniques are applied to improve the physical stability of substrate-bound AuNPs. For instance, thermal treatment close to glass transition temperature enhances the adhesion between the NPs and the substrate for glass-substrate-bound AuNPs [24]. The electrochemical deposition of AuNPs on 4-phenyl-modified glassy carbon (GC) substrate achieved better attachment of AuNPs under sonication and electrochemical treatment [25]. Colloidal AuNPs mixed with chitosan solution and deposited on the substrate are a simple and alternative way to fabricate stable AuNPs-embedded polymer matrix nanocomposites [26].
Substrate-bound Au nanostructures are generally utilized as sensing surfaces in various types of water, food-borne pathogens, and heavy metal ions sensing applications [2, 5, 27]. Well-separated AuNPs immobilized on poly (styrene-b-4-vinyl pyridine) block copolymer-functionalized optical fibers significantly improve protein detection [28]. The label-free detection of bacteria was achieved with AuNPs dispersed on hafnium ditelluride nanosheets and utilized as a nanocomposite surface-enhanced Raman scattering (SERS) substrate [29]. Ochratoxin A aptamer functionalized Au nanorods surface was served as a localized surface plasmon resonance aptasensor chip for the detection of mycotoxin and ochratoxin A [30]. Another interesting paper-based sensing method was developed by immobilizing the antibody attached AuNPs to the bacteria detection zone together with Ag ions for signal enhancement and visualization [31]. AuNPs deposited on an ultramicro interdigital electrode array chip was used as sensor for anodic stripping voltammetry based detection of heavy metal ions in water [32].
Food- and water-borne pathogens (e.g., viruses, bacteria, and parasites) are the biological agents that cause illness and infectious diseases. The symptoms of the infection vary with their hazardous level, which causes diarrhea to death in extreme cases [33]. Therefore, bacteria detection is a critical process to prevent the infectious disease [34]. Among the various detection techniques, the electrochemical sensing technique is the most used in different sensing applications [35]. Lin et al. [36] fabricated an impedimetric biosensor using planar Au- and AuNPs-modified planar Au electrodes followed by self-assembling of thiolated protein G to capture Escherichia coli (E. coli). Ranjbar et al. [37] proposed an electrochemical aptasensor composed of a glassy carbon electrode coated with an AuNPs/carbonNPs/cellulose nanofiber nanocomposite for the detection of Staphylococcus aureus. AuNPs-deposited screen-printed electrodes and peptide PEPTIR-1.0 as a recognition molecule for the electrochemical detection of E. coli were reported by Ropero-Vega et al. [38].
Au nanostructures have been immobilized on different types of substrates and studied the effect of interaction between AuNPs and the substrate, the interparticle spacing upon the sensor sensitivity by optical technique [16, 39]. However, the size and effective surface area of AuNPs on the substrate and the role of interparticle spacing on the current response have not been explored using cyclic voltammetry technique. This study involved three major steps: (1) indium-doped tin oxide (ITO) glass was used as the base substrate, and patterned the electrodes (working electrode (WE), counter electrode (CE), and reference electrode (RE)) was on it; (2) AuNPs were synthesized using two different approaches to achieve two different sizes: trisodium citrate and chitosan-stabilized AuNPs and chitosan-stabilized AuNPs; and (3) synthesized AuNPs were deposited on the working electrode and applied as the detection surface. In this case, two types of gram-negative and gram-positive bacteria, E. coli and E. aurantiacum, were tested using electrochemical measurement in cyclic voltammetry mode. This study aimed to fabricate a low-cost, portable, and disposable sensor for rapid detection of water-/food-borne pathogens without using specific recognition elements such as enzymes, antibodies, aptamers, and nucleic acids because of the lack of facility at the time being.
Experimental
Chemicals and reagents
Sodium chloride (Reagent grade, 99.5%) was obtained from Fluka, Germany. Chitosan (medium molecular weight, 95.6%) was purchased from Sarchem Laboratories, USA. Tetrachloroauric (III) acid trihydrate (HAuCl4.3H2O, Analytical grade, 99%), potassium ferricyanide and potassium ferrocyanide ((K3[Fe(CN)6] and K4[Fe(CN)6]) reagent grade, 99%), and hydrochloric acid (34% w/v, reagent grade) were purchased from Merck, Germany. Trisodium citrate (TSC) (reagent grade, 99.5%) was procured from Fisher Scientific, UK. Nutrient agar was obtained from Difco, USA. Indium tin oxide-coated glass (sheet resistance of ~ 10 Ohms/sq) was procured from Techinstro, India. Standard deionized water (DI) was used throughout the experiment. Sterile distilled water was used for the bacterial culture process.
Synthesis of gold nanoparticles
Synthesis of AuNPs with tri sodium citrate and chitosan
1% (w/v) chitosan solution was prepared using 1% acetic acid (v/v) at room temperature. The prepared chitosan solution (1 mL) was then added to 1 mL of 20 mM HAuCl4.3H2O. 47.5 mL of DI water was added to the gold-chitosan mixture under constant stirring and heated until boiling. When the solution changed from yellow to pale yellow, 2.5 mL of tri sodium citrate (TSC) solution (56 mM) was added and the mixture continued to boil. After 7 min, the color of the solution turned ruby red, known as the AuNP colloid. The colloidal solution was quenched in an ice bath and stored in a refrigerator for further use. The synthesized sample was denoted as CHI-AuNP-TSC.
Synthesis of AuNPs with chitosan
1% chitosan was prepared in 1% acetic acid. The prepared chitosan solution (1 mL) was then added to 1 mL of 20 mM HAuCl4.3H2O. 45.5 mL of DI water was added to the gold-chitosan mixture, which was boiled under constant stirring. After boiling for 15 min, the color of the solution changed from yellow to ruby red. The solution was quenched in an ice bath and stored in a refrigerator for further use. The synthesized sample was denoted as CHI-AuNP.
Sensor fabrication process
Commercially available indium tin oxide (ITO) conducting glass was used as the base substrate, and three sensor electrodes were patterned on it. Four consecutive steps were carried out in this process: (1) design the sensor electrodes according to the position of each electrode (CE, WE, and RE) (see Fig. 1a); (2) transfer the electrode pattern onto masked ITO and thus called mask patterning (see Fig. 1b); (3) etch the unwanted area of the ITO surface to obtain the desired electrode pattern (see Fig. 1c); and (4) lift-off the mask to complete the fabrication process. The sensing areas for the fabricated electrodes were 0.122 cm2 for WE and RE but 0.35 cm2 for CE which was 2.8 times larger than that of WE and RE, respectively. The WE and RE areas were randomly selected, but a larger electrode area for the CE was intentionally made to deliver the required current for the WE. The apparent sensor dimensions were found to be approximately 13 mm in width and 20 mm in length, with a total area of 2.6 cm2 and a thickness of 1 mm. The details of the electrode fabrication processes are provided in supporting information, Fig. S1.
Step-by-step fabrication process of ITO-based three electrodes sensor, a Introducing mask design onto ITO substrate which was being masked with vinyl sticker, b after patterning the mask, c after etching off the unwanted area of ITO surface, and d after lifting off the mask to achieve the base of three-electrode sensor’s pattern
Prior to the sensing performance test, the sensing surfaces were prepared using the following steps. Three microliters of CHI-AuNP-TSC and CHI-AuNP was deposited on the working electrode surfaces, repeated three times, and dried at room temperature. Subsequently, the deposited samples were dried overnight in an oven at 70 °C to enhance the attachment of AuNPs to the working electrode surfaces.
Preparation of bacteria
First, two types of bacteria, gram-negative bacteria E. coli and gram-positive bacteria E. aurantiacum, were prepared on the culture plates. The cultured bacteria were transferred to nutrient broth using loops and incubated at 37 °C for 24 h. Serial dilutions were carried out with sterile water before bacterial counting. Subsequently, 0.1 ml of each bacterium was transferred to sterile nutrient agar and kept in an incubator at 37 °C for 24 h. Subsequently, the colony-forming unit per millimeter was calculated (CFU/ml) using the following equation:
CFU/ml = (no. of colonies × dilution factor) / volume of the culture plate.
Characterizations
Surface morphology of the fabricated EC sensor electrodes was analyzed using a field emission scanning electron microscope (FESEM, JEOL JSM-7800F, Japan). Particle size, morphology, crystal lattice information, and elemental composition were investigated using a high-resolution transmission electron microscopy (HRTEM, JEOL JEM-2100F, Japan). X-ray diffraction was carried out by an X-ray diffractometer (XRD, Rigaku Miniflex-600, Japan), with a step size of 0.02°/s using Cu Kα radiation. The optical absorption property of CHI-AuNP-TSC and CHI-AuNP samples was monitored using a UV–visible spectrophotometer (Ocean Optics USB 4000, USA). Attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR) spectrum was conducted by infrared spectroscopy (PerkinElmer SpectraOne, USA). The spectra were acquired in the range of 500–4000 cm−1 with a signal resolution of 4 cm−1 for 40 scans. The surface and chemical states of the sensor electrodes were studied using X-ray photoelectron spectroscopy (XPS, Scienta Omicron, Germany). Individual components of the obtained XPS spectra were analyzed using a Gaussian Lorentzian with Shirley background function in the Casa XPS software (Casa Software Ltd, UK). The C 1s peak with a binding energy value of 246.8 eV was used as a reference for the calibration process. Electrochemical measurements of cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) were conducted using an Interface 1000 potentiostat (Gamry, USA) in the electrolyte solution containing a mixture of 0.5 M NaCl and 1 mM of (K3[Fe(CN)6]/K4[Fe(CN)6]) (1:1). The CV tests were performed at a scan rate of 250 mV/s with the scan ranges of − 1 V DC to + 1 V DC. The EIS measurement was carried out in the frequency range from 0.1 Hz to 100 kHz.
Three electrodes sensor performance evaluation
CHI-AuNP-TSC- and CHI-AuNP-deposited working electrodes were used as sensing components for the detection of bacteria. E. coli and E. aurantiacum were used as test bacteria. CV was used to study the change in the current response of the sensing surface by varying the concentrations of E. coli and E. aurantiacum in the electrolyte solution containing a mixture of 0.5 M NaCl and 1 mM of (K3[Fe(CN)6]/K4[Fe(CN)6]) (1:1). The CV performance of bacterial sensing was tested at a scan rate of 250 mVs−1 with a scan range of − 1 V to + 1 V DC. A schematic diagram of the three-electrode system sensor test setup and the electrochemical workstation is shown in Fig. 2.
Results and discussion
Surface morphology of AuNPs on ITO substrate
The morphological features of CHI-AuNP-TSC and CHI-AuNP were determined by FESEM. Figure 3 (a-d) shows the surface morphologies of CHI-AuNP-TSC and CHI-AuNP deposited on ITO substrates, which were taken with two different detectors (secondary and backscattered electron detectors). The isotropic shape AuNPs were mostly observed in both CHI-AuNP-TSC and CHI-AuNP, but a few anisotropic shapes were witnessed in the CHI-AuNP sample. The particle size of CHI-AuNP-TSC ranges from 6 to 25 nm, with the highest particle count for a diameter of 10–13 nm (see Fig. 3a inset). Moreover, AuNPs were well dispersed to form a uniform interparticle spacing on the deposited surface probably due to the presence of the chitosan polymer in its vicinity. (The CHI-dominated area is shown with red arrows.) For the CHI-AuNP sample, the particle size distribution ranges from 10 to 60 nm, with the highest particle count for the diameter of 21–30 nm (see Fig. 3b inset). In this case, AuNPs exhibited an aggregated form whereby the interparticle spacing was not uniform (not well dispersed on the surface). In general, the size of the AuNPs for the CHI-AuNP-TSC sample was much smaller than that of the CHI-AuNP.
From the aforementioned observations, it can be suggested that TSC in the AuNPs synthesis process affects two scenarios: size (direct) and particle spacing (indirect). First, the smaller size of NPs in CHI-AuNP-TSC was due to the strong reducing behavior of TSC (main reducing agent), which could favor the faster reduction of AuCl4− to Au0 [40]. Second, chitosan performed as a capping agent for AuNPs during the synthesis process resulting in smaller sizes of NPs, and the excessive chitosan (a portion of chitosan might serve in the reducing process) served as a matrix to attract AuNPs by electrostatic interaction [41] (as illustrated in Fig. 4a). This chitosan matrix also acted as a film to achieve a well-dispersed AuNPs layer on the deposited surface (see Fig. 3a). However, a larger size of AuNPs was witnessed in the CHI-AuNP sample as chitosan served as both a reducing and stabilizing/capping agent at the same time (see Fig. 4b), and the formation of gold nanoparticles took a longer time than the CHI-AuNP-TSC (Two times higher). In this case, most of the chitosan was consumed during the synthesis process, and less chitosan served as a capping agent. Thus, when CHI-AuNP-TSC and CHI-AuNP were deposited on the ITO substrate, the interparticle spacing of the CHI-AuNP-TSC sample was well organized and uniform compared to that of the CHI-AuNP sample. This could be due to the film-forming ability of CHI in CHI-AuNP-TSC whereby it prevents the aggregation of AuNPs on the ITO substrate (see Fig. 3a). On the other hand, most of the chitosan was consumed as both a reducing and capping agent and no additional chitosan was left to form a film in the CHI-AuNP sample. Therefore, less interparticle spacing with aggregation of AuNPs was witnessed on the ITO substrate (see Fig. 3b and d).
The morphology, particle size, and elemental composition of AuNPs
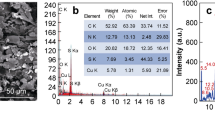
The morphology, crystal structure, size, and composition of the synthesized AuNPs were further investigated using HRTEM. Figure 5 shows the TEM image of CHI-AuNP-TSC and CHI-AuNP samples. CHI-AuNP-TSC sample exhibited isotropic shape nanoparticles with two distinct-size groups (particle size < 6 nm; particle size between 7 and 20 nm), and narrow size distribution was observed. For CHI-AuNP, one type of size group with a bigger particle size was noted compared with CHI-AuNP-TSC. Lattice spacing for both of the samples (inset: Fig. 5a and d) was found to be ~ 0.236 nm which was from (111) interplanar distance of FCC Au crystal [42]. The particle size ranges from 3 to 20 nm with the highest particle count of 10–13 nm for CHI-AuNP-TSC, and 7 nm to 40 nm with the highest particle count of 10–30 nm was observed for CHI-AuNP (Fig. 5a–d inset: particle size distribution graph). In contrast, particle size distribution estimated from the TEM result was slightly different (smaller size in TEM than SEM) from SEM (Fig. 3a–b inset). This could be due to the difference in the resolution (0.8 nm for SEM and 0.1 nm for TEM), imaging technique (secondary electron detection for SEM and transmission electron imaging for TEM), and sample preparation method [43]. However, EDS mapping showed the expected materials like Au (see Fig. 5b, c, e, and f), C, and O from the polymer material and TEM grid (C and O data are not shown here).
Crystalline and surface functional group of synthesized AuNPs
The crystalline structure of AuNPs was analyzed using XRD. Figure 6 displays the XRD patterns of CHI-AuNP-TSC and CHI-AuNP deposited on the ITO substrates. Note that the ITO substrate was included as a reference for the comparison. The XRD patterns of ITO showed the characteristic peaks of ITO located at 2θ = 21.0°, 29.9°, and 50.0° for the indexing angles of reference planes at (211), (222), and (440), respectively [44]. Moreover, two additional peaks at 38.1° and 44.3° were observed in CHI-AuNP-TSC and CHI-AuNP, respectively, which can be assigned to Au (111) and (200) [45]. These observed planes affirmed that AuNPs were deposited on ITO substrates. Besides, Au (111) peak was broadening in CHI-AuNP-TSC, which indicated that AuNPs were smaller in CHI-AuNP-TSC than in CHI-AuNP [46]. These findings were consistent with the FESEM and HRTEM results (see Figs. 3 and 5). The estimated crystalline size of CHI-AuNP-TSC and CHI-AuNP was found to be 0.71 nm and 2.84 nm, respectively.
FTIR spectroscopy was employed to study the functional groups of CHI-AuNP-TSC and CHI-AuNP samples, and pristine CHI was used as a reference/comparison (see Fig. 6b). The omnipresence of chitosan was noted by the exhibition of similar peaks in both the CHI-AuNP-TSC and CHI-AuNP samples when compared with the pristine CHI sample. The stretching vibrations of O–H and N–H groups were observed at around 3380–3340 cm−1, while the absorption band at 2880–2870 cm−1 reflected the symmetric and asymmetric stretching of C–H bonds. The presence of C=O in the amide I group, CH3 in the amide, and C–OH group was also confirmed by absorption bands at 1650–1550 cm−1, 1370–1390 cm−1, and 1250–1260 cm−1, respectively. Furthermore, two bands appearing at 1150–1160 cm−1 and 1552 cm−1 were the bending of N–H bonds and C–N stretching, which affirmed the presence of the ether group of chitosan. C–O stretching of primary alcohol was observed at 1072 cm−1 for CHI-AuNP-TSC and CHI-AuNP samples, while it was also found in pristine CHI sample by detecting the dominant C–O stretching broad bands at 1066 cm−1 and 1018 cm−1, respectively [47,48,49,50]. The presence of AuNPs in the CHI matrix caused interactions by observing the peaks shifting or changing. For instance, the absorption bands of O–H/N–H and C=O of the CHI-AuNP and CHI-AuNP-TSC samples shifted when compared with the pristine CHI (Fig. 6b). Moreover, the formation of the positive absorption frequency around 2000 cm−1 was witnessed for the CHI-AuNP sample, probably owing to the interference of IR excitation with the plasmon resonance frequency of larger AuNPs. The existence of C–H, N–H, C=O, and C–O was also further confirmed by XPS analysis as described in section "Surface properties of AuNPs on ITO substrate."
The optical properties of AuNPs on ITO substrate
The optical properties of AuNPs were studied using UV–visible spectroscopy. Figure 7a and b illustrates the absorption spectra of CHI-AuNP-TSC and CHI-AuNP deposited on ITO substrates. The maximum absorption peak of the CHI-AuNP-TSC (Fig. 7a) emerged at 537 nm due to the localized surface plasmon resonance of AuNPs. The inset in Fig. 7a shows an optical image of AuNPs-deposited ITO sensor substrate and can be visually observed as a red color. The CHI-AuNP in Fig. 7b shows an absorption spectrum with a plateau that starts at a wavelength of 500 nm and continues to a higher wavelength. The blue color of the sample can be seen visually for the CHI-AuNP sample deposited on the ITO sensor substrate (Fig. 7b inset). The difference in the color of the samples indicated a difference in the size and formation of the AuNPs on the ITO substrates. The red color of the CHI-AuNP-TSC sample shows a smaller size of AuNPs with a certain interparticle spacing, while the blue color of the CHI-AuNP sample indicates a larger size of AuNPs with a very small interparticle spacing on ITO substrates (see Fig. 3a and b). The dipole–dipole interactions between AuNPs could lead to a coupling effect in which a larger interparticle distance is associated with a weaker coupling effect, and a narrower SPR absorption band [20, 51]. The UV–visible absorption spectra were consistent with the SEM images, where certain interparticle spaces were witnessed in the CHI-AuNP-TSC (see Fig. 3a). In the CHI-AuNP sample, the NPs were larger and the interparticle distances were also very small due to the aggregated structure of AuNPs on the ITO surface (see Fig. 3b). The experimental results were in good agreement with the reported studies of noble NPs in various host matrices, according to which the position of SPR peak changes upon changing of the surrounding environment [52].
Surface properties of AuNPs on ITO substrate
Figure 8a displays the surface and sub-surface survey spectra for the CHI-AuNP-TSC and CHI-AuNP deposited on ITO substrates. Core-level Au 4f, C 1s, O 1s, and N 1s peaks were detected on both of the samples, but extra peaks of Na 1s and NaKLL (Auger) peaks were observed on TSC reduced AuNPs surface. The presence of a high concentration of C 1s and N 1s peaks revealed the existence of a chitosan matrix in the vicinity of AuNPs, which was further confirmed by the BE shift in core-level Au 4f peaks. Core-level C 1s peaks for both CHI-AuNP-TSC and CHI-AuNP samples (Fig. 8b) composed with C–C/C–H where BE centered at 284.6 eV, C–O at 286.2 eV, and C=O at 287.8 eV, respectively [53]. Figure 8c shows the core-level Au 4f peaks with spin–orbit splitting for Au 4f7/2 at 83.0 eV and Au 4f5/3 at 86.7 eV, respectively. Two obvious differences were witnessed between CHI-AuNP-TSC and CHI-AuNP samples. First, two distinct Au 4f peaks with shake-up satellite peaks positioned at 84.2 eV and 87.2 eV for CHI-AuNP sample which displayed the coexistence of Au0 and Au-CHI (CHI terminated AuNPs) whereby CHI-AuNP-TSC showed very small extra peak at BE value of 84 eV. The enhanced Au-CHI peaks in CHI-AuNP were due to the strong interaction between AuNPs and CHI, where CHI served as both reducing and capping agent, as more CHI interacted with AuNPs in CHI-AuNP sample (see Fig. 4b). Second, the core-level Au 4f peak shifted to higher BE for the CHI-AuNP sample which could be due to the complete electron transfer from AuNPs to the surrounding chitosan matrix [54]. This result affirmed the interaction between AuNPs and chitosan for CHI-AuNP-TSC and CHI-AuNP (see Fig. 4). For instance, AuNPs were surrounded by a chitosan matrix with weakly bound for CHI-AuNP-TSC (see Fig. 4a), but there were complete electrons or charges transferred for CHI-AuNP (see Fig. 4b).
For core-level N 1s peaks (Fig. 8d), two distinct peaks with BE values of 401.8 eV for C=N and 399.7 eV for –NH3+ were observed for CHI-AuNP but very low peak intensity for –NH3+ was detected for CHI-AuNP-TSC. These substantial increases in –NH3+ indicated the presence of chitosan at a very close proximity to AuNPs, especially for aggregated fashion in the CHI-AuNP sample (see Figs. 3b and d and 4b). Conversely, CHI-AuNP-TSC has less nitrogen content due to the presence of TSC on the AuNPs' surface. This phenomenon was confirmed by witnessing the higher content of C and O in the CHI-AuNP-TSC sample (Fig. 8e). The concentration of AuNPs (in terms of atomic % concentration) for both samples was the same, which indicated the total amount of Au present in the sensor surface could be the same for both samples. These above findings shed light that the amount of Au present on the surface did not play a role in improving the bacteria sensing property, but the size and distribution of AuNPs on the surface did the enhancement of bacteria detection, which will be discussed in section "Bacteria detection by electrochemical approach." However, more studies need to be done to prove the aforementioned above claim.
Electrochemical performance of AuNPs on ITO substrate
Voltammetry sweep curves were obtained using bare ITO, CHI-AuNP-TSC and CHI-AuNP deposited on the ITO working electrodes (also called the sensing electrodes). The electrochemical behavior was tested in a mixture of 0.5 M NaCl and 1 mM K3[Fe(CN)6]/K4[Fe(CN)6] electrolyte solution. The CV curve of ITO was used as a reference for a comparison with those of the CHI-AuNP-TSC and CHI-AuNP sensing electrodes. According to the cyclic voltammograms in Fig. 9a, a characteristic pair of redox peaks was obtained for the bare ITO with a peak potential separation (ΔEp = Epa − Epc) of 180 mV, corresponding to the well-known quasi-reversible one-electron transfer redox behavior of the K3[Fe(CN)6]/K4[Fe(CN)6] redox couple. The CHI-AuNP-modified ITO electrode also exhibited the same redox map but with a wider (ΔEp = 195 mV) and lower current response than the bare ITO. For CHI-AuNP-TSC-modified ITO electrode, an improved current response with wider peak potential separation (ΔEp = 225 mV) was obtained. Moreover, a small additional oxidation peak at ~ 0.8 V and a reduction peak at ~ 0.9 V (vs Ag/AgCl) could be due to the ITO substrate–redox probe interaction. After the deposition of CHI-AuNP and CHI-AuNP-TSC on the ITO surface, reduction of these additional peaks was observed because of less interaction between the ITO surface and redox probe. The improvement in the current response of the CHI-AuNP-TSC was an indication of the significant enhancement of the electron transfer kinetics, which could be due to the higher active surface area of AuNPs in the CHI-AuNP-TSC sample. The smaller size of AuNPs with larger interparticle spacing in the CHI-AuNP-TSC sample (see Fig. 3a and c) orchestrated the higher surface area (~ 6.26 × 10–13 m2 per specific area, which is 30% higher than the original surface area, see supporting information) than those of the smaller interparticle spacing with larger NPs sizes in the CHI-AuNP sample (~ 5.57 × 10–13 m2 per specific area, which is 16% higher than the original surface area, see supporting information). This circumstance was one of the key factors to enhance the sensing of bacteria as will be discussed in the further section.
Electrochemical impedance spectroscopy (EIS) is an electrochemical tool for analyzing the interfacial charge transfer properties of the CHI-AuNP-TSC and CHI-AuNP electrodes. In this work, the impedimetric behavior of the prepared electrodes was investigated using a mixture of 0.5 M NaCl as the electrolyte and 1 mM K3[Fe(CN)6]/K4[Fe(CN)6] as a redox probe. The Nyquist plots in Fig. 9b comprise two components: semicircles in the high-frequency region and a linear part in the low-frequency region. The semicircle in the high-frequency attributed to the electron transfer process, while the linear part in the lower-frequency ascribed to transport limitations of electroactive species diffuse to the reaction interface known as Warburg impedance [41]. The diameter of the semicircle represents the charge transfer resistance (Rct) that usually forms at the electrode/electrolyte interface [55]. From the Nyquist plot shown in Fig. 9b, the Rct value of CHI-AuNP-TSC (408 Ω) was lower than that of bare ITO (480 Ω) and AuNP-CHI (724 Ω). This lower Rct could be due to the higher surface area (smaller nanoparticle size) as well as the existence of interparticle spacing between AuNPs in the CHI-AuNP-TSC sample (see Fig. 3a and c), which improved the surface conductivity and heterogeneous electron transfer kinetics activity. This phenomenon was consistent with the CV data shown in Fig. 9a, where the CHI-AuNP-TSC sample achieved the maximum current. In contrast, the series resistance (Rs) of CHI-AuNP (232 Ω) was smaller than that of CHI-AuNP-TSC (401 Ω) and bare ITO (314 Ω). Generally, Rs is the resistance of the electrolyte along with the internal resistance of the electrode (bare ITO electrode and AuNPs deposition layer). In our case, the electrolyte resistance and the current collector were considered to be uniform, but the conductivity of the ITO could be altered according to the area of the working electrodes. Even though all the working electrodes kept the same area, a slight variation occurred during manual masking (mentioned in the electrode preparation section); thus, different Rs values were obtained for the bare ITO electrodes (see Fig. S2, supporting information). However, the remarkable decrease in Rct for the CHI-AuNP-TSC greatly influenced the sensing performance, which will be discussed in the next section. The Nyquist plots were fitted with the equivalent circuit as shown in Fig. 9b, inset.
Bacteria detection by electrochemical approach
To determine the sensing performance of CHI-AuNP-TSC- and CHI-AuNP-deposited working electrodes, two commonly known gram-negative and gram-positive bacteria: E. coli and E. aurantiacum were used. Figure 10a and b shows the CV performance of CHI-AuNP-TSC electrode at different concentrations of E. coli from 14 × 104 CFU/mL to 66 × 104 CFU/mL and E. aurantiacum from 9 × 104 CFU/mL to 44 × 104 CFU/mL, respectively. Before adding the bacteria, the highest anodic and cathodic peak currents were ~ 90 μA for CHI-AuNP-TSC and CHI-AuNP sensors. Although both anodic and cathodic peak currents formed during the oxidation and reduction process, the anodic peak current was mainly used to determine the sensing performance. After adding 50 μl of E. coli (to make 14 × 104 CFU/mL) and E. aurantiacum (to make 9 × 104 CFU/mL) to the electrolyte solution, the values of the anodic peak current (Ipa) reduced from 90 μA to 85 μA for E. coli and 87 μA for E. aurantiacum. When both bacteria concentrations were further increased by adding 250 μl to the electrolyte solution, the current response of both sensing surfaces achieved 57 μA for E. coli and 74 μA for E. aurantiacum, which were reduced by 37% and 20% from that of initial Ipa values. The plot of anodic peak current associated with different concentrations of bacteria exhibited a linear trend as shown in Fig. 10c and d. Moreover, the peak potential separation was more obvious in E. coli (ΔEp ~ 394 mV) than in E. aurantiacum, especially in a higher concentration of bacteria. Therefore, it can be concluded that the CHI-AuNP-TSC sensing surface was more selective toward E. coli than E. aurantiacum. In order to confirm the current response changes due to the detection of bacteria, different volumes of nutrient broth (50 μl to 250 μl) without bacteria were added to the electrolyte solution (Fig. S3). It was clearly seen that no obvious reduction in current response was observed for both CHI-AuNP-CTS and CHI-AuNP samples, confirming that the reduction in current response was triggered mainly due to the presence of bacteria.
Figure 11a and b shows the CV graphs of sensing performance which were conducted in the same way as discussed above using CHI-AuNP electrodes. After introducing different concentrations of E. coli and E. aurantiacum, the reduction of current response was observed in both cases (see Fig. 11a and b). The current response for E. coli decreased from 89 μA (no bacteria) to 84 μA (66 × 104 CFU/mL) and for E. aurantiacum decreased from 92 μA (no bacteria) to 80 μA (44 × 104 CFU/mL), respectively. The anodic peak current decreased by 5.6% for E. coli and 13% for E. aurantiacum from that of the initial Ipa values. CHI-AuNP sensing surface was less selective for E. coli than E. aurantiacum when compared with the CHI-AuNP-TSC sensing performance. Figure 11c and d shows the linear relationship of a slope of the anodic peak current related to the different concentrations of bacteria. The limit of detection (LOD) for CHI-AuNP-TSC and CHI-AuNP was estimated using LOD = 3S/M where S represents the standard deviation of the Ipa and M stands for the slope of the Ipa vs. different concentrations of both bacteria [56]. The LOD for E. coli and E. aurantiacum of the CHI-AuNP-TSC sensor was 1.07 CFU/mL and 2.41 CFU/mL, and the CHI-AuNP sensor was 12.68 CFU/mL and 5.22 CFU/mL, respectively. In addition, a comparison of the detection limits between this work and the reported studies using electrochemical techniques is presented in Table 1.
From the sensing performances of CHI-AuNP-TSC and CHI-AuNP shown in Fig. 10 and Fig. 11, the reduction of peak current response after adding the bacteria was due to the charge transfer inhibiting molecules (bacteria) toward the vicinity of the sensing electrode [13]. In other words, when the K3[Fe(CN)6]/K4[Fe(CN)6] was oxidized/reduced at the surface of the electrode, the redox species along with bacteria were moving toward the sensing surface. The flux of the redox species was disturbed by the presence of bacteria near or on the sensing electrode surface resulting in the reduction of the peak current was obtained. The presence of bacteria on the electrode surface was further confirmed by SEM which will be discussed in detail in a later section. However, the current response in CHI-AuNP-TSC (Fig. 10) was higher than CHI-AuNP (Fig. 11) for both types of bacteria. This could be due to the combined effect of CHI and AuNPs in the CHI-AuNP-TSC sample where smaller AuNPs with interparticle spacing could lead to a higher electroactive surface area (see Fig. 3a and c). This phenomenon was confirmed by observing the improvement in surface conductivity and good electron transfer ability (confirmed by the small Rct value in Fig. 9b) of the CHI-AuNP-TSC sensing surface and thus a higher redox current response. In addition, both bacteria have negative charge although they have different nature of outer cell membrane. Gram-negative bacteria (E. coli) are composed of a thin layer of lipopolysaccharide at the outer surface, followed by another peptidoglycan layer of ~ 7 to 8 nm. The cell walls of gram-negative bacteria lack strength and rigidity which can provide more anchoring sites to promote more negative charges on them. In contrast, the cell walls of gram-positive bacteria (E. aurantiacum) are rigid and comprise a thick outer membrane peptidoglycan layer of ~ 20 to 80 nm. The thicker membrane provides fewer anchoring sites resulting in fewer negative charges on gram-positive bacteria [63, 64]. The AuNPs-embedded-CHI matrix on the sensing surface in CHI-AuNP-TSC sample (see Figs. 3a and 4a) might attract bacteria through electrostatic attraction, thus enhancing the bacterial sensing activity, especially for gram-negative bacteria (E. coli). In contrast, the aggregated structure with larger AuNPs promotes less electroactive surface area in the CHI-AuNP sample, which led to lower redox current response and sensitivity toward both types of bacteria.
Post-analysis of the sensing surfaces
Post-analysis of used CHI-AuNP-TSC and CHI-TSC sensing surfaces was conducted by using FESEM. Prior to the analysis, the used sensing surfaces were gently cleaned with DI water to remove the deposited salt from the electrolyte, and dried at room temperature. Figure 12a-d clearly exhibits the presence of bacteria (both E. coli and E. aurantiacum) on both of the CHI-AuNP-TSC and CHI-TSC sensing surfaces (shown by red arrows). This observation of bacteria confirmed our previous statement of the movement of bacteria along with the redox species toward the sensing surfaces during oxidizing/reducing reaction. However, the number of bacteria on the CHI-AuNP-TSC and CHI-AuNP sensing surfaces do not reflect the redox current response (Figs. 10 and 11). Moreover, bacteria were still intact (without disintegrating) on both sensing surfaces, suggesting that our sensor's detection phenomenon was non-destructive. After utilizing the sensor for bacteria detection, a noticeable amount of AuNPs was still present on both sensing surfaces. Nevertheless, fewer amounts of AuNPs were observed on the used CHI-AuNP (Fig. 12c and d) sensing surface compared to that of the used CHI-AuNP-TSC (Fig. 12a and b), probably due to the poor adhesion of CHI-AuNP on ITO surface which could detach some AuNPs from the electrode surface. Therefore, it can conclude that the CHI-AuNP-TSC sensing surface was not only outperformed but also more stable than the CHI-AuNP surface.
Conclusions
Systematic development of a simple and inexpensive portable sensor was carried out on ITO conducting substrate and used for the detection of E. coli and E. aurantiacum bacteria. AuNPs were synthesized by two different approaches: trisodium citrate and chitosan-stabilized AuNPs and chitosan-stabilized AuNPs. Two different types of AuNPs were immobilized on the working electrode of the fabricated sensor surface. From FESEM and HRTEM results, the difference in size, morphology, and formation of AuNPs on the sensor surfaces was witnessed whereby affected the sensing performance. Optical absorption exhibited the narrower in the SPR absorption band for CHI-AuNP-TSC due to smaller size with larger interparticle spacing. XRD analysis further confirmed the smaller crystal size of CHI-AuNP-TSC than CHI-AuNP. FTIR and XPS analysis further yielded the qualitative and quantitative estimation of the synthesized AuNPs. Electrochemical studies demonstrated that the CHI-AuNP-TSC electrode offered a higher current response with lower charge transfer resistance than the CHI-AuNP electrode. CHI-AuNP-TSC sensor achieved the lowest detection limit of 1.07 CFU/mL and 2.41 CFU/mL with higher sensitivity for the detection of E. coli and E. aurantiacum and showed better selectivity toward E. coli. Moreover, the CHI-AuNP-TSC sensor surface was more stable than CHI-AuNP, which could be a good candidate for further development. In future, the presented sensor can be modified with specific recognition elements such as enzymes, antibodies, and aptamers for specific detection and used as a portable biosensor chip for rapid detection of water and food-borne diseases, environmental monitoring, and point-of-care device.
Data availability
All data generated or analyzed during this study are included in this article [and its supplementary information file].
References
Liu G, Lu M, Huang X, Li T, Xu D. Application of gold-nanoparticle colorimetric sensing to rapid food safety screening. Sensors. 2018;18(12):4166.
Goel P, Arora M. Fabrication of chemical sensor for organochlorine pesticide detection using colloidal gold nanoparticles. MRS Commun. 2018;8(3):1000–7.
Mubeen S, Zhang T, Chartuprayoon N, Rheem Y, Mulchandani A, Myung NV, Deshusses MA. Sensitive detection of H2S using gold nanoparticle decorated single-walled carbon nanotubes. Anal Chem. 2010;82(1):250–7.
Silva-De Hoyos LE, Sánchez-Mendieta V, Camacho-López MA, Trujillo-Reyes J, Vilchis-Nestor AR. Plasmonic and fluorescent sensors of metal ions in water based on biogenic gold nanoparticles. Arab J Chem. 2020;13(1):1975–85.
Bu T, Jia P, Liu J, Liu Y, Sun X, Zhang M, Tian Y, Zhang D, Wang J, Wang L. Diversely positive-charged gold nanoparticles based biosensor: A label-free and sensitive tool for foodborne pathogen detection. Food Chem. 2019;3:100052–100052.
Jain PK, Lee KS, El-Sayed IH, El-Sayed MA. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: applications in biological imaging and biomedicine. J Phys Chem B. 2006;110(14):7238–48.
Pérez-Juste J, Pastoriza-Santos I, Liz-Marzán LM, Mulvaney P. Gold nanorods: Synthesis, characterization and applications. Coord Chem Rev. 2005;249(17–18):1870–901.
Skrabalak SE, Chen J, Sun Y, Lu X, Au L, Cobley CM, Xia Y. Gold nanocages: synthesis, properties, and applications. Acc Chem Res. 2008;41(12):1587–95.
Samal AK, Polavarapu L, Rodal-Cedeira S, Liz-Marzán LM, Pérez-Juste J, Pastoriza-Santos I. Size tunable Au@Ag core-shell nanoparticles: synthesis and surface-enhanced raman scattering properties. Langmuir. 2013;29(48):15076–82.
Chang C-C, Chen C-P, Wu T-H, Yang C-H, Lin C-W, Chen C-Y. Gold nanoparticle-based colorimetric strategies for chemical and biological sensing applications. Nanomaterials. 2019;9(6):861.
Siddique S, Chow JCL. Gold nanoparticles for drug delivery and cancer therapy. Appl Sci. 2020;10(11):3824.
Szekeres GP, Kneipp J. SERS Probing of proteins in gold nanoparticle agglomerates. Front. Chem. 2019; 7.
Wang F, Zhang Y, Chen D, Zhang Z, Li Z. Ultrasensitive detection of tumor-specific exosomal proteins by a single microbead-based aptasensor coupled with terminal deoxynucleotidyl transferase-initiated DNA amplification (SMAT). Sens Actuators B Chem. 2021;341: 130034.
Beaton G, Zacks J, Stamplecoskie K. Al2O3 anchored silver and gold nanoparticles as accessible, stable, and re-usable catalysts. Colloids Surf A Physicochem Eng Asp. 2022;646: 128972.
German N, Ramanavicius A, Ramanaviciene A. Electrochemical deposition of gold nanoparticles on graphite rod for glucose biosensing. Sens Actuators B Chem. 2014;203:25–34.
Kyaw HH, Zar Myint MT, Al-Harthi SH, Maekawa T, Yanagisawa K, Sellai A, Dutta J. Observation of exchanging role of gold and silver nanoparticles in bimetallic thin film upon annealing above the glass transition temperature. Mater Res Express. 2017;4(8): 086409.
Kyaw HH, Myint MTZ, Al-Harthi SH, Maekawa T, Yanagisawa K, Sellai A, Dutta J. The influence of initial gold nanoparticles layer on migration of silver nanoparticles in silver/glass matrix. Thin Solid Films. 2019;685:216–24.
McCann R, Hughes C, Bagga K, Stalcup A, Vázquez M, Brabazon D. Pulsed laser deposition of plasmonic nanostructured gold on flexible transparent polymers at atmospheric pressure. J Phys D: Appl Phys. 2017;50(24): 245303.
Nishi M. Focused-ion-beam-enabled electroless growth of gold nanoparticles on silicon. J Ceram Soc Jpn. 2018;126(8):614–24.
Kyaw HH, Al-Harthi SH, Sellai A, Dutta J. Self-organization of gold nanoparticles on silanated surfaces. Beilstein J Nanotechnol. 2015;6:2345–53.
Matsumoto M, Kaneko K, Hara M, Matsui M, Morita K, Maruyama T. Covalent immobilization of gold nanoparticles on a plastic substrate and subsequent immobilization of biomolecules. RSC Adv. 2021;11(38):23409–17.
Yuan J, Hajebifard A, George C, Berini P, Zou S. Ordered gold nanoparticle arrays on glass and their characterization. J Colloid Interface Sci. 2013;410:1–10.
Liu Z, Bai L, Zhao G, Liu Y. Sandwich-like layer-by-layer assembly of gold nanoparticles with tunable SERS properties. Beilstein J Nanotechnol. 2016;7:1028–32.
Karakouz T, Maoz BM, Lando G, Vaskevich A, Rubinstein I. Stabilization of gold nanoparticle films on glass by thermal embedding. ACS Appl Mater Interfaces. 2011;3(4):978–87.
Liu G, Luais E, Gooding JJ. The fabrication of stable gold nanoparticle-modified interfaces for electrochemistry. Langmuir. 2011;27(7):4176–83.
Xiang C, Li R, Adhikari B, She Z, Li Y, Kraatz H-B. Sensitive electrochemical detection of Salmonella with chitosan–gold nanoparticles composite film. Talanta. 2015;140:122–7.
Wang F, Zhang Y, Lu M, Du Y, Chen M, Meng S, Ji W, Sun C, Peng W. Near-infrared band gold nanoparticles-Au film “hot spot” model based label-free ultratrace lead (II) ions detection via fiber SPR DNAzyme biosensor. Sens Actuators B Chem. 2021;337: 129816.
Lu M, Zhu H, Bazuin CG, Peng W, Masson J-F. Polymer-templated gold nanoparticles on optical fibers for enhanced-sensitivity localized surface plasmon resonance biosensors. ACS Sens. 2019;4(3):613–22.
Li Y, Guo Y, Ye B, Zhuang Z, Lan P, Zhang Y, Zhong H, Liu H, Guo Z, Liu Z. Rapid label-free SERS detection of foodborne pathogenic bacteria based on hafnium ditelluride-Au nanocomposites. J Innov Opt Health Sci. 2020;13(05):2041004.
Park J-H, Byun J-Y, Mun H, Shim W-B, Shin Y-B, Li T, Kim M-G. A regeneratable, label-free, localized surface plasmon resonance (LSPR) aptasensor for the detection of ochratoxin A. Biosens Bioelectron. 2014;59:321–7.
Ma S, Tang Y, Liu J, Wu J. Visible paper chip immunoassay for rapid determination of bacteria in water distribution system. Talanta. 2014;120:135–40.
Wang Y, Xu Y, Jiang J, Li Y, Tong J, Bian C. A portable sensor system with ultramicro electrode chip for the detection of heavy-metal ions in water. Micromachines. 2021;12(12):1468.
Cesewski E, Johnson BN. Electrochemical biosensors for pathogen detection. Biosens Bioelectron. 2020;159: 112214.
Ali AA, Altemimi AB, Alhelfi N, Ibrahim SA. Application of biosensors for detection of pathogenic food bacteria: a review. Biosensors. 2020;10(6):58.
Bansod B, Kumar T, Thakur R, Rana S, Singh I. A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosens Bioelectron. 2017;94:443–55.
Lin D, Pillai RG, Lee WE, Jemere AB. An impedimetric biosensor for E. coli O157:H7 based on the use of self-assembled gold nanoparticles and protein G. Mikrochim Acta. 2019;186:1–9.
Ranjbar S, Shahrokhian S. Design and fabrication of an electrochemical aptasensor using Au nanoparticles/carbon nanoparticles/cellulose nanofibers nanocomposite for rapid and sensitive detection of Staphylococcus aureus. Bioelectrochemistry. 2018;123:70–6.
Ropero-Vega JL, Redondo-Ortega JF, Galvis-Curubo YJ, Rondón-Villarreal P, Flórez-Castillo JM. A bioinspired peptide in TIR protein as recognition molecule on electrochemical biosensors for the detection of E. coli O157:H7 in an aqueous matrix. Molecules. 2021;26(9):2559.
Badilescu S, Raju D, Bathini S, Packirisamy M. Gold nano-island platforms for localized surface plasmon resonance sensing: a short review. Molecules. 2020;25(20):4661.
Nguyen Ngoc L, Le Van V, Chu Dinh K, Sai Cong D, Cao Thi N, Pham Thi H, Nguyen Duy T, Luu MQ. Synthesis and optical properties of colloidal gold nanoparticles. J Phys Conf Ser. 2009;187(1): 012026.
Wan J, Ai J, Zhang Y, Geng X, Gao Q, Cheng Z. Signal-off impedimetric immunosensor for the detection of Escherichia coli O157:H7. Sci Rep. 2016;6:19806.
Zhang J, Zhao B, Liang W, Zhou G, Liang Z, Wang Y, Qu J, Sun Y, Jiang L. Three-phase electrolysis by gold nanoparticle on hydrophobic interface for enhanced electrochemical nitrogen reduction reaction. Adv Sci. 2020;7(22):2002630.
Tuoriniemi J, Johnsson A-CJH, Holmberg JP, Gustafsson S, Gallego-Urrea JA, Olsson E, Pettersson JBC, Hassellöv M. Intermethod comparison of the particle size distributions of colloidal silica nanoparticles. Sci Technol Adv Mate. 2014;15(3):035009.
Thirumoorthi M, Thomas Joseph Prakash J. Structure, optical and electrical properties of indium tin oxide ultra thin films prepared by jet nebulizer spray pyrolysis technique. J Asian Ceram Soc. 2016;4(1):124–32.
Krishnamurthy S, Esterle A, Sharma NC, Sahi SV. Yucca-derived synthesis of gold nanomaterial and their catalytic potential. Nanoscale Res Lett. 2014;9(1):627.
Madhavi J. Comparison of average crystallite size by X-ray peak broadening and Williamson-Hall and size–strain plots for VO2+ doped ZnS/CdS composite nanopowder. SN Appl Sci. 2019;1(11):1509.
Mohan JC, Praveen G, Chennazhi KP, Jayakumar R, Nair SV. Functionalised gold nanoparticles for selective induction of in vitro apoptosis among human cancer cell lines. J Exp Nanosci. 2013;8(1):32–45.
Fernandes Queiroz M, Melo KRT, Sabry DA, Sassaki GL, Rocha HAO. Does the use of chitosan contribute to oxalate kidney stone formation? Mar Drugs. 2015;13(1):141–58.
Mohan CO, Gunasekaran S, Ravishankar CN. Chitosan-capped gold nanoparticles for indicating temperature abuse in frozen stored products. npj Sci Food. 2019;3(1):2.
Wang Y-C, Mohan CO, Guan J, Ravishankar CN, Gunasekaran S. Chitosan and gold nanoparticles-based thermal history indicators and frozen indicators for perishable and temperature-sensitive products. Food Control. 2018;85:186–93.
Jia K, Bijeon JL, Adam PM, Ionescu RE. Large scale fabrication of gold nano-structured substrates via high temperature annealing and their direct use for the LSPR detection of Atrazine. Plasmonics. 2013;8(1):143–51.
Badeggi UM, Ismail E, Adeloye AO, Botha S, Badmus JA, Marnewick JL, Cupido CN, Hussein AA. Green synthesis of gold nanoparticles capped with procyanidins from leucosidea sericea as potential antidiabetic and antioxidant agents. Biomolecules. 2020;10(3):452.
Smith M, Scudiero L, Espinal J, McEwen J-S, Garcia-Perez M. Improving the deconvolution and interpretation of XPS spectra from chars by ab initio calculations. Carbon. 2016;110:155–71.
Pramanik G, Humpolickova J, Valenta J, Kundu P, Bals S, Bour P, Dracinsky M, Cigler P. Gold nanoclusters with bright near-infrared photoluminescence. Nanoscale. 2018;10(8):3792–8.
Cho KT, Lee SB, Lee JW. Facile synthesis of highly electrocapacitive nitrogen-doped graphitic porous carbons. J Phys Chem C. 2014;118(18):9357–67.
Narayana PV, Reddy TM, Gopal P, Naidu GR. Electrochemical sensing of paracetamol and its simultaneous resolution in the presence of dopamine and folic acid at a multi-walled carbon nanotubes/poly(glycine) composite modified electrode. Anal Methods. 2014;6(23):9459–68.
Al-Fandi MG, Alshraiedeh NaH, Oweis RJ, Hayajneh RH, Alhamdan IR, Alabed RA, Al-Rawi OF. Direct electrochemical bacterial sensor using ZnO nanorods disposable electrode. Sens Rev. 2018;38(3):326–34.
Sun Q, Liu X, Tang H, Qian Y, Gu H, He H. A sandwich-type electrochemical immunosensor for the sensitive determination of salmonella typhimurium in food. Electroanalysis. 2022;34(5):911–8.
Kwon J, Cho E-M, Nandhakumar P, Yang SI, Yang H. Rapid and sensitive detection of aspergillus Niger using a single-mediator system combined with redox cycling. Anal Chem. 2018;90(22):13491–7.
Khalid SA, Hassan RYA, El Nashar RM, El-Sherbiny IM. Voltammetric determination of Salmonella typhimurium in minced beef meat using a chip-based imprinted sensor. RSC Adv. 2022;12(6):3445–53.
Feng K, Li T, Ye C, Gao X, Yang T, Liang X, Yue X, Ding S, Dong Q, Yang M, Xiong C, Huang G, Zhang J. A label-free electrochemical immunosensor for rapid detection of salmonella in milk by using CoFe-MOFs-graphene modified electrode. Food Control. 2021;130: 108357.
Fei J, Dou W, Zhao G. A sandwich electrochemical immunosensor for Salmonella pullorum and Salmonella gallinarum based on a screen-printed carbon electrode modified with an ionic liquid and electrodeposited gold nanoparticles. Mikrochim Acta. 2015;182(13):2267–75.
Shrivastava S, Bera T, Roy A, Singh G, Ramachandrarao P, Dash D. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology. 2007;18(22): 225103.
Abbaszadegan A, Ghahramani Y, Gholami A, Hemmateenejad B, Dorostkar S, Nabavizadeh M, Sharghi H. The effect of charge at the surface of silver nanoparticles on antimicrobial activity against gram-positive and gram-negative bacteria: a preliminary study. J Nanomater. 2015;2015: 720654.
Acknowledgements
The authors would like to acknowledge the partial support of Nanotechnology Research Center, Department of Physics (Surface Science Lab), College of Science and Electron Microscope Unit, College of Medicine, Sultan Qaboos University.
Funding
This research was funded by Sultan Qaboos University internal grant project IG/DVC/NRC/20/01.
Author information
Authors and Affiliations
Contributions
K Al-Yahmadi contributed to methodology and formal analysis and wrote the original draft. HH Kyaw and MTZ Myint contributed to conceptualization, methodology, data curation, formal analysis, writing, and review and editing. M Al-Abri and S Dobretsov contributed to data curation, validation, and review and editing. R Al-Mamari contributed to the preparation of bacteria. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Al-Yahmadi, K., Kyaw, H.H., Myint, M.T.Z. et al. Development of portable sensor for the detection of bacteria: effect of gold nanoparticle size, effective surface area, and interparticle spacing upon sensing interface. Discover Nano 18, 45 (2023). https://doi.org/10.1186/s11671-023-03826-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11671-023-03826-4