Abstract
Porous TiO2 were assembled by evaporating or refluxing TiO2 colloid, which was obtained by dispersing the TiO2 nanoparticles with a crystallite size (d XRD) of 3.2 nm into water or ethanol without any additives. Porous transparent bulk TiO2 was obtained by evaporating the TiO2-C2H5OH colloid at room temperature for 2 weeks, while porous TiO2 nanospheres were assembled by refluxing the TiO2-H2O colloid at 80 °C for 36 h. Both of the porous TiO2 architectures were pore-size-adjustable depending on the further treating temperature. Porous TiO2 nanospheres exhibited enhanced photocatalysis activity compared to the nanoparticles.
Similar content being viewed by others
Background
Size plays an outstanding role in determining the properties of materials. After several decades of intense research on size effects of single nanoparticle or small nanoparticle clusters, the trend goes in the direction of meso- and macroscale structures consisting of nanoscale building blocks [1]. Nanoparticles, which possess the characters of small size and large surface area, have received widely attention due to their unique properties of optics, magnetics, electrics and catalysis [2–5]. To expand the research on nanoparticles, there has been growing interest in constructing hierarchical structures consisting of aggregated nanoparticles [6–8]. The combination of size effects and collective properties gives rise to fascinating opportunities for the creation of unprecedented materials. It is important to control the organization and dispersion states to assemble nanomaterials [9–11]. For example, the homogeneous and disordered assembly of densely packed nanoparticles can result in bulk transparent film-like objects through gradually evaporating the high-quality homogeneous nanoparticle colloid [12, 13]. The high transmittance benefits from the homogeneous particles, which are quite small and do not scatter visible light. The homogeneous assembly of densely packed nanoparticles results in a narrow range of pore sizes that are highly dependent on the size and uniformity of the nanoparticles [14, 15].
As a most promising photocatalyst, anatase TiO2 has been widely investigated to improve its photocatalytic performance. Porous TiO2 has performed much higher photocatalytic activity than solids due to their stronger adsorption to substrates and/or higher light-harvesting ability [16–19]. The void space can modulate the refractive index, lower the density, increase the active area for catalysis and improve the catalysts’ ability to withstand cyclic changes in volume. Furthermore, void space in hollow structures can be used to encapsulate and control release of sensitive materials such as drugs and cosmetics. TiO2 nanomaterials with different morphologies have been reported, and it is still a challenge to assemble TiO2 nanoparticles (TiO2-NPs) to form a porous structure [20–22].
In our study, monodispersed anatase TiO2-NPs were prepared by the hydrolysis of tetrabutyltitanate (TBT) in a cyclohexane/ethanol medium. The assembly of TiO2-NPs was investigated under different conditions and resulted in transparent bulk TiO2 and porous TiO2 nanospheres (TiO2-NSs).
Methods
Preparation of TiO2-NPs
All of the chemicals were of analytic grade and used without further purification. We prepared TiO2 nanoparticles by the hydrolysis of tetrabutyltitanate (TBT) in a cyclohexane (C6H12)/ethanol (C2H5OH) medium under refluxing conditions as reported [23]. Briefly, 36 mL of TBT was dissolved in the mixed solvents (90 mL of ethanol and 90 mL of cyclohexane), and 9 mL of HCl (TBT/HCl (v/v) = 1:0.25) was added to it. The final homogeneous solution was refluxed at 67 °C for 10 h and then cooled to room temperature. To precipitate the TiO2-NPs, 600 mL of ethanol was added dropwise to the solution under stirring. After standing overnight, a white precipitate was harvested by centrifugation. The powder was obtained by washing with ethanol three times and dried in air at room temperature. (Additional file 1 shows the X-ray diffraction (XRD) pattern, transmission electron microscopy (TEM) image and the size distribution histogram of TiO2-NPs).
Preparation of the Porous Transparent Bulk TiO2
Ten milligrams of freshly prepared TiO2-NPs was added to 10 mL of ethanol in a 1000-mL beaker covered with plastic wrap. After standing for 2 days, the turbid suspension gradually changed to a transparent TiO2-C2H5OH colloid. Porous transparent bulk TiO2 was obtained by evaporating the TiO2-C2H5OH colloid at room temperature for 2 weeks. To investigate the thermal stability, porous transparent bulk TiO2 was heated at different temperatures for 2 h with a heating rate of 5 °C/min in air.
Preparation of TiO2-NSs
The freshly prepared TiO2-NPs were dispersed into deionized water to form a TiO2-H2O colloid. Typically, 100 mL of a 10-g/L TiO2-H2O colloid was heated at 80 °C for 36 h. Then, the precipitate was collected by centrifugation, washed with deionized water several times, and dried in air at room temperature. The thermal stability of the TiO2-NSs was studied by heating at different temperatures for 2 h with a rate of 5 °C/min in air.
Characterization
Powder XRD patterns were recorded on a PANalytical B.V. Empyrean X-ray diffractometer with graphite-filtered Cu Kα radiation at 40 kV and 40 mA. A SU8020 electron microscope was applied to get scanning electron microscopy (SEM) images. High-resolution transmission electron microscopy (HRTEM) was performed on a Tecnai G2 S-TWIN F20 at an accelerating voltage of 200 kV. Before being studied by HRTEM, porous transparent bulk TiO2 specimens were fabricated on an FEI Helios NanoLab 600i FIB/SEM dual-beam system. Infrared (IR) spectra of the samples were recorded on a Bruker IFS-66V/S FT-IR spectrometer with a resolution of 2 cm−1. Thermogravimetric analysis (TG) was carried out on a NETZSCH STA 449 F3 with a heating rate of 20 K/min from room temperature to 800 °C. Brunauer-Emmett-Teller (BET) measurements were performed via the nitrogen adsorption method on a Micromeritics ASAP 2420, and UV-Vis absorption was measured on a UV-2450 spectrophotometer.
Photodegradation
The photocatalytic activity of TiO2-NPs and TiO2-NSs was tested by measuring the degradation rate of methylene blue (MB) under UV light irradiation. Typically, 50 mg of the as-prepared TiO2 catalyst was added to 50 mL of an aqueous solution containing 10 mg/L MB. The mixture was magnetically stirred in the dark at ambient temperature for 1 h to achieve adsorption-desorption equilibrium of TiO2 with MB followed by exposure to UV light from a 125-W high-pressure mercury vapour lamp. Five milliliters of the suspension was extracted every 10 min, and the suspended solid was immediately separated by centrifugation. UV-Vis absorption spectra were measured to monitor the concentration of MB remaining in the aqueous system. For comparison, Degussa P25 was adopted to investigate the photocatalytic activity under the same condition.
Results and Discussion
The Assembly of Porous Transparent Bulk TiO2
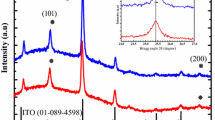
When freshly prepared TiO2-NPs with a crystallite size (d XRD) of 3.2 nm (see Additional file 1) were dispersed in ethanol, the turbid suspension gradually became a transparent TiO2-C2H5OH colloid after standing for 2 days. The digital photographic image is shown in Fig. 1a. After evaporating at room temperature, the colloid slowly condensed and formed porous transparent objects with size of 1–3 cm as shown in Fig. 1b. The IR spectrum and TG analysis of the porous transparent bulk TiO2 are present in Additional file 1: Figure S4 and Figure S5. IR spectrum (Additional file 1: Figure S4) shows the absorption peaks corresponding to the surface hydroxy group (Ti-O-H) and/or water molecules (O-H stretching mode at ~3200 cm−1 and O-H bending mode at ~1620 cm−1). TG analysis (Additional file 1: Figure S5) shows the weight losses from room temperature to 200 °C or higher, which indicates the loss of physically adsorbed water and the surface hydroxy groups. The hydrated layer formed around the nanoparticles and their hydrophilic nature leads to the formation of homogeneous and closely packed nanocrystals [12]. The nanoparticles dispersed in ethanol were homogeneously assembled into bulk materials after the evaporation of ethanol without the formation of inhomogeneous aggregates. During evaporation, TiO2-NPs in the colloid were drawn to the meniscus by convective transport; capillary forces pushed the organization of close-packed structures [24]. Figure 2a shows the SEM image of the smooth surface; no grain boundaries or cracks were observed on micrometre scale of the bulk objects. The micropores in porous transparent bulk TiO2 are confirmed by TEM (Fig. 2d) and N2 physisorption measurements and are formed in the interspaces of the densely packed TiO2-NPs.
Figure 3b shows the N2 adsorption-desorption isotherms and the corresponding pore size distribution curves of porous transparent bulk TiO2. The curve indicates a sharp uptake at low relative pressures and gradually increasing uptake at higher relative pressures. The adsorption isotherm is of type I according to the IUPAC classification, which indicates the presence of micropores [25]. The specific surface area of porous transparent bulk TiO2 was 197 m2/g, and the main pore size was located around 1.3 nm. This result agrees well with the homogenous and disordered assembly of nanoparticles in the porous transparent bulk TiO2.
The as-prepared porous transparent bulk TiO2 was thermally stable up to 500 °C. No grain boundaries or cracks on micrometre scale were observed (Fig. 2b). After heating at 800 °C for 2 h, boundaries were observed on the surface of the objects using SEM, as shown in Fig. 2c, and the phase was transformed from anatase to rutile during the thermal treatment (Fig. 3a). The BET specific surface decreased, and the pore size gradually increased with heating temperature. The shape of the isotherm of the sample heated above 400 °C turns to type IV with type H2 hysteresis as shown in Fig. 3e, f. This result is consistent with the transformation of pore size from microporosity to mesoporosity as given in Table 1. After heating at 500 °C, the BET specific surface area decreased to 39 m2/g, and the pore size was 6.9 nm, which indicates that the pore size of porous transparent bulk TiO2 was adjustable from microporous to mesoporous via thermal treatment.
The Assembly of TiO2-NSs
Freshly prepared TiO2-NPs can be dispersed into deionized water to form a colloid without any additives [23], and porous TiO2-NSs can be prepared by heating 10 g/L TiO2-H2O colloid at 80 °C for 36 h. The SEM image of the TiO2-NSs is shown in Fig. 4a, and the results indicate that the nanoparticles assembled into spheres due to the heat treatment. The assembled spheres had a spherical diameter of 100–270 nm. Detailed information of a single TiO2-NS was provided by TEM (Fig. 4b). The nanospheres had a rough surface and were assembled by nanoparticles (Fig. 4c). In addition, the porous interior is shown in Fig. 4b, c. As revealed in the TEM images, the spheres were composed of TiO2-NPs.
The HRTEM micrograph shown in Fig. 4d indicates that each nanoparticle was fully crystallized with a grain size (d TEM) of ca. 5 nm. The result is consistent with the analysis of XRD data of untreated TiO2-NSs shown in Fig. 5a, which is assigned to anatase TiO2 (JCPDS card no. 21-1276) with crystallite size (d XRD) of 4.6 nm. Heating treatment in the water encouraged the grain growth due to the Ostwald ripening mechanism and resulted in a larger crystallite size of 4.6 nm compared to the original size of 3.2 nm.
The pore size of the TiO2-NSs was confirmed by N2 physisorption measurements. Figure 5b–f shows the N2 adsorption-desorption isotherms (type IV) [25] and the corresponding pore size distribution curves of the samples heated under different temperatures. The BET specific surface area of the untreated sample was 169 m2/g, and the average pore size was 1.9 nm (Fig. 5b), which was larger than the transparent bulk TiO2 due to the bigger crystallite size of the units. After heating at different temperatures, the BET specific surface area and pore size of the assembled nanospheres changed gradually, which can also be ascribed to the increase of the crystallite size, as shown in Table 2. The TiO2-NSs heated at 600 °C exhibited the largest pore size (6.4 nm), and the untreated TiO2-NSs exhibited the largest BET specific surface area (169 m2/g).
Photocatalytic Activities of TiO2-NPs and TiO2-NSs
The photocatalytic activities of TiO2-NPs and TiO2-NSs were evaluated by the degradation of MB under UV light irradiation. As shown in Fig. 6, the photocatalytic result indicates that the photocatalytic performance was substantially improved after the assembly of TiO2-NPs into TiO2-NSs. MB was almost completely removed in 40 min by the TiO2-NSs heated at 400 °C, which exhibit superior performance among the photocatalysts we studied, including P25.
The number of effective active sites on the surface is an important character of the activity of a photocatalyst, which would be affected by many factors. Photocatalysts with smaller particle size usually exhibit higher activity. The reason may be that when the particle size is smaller, the specific surface area is larger, and more photocatalysis active sites are available. Whereas, when the particle size is too small, most of the electrons and holes are generated close to the surface and surface recombination is faster than interfacial charge carrier transfer processes. It is reported that when the particle size of TiO2 is smaller than 10 nm, the photocatalytic activity will decrease [26, 27]. But the crystallite size (d XRD) of as-prepared TiO2-NSs in this work was 4.6 nm which is far less than 10 nm. In addition, the spheres were assembled from nanoparticles by the heat treatment. Surface states play an important role in the recombination process of electron and hole [28]. We believe that the effective contact among nanoparticles affected the surface states which could reduce the recombination chance of electron and hole. Therefore, the photocatalytic activity of TiO2-NSs improved compared to TiO2-NPs.
Moreover, the defect in nano semiconductor photocatalysts plays an important role in photocatalysis [29–31]. The photocatalytic enhancement upon the calcination is attributed to the fact that increasing calcination temperature leads to a decrease in the concentration of bulk defects in TiO2 due to the increased crystallinity evidenced by XRD (Fig. 5a), thus reducing the recombination of photogenerated electrons and holes. As can be seen from Fig. 6 and Table 2, the specific surface area of the TiO2-NSs samples decreases with increasing calcination temperature, but actually it should be understood as the increase of the specific photocatalytic activity (per unit surface area), that leads the photocatalytic activity of TiO2-NSs heated at 400 °C higher than that of uncalcined TiO2-NSs and other calcinated TiO2-NSs.
Conclusions
Porous transparent bulk TiO2 was formed upon slow evaporation of TiO2-C2H5OH colloid. The crystalline building blocks are clearly distinguishable in the free-standing macroscopic bodies. Porous TiO2-NSs were assembled by refluxing the TiO2-H2O colloid and exhibited enhanced photocatalysis activity compared to the nanoparticles. Both of the porous TiO2 architectures were pore-size-adjustable depending on the further treating temperature. The current strategy and resulting materials have potentials of designing a variety of bulk objects. The controlled assembly of nanoparticles might open up the pathway to a variety of macroscopic materials with hierarchical architectures and complex morphologies.
References
Dorota K, Alessandro L, Markus N (2014) Metal oxide particles in materials science: addressing all length scales. Adv Mater 26:235–56
Pacholski C, Kornowski A, Weller H (2002) Self-assembly of ZnO: from nanodots, to nanorods. Angew Chem Int Ed 41:1188–91
Yang J, Peng JJ, Zhang QB, Peng F, Wang HJ, Yu H (2009) One-step synthesis and characterization of gold-hollow PbSx hybrid nanoparticles. Angew Chem Int Ed 48:3991–5
Wang X, Fu HB, Peng AD, Zhai TY, Ma Y, Yuan FL, Yao JN (2009) One-pot solution synthesis of cubic cobalt nanoskeletons. Adv Mater 21:1636–40
Khalavka Y, Sonnichsen C (2008) Growth of gold tips onto hyperbranched CdTe nanostructures. Adv Mater 20:588–91
Sun W, Chen M, Zhou SX, Wu LM (2014) Synthesis of hierarchically nanostructured TiO2 spheres with tunable morphologies based on a novel amphiphilic polymer precursor and their use for heavy metal ion sequestration. J Mater Chem A 2:14004–13
Feng JW, Hong Y, Zhang J, Wang PQ, Hu ZY, Wang Q, Han LY, Zhu YJ (2014) Novel core-shell TiO2 microsphere scattering layer for dye-sensitized solar cells. J Mater Chem A 2:1502–8
Pan JH, Cai ZY, Yu Y, Zhao XS (2011) Controllable synthesis of mesoporous F-TiO2 spheres for effective photocatalysis. J Mater Chem 21:11430–8
Yeom J, Yeom B, Chan H, Smith KW, Dominguez-Medina S, Bahng JH, Zhao GP, Chang WS, Chang SJ, Chuvilin A, Melnikau D, Rogach AL, Zhang PJ, Link S, Kral P, Kotov NA (2015) Chiral templating of self-assembling nanostructures by circularly polarized light. Nat Mater 14:66–72
Zhong YR, Yang M, Zhou XL, Luo YT, Wei JP, Zhou Z (2015) Orderly packed anodes for high-power lithium-ion batteries with super-long cycle life: rational design of MnCO3/large-area graphene composites. Adv Mater 27:806–12
King NP, Bale JB, Sheffler W, McNamara DE, Gonen S, Gonen T, Yeates TO, Baker D (2014) Accurate design of co-assembling multi-component protein nanomaterials. Nature 510:103–8
Oaki Y, Anzai T, Imai H (2010) Homogeneous and disordered assembly of densely packed nanocrystals. Adv Funct Mater 20:4127–32
Oaki Y, Nakamura K, Imai H (2012) Homogeneous and disordered assembly of densely packed titanium oxide nanocrystals: an approach to coupled synthesis and assembly in aqueous solution. Chem-Eur J 18:2825–31
Shopsowitz KE, Qi H, Hamad WY, MacLachlan MJ (2010) Free-standing mesoporous silica films with tunable chiral nematic structures. Nature 468:422–U246
Ostomel TA, Stucky GD (2004) Free-standing mesoporous titania films with anatase nanocrystallites synthesized at 80 degrees C. Chem Commun 8:1016–7
Lou XW, Archer LA (2008) A general route to nonspherical anatase TiO2 hollow colloids and magnetic multifunctional particles. Adv Mater 20:1853–8
Eiden-Assmann S, Widoniak J, Maret G (2004) Synthesis and characterization of porous and nonporous monodisperse colloidal TiO2 particles. Chem Mater 16:6–11
Lv KL, Yu JG, Fan JJ, Jaroniec M (2011) Rugby-like anatase titania hollow nanoparticles with enhanced photocatalytic activity. Cryst Eng Comm 13:7044–8
Li HX, Bian ZF, Zhu J, Zhang DQ, Li GS, Huo YN, Li H, Lu YF (2007) Mesoporous titania spheres with tunable chamber structure and enhanced photocatalytic activity. J Am Chem Soc 129:8406–7
Li XF, Lv KL, Deng KJ, Tang JF, Su R, Sun J, Chen LQ (2009) Synthesis and characterization of ZnO and TiO2 hollow spheres with enhanced photoreactivity. Mater Sci Eng B-Adv 158:40–7
Liu SW, Yu JG, Mann S (2009) Spontaneous construction of photoactive hollow TiO2 microspheres and chains. Nanotechnology 20:325606
Huang ZA, Wang ZY, Lv KL, Zheng Y, Deng KJ (2013) Transformation of TiOF2 cube to a hollow nanobox assembly from anatase TiO2 nanosheets with exposed {001} facets via solvothermal strategy. ACS Appl Mater Interface 5:8663–9
Innocenzi P, Malfatti L, Kldchob T, Falcaro P (2009) Order-disorder in self-assembled mesostructured silica films: a concepts review. Chem Mater 21:2555–64
Wang DP, Zeng HC (2009) Multifunctional roles of TiO2 nanoparticles for architecture of complex core-shells and hollow spheres of SiO2-TiO2-polyaniline system. Chem Mater 21:4811–23
Chen DH, Cao L, Huang FZ, Imperia P, Cheng YB, Caruso RA (2010) Synthesis of monodisperse mesoporous titania beads with controllable diameter, high surface areas, and variable pore diameters (14-23 nm). J Am Chem Soc 132:4438–44
Zhang Z, Wang CC, Zakaria R, Ying JY (1998) Role of particle size in nanocrystalline TiO2-based photocatalysts. J Phy Chem B 102:10871–8
Wang CC, Zhang Z, Ying JY (1997) Photocatalytic decomposition of halogenated organics over nanocrystalline titania. Nanostruct Mater 9:583–6
Bisquert J, Zaban A, Salvador P (2002) Analysis of the mechanisms of electron recombination in nanoporous TiO2 dye-sensitized solar cells. Nonequilibrium steady-state statistics and interfacial electron transfer via surface states. J Phy Chem B 106:8774–82
Kong M, Li YZ, Chen X, Tian TT, Fang PF, Zheng F, Zhao XJ (2011) Tuning the relative concentration ratio of bulk defects to surface defects in TiO2 nanocrystals leads to high photocatalytic efficiency. J Am Chem Soc 133:16414–7
Li YZ, Xie W, Hu XL, Shen GF, Zhou X, Xiang Y, Zhao XJ, Fang PF (2010) Comparison of dye photodegradation and its coupling with light-to-electricity conversion over TiO2 and ZnO. Langmuir 26:591–7
Xie W, Li YZ, Sun W, Huang JC, Xie H, Zhao XJ (2010) Surface modification of ZnO with Ag improves its photocatalytic efficiency and photostability. J Photoch Photobio A 216:149–55
Acknowledgements
This work is supported by the National Natural Science Foundation of China (No. 21071058, 21371066, 21301067).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing Interests
The authors declare that they have no competing interests.
Authors’ Contributions
XL undertook the whole research work and prepared the manuscript. WD was involved in the XRD and TEM characterization and analysis of TiO2 nanoparticles. YC presented the opinion of the thermal stability of porous transparent bulk TiO2 and was involved in the analysis and measurement of photocatalytic activity. SJ and WX were involved in the analysis and measurement of N2 adsorption-desorption isotherms. YZ and YK were involved in characterization and preparation of porous transparent bulk TiO2 and TiO2-NSs. LL and ZF involved in the SEM and TEM characterization and analysis of the samples. GP designed the experiments and is responsible for the manuscript. All authors read and approved the final manuscript.
Additional File
Additional file 1:
Supporting information. Figure S1. XRD patterns of TiO2-NPs. Figure S2. TEM image of TiO2-NPs. Figure S3. Size distribution histogram of TiO2-NPs. Figure S4. IR spectrum of the porous transparent bulk TiO2. Figure S5. TG analysis of the porous transparent bulk TiO2.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Liu, X., Duan, W., Chen, Y. et al. Porous TiO2 Assembled from Monodispersed Nanoparticles. Nanoscale Res Lett 11, 159 (2016). https://doi.org/10.1186/s11671-016-1372-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11671-016-1372-2