Abstract
Introduction
Advanced neuroimaging techniques have extensively contributed to elucidate the complex mechanisms underpinning the pathophysiology of migraine, a neurovascular disorder characterized by episodes of headache associated with a constellation of non-pain symptoms. The present manuscript, summarizing the most recent progresses of the arterial spin labelling (ASL) MRI techniques and the most significant findings from ASL studies conducted in migraine, is aimed to clarify how ASL investigations are contributing to the evolving insight on migraine pathophysiology and their putative role in migraine clinical setting. ASL techniques, allowing to quantitatively demonstrate changes in cerebral blood flow (CBF) both during the attacks and in the course of interictal period, could represent the melting point between advanced neuroimaging investigations, conducted with pure scientific purposes, and conventional neuroimaging approaches, employed in the diagnostic decision-making processes.
Main body
Converging ASL evidences have demonstrated that abnormal CBF, exceeding the boundaries of a single vascular territory, with biphasic trend dominated by an initial hypoperfusion (during the aura phenomenon but also in the first part of the headache phase) followed by hyperperfusion, characterizes migraine with aura attack and can represent a valuable clinical tool in the differential diagnosis from acute ischemic strokes and epileptic seizures.
Studies conducted during migraine without aura attacks are converging to highlight the involvement of dorsolateral pons and hypothalamus in migraine pathophysiology, albeit not able to disentangle their role as “migraine generators” from mere attack epiphenomenon. Furthermore, ASL findings tend to support the presence of perfusion abnormalities in brain regions known to be involved in aura ignition and propagation as well as in areas involved in multisensory processing, in both patients with migraine with aura and migraine without aura.
Conclusion
Although ASL studies have dramatically clarified quality and timing of perfusion abnormalities during migraine with aura attacks, the same cannot be said for perfusion changes during migraine attacks without aura and interictal periods. Future studies with more rigorous methodological approaches in terms of study protocol, ASL technique and sample selection and size are mandatory to exploit the possibility of better understanding migraine pathophysiology and identifying neuroimaging biomarkers of each migraine phase in different migraine phenotypes.
Similar content being viewed by others
Introduction
Migraine is a genetically determined neurovascular disorder featuring recurrent episodes of headache accompanied by a constellation of non-pain symptoms including sensory hypersensitivity, neurovegetative symptoms as well as cognitive and emotional changes [1]. A non-negligible percentage of patients (about one third of patients with migraine) experiences focal neurological symptoms (the so-called aura), usually starting before the headache onset, gradually spreading up to slowly disappear. Interestingly, besides the ictal burden, compelling evidences support clinical and psychosocial impact also during interictal periods in these patients [2].
The diagnosis of migraine is based on the different features of headache (e.g., quality, location, duration, timing, severity), the presence of accompanying symptoms as phono/photophobia, nausea and vomiting, along with attacks-related disability and precipitating events [3]. Therefore, due to the unavailability of specific neuroimaging biomarkers, in the current clinical practice, migraine diagnosis is an eminently clinical process where instrumental examinations take a part only in excluding causes of secondary headaches or, eventually, migraine-worsening disorders [4].
In the last decades, among the advanced functional, structural and microstructural neuroimaging approaches, arterial spin labelling (ASL) techniques, able to quantitatively investigate, with clinical high field MRI, both global and local changes in cerebral blood flow (CBF), represented a substantial breakthrough in the knowledge of human brain processes as well as in other brain disorders [5].
Specifically, ASL techniques have contributed to elucidate the complex mechanisms underlying migraine pathophysiology by the identification of CBF abnormalities both during attacks and in the course of interictal period.
Furthermore, while until few years ago the idea that advanced neuroimaging techniques could play a role in the evaluation of patients with migraine in clinical setting was “heretical”, nowadays ASL techniques, by providing quantitative MRI biomarkers, could represent the melting point between advanced neuroimaging investigations, often conducted on a group-level with pure scientific purposes, and conventional neuroimaging approaches, already employed in the diagnostic decision-making process applied to single patients with migraine [6]. Similarly, in a futuristic way, ASL techniques could be putatively able to provide prognostic critical information in order to support “tailored” therapeutic strategies in every single patient with migraine, overcoming the limitations related to the variability of the judgment and experience of clinicians.
The present narrative review focuses on the progresses of the ASL techniques over time, their role in the evolution of understanding migraine pathophysiology and their applicability in migraine clinical setting.
Methods
Two of the authors (AR and MS) performed searches on the PubMed.com website to identify all the original articles by entering the key word "migraine without aura" or “migraine with aura” combined with "arterial spin labeling". The literature search was finalized on Pubmed.com March 20th, 2023 and was restricted to human studies written in English language. Our search approach resulted, after removing duplicates, in a total of 57 papers. Among these, 25 reviews, letters, experiments testing treatment effects or studies not focused on migraine as well as articles in which methods were not properly described were excluded.
To identify additional relevant studies, we thoroughly checked the reference list for each considered article. Finally, the author neurologists (AR, AT and GT) reviewed the papers found in the preliminary phase to screen for inclusion and exclusion criteria. We ended up with a total of 32 peer-reviewed studies.
Arterial spin labelling
ASL is a non-contrast MRI technique able to perform a measurement of brain perfusion by means of magnetically labelled arterial blood water working as an endogenous freely diffusible tracer [7,8,9]. Radiofrequency inversion pulses are used to modify the T1 signal longitudinal magnetizing (“label”) moving protons within the carotid and vertebral arteries at the skull base. Image acquisition occurs after a time delay to allow the labeled blood to flow into the cerebral arteries (i.e. arterial capillaries) where imaging slices are acquired. Perfusion characteristics are quantitatively determined by pairwise comparison of imaging data with and without labeling (the latter working as control) [7,8,9].
The advantages of ASL compared with conventional (e.g. 133Xe-SPECT, H215O-PET) as well as advanced (e.g. phase-contrast MRI) perfusion-based techniques include absolute quantification, repeatability, avoidance of intravenous contrast administration and, overall, superior spatial resolution and sensitivity. More specifically, all the major methods that have been developed for measuring CBF are based on the principles of compartmental modeling and tracer kinetics describing the dynamics of a tracer as it crosses the arterial tree into the brain’s microvasculature (non-diffusible tracers) and into the tissue (diffusible tracers) prior to venous washout. Contrariwise, ASL fMRI uses arterial water as an endogenous tracer and thus does not require injection of exogenous tracers that can be uncomfortable and potentially harmful. Therefore, ASL, being noninvasive, is safe to repeat over time and can be used to track changes in CBF in the course of disease progression or treatment. Furthermore, ASL yields an absolute measurement of CBF and therefore any change in flow can be expressed in physiologically meaningful units rather than as a percentage of change. Finally, ASL yields CBF images with higher spatial and temporal resolution than any other current technique.
Based on the labeling scheme, ASL can be divided into 3 categories: pulsed ASL (pASL), continuous ASL (cASL) and the more recently introduced pseudo-continuous ASL (pcASL).
The pASL was the first approach developed and is characterized by the application of a single inversion pulse providing high and stable labeling efficiency (e.g., up to 95%), although this approach is burdened by a suboptimal SNR and a limited spatial coverage.
Contrariwise, cASL, characterized by the constant application of a pulse gradient to label arterial blood flowing through a given plane, provides a higher SNR (about 30–50% higher than pASL) although with suboptimal labeling efficiency (e.g., up to 68%).
Finally, the pcASL, using a long series of radiofrequency and gradient pulses, provides a more robust measure of CBF, even at low flows, and is able to offer both higher SNR and labeling efficiency compared with pASL and cASL respectively.
In order to maximize the SNR and temporal stability of ASL scans, background suppression techniques and three-dimensional (3D) fast imaging sequences have been developed. 3D acquisitions not only offer increased SNR compared with two-dimensional (2D) acquisitions but they are also ideally suited for background suppression. A recent consensus recommended pcASL with background-suppressed 3D acquisitions as the preferred strategy for ASL clinical applications [5].
It is noteworthy that using the ASL techniques the selection of post-labeling delay (PLD) (that is the time interval between the labeling and the slice acquisition) is crucial to obtain reliable measurements. Specifically, the time interval must be long enough to allow labeled protons to flow into the imaging slice, but not so long that the majority of measurable tracer has decayed. For this reason, until few years ago, the above-mentioned ASL sequences, characterized by a single PLD between the labeling plane and the image acquisition, were burdened by a poor accuracy. More specifically, the variability of regional arterial transit time (ATT), depending on physiological and anatomical parameters (including, e.g., the cardiac output) as well as on the coexistence of some cerebrovascular conditions (e.g., stenotic or low calibre arteries, or collateral circulation), resulted in a potential mismatch between labeling plane (radiofrequency-labeled local blood flow) and 3D image acquisition (PLD sequences) [10]. Another limitation of ASL sequences is related to the fact that settings cannot be easily adjusted or optimized to achieve the highest sensitivity for regional CBF changes, considering single or multiple perfusion territories, within functional relevant domains of interest. This is a momentous issue whenever the highest effects of reduced or increased CBF may encompass adjacent perfusion territories characterized by different ATT or when specific pathological conditions, systematically affecting CBF parameters, may occur.
In order to surmount the above-mentioned constraints a modified ASL technique has been developed, the so-called multi-delay-3D-pcASL, adding to the noteworthy advantages of 3D-pcASL: i) the proficiency of acquiring volumes with multiple different post-labeling delay times within the same series (i.e. multi-delay) increasing the reliability of whole brain CBF analyses ii) the possibility to contextual estimate adjunctive hemodynamic parameters as “mean arterial transit time” (ATT), arterial cerebral blood volumes and visualize the collateral flow through dynamic image series [11]. As a proof of the concept, previous multi-delay-3D-pcASL studies have demonstrated that the use of multiple post-labeling times may lead to sufficiently accurate ATT estimation, thus improving the overall perfusion quantification models and, consequently, providing CBF maps with reduced errors from ATT and tissue T1 variations [12].
Beyond the mere evaluation of regional CBF, ASL techniques allow broader applications to assess brain function during both resting and task condition [13]. Specifically, ASL can be used to explore the brain resting-state functional connectivity (as an alternative way than the blood oxygen level-dependent (BOLD) technique) for a functional connectome analysis), and neurovascular coupling (if CBF and BOLD signals are properly combined from concurrent ASL and BOLD sequences) [14]. The neurovascular coupling is the complex biological process that underlies use-dependent increases in blood flow in response to neural activation.
ASL application to migraine models
It is known that migraine is not simply headache but a complex and multifaceted neurological disease. Although head pain is the most characterizing symptom, patients with migraine also experience a plethora of non-headache symptoms starting before headache onset or persisting after its resolution, classically divided into the preictal, aura, headache and postdromal as well as interictal phases, overall configuring the so-called migraine cycle. In the last years, advanced neuroimaging studies have played a critical role in demonstrating, during the preictal phase but persisting throughout the whole headache timeline, the early activation of the hypothalamus as well as trigeminal, pontine and thalamo-cortical pathways [15]. Along with the elucidation of the involvement of neuronal pathways underpinning each phase of migraine cycle, the ASL techniques have further contributed to clarify the neurovascular substrates of migraine disorder (See Table 1 and Fig. 1 for further information).
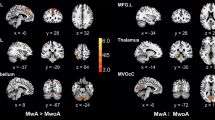
ASL perfusion changes for each phase of migraine cycle. A and B CBF maps showing an early left hemisphere hypoperfusion followed by hyperperfusion in areas congruous with aura symptoms (exceeding the boundaries of single vascular territories) (modified from Boulouis et al., 2016 [18]). C Reduced regional CBF in lateral hypothalamus and visual cortex during the preictal phase of migraine attack (modified from Meylakh et al., 2020 [38]). D Increased regional CBF in limbic circuit during the headache phase of migraine (migraine without aura attack) (modified from Stankewitz et al., 2021 [43]). E Increased regional CBF in extrastriate cortex and superior temporal gyrus during the interictal period (migraine with aura) (modified from Michels et al., 2019 [28])
ASL investigation during aura and headache phases of migraine with aura attacks
About 30% of patients with migraine reports fully reversible focal neurological symptoms, constituting the so-called aura, gradually spreading in about 5 min, most often before the headache onset (more rarely starting along with headaches), until slowly disappearing between 5 to 60 min.
Visual symptoms, either negative or positive, are the most frequently reported in the course of aura, followed by somatosensory symptoms (frequently manifesting as cheiro-oral paraesthesias) and dysphasic symptoms respectively in about one third and 10% of patients [44, 45]. Nevertheless, patients may rarely report other higher cortical dysfunctions such as memory disturbances, prosopagnosia, dyscalculia and manual dyspraxia in the course of aura phenomenon. The cortical spreading depolarization (CSD), a wave of initial increase, followed by a sustained decrease, was invoked as the pathophysiologic mechanism underlying the aura phenomenon based on well-known electrophysiological studies on rabbit brain [46]. Only several decades later, the pioneering studies of Olesen and colleagues demonstrated, using 133Xe-SPECT during aura phase of provoked or spontaneous migraine attacks, a cortical oligemia spreading from occipital areas to the anterior regions over 15–45 min, not congruent with large cerebral arteries vascular territories [47, 48]. Interestingly, the regional hypoperfusion involving cerebral hemisphere during the aura symptoms kept being detectable by SPECT sequences also after 30 and 150 min from the onset of spontaneous migraine with aura and the same cerebral regions were then overwhelmed by hyperemia [49]. Subsequently, Hadjikhani and colleagues by means of BOLD-fMRI experiments in patients with migraine during the aura symptoms showed that perfusion changes, far from being the “primum movens” of aura, resulted from autoregulatory mechanisms secondary to the neuronal spreading depression [50,51,52]. These findings stimulated the interest of researchers and clinicians so that several ASL studies have been conducted to explore the perfusion pattern of aura phenomenon in order to elucidate the underpinning mechanisms and to improve the differential diagnosis between the manifestations of aura spectrum and cerebral ischemic events.
Among these studies, patients with migraine were investigated with 2D-pASL within 6 h from the onset of aura phenomenon to identify CBF changes correlated with the diagnosis of migraine with aura (MwA). The results showed asymmetrical hypoperfusion, predominantly in the parieto-occipital regions, not confined to specific vascular territories [52]. The aura-related perfusion changes were associated, in the majority of patients (74%), with intracranial vasculature vasospasm as detected by TOF-MRA images. Nevertheless, both perfusion and vasculature features were only qualitatively evaluated based on a mere visual assessment [16]. Pathophysiological and clinical insights on aura-related cerebral hypoperfusion could be even more relevant in complex aura phenotypes (such as hemiplegic migraine, atypical aura or confusional migraine attacks) often representing a diagnostic challenge, difficult to be differentiated from acute ischaemic strokes. Focusing on hemiplegic migraine attacks, a pattern of cerebral hypoperfusion involving the hemisphere contralateral to motor dysfunction was detected since the first ASL approaches [17]. However, over time, specific and accurate data arose from studies employing ASL technique evolutions. For instance, using 3D-pcASL, abnormal CBF has been demonstrated in paediatric patients with MwA manifesting complex aura phenotypes [18]. Interestingly, patients investigated within 14 h from the onset of the aura exhibited a decreased CBF, involving the hemisphere contralateral to aura symptoms, whereas after 17 h from the onset of the aura, an increased CBF was highlighted in the same areas, independently from the symptoms or the persistence of either aura or headache at the time of the scan [18]. This biphasic perfusion evolution characterized by an initial regional hypoperfusion followed by hyperperfusion, seems to represent the typical pattern of MwA attack since it has been observed also in patients experiencing typical visual MwA when investigated after the onset of aura phenomenon as well as when explored several hours after the aura resolution during the headache phase [19,20,21]. The shift from regional cerebral hypoperfusion to hyperperfusion (due to delayed arrival of labelled blood in the territories involved) justify the so-called “arterial transit artefact” in patients explored during the transition from the aura to the headache phase, as already demonstrated in the early reperfusion following intravenous thrombolysis in patients with ischaemic stroke [21].
While the presence of a biphasic CBF changes involving aura and early headache phases is an indisputable fact, the time-course of the perfusion phenomenon seems difficult to be consistently delineated. Therefore, 3D-pcASL quantitative and qualitative analyses, along with MRA and SWI qualitative analyses, have been conducted in patients with MwA or hemiplegic migraine within 12 h from symptoms onset. Consistently with previous studies, initial hypoperfusion (with concomitant apparent constriction of distal vessels on MRA and deoxygenated blood in cerebral veins on SWI) followed by a rebound hyperperfusion in the affected hemisphere were demonstrated [23]. Focusing on the time-course of perfusion changes, the duration of cerebral regional hypoperfusion lasted over 11 h (since the aura onset) in the majority of patients, although more rapid transition to the rebound hyperperfusion could be noticed in some patients. Interestingly, a discordance between increased regional CBF and reduction of vessel calibres was observed during the hyperperfusion in a small percentage of patients, suggesting reversible disturbances of neurovascular coupling (the mutual relation between neuronal activity and perfusion) during the transition from aura and headache phases.
Considering the cerebral topography of the biphasic hypoperfusion-hyperperfusion phenomenon, the involvement of posterior regions during the attack of sporadic hemiplegic migraine was highlighted in an early anecdotal report [17]. Subsequently, a pASL study with a larger sample-size confirmed that occipital and parietal cortices were the most frequently encompassed by hypoperfusion in patients with MwA compared to patients with MwoA, whit a significant correlation between the hyperperfusion magnitude and the complexity of aura phenotype [24]. Although limited, these findings suggest that i) the shift from regional cerebral hypoperfusion to hyperperfusion may occur in some patients sooner than expected according to the findings of previous ASL studies ii) the time-gap between the clinical onset of aura and MRI images acquisition is crucial more than the actual presence of symptoms at the time of imaging scan, considering the risk of detecting aura-related hypoperfusion also in the course of the following headache, when the aura symptoms are clinically extinguished iii) posterior cerebral areas, especially parietal and occipital cortices, are preferentially involved by perfusion changes in the course of both aura and headache phases of migraine attacks.
Altogether the above-mentioned ASL findings, beyond the insight provided about the pathophysiological mechanisms underlying the different phases of migraine cycle, give some critical pieces of advice with potential impact in the clinical practice. First, the presence of hypoperfusion during the first phases of MwA attacks, especially in patients with hemiplegic migraine, may suggest avoiding triptans during the aura symptoms in these patients due to the triptans vasoconstrictive properties and related concerns about ischaemic events. Moreover, ASL techniques, detecting in patients presenting with aura symptoms the presence of biphasic CBF changes involving vascular territories belonging to different vessels in the absence of DWI abnormalities, may allow us to confute alternative diagnoses such as ischaemic attacks and epileptic seizures.
ASL investigation during migraine without aura attacks
It should be underlined that there is a scarcity of brain perfusion studies based on ASL sequences focusing on the headache phase of MwoA attacks and those conducted have shown inconsistent results. Indeed, it has been reported, by anecdotic observations, a significant relative hypoperfusion in the bilateral median thalamic structures (including hypothalamus) as well as in the frontal cortex during the headache phase of MwoA when compared to the interictal phase [25]. Interestingly, after 30 min from rescue therapy with rizatriptan, the observed hypoperfusion partially normalized. These findings support the involvement of hypothalamus and surrounding brain structures as a critical mechanism in the pathogenesis of migraine attacks, as widely demonstrated by means of BOLD investigations [53].
Nevertheless, other observations tend to be in line with the pioneering observations of Weiller showing, by means of H215O PET, a higher regional CBF values in the dorsal pons during a spontaneous MwoA attack, persistent after sumatriptan-related pain relief, compared to the pain-free state [54]. In particular, a multiparametric study evidenced a significant CBF increase in the dorsolateral pons (ipsilaterally to the headache side) concomitantly with increase in creatine levels (in the absence of changes in glutamate concentration), during provoked MwoA attacks (by CGRP or sildenafil) compared to the interictal period and to healthy subjects [26]. Contrariwise, by using 3D-pcASL, no global or regional perfusion differences were identified during spontaneous migraine attacks and migraine-free phases [27].
It should be underlined that the results of the above-mentioned longitudinal studies, aimed to investigate different phases of migraine attacks in the same patient (e.g. ictal versus interictal scans), may be potentially biased by the well-known influence of caffeine intake, tobacco smoking, and hematocrit changes on CBF, as well as by the physiologic circadian variation of CBF values.
Despite the paucity of studies, the above-mentioned data highlight the momentous involvement of dorsolateral pons and hypothalamus in the headache phases of migraine attacks. However, they are not able to disentangle which structure can assume the role of “migraine generator” or, whether, alternatively, the activation of both hypothalamus and dorsolateral pons could represent an epiphenomenon of the persistence of hypothalamus involvement (started in the so-called preictal phases of migraine attacks) and the migraine-related pain perception, respectively [55,56,57].
ASL investigation during the interictal period
Similarly to the lack of converging evidences about the presence of perfusion abnormalities during the headache (i.e. ictal) phases in patients experiencing MwoA, many discrepancies emerged from ASL studies investigating the interictal period of both patients with Mwa and patients with MwoA.
Patients with episodic migraine (both with and without aura) showed extrastriate cortex (V5 area) and superior temporal gyrus hyperperfusion compared to healthy subjects when explored by means of 2D-pcASL during the interictal period [28]. Interestingly, the perfusion abnormalities were more pronounced in MwA patients [28]. The involvement of posterior brain areas, in the terms of asymmetries of blood flow in the posterior cerebral artery territories, has been also demonstrated, by exploring a large sample of 170 subjects, in MwA patients (but not MwoA) during the interictal period [29]. Interestingly, the hypoperfusion in the territories supplied by the posterior cerebral artery was significantly associated with a higher prevalence of abnormalities of the circle of Willis.
A significant increase in regional CBF has been demonstrated in the primary somatosensory cortex (S1) in patients with migraine experiencing ictal cutaneous allodynia investigated during the interictal period (subjects were headache-free from at least 48 h at the time of scan acquisition) [31]. Moreover, the authors found a positive correlation between regional CBF degree and headache attack frequency, as already observed also in a cohort of paediatric and adolescent patients with migraine [32]. Increased regional CBF has been also demonstrated by means 3D-pc-ASL in the temporal areas (Brodmann 38), supposed to represent a convergence zone of information from auditory, somatosensory, visual, and olfactory inputs and, therefore, involved in multisensory integration [33].
Finally, interictal regional CBF abnormalities have been recently investigated by means of 3D-pcASL in a large cohort of patients with migraine. After whole brain voxel-based CBF comparison between patients with migraine and healthy subjects, the areas showing significant differences between the groups were extracted as ROIs to compare the normalized regional CBF values among all the subjects [34]. Over and above the visual, somatosensory and multisensory integration cortices observed in the previous studies, higher CBF levels in superior frontal and postcentral gyri and cerebellum, and lower CBF levels in the bilateral middle frontal gyrus, thalamus and medioventral occipital cortex have been demonstrated in patients with MwA compared with patients with MwoA and healthy subjects. Interestingly, based solely on CBF values of these six cerebral regions, by using a machine learning approach, it was possible to correctly classify more than 80% of patients with MwA or MwoA [34].
In this scenario, although aimed to investigate the CBF pattern underpinning visual snow, a syndrome characterized by the presence of continuous and unremitting visual disturbances often, but not exclusively, found as migraine comorbidity [30, 58], a very recent 3D-pcASL study is worth to be mentioned due to the enrolment of patients with MwA and MwoA. While a higher regional CBF encompassing an extensive brain network has been demonstrated in patients with visual snow (regardless of concomitant migraine) during both’snow-like’ visual stimuli and rest condition, a post-hoc analysis has suggested that the involvement of bilateral cuneus, posterior cingulate cortex, primary auditory cortex, fusiform gyrus, and cerebellum might be more closely related to the interictal phases of migraine. Visual symptoms, and specifically visual sensitivity to light (explored by Visual Light Sensitivity Questionnaire-8), along with migraine frequency have demonstrated a positive correlation with reduced regional CBF of frontal areas in a wide sample of patients with MwoA compared with healthy subjects [35]. Moreover, frontal ROI were characterized by a disrupted CBF connectivity with several cortical (i.e., contralateral frontal and angular gyri and calcarine cortex) and subcortical (i.e., putamen and caudate) brain areas, further supporting the involvement of an “advanced neurolimbic visual pain network” in patients with MwoA as well as in patients with MwA [59].
A reduced CBF of nucleus accumbens (identified as ROI using a specific nucleus accumbens perfusion mask) has been demonstrated by 3D-pcASL in patients with chronic migraine during the interictal period when compared with healthy subjects [36], further corroborated by the ROC curve analysis enabling the identification of patients with chronic migraine from healthy subjects (sensitivity of 50.00% but with a specificity over 90%). Apart from the known role played by the nucleus accumbens in pain modulation and reward/addiction mechanisms, no specific information have been reported about the presence of medication overuse headache in these patients. Moreover, the lack of a comparison group (e.g., patients with episodic migraine) made not possible to clarify the specificity of nucleus accumbens hypoperfusion in chronic migraine. CBF changes have been recently investigated by the same research group in patients with chronic migraine compared with healthy subjects, using the novel multi-delay-3D-pcASL approach, showing increased regional CBF and arterial cerebral blood volumes in bilateral ventral lateral nuclei of thalamus [37]. Being the thalamus a relay centre of ascending trigemino-cortical nociceptive information, characterized by increased activation during migraine attacks as well as by abnormal functional connectivity in the interictal period in patients with episodic migraine [60], the authors suggested that the chronification process, towards a never-ending attack, could be underpinned by a constant increase of neuronal thalamic activation and, in turns, of regional CBF. However, this riveting hypothesis is burdened by a low sample size and spatial resolution making impossible to clearly discriminate among thalamic nuclei (being the ASL imaging resolution of about 1.6 mm × 1.6 mm × 4.0 mm).
Totally different are the results from three recent studies, finding no CBF changes in both patients with MwoA and MwA [38,39,40], although a significantly lower global CBF characterizes patients with MwA with high white matter hyperintensities load explored during interictal period when compared to healthy subjects [40]. Albeit the findings do not allow a univocal interpretation, the authors argued that the well-known CBF fluctuations observed during the MwA attacks might affect microvascular haemodynamic leading to microischaemic injury that can be observed also during the interictal period. Nevertheless, the possibility that the decreased CBF could be the result, rather than the cause, of white matter abnormalities in patients with MwA cannot be excluded, to date [40].
The evidences of both impaired cerebral perfusion and functional neuronal changes tend to support the hypothesis of an altered relationship between neuronal activity and cerebral perfusion maps (i.e. neurovascular coupling) during the different phases of migraine cycle. On the other hand, neurovascular coupling abnormalities have been demonstrated in patients with chronic migraine investigated by using 3D-pcASL technique [41]. Specifically, significantly lower neurovascular coupling indices in inferior parietal, superior marginal and left angular gyri, as well as significantly higher neurovascular coupling indices in precuneus, superior occipital and parietal gyri were identified, probably underpinning migraine-related abnormalities in visual and sensory processing.
The 3D-pcASL has been recently employed, along with DWI sequences, to investigate global functional and structural brain connectome parameters in patients with MwoA patient compared to patients with MwA [42] showing no differences in network measures of global structural connectivity but significantly lower assortative coefficient in patients with MwA compared to patients with MwoA. Based on the fact that assortativity coefficient is a parameter of network resilience able to quantify the mutually interconnection among cortical hubs (so-called nodes), networks with low assortativity, as observed in patients with MwA, are more vulnerable to insult, easily disrupted, and more synchronized than those with high assortativity [61]. Unfortunately, the low sample size as well as the absence of a healthy subjects group strongly affect both the specificity and the generalizability of the results.
Although the above-mentioned studies suffer from several limitations such as differences in patients homogeneity, disparities in ASL techniques (with consequent divergences in labeling schemes, background suppressions, dimensional resolutions, etc.), as well as in methodological procedures (e.g. whole-brain analysis or ROI-based approaches), altogether they support disease-specific functional changes in trigemino-cortical pathway and in brain regions known to be involved in somatosensory, auditory and visual processing suggesting abnormal pain perception and modulation as well as dysfunctional mechanisms of multisensory integration in both patients with MwoA and MwA, also during the interictal phase. To the same extent, ASL findings, consistently with data from advanced neuroimaging literature [62], further corroborate the critical role of brain structures related to cortical spreading depression ignition and propagation also between MwA attacks.
ASL investigation during the preictal phase
In the last years, besides with the insight about the differences within the phases of migraine cycle the preictal phase of migraine attacks has been specifically investigated with even more advanced ASL approaches as well as using methodologically rigorous study paradigms. Unfortunately, due to the unpredictability of migraine attacks, it is quite difficult to collect MRI scans during the period immediately prior to migraine attacks, frequently only by chance and, therefore, in a low sample of patients.
By means of 3D-pcASL, immediately before the onset of headache in patients with MwoA (within at least 24 h), a decreased regional CBF has been observed in the lateral hypothalamus that was also characterized by a reduced functional connectivity with several regions within the pain processing pathway (e.g. midbrain periaqueductal grey, dorsal pons, rostral ventromedial medulla and cingulate cortex) [38]. These findings are in line with literature data supporting the role played by the hypothalamus in migraine attack ignition previously suggested by both PET and fMRI studies showing an increased hypothalamic activity during the hours preceding the headache of the migraine attack [56, 57].
The rhythmicity of the cerebral perfusion and changes of hypothalamic functional connectivity over migraine cycle have been explored by daily longitudinal intra-individual scanning (82 scans to cover the course of migraine cycles of twelve patients with migraine) [43]. Interestingly, during the headache phases, the highest perfusion of the limbic circuit (i.e., insula and nucleus accumbens) was detected along with a collapse of functional connectivity between the hypothalamus and the limbic areas. More in depth, the functional connectivity between hypothalamus and limbic structures progressively increased, over the migraine cycle towards the next attack, up to rapidly drop during the headache phase. According with these data, while the hypothalamus seems to act as a metronome of internal processes, able to control limbic pathways, the migraine attacks may represent the result of the lost hypothalamic inhibition on the circuit.
Whether the functional and perfusion hypothalamic abnormalities may represent the correlates of the brain changes closely preceding the headache phases of migraine attacks, reflecting modulatory input to the trigeminovascular system from several cortical and subcortical areas, or if they represent the neuronal and perfusion correlates of preictal symptoms is still matter of debate, although the current trend to accept that premonitory symptoms as due to hypothalamic dysfunction seems to be premature [63]. Future insight on postdrome phase could further improve our knowledge about migraine cyclicity shedding a light on the mechanisms underlying not only the ignition of migraine attacks but also the self-limitation of migraine attacks in a way characterizing the typical migraine attack duration (i.e., the well know duration of about 4–72 h) and that, on the other hand, may set up the neuronal patterns towards the interictal changes predisposing the next migraine attack.
Final considerations
Taken together, the data emerging from the ASL studies in patients with migraine allow to achieve some conclusions:
-
1) During MwA attacks the areas congruous with aura symptoms are characterized by an altered cerebral perfusion, exceeding the boundaries of a single vascular territory, with a biphasic trend dominated by an initial hypoperfusion (persisting even during the first hours of the headache attack) followed by an hyperperfusion phase. However, future studies are mandatory to determine the duration of the hypoperfusion, over different phases of migraine cycle, as precise as possible. Nevertheless, beyond the role of ASL techniques to explore migraine pathophysiological mechanisms, they could represent a valuable clinical tool to be applied in the differential diagnosis of migraine aura from acute ischemic strokes (in particular in the cases of atypical aura, hemiplegic migraine, confusional migraine, HANDL).
-
2) Although perfusion abnormalities have been observed in the visual associative and primary somatosensory cortices as well as in areas involved in the processing and modulation of the painful experience, the scarcity of data does not allow univocal and conclusive insight on regional CBF changes found in the intercritical period, as witnessed by the conflicting results and most likely due to several methodological issues. Among these, i) different ASL techniques adopted in the perfusion studies, from the most pioneering and rudimental to the most advanced and accurate approaches, up to the more complete multi delay-3D-pcASL which has been only recently sufficiently developed for clinical studies; ii)) heterogeneous patients samples (e.g. groups of patients including different migraine phenotype, with or without concomitant preventive treatments, with no reference to the intake of medications for the acute phase).
References
Noseda R, Burstein R (2013) Migraine pathophysiology: anatomy of the trigeminovascular pathway and associated neurological symptoms, cortical spreading depression, sensitization, and modulation of pain. Pain 154(Suppl 1):S44–S53
Vincent M, Viktrup L, Nicholson RA, Ossipov MH, Vargas BB (2022) The not so hidden impact of interictal burden in migraine: a narrative review. Front Neurol 13:1032103
Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition (2018) Cephalalgia: an international journal of headache, 38(1), 1–211
Evans RW, Burch RC, Frishberg BM, Marmura MJ, Mechtler LL, Silberstein SD, Turner DP (2020) Neuroimaging for Migraine: the american Headache Society systematic review and evidence-based Guideline. Headache 60(2):318–336
Alsop DC, Detre JA, Golay X, Günther M, Hendrikse J, Hernandez-Garcia L, Lu H, MacIntosh BJ, Parkes LM, Smits M, van Osch MJ, Wang DJ, Wong EC, Zaharchuk G (2015) Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the european consortium for ASL in dementia. Magn Reson Med 73(1):102–116
Russo A, Silvestro M, Tessitore A, Tedeschi G (2019) Functional neuroimaging biomarkers in Migraine: Diagnostic, Prognostic and therapeutic implications. Curr Med Chem 26(34):6236–6252
Nezamzadeh M, Matson GB, Young K, Weiner MW, Schuff N (2010) Improved pseudo-continuous arterial spin labelling for mapping brain perfusion. J Magn Reson imaging: JMRI 31(6):1419–1427
Wu WC, Lawrence St, Licht KS, D. J., Wang DJ (2010) Quantification issues in arterial spin labelling perfusion magnetic resonance imaging. Top Magn Reson imaging: TMRI 21(2):65–73
van Osch MJ, Teeuwisse WM, Chen Z, Suzuki Y, Helle M, Schmid S (2018) Advances in arterial spin labelling MRI methods for measuring perfusion and collateral flow. J Cereb blood flow metabolism: official J Int Soc Cereb Blood Flow Metabolism 38(9):1461–1480
Wolf RL, Alsop DC, McGarvey ML et al (2003) Susceptibility contrast and arterial spin labelling perfusion MRI in cerebrovascular disease. J Neuroimaging 13:17Y27
Golay X, Ho ML (2022) Multidelay ASL of the pediatric brain. Br J Radiol 95(1134):20220034
Johnston ME, Lu K, Maldjian JA, Jung Y (2015) Multi-TI arterial spin labeling MRI with variable TR and Bolus Duration for Cerebral Blood Flow and arterial transit time mapping. IEEE Trans Med Imaging 34(6):1392–1402
Hernandez-Garcia L, Aramendía-Vidaurreta V, Bolar DS, Dai W, Fernández-Seara MA, Guo J, Madhuranthakam AJ, Mutsaerts H, Petr J, Qin Q, Schollenberger J, Suzuki Y, Taso M, Thomas DL, van Osch MJP, Woods J, Zhao MY, Yan L, Wang Z, Zhao L, ... Okell TW (2022) Recent Technical Developments in ASL: A Review of the State of the Art. Magn Reson Med 88(5):2021–2042
Chen JJ, Jann K, Wang DJ (2015) Characterizing resting-state brain function using arterial spin labeling. Brain Connect 5(9):527–542
Messina R, Cetta I, Colombo B, Filippi M (2022) Tracking the evolution of non-headache symptoms through the migraine attack. J Headache Pain 23(1):149 Published 2022 Nov 23
Cadiot D, Longuet R, Bruneau B, Treguier C, Carsin-Vu A, Corouge I, Gomes C, Proisy M (2018) Magnetic resonance imaging in children presenting migraine with aura: Association of hypoperfusion detected by arterial spin labelling and vasospasm on MR angiography findings. Cephalalgia: an international journal of headache 38(5):949–958
Kim S, Kang M, Choi S (2016) A case report of sporadic hemiplegic migraine associated cerebral hypoperfusion: comparison of arterial spin labeling and dynamic susceptibility contrast perfusion MR imaging. Eur J Pediatrics 175(2):295–298
Boulouis G, Shotar E, Dangouloff-Ros V, Grévent D, Calmon R, Brunelle F, Naggara O, Kossorotoff M, Boddaert N (2016) Magnetic resonance imaging arterial-spin-labelling perfusion alterations in childhood migraine with atypical aura: a case-control study. Dev Med Child Neurol 58(9):965–969
Pollock JM, Deibler AR, Burdette JH, Kraft RA, Tan H, Evans AB, Maldjian JA (2008) Migraine associated cerebral hyperperfusion with arterial spin-labeled MR imaging. AJNR Am J Neuroradiol 29(8):1494–1497
Burns R, De Malherbe M, Chadenat ML, Pico F, Buch D (2017) Arterial spin-labeled MR Imaging detecting biphasic neurovascular changes in Migraine with Persistent Aura. Headache 57(10):1627–1628
Law-Ye B, Fargeot G, Leclercq D (2017) Arterial spin labeling hypoperfusion in Migraine Aura. Headache 57(6):935–936
Wolf ME, Okazaki S, Eisele P, Rossmanith C, Gregori J, Griebe M, Günther M, Gass A, Hennerici MG, Szabo K, Kern R (2018) Arterial spin labeling cerebral perfusion magnetic resonance imaging in Migraine Aura: an observational study. J stroke Cerebrovasc diseases: official J Natl Stroke Association 27(5):1262–1266
Cobb-Pitstick KM, Munjal N, Safier R, Cummings DD, Zuccoli G (2018) Time course of cerebral perfusion changes in children with migraine with aura mimicking stroke. AJNR Am J Neuroradiol 39(9):1751–1755
Uetani H, Kitajima M, Sugahara T, Kikuchi H, Muto Y, Hirahara T, Tateishi M, Kuroki Y, Yamashita Y (2018) Perfusion abnormality on three-dimensional arterial spin labeling with a 3T MR system in pediatric and adolescent patients with migraine. J Neurol Sci 395:41–46
Kato Y, Araki N, Matsuda H, Ito Y, Suzuki C (2010) Arterial spin-labeled MRI study of migraine attacks treated with rizatriptan. J Headache Pain 11(3):255–258
Younis S, Christensen CE, Vestergaard MB, Lindberg U, Tolnai D, Paulson OB, Larsson HB, Hougaard A, Ashina M (2021) Glutamate levels and perfusion in pons during migraine attacks: a 3T MRI study using proton spectroscopy and arterial spin labeling. J Cereb blood flow metabolism: official J Int Soc Cereb Blood Flow Metabolism 41(3):604–616
Gil-Gouveia R, Pinto J, Figueiredo P, Vilela PF, Martins IP (2017) An arterial spin labeling MRI perfusion study of migraine without aura attacks. Front Neurol 8:280
Michels L, Villanueva J, O’Gorman R, Muthuraman M, Koirala N, Büchler R, Gantenbein AR, Sandor PS, Luechinger R, Kollias S, Riederer F (2019) Interictal Hyperperfusion in the higher visual cortex in patients with episodic migraine. Headache 59(10):1808–1820
Cucchiara B, Wolf RL, Nagae L, Zhang Q, Kasner S, Datta R, Aguirre GK, Detre JA (2013) Migraine with aura is associated with an incomplete circle of Willis: results of a prospective observational study. PLoS ONE 8(7)
Puledda F, Schankin CJ, O’Daly O, Ffytche D, Eren O, Karsan N, Williams SCR, Zelaya F, Goadsby PJ (2021) Localised increase in regional cerebral perfusion in patients with visual snow syndrome: a pseudo-continuous arterial spin labelling study. J Neurol Neurosurg Psychiatry 92(9):918–926
Hodkinson DJ, Veggeberg R, Wilcox SL, Scrivani S, Burstein R, Becerra L, Borsook D (2015) Primary somatosensory cortices contain altered patterns of Regional Cerebral Blood Flow in the Interictal phase of Migraine. PLoS ONE 10(9)
Youssef AM, Ludwick A, Wilcox SL, Lebel A, Peng K, Colon E, Danehy A, Burstein R, Becerra L, Borsook D (2017) In child and adult migraineurs the somatosensory cortex stands out ... again: An arterial spin labeling investigation. Hum Brain Mapp 38(8):4078–4087
Chen Z, Chen X, Liu M, Liu M, Ma L, Yu S (2018) Evaluation of gray matter perfusion in episodic migraine using voxel-wise comparison of 3D pseudo-continuous arterial spin labeling. J Headache Pain. 19(1):36
Fu T, Liu L, Huang X, Zhang D, Gao Y, Yin X, Lin H, Dai Y, Wu X (2022) Cerebral blood flow alterations in migraine patients with and without aura: an arterial spin labeling study. J Headache Pain 23(1):131
Zhang D, Huang X, Mao C, Chen Y, Miao Z, Liu C, Xu C, Wu X, Yin X (2021) Assessment of normalized cerebral blood flow and its connectivity with migraines without aura during interictal periods by arterial spin labeling. J Headache Pain 22(1):72
Liu M, Sun Y, Li X, Chen Z (2022) Hypoperfusion in nucleus accumbens in chronic migraine using 3D pseudo-continuous arterial spin labeling imaging MRI. J Headache Pain 23(1):72
Bai X, Wang W, Zhang X, Hu Z, Zhang Y, Li Z, Zhang X, Yuan Z, Tang H, Zhang Y, Yu X, Zhang P, Wang Y, Sui B (2022) Cerebral perfusion variance in new daily persistent headache and chronic migraine: an arterial spin-labeled MR imaging study. J Headache Pain 23(1):156
Meylakh N, Marciszewski KK, Di Pietro F, Macefield VG, Macey PM, Henderson LA (2020) Altered regional cerebral blood flow and hypothalamic connectivity immediately prior to a migraine headache. Cephalalgia: an international journal of headache 40(5):448–460
Datta R, Aguirre GK, Hu S, Detre JA, Cucchiara B (2013) Interictal cortical hyperresponsiveness in migraine is directly related to the presence of aura. Cephalalgia: an international journal of headache 33(6):365–374
Zhang Q, Datta R, Detre JA, Cucchiara B (2017) White matter lesion burden in migraine with aura may be associated with reduced cerebral blood flow. Cephalalgia: an international journal of headache 37(6):517–524
Hu B, Yu Y, Dai YJ, Feng JH, Yan LF, Sun Q, Zhang J, Yang Y, Hu YC, Nan HY, Zhang XN, Zheng Z, Qin P, Wei XC, Cui GB, Wang W (2019) Multi-modal MRI reveals the neurovascular coupling dysfunction in chronic migraine. Neuroscience 419:72–82
Park S, Lee DA, Lee HJ, Shin KJ, Park KM (2022) Brain networks in migraine with and without aura: an exploratory arterial spin labeling MRI study. Acta Neurol Scand 145(2):208–214
Stankewitz A, Keidel L, Rehm M, Irving S, Kaczmarz S, Preibisch C, Witkovsky V, Zimmer C, Schulz E, Toelle TR (2021) Migraine attacks as a result of hypothalamic loss of control. NeuroImage Clin 32:102784
Petrusic I, Viana M, Dakovic M, Zidverc-Trajkovic J (2019) Application of the Migraine Aura Complexity score (MACS): clinical and Neuroimaging Study. Front Neurol 10:1112
Viana M, Sances G, Linde M, Ghiotto N, Guaschino E, Allena M, Terrazzino S, Nappi G, Goadsby PJ, Tassorelli C (2017) Clinical features of migraine aura: results from a prospective diary-aided study. Cephalalgia: an international journal of headache 37(10):979–989
Fraser CL, Hepschke JL, Jenkins B, Prasad S (2019) Migraine aura: pathophysiology, mimics, and Treatment Options. Semin Neurol 39(6):739–748
Olesen J, Larsen B, Lauritzen M (1981) Focal hyperemia followed by spreading oligemia and impaired activation of rCBF in classic migraine. Ann Neurol 9(4):344–352
Lauritzen M, Olesen J (1984) Regional cerebral blood flow during migraine attacks by Xenon-133 inhalation and emission tomography. Brain 107(Pt 2):447–461
Andersen AR, Friberg L, Olsen TS, Olesen J (1988) Delayed hyperemia following hypoperfusion in classic migraine. Single photon emission computed tomographic demonstration. Arch Neurol 45(2):154–159
Silberstein SD (2004) Migraine pathophysiology and its clinical implications. Cephalalgia: an international journal of headache 24(Suppl 2):2–7
Hadjikhani N, Rio SD, Wu M, Schwartz O, Bakker D, Fischl D, Kwong B, Cutrer KK, Rosen FM, Tootell BR, Sorensen RB, A. G., Moskowitz MA (2001) Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci USA 98(8):4687–4692
Cao Y, Welch KM, Aurora S, Vikingstad EM (1999) Functional MRI-BOLD of visually triggered headache in patients with migraine. Arch Neurol 56(5):548–554
May A (2017) Understanding migraine as a cycling brain syndrome: reviewing the evidence from functional imaging. Neurol Sciences: official J Italian Neurol Soc Italian Soc Clin Neurophysiol 38(Suppl 1):125–130
Weiller C, May A, Limmroth V, Jüptner M, Kaube H, Schayck RV, Coenen HH, Diener HC (1995) Brain stem activation in spontaneous human migraine attacks. Nat Med 1(7):658–660
Borsook D, Burstein R (2012) The enigma of the dorsolateral pons as a migraine generator. Cephalalgia: an international journal of headache 32(11):803–812
Denuelle M, Fabre N, Payoux P, Chollet F, Geraud G (2007) Hypothalamic activation in spontaneous migraine attacks. Headache 47(10):1418–1426
Schulte LH, Mehnert J, May A (2020) Longitudinal neuroimaging over 30 days: temporal characteristics of Migraine. Ann Neurol 87(4):646–651
Schankin CJ, Maniyar FH, Digre KB, Goadsby PJ (2014) Visual snow’ - a disorder distinct from persistent migraine aura. Brain 137(Pt 5):1419–1428
Russo A, Tessitore A, Silvestro M, Di Nardo F, Trojsi F, Del Santo T, De Micco R, Esposito F, Tedeschi G (2019) Advanced visual network and cerebellar hyperresponsiveness to trigeminal nociception in migraine with aura. J Headache Pain 20(1):46
Russo A, Esposito F, Conte F, Fratello M, Caiazzo G, Marcuccio L, Giordano A, Tedeschi G, Tessitore A (2017) Functional interictal changes of pain processing in migraine with ictal cutaneous allodynia. Cephalalgia: an international journal of headache 37(4):305–314
Wang Y, Rheault F, Schilling KG, Beason-Held LL, Shafer AT, Resnick SM, Landman BA (2022) Longitudinal changes of connectomes and graph theory measures in aging. Proceedings of SPIE–the International Society for Optical Engineering, 12032, 120321U
Russo A, Silvestro M, Tessitore A, Tedeschi G (2019) Shedding light on migraine with aura: the clarifying role of advanced neuroimaging investigations. Expert Rev Neurother 19(8):739–750
Gollion C, De Icco R, Dodick DW, Ashina H (2022) The premonitory phase of migraine is due to hypothalamic dysfunction: revisiting the evidence. J Headache Pain 23(1):158
Acknowledgements
N.A.
Funding
Work supported by #NEXTGENERATIONEU (NGEU) and funded by the Ministry of University and Research (MUR), National Recovery and Resilience Plan (NRRP), project MNESYS (PE0000006) – A Multiscale integrated approach to the study of the nervous system in health and disease (DN. 1553 11.10.2022).
Author information
Authors and Affiliations
Contributions
AR: literature review, manuscript drafting and revisionMS: literature review, manuscript drafting and revision; AT: manuscript revision; IO: table and figure drafting; ADR: manuscript revision; RDM: manuscript revision; GT: manuscript revision; FE: manuscript drafting and revisionMC: literature review, manuscript drafting and revision. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Russo, A., Silvestro, M., Tessitore, A. et al. Arterial spin labeling MRI applied to migraine: current insights and future perspectives. J Headache Pain 24, 71 (2023). https://doi.org/10.1186/s10194-023-01597-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10194-023-01597-y