Abstract
Background
A previous European Headache Federation (EHF) guideline addressed the use of monoclonal antibodies targeting the calcitonin gene-related peptide (CGRP) pathway to prevent migraine. Since then, randomized controlled trials (RCTs) and real-world evidence have expanded the evidence and knowledge for those treatments. Therefore, the EHF panel decided to provide an updated guideline on the use of those treatments.
Methods
The guideline was developed following the Grading of Recommendation, Assessment, Development, and Evaluation (GRADE) approach. The working group identified relevant questions, performed a systematic review and an analysis of the literature, assessed the quality of the available evidence, and wrote recommendations. Where the GRADE approach was not applicable, expert opinion was provided.
Results
We found moderate to high quality of evidence to recommend eptinezumab, erenumab, fremanezumab, and galcanezumab in individuals with episodic and chronic migraine. For several important clinical questions, we found not enough evidence to provide evidence-based recommendations and guidance relied on experts’ opinion. Nevertheless, we provided updated suggestions regarding the long-term management of those treatments and their place with respect to the other migraine preventatives.
Conclusion
Monoclonal antibodies targeting the CGRP pathway are recommended for migraine prevention as they are effective and safe also in the long-term.
Similar content being viewed by others
Background
The landscape of migraine prevention has experienced relevant changes since the introduction of the monoclonal antibodies (mAbs) targeting the calcitonin gene-related (CGRP) peptide or the CGRP receptor (together referred to as CGRP-mAbs). These substances form a new class of drugs specifically developed for migraine prevention. In 2019 the European Headache Federation (EHF) issued the first guideline for the use of CGRP-mAbs for migraine prevention in adults [1]. The guideline was published to provide a first guidance on the use of CGRP-mAbs to clinicians. Since then, new drugs and randomized controlled trials (RCTs) were published together with several real-world studies. CGRP-mAbs entered the market with different prescription and reimbursement regulations for their use across countries.
Considering the new knowledge on the topic, the EHF council decided to update the 2019 guideline.
Methods
The EHF identified a Panel of Experts consisting of the members of the working group contributing to the first guideline plus members of the EHF council; one junior member who did not participate in voting provided support for data extraction and statistical analyses. All but one member are physicians with expertise in migraine treatment; one member (AMVDB) is a pharmacologist with expertise in migraine treatment.
This guideline was organized into two parts. The first part provides evidence-based recommendations, and the second part provides Statements based on Experts Consensus.
For both parts, members of the Panel group reconsidered the clinical questions formulated in the previous guideline. Additional clinical questions were added for aspects consensually considered relevant by panel members.
Review of the literature
The systematic review of the literature was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [2, 3] from the beginning of indexing up to February 2022. We identified key papers on the use of CGRP-mAbs in individuals with migraine.
The following search string was used in both databases: “(migraine OR headache) AND (CGRP OR eptinezumab OR erenumab OR fremanezumab OR galcanezumab)”. Two investigators (SS and RO) independently screened the titles and abstracts of the publications to verify study eligibility. In the assessment of clinical questions for evidence-based reommendations, we included Phase II and Phase III primary RCTs using commercially available doses of CGRP-mAbs; we excluded reviews, other non-original articles (letters, comments, corrections to original articles), real-world studies, phase I RCTs, dose-ranging studies not using commercially available doses of CGRP-mAbs, and post-hoc and subgroup analyses of primary RCTs. For the assessment of additional questions subjected to consensus, we considered primary RCTs, their post-hoc and subgroup analyses, and real-world studies, which were selected by the Authors on the basis of clinical relevance.
Literature screening was conducted in two steps. In the first step, studies were excluded after reading the title and the abstract for clear exclusion criteria. For studies that passed the first step, the full text was assessed to decide about inclusion/exclusion. Disagreements were resolved by consensus. The reasons for exclusion were recorded and summarized. To summarize the search results, a data extraction sheet was developed including the information of interest. Papers retrieved from the literature search as well as summary tables were shared among the panelists.
Development of evidence-based recommendations
The evidence—based recommendations were developed according to the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system [4] as the method of choice to establish recommendations.
Clinical questions were developed according to the GRADE system as Patients; Intervention; Comparison and Outcome (PICO) [4]. Outcome parameters rated as important or critical by members of the group were considered. The selected outcome parameters were reduction in monthly migraine days and proportion of individuals with migraine having at least a 50% reduction from baseline in monthly migraine days. For the studies not reporting monthly migraine days, we considered monthly headache days. For the present guideline we did not include patients-reported outcomes in the quantitative analyses. The reasons which were considered for this decision were heterogeneity of the different instruments across studies, lack of adequate information on the clinical meaningfulness of the scores for some of the instruments and the assumption that improvement in patients-reported outcomes tends to follow the improvement in monthly migraine days and responder rate.
For RCTs, the general description of the study was extracted for each publication. We extracted first author name and year of publication, full citation, study design and setting, study period, number of included individuals with migraine, diagnostic criteria for migraine, definition of migraine and headache day, migraine type, treatment type, duration of observations and treatments, and study results. Data extraction was performed by two researchers (SS and RO) and double checked by other panel members (CD, RGG, MS, JV).
For each of the selected studies one author (SS) addressed the presence of possible bias; this was checked by one panel member (JV). Thereafter, quality of evidence was addressed for selected outcomes according to the GRADE approach [4]. Information derived from RCTs was considered as high quality of evidence but the quality of single studies was downgraded in the case of study limitations such as lack of allocation concealment, lack of blinding, incomplete accounting for individuals with migraine and outcome events, selective outcome reporting bias, or other limitations such as inadequate sample or lack of sample size calculation [5]. Final quality of evidence for each of the considered outcomes was rated as high, medium, low or very low based on study design, study limitations, inconsistency, indirectness, imprecision, publication bias, effect size, dose response, and confounding factors (Table 1) [4]. Summary of findings tables were drafted using the GRADE pro statistical software considering all the outcomes considered important or critical. For the analysis of extracted data we used R, version 4.1.2 [6], and RevMan software, version 5.4. Data analysis was performed on a fixed-effects basis and results were summarized as risk ratio (RR) or risk difference (RD) and 95% confidence intervals (CI). The quality of evidence tables were prepared by a single author (SS) and then discussed and agreed in a panel meeting.
Development of the expert consensus
Questions relevant to clinical practice were drafted by experts. Questions included in the previous report were reconsidered and additional questions were added. This process was done by filling a web-based questionnaire where panel members were inquired about their opinion referring to the available clinical questions and for suggestions of new topics.
For those clinical questions the GRADE approach was not applicable, recommendations were developed as expert statements. For addressing the clinical question, information from RCTs and from observational studies was considered.
To reach a consensus on the different statements, panel discussion meetings were performed to exchange information and opinions. During the panel meeting a proposal of statements was drafted and each panel member was requested to vote on the proposed statement. Statements reaching at least a 70% agreement of the panel members were reported in the paper.
Drafting of the statements
For evidence-based recommendations, strength (strong or weak) and direction (in favor or against) of the recommendation was determined on basis of balance between desirable and undesirable effects (Table 1) [4]. The recommendations were made exclusively based on clinical criteria. The issues of cost, reimbursement, marketing, and distribution of drugs were not considered when making the statements.
For Expert consensus statements the same wording frame of evidence-based recommendations was followed where possible. No formal rating of the quality of evidence was performed in this case.
Final approval of the document
The guideline document underwent several rounds of revisions among the Panel members until an agreement on all the content was reached. The final version of the document was approved by all contributing authors.
Results
This guideline is structured into two parts; the first part reports the evidence-based recommendations, and the second part reports the Expert Consensus Statements.
Evidence-based recommendations
For the evidence-based recommendations, three PICO questions were selected. We considered phase II and phase III RCTs comparing any CGRP-mAb with placebo. Only doses finally available on the market were considered to provide evidence-based recommendations, with the only exception of fremanezumab 225 mg monthly for chronic migraine, which in RCTs had a 675 mg loading dose not used in clinical practice.
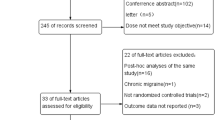
We selected 23 studies eligible for those PICO questions [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29]. Study selection is reported in Fig. 1, while the assessment of the risk of bias of each study is reported in Fig. 2.
The summary evidence-based recommendations are reported in Table 2.
Evidence-based recommendation – question 1
In individuals with episodic migraine, is preventive treatment with monoclonal antibodies targeting the CGRP pathway as compared to placebo, effective and safe? |
Population: individuals with episodic migraine
Intervention: eptinezumab, erenumab, fremanezumab, galcanezumab
Comparison: placebo
Outcome: reduction in migraine days, responder rate (individuals with migraine with at least 50% reduction in migraine days), reduction in the use of acute attack medication, safety (serious adverse events or mortality)
Fifteen studies were considered for this question [7,8,9,10, 15, 16, 18, 21, 24,25,26, 28, 29]. The list of selected studies for question 1 is reported in Table 3. The overall results of the studies considered for question 1 are reported in Fig. 3. All the considered CGRP-mAbs (eptinezumab, erenumab, galcanezumab and fremanezumab) were associated with significant benefits considering the pre-defined outcomes as compared to placebo. No significant safety concerns were found in the different studies.
The quality of evidence for the available compounds and for the different outcomes ranged from moderate to high (Table 4). The evidence-based recommendations for question 1 are reported in Table 2.
In individuals with episodic migraine, we recommend eptinezumab, erenumab, fremanezumab and galcanezumab as preventive treatment Quality of evidence: moderate to high Strength of the recommendation: strong |
Evidence-based recommendation – question 2
In individuals with chronic migraine, is preventive treatment with monoclonal antibodies targeting the CGRP pathway as compared to placebo, effective and safe? |
Population: individuals with chronic migraine
Intervention: eptinezumab, erenumab, fremanezumab, galcanezumab
Comparison: placebo
Outcome: reduction in migraine days, responder rate (individuals with migraine with at least 50% reduction in migraine days), reduction in the use of acute attack medication, safety (serious adverse events or mortality)
Ten studies were considered for this question [8, 11, 13, 17, 19, 20, 22, 23, 27, 29]. The list of selected studies for question 2 is reported in Table 5. The overall results of the studies considered for question 2 are reported in Fig. 4. All the considered CGRP-mAbs (eptinezumab, erenumab, galcanezumab and fremanezumab) were associated with significant benefits considering the pre-defined outcomes as compared to placebo. No significant safety concerns were found in the different studies.
The quality of evidence for the available compounds and for the different outcomes ranged from moderate to high (Table 6). The evidence-based recommendations for question 2 are reported in Table 2.
In individuals with chronic migraine, we recommend eptinezumab, erenumab, fremanezumab and galcanezumab as preventive treatment Quality of evidence: moderate to high Strength of the recommendation: strong |
Evidence-based recommendation – question 3
In individuals with migraine, is preventive treatment with monoclonal antibodies targeting the CGRP pathway, as compared to another migraine preventive treatment, more effective and/or tolerable? |
Population: individuals with migraine
Intervention: eptinezumab, erenumab, fremanezumab, galcanezumab
Comparison: antiepileptics (topiramate, valproate), antidepressants (amitriptyline), beta-blockers (atenolol, metoprolol, propranolol, timolol), calcium-channel blockers (flunarizine), onabotulinumtoxinA, renin-angiotensin system inhibitors (candesartan, lisinopril)
Outcome: reduction in migraine days, responder ratio (individuals with migraine with at least 50% reduction in migraine days), reduction in the use of acute attack medication, discontinuation, due to adverse events, safety (serious adverse events or mortality)
We found only one RCT which compared a CGRP-mAb versus another migraine preventive agent [14] (Table 7). In this trial erenumab (70 to 140 mg/month) was compared with topiramate (50 to 100 mg/day). The primary endpoint was medication-discontinuation due to an adverse event. The predefined secondary endpoint was set to the 50% responder rate. This study was performed in Germany only. Summary of findings for this study are reported in Table 8. Based on an intention-to-treat analysis, over the 24-week study period, there was a higher reduction in monthly migraine days with erenumab (− 5.86, SE 0.24) than with topiramate (− 4.02, SE 0.24; p < 0.001). More individuals with migraine achieved a > 50% reduction in monthly migraine days with erenumab than with topiramate (55.4% vs. 31.2%; odds ratio 2.76; 95% confidence interval 2.06–3.71; p < 0.001). In the erenumab group, 10.6% discontinued medication due to adverse events compared to 38.9% in the topiramate group (odds ratio, 0.19; 95% confidence interval 0.13–0.27; p < 0.001). No relevant safety concerns were observed for erenumab. The evidence-based recommendation for question 3 is reported in Table 2.
In individuals with episodic or chronic migraine we recommend erenumab over topiramate as preventive treatment Quality of evidence: low Strength of the recommendation: strong |
Expert consensus statements
The summary of statements is reported in Table 9.
Expert consensus statement – question 1
When should treatment with monoclonal antibodies targeting the CGRP pathway be offered to individuals with migraine? |
Clinical guidance
The previous EHF guideline recommended CGRP-mAbs as a third line treatment for migraine prevention in individuals with migraine and inadequate response, lack of tolerability or lack of compliance to at least two categories of migraine preventatives [1]. Of note, in phase II and phase III trials on CGRP-mAbs, 46.3% of individuals with migraine were treatment naive or without a previous history of drug failure [7,8,9,10, 16, 17, 19, 20, 24, 26].
After the publication of the previous guideline, CGRP-mAbs became available in Europe and real-world observational studies confirmed the effectiveness of those drugs outside RCTs [31,32,33,34]. Tolerability and safety profiles were confirmed to be excellent and the adherence to treatment was not reported as a critical issue as it was with oral treatments [35,36,37].
CGRP-mAbs have an efficacy which is at least comparable to the efficacy of the formerly used preventive drugs. Among the oral prophylactics, high dropout rates were reported especially for amitriptyline, valproate or topiramate [37]. The major added value of CGRP-mAbs, compared to the classical preventatives, seems to be their unprecedented favorable adverse effect profile that is also associated with ease of use and high efficacy. These characteristics lead most individuals with migraine to express a clear preference for CGRP-mAbs as a first-line option [38]. Poor response in individuals with migraine may also be attributed to lack of compliance to available medical treatments because of the need of taking multiple doses of the drugs or adverse events. Additionally, CGRP-mAbs may represent a suitable option for individuals with migraine who have contraindications to other preventive treatments or in whom adverse events may be particularly challenging. Considering the overall evidence of benefits regarding the CGRP-mAbs, their ease of use, and the lack of reasons to make their use undesirable from a clinical point of view, the panel was in favor of offering those drugs within the other available options which are usually considered when choosing a migraine preventive treatment. There are no reasons on clinical grounds to postpone the initiation of this treatment. However, first line treatment option should be carefully chosen by physicians considering the patient’s history, comorbidities, and burden of the disease. Headache experts must be able to choose, after discussion with the patient, the therapy that is most appropriate. Comorbid depression and migraine may make preferrable the choice of an antidepressant, comorbid uncontrolled hypertension may favor a beta-blocker or renin angiotensin system inhibitors. Postponing the initiation of CGRP-mAbs, being forced to use strategies which cannot be considered ideal in a patient is a suboptimal treatment paradigm, which does not lead to immediate advantages to individuals with migraine and may favor disease progression and chronicity.
In individuals with migraine who require preventive treatment, we suggest monoclonal antibodies targeting the CGRP pathway to be included as a first line treatment option. |
Expert consensus statement – question 2
How should other preventive treatments be managed when using monoclonal antibodies targeting the CGRP pathway in individuals with migraine? |
Clinical guidance
We have scarce information on how to manage other oral preventive treatments in association with CGRP-mAbs in individuals with migraine. Individuals with migraine who are considered for starting a CGRP-mAb may already be taking other preventive drugs. In this case there is the option to stop the ongoing preventative when starting a CGRP-mAb or to continue the oral preventatives and decide later whether to stop. Benefits and risks of the two options should be considered and discussed with individuals with migraine. Polytherapy can also be considered at a later stage in individuals with migraine who still have a relevant residual migraine burden despite having a clinically meaningful relief with a CGRP-mAb. So far, there is no robust evidence either to support or discard the combination of different migraine preventatives. Withdrawal of other preventive drugs can be done early or later in individuals with migraine showing a favorable clinical response after starting the CGRP-mAb. While as general concept monotherapy is preferrable, some individuals with migraine do not have adequate pain relief with a single drug. In those cases, a combination of different drugs might be considered referring to the previous pharmacological history and comorbidities. Combined treatment might be particularly suitable for patients achieving a substantial relative response (e.g. 50% reduction in monthly migraine days) with CGRP-mAbs with a relevant number of residual migraine or headache days [39]. Due to these considerations, the panel decided not to make an explicit statement either in favor or against combination therapy. and to leave this option to individual considerations.
In individuals with episodic or chronic migraine there is insufficient evidence to make suggestions regarding the combination of monoclonal antibodies targeting the CGRP with other preventatives to improve migraine clinical outcomes |
Expert consensus statement – question 3
When should treatment efficacy in patients on treatment with anti-CGRP monoclonal antibodies be firstly evaluated? |
Clinical guidance
As a rule, treatment can be stopped if it is considered not effective. Available date from RCTs and from observational studies indicated that CGRP-mAbs have a quick onset of action [33, 40,41,42,43,44,45,46,47,48] as benefits may be evident in some individuals with migraine even in the first days or first week after starting treatment. Data from randomized and real-world studies also showed that there may be an increase in the responder rate over time as a variable proportion of individuals with migraine who do not have an immediate response start to have a favorable response later on with the ongoing treatment [33, 46,47,48,49,50]. The majority of individuals with migraine who can be considered responders can be identified after 3 months [33, 46, 47, 49, 51]. For those reasons we suggest the first evaluation of individuals with migraine to address efficacy to take place after a minimum of three consecutive months of treatment. We recognize that some individuals with migraine may take more time to achieve a relevant benefit. In selected cases decision on treatment maintenance can be readdressed after an additional period of 3 months.
In individuals with episodic or chronic migraine who start a new treatment with one monoclonal antibody targeting the CGRP pathway we suggest evaluating efficacy after a minimum of 3 consecutive months on treatment |
Expert consensus statement – question 4
When should treatment with anti-CGRP monoclonal antibodies be paused in individuals with migraine? |
Clinical guidance
The CGRP-mAbs are challenging the conventional temporal paradigm of migraine prevention. With the conventional oral preventative drugs, individuals with migraine were typically treated for 6 to 12 months in order to minimize side effects and to re-evaluate the underlying disease burden given the intrinsic cyclic course of migraine. Treatments were then repeated over time for a variable duration according to clinical needs. Monthly or quarterly administration of CGRP mAbs is more accepted by individuals with migraine than the daily oral regimen. Moreover, the excellent tolerability profile makes the CGRP-mAbs more suitable for prolonged treatments. So far, there are no studies which provide a clear guidance on the optimal duration of migraine preventive treatments. It is highly probable that a broadly generalizable approach does not exist and that also treatment duration needs to be adapted on a case-by-case strategy or considering homogeneous groups of individuals with migraine. One question is still open as to whether a longer duration of treatment may have a disease-modifying effect in individuals with a long history of chronic migraine and be able to provide a stable reduction of migraine or headache days, even after stopping the treatment.
In individuals with episodic or chronic migraine we suggest considering a pause in the treatment with monoclonal antibodies targeting the CGRP pathway after 12-18 months of continuous treatment. If deemed necessary, treatment should be continued as long as needed. In individuals with migraine who pause treatment, we suggest restarting the treatment if migraine worsens after treatment withdrawal. |
Expert consensus statement – question 5
Should individuals with migraine and medication overuse be offered treatment with monoclonal antibodies targeting the CGRP pathway? |
Clinical guidance
All the available RCTs on chronic migraine included individuals with migraine and medication overuse [13, 17, 20, 22, 23]. CGRP-mAbs were started without specific strategies in the population of individuals with migraine and medication overuse. In those studies, the efficacy of all four mAbs seemed to be independent of whether the patient had medication overuse [52,53,54]. We have, at this moment, no evidence to indicate that the effect of CGRP mAbs will be different, if preceded or not by detoxification. In clinical practice, some adopt withdrawal strategies before offering preventive medications to individuals with medication overuse and there is some evidence indicating that detoxification is feasible and effective [55]. However, detoxification is not easy and feasible in all individuals with migraine and dedicated resources are needed.
In addition to evidence from RCTs, there is also evidence from real-world studies suggesting that CGRP-mAbs are highly effective even in the absence of prior detoxification in individuals with medication overuse [31, 56] and that the response to CGRP-mAbs does not depend on detoxification [57, 58].
In individuals with migraine and medication overuse there is need of well-designed clinical trials to evaluate the effect of treatment CGRP-mAbs before and after withdrawal of acute medication. Additionally, it should be clarified whether individuals with migraine and medication overuse who do not respond to CGRP-mAbs may benefit from detoxification strategies before initiation of CGRP-mAbs and whether detoxification may change the responder status.
In individuals with migraine and medication overuse, we suggest offering monoclonal antibodies targeting the CGRP pathway. |
Expert consensus statement – question 6
In individuals with migraine who are non-responders to one monoclonal antibody targeting the CGRP pathway, is switching to a different antibody an option? |
Clinical guidance
All the CGRP-mAbs have an excellent tolerability profile. Nevertheless, tolerability issues may appear and make stopping of one CGRP-mAb necessary. If the reported side effect is specific for a given CGRP-mAb (e.g. constipation related to erenumab) [59], switching to a different CGRP-mAb may be appropriate based on clinical experience. Much more complex is the issue of a CGRP-mAb switch for efficacy reasons. Indeed, there is a non-negligible proportion of individuals with migraine who do not have a clinical response after maintaining the treatment for an adequate period [39, 60]. In those individuals with migraine, a switch to a different CGRP-mAb may represent an option. Considerations to support the switch from one CGRP-mAb to another, include differences in the mechanism of action (action on the ligand or on the receptor), difference in administration schedule (monthly versus quarterly) and to a lesser extent difference in formulations (subcutaneous versus intravenous) Eptinezumab is the only CGRP mAb available in an intravenous formulation. From a pharmacological perspective, eptinezumab only requires hours (theoretically even only minutes, given its intravenous administration) to reach its maximum serum concentrations, which is as fast as the gepants, but considerably faster than the other antibodies, which require up to 1 week to reach their maximum levels [61]. So far, there are no RCTs which addressed whether switching between different CGRP-mAbs may offer benefits to non-responder individuals with migraine. Some observational studies provide information to support this possibility [62,63,64]; however, bias cannot be excluded, and those data cannot be considered sufficient to recommend a switch. We also have to consider that many individuals with migraine, who are non-responders to CGRP-mAbs, have already failed all the other treatment options and so the switch to a different CGRP-mAb may represent the only viable strategy. It is worth to know that in the migraine treatment setting, switch to other drugs in the same class is an accepted strategy for some classes (e.g. switch to one triptan to a different triptan).
Considering the above reported reasons, the panel expressed a consensus statement to recognize the lack of adequate scientific evidence but at the same time we acknowledge that, for some individuals with migraine, a switch may represent the best therapeutic option. RCTs to test a CGRP-mAb switch in individuals with inadequate response to the first CGRP-mAb are needed to provide information on this issue.
In individuals with migraine with inadequate response to one monoclonal antibody targeting the CGRP pathway, there is insufficient evidence on the potential benefits of antibody switch but switching may be an option. |
Expert consensus statement – question 7
In which individuals with migraine is caution suggested when considering treatment with monoclonal antibodies targeting the CGRP pathway? |
Clinical guidance
CGRP-mAbs are unlikely to produce drug interactions which may be particularly relevant in individuals with migraine with comorbidities. Pregnant and nursing women were excluded from RCTs and there is no robust information on the risk for the fetus or the newborn driven by CGRP-mAbs. The limited real-life data available so far have not shown major concerns with the accidental and short-lived exposure to erenumab, galcanezumab, and fremanezumab in pregnancy and lactation [65]. However, caution is needed because experimental data indicate that erenumab crosses the placenta [66]. Moreover, CGRP has an important role in the regulation of uteroplacental circulation; its levels are increased during physiological pregnancy and decreased in pre-eclampsia [67]. Concerns in the use of those drugs in women of childbearing potential are related also to the long (around 1 month) half-life of the CGRP-mAbs that implies that these drugs can only be considered as eliminated from the circulation 6 months after stopping [61]. Information about the potential risk related to an unplanned pregnancy are to be discussed with female individuals with migraine of childbearing potential.
Concerns regarding vascular safety of these drugs were raised considering that CGRP is among the most potent vasodilators in animals and humans and that CGRP-mediated vasodilation is a rescue mechanism in brain as well as cardiac ischemia [68,69,70]. Additionally, there is experimental evidence that blockade of the CGRP pathway by a small molecule CGRP antagonist may worsen an ischemic stroke [71]. Although, one study did not show an increased risk after the administration of erenumab in individuals with migraine and stable angina [72], data should be taken with caution because of methodological issues [73]. Results from RCTs have not shown potential risks even in longer follow-up; however, it should be considered that patients considered at high vascular risk were generally excluded [74]. So far in real-world studies, no reliable evidence of an association between CGRP-mAbs and vascular events has emerged; but again, in those studies most of the patients were at low vascular risk. Retrospective analysis of postmarketing (spontaneous) case reports of erenumab-associated adverse events, indicated an association between erenumab use and high blood pressure [75] which has led to change in the label for this drug. Given those premises, a case-by-case evaluation is needed when considering the use of CGRP-mAbs in individuals with migraine considered at high vascular risk of with overt history of vascular events. The Expert panel also decided to suggest caution in the use in individuals with migraine with a history of Raynaud phenomenon as some reports have linked the use of CGRP-mAbs to this phenomenon [76,77,78].
Constipation could be related to CGRP-mAb use due to potential inhibition of gastrointestinal motility, which is regulated by CGRP [79, 80]. Constipation emerged as a frequent adverse event of treatment with galcanezumab and mostly with erenumab, as reported in real-world studies [33, 46, 47]; however, the vast majority of cases was mild and did not lead to treatment stopping. There is a single reported case of paralytic ileus after abdominal surgery in a patient treated with erenumab and with a history of constipation [81]. In the absence of further safety data, caution might be needed when using erenumab in patients with a history of constipation.
We suggest avoiding monoclonal antibodies targeting the CGRP pathway in pregnant or nursing women. We suggest caution and decision on a case-by-case basis in the presence of vascular disease or risk factors and Raynaud phenomenon. We suggest caution in erenumab use in individuals with migraine and history of severe constipation. |
Conclusions
The available data confirm that monoclonal antibodies targeting the CGRP pathway appear to be effective and safe for migraine prevention even in the long term. Objective biomarkers of treatment response are still lacking; nevertheless, the available RCTs and real-world data can provide insights on treatment individualization, including treatment duration, combination with other treatments, and the management of safety issues.
Availability of data and materials
There are no original data.
Abbreviations
- CGRP:
-
Calcitonin gene-related peptide
- CM:
-
Chronic migraine
- EHF:
-
European Headache Federation
- EM:
-
Episodic migraine
- GRADE:
-
Grading of Recommendation Assessment, Development, and Evaluation
- mAb(s):
-
Monoclonal antibody(ies)
- RCT(s):
-
Randomized controlled trial(s)
References
Sacco S, Bendtsen L, Ashina M, Reuter U, Terwindt G, Mitsikostas DD et al (2019) European headache federation guideline on the use of monoclonal antibodies acting on the calcitonin gene related peptide or its receptor for migraine prevention. J Headache Pain 20(1):6
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71
The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system. Available from: http://gdt.guidelinedevelopment.org/app/handbook/handbook.html. Accessed 15 Feb 2022.
Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P et al (2011) GRADE guidelines: 4. Rating the quality of evidence--study limitations (risk of bias). J Clin Epidemiol 64(4):407–415
Team RC (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna Available from: https://www.R-project.org/
Dodick DW, Ashina M, Brandes JL, Kudrow D, Lanteri-Minet M, Osipova V et al (2018) ARISE: a phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia 38(6):1026–1037
Mulleners WM, Kim BK, Lainez MJA, Lanteri-Minet M, Pozo-Rosich P, Wang S et al (2020) Safety and efficacy of galcanezumab in patients for whom previous migraine preventive medication from two to four categories had failed (CONQUER): a multicentre, randomised, double-blind, placebo-controlled, phase 3b trial. Lancet Neurol 19(10):814–825
Skljarevski V, Matharu M, Millen BA, Ossipov MH, Kim BK, Yang JY (2018) Efficacy and safety of galcanezumab for the prevention of episodic migraine: results of the EVOLVE-2 phase 3 randomized controlled clinical trial. Cephalalgia 38(8):1442–1454
Stauffer VL, Dodick DW, Zhang Q, Carter JN, Ailani J, Conley RR (2018) Evaluation of galcanezumab for the prevention of episodic migraine: the EVOLVE-1 randomized clinical trial. JAMA Neurol 75(9):1080–1088
Ferrari MD, Diener HC, Ning X, Galic M, Cohen JM, Yang R et al (2019) Fremanezumab versus placebo for migraine prevention in patients with documented failure to up to four migraine preventive medication classes (FOCUS): a randomised, double-blind, placebo-controlled, phase 3b trial. Lancet 394(10203):1030–1040
Dodick DW, Silberstein SD, Bigal ME, Yeung PP, Goadsby PJ, Blankenbiller T et al (2018) Effect of Fremanezumab compared with placebo for prevention of episodic migraine: a randomized clinical trial. JAMA 319(19):1999–2008
Silberstein SD, Dodick DW, Bigal ME, Yeung PP, Goadsby PJ, Blankenbiller T et al (2017) Fremanezumab for the preventive treatment of chronic migraine. N Engl J Med 377(22):2113–2122
Reuter U, Ehrlich M, Gendolla A, Heinze A, Klatt J, Wen S et al (2022) Erenumab versus topiramate for the prevention of migraine - a randomised, double-blind, active-controlled phase 4 trial. Cephalalgia 42(2):108–118
Reuter U, Goadsby PJ, Lanteri-Minet M, Wen S, Hours-Zesiger P, Ferrari MD et al (2018) Efficacy and tolerability of erenumab in patients with episodic migraine in whom two-to-four previous preventive treatments were unsuccessful: a randomised, double-blind, placebo-controlled, phase 3b study. Lancet 392(10161):2280–2287
Sun H, Dodick DW, Silberstein S, Goadsby PJ, Reuter U, Ashina M et al (2016) Safety and efficacy of AMG 334 for prevention of episodic migraine: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol 15(4):382–390
Bigal ME, Edvinsson L, Rapoport AM, Lipton RB, Spierings EL, Diener HC et al (2015) Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of chronic migraine: a multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol 14(11):1091–1100
Bigal ME, Dodick DW, Rapoport AM, Silberstein SD, Ma Y, Yang R et al (2015) Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of high-frequency episodic migraine: a multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol 14(11):1081–1090
Tepper S, Ashina M, Reuter U, Brandes JL, Dolezil D, Silberstein S et al (2017) Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol 16(6):425–434
Dodick DW, Lipton RB, Silberstein S, Goadsby PJ, Biondi D, Hirman J et al (2019) Eptinezumab for prevention of chronic migraine: a randomized phase 2b clinical trial. Cephalalgia 39(9):1075–1085
Ashina M, Saper J, Cady R, Schaeffler BA, Biondi DM, Hirman J et al (2020) Eptinezumab in episodic migraine: a randomized, double-blind, placebo-controlled study (PROMISE-1). Cephalalgia 40(3):241–254
Lipton RB, Goadsby PJ, Smith J, Schaeffler BA, Biondi DM, Hirman J et al (2020) Efficacy and safety of eptinezumab in patients with chronic migraine: PROMISE-2. Neurology 94(13):e1365–e1377
Detke HC, Goadsby PJ, Wang S, Friedman DI, Selzler KJ, Aurora SK (2018) Galcanezumab in chronic migraine: the randomized, double-blind, placebo-controlled REGAIN study. Neurology 91(24):e2211–e2221
Goadsby PJ, Reuter U, Hallstrom Y, Broessner G, Bonner JH, Zhang F et al (2017) A controlled trial of erenumab for episodic migraine. N Engl J Med 377(22):2123–2132
Sakai F, Takeshima T, Tatsuoka Y, Hirata K, Lenz R, Wang Y et al (2019) A randomized phase 2 study of erenumab for the prevention of episodic migraine in Japanese adults. Headache 59(10):1731–1742
Wang SJ, Roxas AA Jr, Saravia B, Kim BK, Chowdhury D, Riachi N et al (2021) Randomised, controlled trial of erenumab for the prevention of episodic migraine in patients from Asia, the Middle East, and Latin America: the EMPOwER study. Cephalalgia 41(13):1285–1297
Sakai F, Suzuki N, Kim BK, Igarashi H, Hirata K, Takeshima T et al (2021) Efficacy and safety of fremanezumab for chronic migraine prevention: multicenter, randomized, double-blind, placebo-controlled, parallel-group trial in Japanese and Korean patients. Headache 61(7):1092–1101
Sakai F, Suzuki N, Kim BK, Tatsuoka Y, Imai N, Ning X et al (2021) Efficacy and safety of fremanezumab for episodic migraine prevention: multicenter, randomized, double-blind, placebo-controlled, parallel-group trial in Japanese and Korean patients. Headache 61(7):1102–1111
Takeshima T, Sakai F, Hirata K, Imai N, Matsumori Y, Yoshida R et al (2021) Erenumab treatment for migraine prevention in Japanese patients: efficacy and safety results from a phase 3, randomized, double-blind, placebo-controlled study. Headache 61(6):927–935
Skljarevski V, Oakes TM, Zhang Q, Ferguson MB, Martinez J, Camporeale A et al (2018) Effect of different doses of galcanezumab vs placebo for episodic migraine prevention: a randomized clinical trial. JAMA Neurol 75(2):187–193
Caronna E, Gallardo VJ, Alpuente A, Torres-Ferrus M, Pozo-Rosich P (2021) Anti-CGRP monoclonal antibodies in chronic migraine with medication overuse: real-life effectiveness and predictors of response at 6 months. J Headache Pain 22(1):120
McAllister P, Lamerato L, Krasenbaum LJ, Cohen JM, Tangirala K, Thompson S et al (2021) Real-world impact of fremanezumab on migraine symptoms and resource utilization in the United States. J Headache Pain 22(1):156
Ornello R, Casalena A, Frattale I, Gabriele A, Affaitati G, Giamberardino MA et al (2020) Real-life data on the efficacy and safety of erenumab in the Abruzzo region, central Italy. J Headache Pain 21(1):32
Straube A, Stude P, Gaul C, Schuh K, Koch M (2021) Real-world evidence data on the monoclonal antibody erenumab in migraine prevention: perspectives of treating physicians in Germany. J Headache Pain 22(1):133
Hepp Z, Dodick DW, Varon SF, Chia J, Matthew N, Gillard P et al (2017) Persistence and switching patterns of oral migraine prophylactic medications among patients with chronic migraine: a retrospective claims analysis. Cephalalgia 37(5):470–485
Hepp Z, Dodick DW, Varon SF, Gillard P, Hansen RN, Devine EB (2015) Adherence to oral migraine-preventive medications among patients with chronic migraine. Cephalalgia 35(6):478–488
Vandervorst F, Van Deun L, Van Dycke A, Paemeleire K, Reuter U, Schoenen J et al (2021) CGRP monoclonal antibodies in migraine: an efficacy and tolerability comparison with standard prophylactic drugs. J Headache Pain 22(1):128
Dapkute A, Vainauskiene J, Ryliskiene K (2022) Patient-reported outcomes of migraine treatment with erenumab: results from a national patient survey. Neurol Sci 43(5):3305–3312
Ornello R, Baraldi C, Guerzoni S, Lambru G, Andreou AP, Raffaelli B et al (2022) Comparing the relative and absolute effect of erenumab: is a 50% response enough? Results from the ESTEEMen study. J Headache Pain 23(1):38
Detke HC, Millen BA, Zhang Q, Samaan K, Ailani J, Dodick DW et al (2020) Rapid onset of effect of galcanezumab for the prevention of episodic migraine: analysis of the EVOLVE studies. Headache 60(2):348–359
Igarashi H, Shibata M, Ozeki A, Day KA, Matsumura T (2021) Early onset and maintenance effect of galcanezumab in Japanese patients with episodic migraine. J Pain Res 14:3555–3564
Schwedt T, Reuter U, Tepper S, Ashina M, Kudrow D, Broessner G et al (2018) Early onset of efficacy with erenumab in patients with episodic and chronic migraine. J Headache Pain 19(1):92
Schwedt TJ, Kuruppu DK, Dong Y, Standley K, Yunes-Medina L, Pearlman E (2021) Early onset of effect following galcanezumab treatment in patients with previous preventive medication failures. J Headache Pain 22(1):15
Takeshima T, Nakai M, Shibasaki Y, Ishida M, Kim BK, Ning X et al (2022) Early onset of efficacy with fremanezumab in patients with episodic and chronic migraine: subanalysis of two phase 2b/3 trials in Japanese and Korean patients. J Headache Pain 23(1):24
Winner PK, Spierings ELH, Yeung PP, Aycardi E, Blankenbiller T, Grozinski-Wolff M et al (2019) Early onset of efficacy with fremanezumab for the preventive treatment of chronic migraine. Headache 59(10):1743–1752
Lambru G, Hill B, Murphy M, Tylova I, Andreou AP (2020) A prospective real-world analysis of erenumab in refractory chronic migraine. J Headache Pain 21(1):61
Russo A, Silvestro M, Scotto di Clemente F, Trojsi F, Bisecco A, Bonavita S et al (2020) Multidimensional assessment of the effects of erenumab in chronic migraine patients with previous unsuccessful preventive treatments: a comprehensive real-world experience. J Headache Pain 21(1):69
Goadsby PJ, Dodick DW, Martinez JM, Ferguson MB, Oakes TM, Zhang Q et al (2019) Onset of efficacy and duration of response of galcanezumab for the prevention of episodic migraine: a post-hoc analysis. J Neurol Neurosurg Psychiatry 90(8):939–944
McAllister PJ, Turner I, Reuter U, Wang A, Scanlon J, Klatt J et al (2021) Timing and durability of response to erenumab in patients with episodic migraine. Headache 61(10):1553–1561
Kuruppu DK, North JM, Kovacik AJ, Dong Y, Pearlman EM, Hutchinson SL (2021) Onset, maintenance, and cessation of effect of galcanezumab for prevention of migraine: a narrative review of three randomized placebo-controlled trials. Adv Ther 38(3):1614–1626
Tepper SJ, Lucas S, Ashina M, Schwedt TJ, Ailani J, Scanlon J et al (2021) Timing and durability of response to erenumab in patients with chronic migraine. Headache 61(8):1255–1263
Silberstein SD, Cohen JM, Seminerio MJ, Yang R, Ashina S, Katsarava Z (2020) The impact of fremanezumab on medication overuse in patients with chronic migraine: subgroup analysis of the HALO CM study. J Headache Pain 21(1):114
Dodick DW, Doty EG, Aurora SK, Ruff DD, Stauffer VL, Jedynak J et al (2021) Medication overuse in a subgroup analysis of phase 3 placebo-controlled studies of galcanezumab in the prevention of episodic and chronic migraine. Cephalalgia 41(3):340–352
Diener HC, Marmura MJ, Tepper SJ, Cowan R, Starling AJ, Diamond ML et al (2021) Efficacy, tolerability, and safety of eptinezumab in patients with a dual diagnosis of chronic migraine and medication-overuse headache: subgroup analysis of PROMISE-2. Headache 61(1):125–136
Pijpers JA, Louter MA, de Bruin ME, van Zwet EW, Zitman FG, Ferrari MD et al (2016) Detoxification in medication-overuse headache, a retrospective controlled follow-up study: does care by a headache nurse lead to cure? Cephalalgia 36(2):122–130
Ornello R, Casalena A, Frattale I, Caponnetto V, Gabriele A, Affaitati G et al (2020) Conversion from chronic to episodic migraine in patients treated with erenumab: real-life data from an Italian region. J Headache Pain 21(1):102
Baraldi C, Castro FL, Cainazzo MM, Pani L, Guerzoni S (2021) Predictors of response to erenumab after 12 months of treatment. Brain Behav 11(8):e2260
Cainazzo MM, Baraldi C, Ferrari A, Lo Castro F, Pani L, Guerzoni S (2021) Erenumab for the preventive treatment of chronic migraine complicated with medication overuse headache: an observational, retrospective, 12-month real-life study. Neurol Sci 42(10):4193–4202
Pham A, Burch R (2020) A real-world comparison of erenumab and galcanezumab in a tertiary headache center. Headache 60:4–5
Sacco S, Lampl C, Maassen van den Brink A, Caponnetto V, Braschinsky M, Ducros A et al (2021) Burden and attitude to resistant and refractory migraine: a survey from the European Headache Federation with the endorsement of the European Migraine & Headache Alliance. J Headache Pain 22(1):39
Al-Hassany L, Goadsby PJ, Danser AHJ, MaassenVanDenBrink A (2022) Calcitonin gene-related peptide-targeting drugs for migraine: how pharmacology might inform treatment decisions. Lancet Neurol 21(3):284–294
Overeem LH, Peikert A, Hofacker MD, Kamm K, Ruscheweyh R, Gendolla A et al (2022) Effect of antibody switch in non-responders to a CGRP receptor antibody treatment in migraine: a multi-center retrospective cohort study. Cephalalgia 42:291–301
Patier Ruiz I, Sanchez-Rubio Ferrandez J, Carcamo Fonfria A, Molina Garcia T (2022) Early experiences in switching between monoclonal antibodies in patients with nonresponsive migraine in Spain: a case series. Eur Neurol 85:132–135
Ziegeler C, May A (2020) Non-responders to treatment with antibodies to the CGRP-receptor May profit from a switch of antibody class. Headache 60(2):469–470
Noseda R, Bedussi F, Gobbi C, Zecca C, Ceschi A (2021) Safety profile of erenumab, galcanezumab and fremanezumab in pregnancy and lactation: analysis of the WHO pharmacovigilance database. Cephalalgia 41(7):789–798
Bussiere JL, Davies R, Dean C, Xu C, Kim KH, Vargas HM et al (2019) Nonclinical safety evaluation of erenumab, a CGRP receptor inhibitor for the prevention of migraine. Regul Toxicol Pharmacol 106:224–238
Yadav S, Yadav YS, Goel MM, Singh U, Natu SM, Negi MP (2014) Calcitonin gene- and parathyroid hormone-related peptides in normotensive and preeclamptic pregnancies: a nested case-control study. Arch Gynecol Obstet 290(5):897–903
Favoni V, Giani L, Al-Hassany L, Asioli GM, Butera C, de Boer I et al (2019) CGRP and migraine from a cardiovascular point of view: what do we expect from blocking CGRP? J Headache Pain 20(1):27
MaassenVanDenBrink A, Meijer J, Villalon CM, Ferrari MD (2016) Wiping out CGRP: potential cardiovascular risks. Trends Pharmacol Sci 37(9):779–788
Russell FA, King R, Smillie SJ, Kodji X, Brain SD (2014) Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev 94(4):1099–1142
Mulder IA, Li M, de Vries T, Qin T, Yanagisawa T, Sugimoto K et al (2020) Anti-migraine calcitonin gene-related peptide receptor antagonists worsen cerebral ischemic outcome in mice. Ann Neurol 88(4):771–784
Depre C, Antalik L, Starling A, Koren M, Eisele O, Lenz RA et al (2018) A randomized, double-blind, placebo-controlled study to evaluate the effect of erenumab on exercise time during a treadmill test in patients with stable angina. Headache 58(5):715–723
Maassen van den Brink A, Rubio-Beltran E, Duncker D, Villalon CM (2018) Is CGRP receptor blockade cardiovascularly safe? Appropriate studies are needed. Headache 58(8):1257–1258
Ashina M, Goadsby PJ, Reuter U, Silberstein S, Dodick DW, Xue F et al (2021) Long-term efficacy and safety of erenumab in migraine prevention: results from a 5-year, open-label treatment phase of a randomized clinical trial. Eur J Neurol 28(5):1716–1725
Saely S, Croteau D, Jawidzik L, Brinker A, Kortepeter C (2021) Hypertension: a new safety risk for patients treated with erenumab. Headache 61(1):202–208
Breen ID, Brumfiel CM, Patel MH, Butterfield RJ, VanderPluym JH, Griffing L et al (2021) Evaluation of the safety of calcitonin gene-related peptide antagonists for migraine treatment among adults with Raynaud phenomenon. JAMA Netw Open 4(4):e217934
Evans RW (2019) Raynaud's phenomenon associated with calcitonin gene-related peptide monoclonal antibody antagonists. Headache 59(8):1360–1364
Manickam AH, Buture A, Tomkins E, Ruttledge M (2021) Raynaud’s phenomenon secondary to erenumab in a patient with chronic migraine. Clin Case Rep 9(8):e04625
Haanes KA, Edvinsson L, Sams A (2020) Understanding side-effects of anti-CGRP and anti-CGRP receptor antibodies. J Headache Pain 21(1):26
Holzer P, Holzer-Petsche U (2021) Constipation caused by anti-calcitonin gene-related peptide migraine therapeutics explained by antagonism of calcitonin gene-related Peptide's motor-stimulating and prosecretory function in the intestine. Front Physiol 12:820006
Frattale I, Ornello R, Pistoia F, Caponnetto V, Colangeli E, Sacco S (2021) Paralytic ileus after planned abdominal surgery in a patient on treatment with erenumab. Intern Emerg Med 16(1):227–228
Acknowledgments
None.
Funding
This work is supported by a waiver for the European Headache Federation collection 2022.
Author information
Authors and Affiliations
Contributions
SS led the consensus process and drafted the manuscript. All other authors except RO participated in the development of the evidence-based recommendations and of the expert consensus statements. RO performed the statistical analyses. MSDR, JV, CD, RGG checked data accuracy. All authors revised the manuscript and the statements for content. Each author participated sufficiently in the work to take public responsibility for appropriate portions of the content; and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval and consent to participate was not needed for this consensus.
Consent for publication
All authors have reviewed the final version and gave their approval for publication.
Competing interests
Competing interests are reported in Supplemental File S1.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Conflicts of interest disclosures.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sacco, S., Amin, F.M., Ashina, M. et al. European Headache Federation guideline on the use of monoclonal antibodies targeting the calcitonin gene related peptide pathway for migraine prevention – 2022 update. J Headache Pain 23, 67 (2022). https://doi.org/10.1186/s10194-022-01431-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10194-022-01431-x