Abstract
Background
Occipital transcranial direct current stimulation (tDCS) is an effective and safe treatment for migraine attack prevention. Structural brain alterations have been found in migraineurs in regions related to pain modulation and perception, including occipital areas. However, whether these structural alterations can be dynamically modulated through tDCS treatment is understudied.
Objective
To track longitudinally grey matter volume changes in occipital areas in episodic migraineurs during and up to five months after occipital tDCS treatment in a single-blind, and sham-controlled study.
Methods
24 episodic migraineurs were randomized to either receive verum or sham occipital tDCS treatment for 28 days. To investigate dynamic grey matter volume changes patients underwent structural MRI at baseline (prior to treatment), 1.5 months and 5.5 months (after completion of treatment). 31 healthy controls were scanned with the same MRI protocol. Morphometry measures assessed rate of changes over time and between groups by means of tensor-based morphometry.
Results
Before treatment, migraineurs reported 5.6 monthly migraine days on average. A cross-sectional analysis revealed grey matter volume increases in the left lingual gyrus in migraineurs compared to controls. Four weeks of tDCS application led to a reduction of 1.9 migraine days/month and was paralleled by grey matter volume decreases in the left lingual gyrus in the treatment group; its extent overlapping with that seen at baseline.
Conclusion
This study shows that migraineurs have increased grey matter volume in the lingual gyrus, which can be modified by tDCS. Tracking structural plasticity in migraineurs provides a potential neuroimaging biomarker for treatment monitoring.
Trial registration
ClinicalTrials.gov, NCT03237754. Registered 03 August 2017 – retrospectively registered, https://clinicaltrials.gov/ct2/show/NCT03237754.
Similar content being viewed by others
Background
Migraine is a common relapsing headache disease with a huge socioeconomic burden. After lower back pain, it is the second most disabling condition [1]. The 2016 Global Burden of Disease Study measured a worldwide prevalence of 14.4% with 18.8% of women and 9.8% of men suffering from this disease highlighting its impact on the society [2]. Migraine attacks affect patients’ daily life considerably and have a significant negative impact on health-related quality of life in a frequency-dependent manner with higher attack frequency being associated with more severe disability [3,4,5]. Moreover, migraine is associated with numerous comorbidities including sleep disorders and various psychiatric diseases such as depression and anxiety disorders [6, 7]. Hence, migraine poses a substantial socioeconomic burden by both direct costs through specific migraine treatment and the associated comorbidities, and indirect costs due to work-absenteeism and loss in productivity [8].
Current treatment options for acute migraine attacks comprise mostly medication such as common non-steroidal anti-inflammatory drugs (NSAIDs) and triptans. Many patients remain unsatisfied with their medication during acute migraine attacks what highlights the importance of prevention of migraine attacks [9]. Attack prevention is multifaceted and includes lifestyle modifications, behavioral therapy, medication such as beta blockers, calcium channel blockers, antiepileptic drugs or antibodies against calcitonin gene-related peptide (CGRP) or its receptor, and neuromodulation. However, many patients do not desire prophylactic treatment with medication which is represented in relatively low adherence rates [10].
Several studies have investigated the efficiency of transcranial direct current stimulation (tDCS) in migraine prevention [11,12,13,14,15,16]. Most of them reported significant reduction in migraine days when applying tDCS to the occipital cortex, primary motor cortex, or the dorsolateral prefrontal cortex [11, 12, 14,15,16]. The advantages of tDCS treatment are its relatively low cost compared to other neurostimulation methods, safety and generally mild side effects [17]. tDCS acts by modifying cortical excitability through hyperpolarization or subthreshold depolarization of neurons depending on direction of current flow [18,19,20,21]. This effect can be used in migraine treatment as the brains of migraineurs exhibit altered cortical excitability and information processing over the migraine cycle [22,23,24,25,26].
The results from several studies indicate that additionally to having altered functionality in terms of cortical excitability, migraineurs undergo structural changes in several cortical and subcortical areas related to perception and pain processing, including the occipital cortex, when comparing them to a healthy population [27]. Moreover, longitudinal observations suggest that these alterations are dynamic over time and respond to beneficial migraine treatment [28,29,30].
A clinical trial, investigating the efficiency of a 28-day occipital anodal tDCS stimulation found a reduction of monthly migraine days of 2.6 days during the third month after tDCS treatment, while at earlier and later time points the tDCS and sham cohort did not differ significantly. Based on this clinical report the present study aims to investigate structural differences between episodic migraineurs and healthy controls by means of voxel-based morphometry (VBM) and at tracking these morphological alterations up to 5 months after a 28-day treatment period with occipital tDCS using tensor-based morphometry (TBM) for assessing the temporal dynamics of structural plasticity. Given the location of tDCS treatment and results from literature, we focused on structural changes in occipital cortical areas. Based on literature we hypothesized that (i) episodic migraine patients show structural alterations in occipital areas compared to a healthy control population; (ii) these alterations are dynamic over time and can be reversed by successful occipital tDCS treatment.
Materials and methods
Participants and exclusion/inclusion criteria
In this study, we enrolled patients between the age of 18 and 80 that had a preexisting diagnosis of “episodic migraine (EM) without aura” or “EM with and without aura” according to the International Classification of Headache Disorders, 3rd edition (ICHD-3) criteria. All headaches were diagnosed by an experienced neurologist [31]. Exclusion criteria included pregnancy, presence of a neurodegenerative disease, and contraindications against magnetic resonance imaging (MRI).
We also recruited healthy individuals as a control group who underwent the same imaging protocol as the patients.
All participants gave their informed consent and the study was approved by the local ethics committee.
Study design
This longitudinal, single-blind, randomized and sham-controlled trial was conducted at the University Hospital Zurich [32]. The migraine patients were randomized to either receive verum or sham tDCS treatment by using a block randomization technique with block sizes of ten (five verum and five sham per block).
During the whole study period, patients kept a headache diary and recorded the following parameters: occurrence, duration, quality, and intensity of headache attacks, as well as medication intake and accompanying features. The study period was divided into six subsequent blocks of 28 days, which will be referred to in the following as baseline period, T1, T2, T3, T4, and T5 (see Fig. 1). The baseline period served to assess patient’s migraine characteristics using the same headache diary and to validate the diagnosis of EM. During T1, directly following the baseline visit, the 28-day lasting tDCS treatment was performed. At three time points, migraine patients underwent clinical examination as well as structural MR imaging. These time points (baseline visit, FUP1, FUP2) are indicated in Fig. 1. The clinical outcome of this study is reported separately [32].
Overview of the study design. The observation period was subdivided into six 28-day periods (Baseline, T1–5). The tDCS treatment was performed during T1 and was initiated immediately after the baseline visit. FUP1 was scheduled shortly after the end of the stimulation period and FUP2 after T5. Modified from Pohl et al. [32]
Transcranial direct current stimulation
In this study, patients themselves applied anodal stimulation over the visual cortex by using a one-channel stimulator and standard rubber tDCS electrodes (DC-STIMULATOR PLUS, NeuroConn, Ilmenau, Germany). The active electrode (electrode size 5 × 7 cm2, current density 0.029 mA/cm2) was placed at Oz, located at the inion. A reference electrode (electrode size 10 × 10 cm2, current density 0.01 mA/cm2) was placed at Cz at the intersection between a sagittal line connecting the nasion and the inion and a coronal line between the tragus of both ears. Whereas the active electrode consisted of a more focal electrode resulting in a higher current density, a larger electrode was chosen as reference. This allowed maximizing the current density over the visual cortex while rendering the reference electrode functionally ineffective.
Patients were instructed on placing of the electrodes and handling of the device at the baseline visit.
The tDCS treatment was performed daily for 20 min during the 28-day T1 period (compare Fig. 1). The verum tDCS consisted of applying 1 mA over the 20-min session, while during sham stimulation 1 mA intensity was maintained only for 30 s with intermittent impedance check in the remaining 1170 s.
MRI measurements
We scanned patients with a 3 Tesla Philips Ingenia scanner (Philips Healthcare, Best, The Netherlands) with a 32-channel receive-only head coil at the Neuroimaging Center of the University Hospital Zurich. Apart from other sequences, a 3D T1-weighted magnetization prepared rapid gradient echo (MPRAGE) sequence was acquired for each subject. Scanning parameters were as follows: 160 slices, repetition time: 8.1 ms, echo time: 3.7 ms, flip angle: 8°, voxel dimensions: 1 × 1 × 1 mm, field of view: 240 × 240 mm2, scan time: 4:32 min. An experienced neuroradiologist examined all structural images for the presence of any brain abnormalities.
This imaging protocol was conducted for each patient at three time points, baseline and two follow-up sessions. The first follow-up measurement was scheduled at 1.5 months following the baseline scan and the second measurement approximately 7 months after baseline (compare Fig. 1).
Controls were scanned with the same imaging protocol at baseline and one follow-up session at 1.5 months post-baseline.
MRI processing
A total of 3 structural T1w images were acquired for each patient and 2 images for healthy controls on which voxel based morphometry methods were applied for estimation of grey matter (GM) and white matter (WM) changes in the brain [33].
The volumetric analysis was subdivided into two separate analyses, one cross-sectional comparison at baseline between healthy subjects and migraine patients and one longitudinal analysis of migraine patients for the time points baseline, FUP1 and FUP2, for which slightly different imaging processing pipelines were employed.
For the assessment of volumetric differences between EM patients and healthy controls, we used voxel-based morphometry within SPM12 (University College London, London, UK). First, the baseline T1w-MPRAGE images of each subject were segmented into GM, WM and cerebrospinal fluid (CSF) by using unified segmentation [34] which produced three probabilistic maps of these respective tissue types. This step was followed by spatial normalization of these probabilistic maps into standard Montreal Neurological Institute (MNI) space with a diffeomorphic Anatomical Registration using Exponentiated Lie algebra (DARTEL) algorithm [35] and application of smoothing with an isotropic Gaussian kernel of 3 mm full width at half maximum (FWHM).
For assessing regional longitudinal volumetric changes in migraine patients, we used TBM inside the SPM12 framework. First, the T1w MR images of the three time points were longitudinally co-registered, based on a registering of each imaging volume to a subject-specific average map. This step includes non-linear and rigid-body registration with corrections for intensity bias artifacts. The results of this step were subject-specific average maps representing a midpoint image, corresponding deformation fields and Jacobian Determinant Maps from each time point to this average map. This average map was segmented into the different tissue components using the same unified segmentation as described above. By applying the Jacobian Determinant Maps on the midpoint image, the respective tissue probability maps of each separate time point were obtained. For improved normalization into standard MNI space, the DARTEL algorithm was applied to the midpoint images and the produced deformation fields were used to register each individual time point to MNI space. Finally, an isotropic smoothing kernel of 3 mm FWHM was applied.
The total intracranial volume (TIV), used as covariate in the statistical models, was calculated as the sum of brain GM, WM and CSF volumes defined by a cut-off at the lowest slice including the cerebellum.
Statistical analysis
Descriptive analysis of demographic data was performed by using parametric and non-parametric statistics in STATA 17.0 (StataCorp LLC, College Station, TX, USA) and included two-sided t-tests, Wilcoxon rank-sum test, Kruskal-Wallis test and Fisher’s exact test.
To assess volumetric differences in GM and WM of the brain and between patients and controls at baseline general linear models were fitted with the covariates TIV, sex and age inside the SPM12 framework. Then, voxel-wise two-sample T-tests were conducted for comparison of volumetric increases or decreases between patients and controls. In order to assess the effect of the presence of aura on these observed volumetric changes we performed one-way ANOVA at baseline in the identified regions including the groups “Controls”, “EM with aura”, “EM without aura”. Furthermore, we assessed whether the observed structural alterations correlated with clinical factors representing disease severity such as monthly migraine days and migraine intensity, and the total number of years that patients are suffering from migraine. This correlation analysis was implemented with a similar model in SPM12 by adding the respective clinical factor as an additional covariate. Significance threshold was set at p < 0.001 uncorrected for multiple comparison at voxel-level with a cluster extent (CE) threshold of 20 contiguous voxels for all analyses. Only significant results with p < 0.05, corrected for family-wise error (FWE) at the cluster level using Random Field Theory are considered.
For the assessment of longitudinal volumetric changes, we used the SPM Sandwich Estimator Toolbox (SwE) for Longitudinal & Repeated Measures Data, which is based on a marginal model where the expected variability is described as a function of predictors (defined in the design matrix) and additionally accounts for correlations due to repeated measurements and unexplained variations across individuals. It has the following form for subject i:
where yi represents the tissue volumes at multiple timepoints, Xi denotes the design matrix and ϵi∗ the random effects modelled by individual marginal error terms with between-subject variance components with mean 0 [36]. The design matrix Xi consisted of the predictors intercept, time and time2 as well as the covariates age, sex, TIV, number of migraine days during baseline period, number of stimulation days, the Depression subscale of the Hospital Anxiety and Depression Scale (HADS-D), and the presence of aura. We chose to introduce a quadratic term due to the observed quadratic behavior of the clinical effect reported by Pohl et al. [32] and the assumption that potential volumetric changes would behave likewise. The HADS-D score was included because there is evidence that depression alters cortical excitability and hence might influence the efficiency of tDCS treatment [37,38,39,40]. We tested for significant differences in the quadratic component between tDCS and sham patients with a cluster inference threshold of p < 0.001 and a CE threshold of 20 contiguous voxels. Results with q < 0.05 (FDR-corrected) were considered as significant.
A correlation analysis was additionally performed to assess whether the GM changes are associated with the clinical effect of tDCS therapy represented by changes in the number of monthly migraine days. This correlation analysis was conducted by using a similar model within the SwE framework and adding the number of migraine days at baseline, T2 and T5 as an additional covariate.
Results
Demographics, baseline characteristics, and clinical outcome
We enrolled 31 healthy controls and 24 episodic migraineurs in this study. One control was excluded from further analysis due to severe artifacts on MRI. The baseline characteristics of the control and patient cohorts including subdivision of patients into migraineurs with (MwA) and without aura (MwoA) were not different in age, sex, number of migraine days, migraine intensity and duration of migraine disease (Table 1).
Likewise, in both randomized groups (tDCS and sham), migraine patients did not differ with respect to age, sex, average number of migraine days, average migraine intensity, disease duration, patients with aura, number of stimulation days, HADS-D score and timing of the follow-up measurements (Table 2). However, the timing of the second follow-up measurement displayed a relatively high variation ranging from 98 days to 373 days post-baseline.
The clinical outcome of this study is reported separately [32]. A 28-day treatment with occipital tDCS led to a mean reduction of 1.9 migraine days/month compared to the control group. At the beginning and the end of the observation period no antimigraine effect of tDCS treatment was detected, but the effect gradually developed over time and reached its maximum at four months after the initiation of the treatment.
Cross-sectional VBM analysis
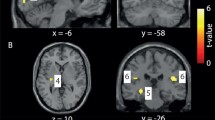
When comparing healthy controls with migraineurs at baseline, migraineurs showed significantly increased GM volume in the left lingual gyrus (z-score = 4.53, x = − 6, y = − 92, z = − 17, p = 0.003, CE = 228) (see Fig. 2). There were no significant differences in WM volume. The subgroup analysis did not show any significant volumetric differences between migraineurs with aura and migraineurs without aura in this identified region.
Correlation of structural alterations with clinical parameters
The correlations of structural brain changes with the following clinical parameters were assessed: monthly migraine days, migraine severity and disease duration. We did not detect any significant associations between structural alterations and the clinical factors in this patient cohort.
Longitudinal TBM analysis
Investigating dynamic volumetric changes over the observation period of approximately 170 days after the baseline visit we found a significant difference in the rate of quadratic GM change in the left lingual gyrus between the sham and verum tDCS group (z-score = 3.62, x = − 2, y = − 90, z = − 12, q = 0.039, CE = 23) (see Fig. 3A). Note, this cluster of dynamic GM volume change overlaps with the identified cluster in the cross-sectional analysis at baseline where migraineurs possessed increased cortical volume when compared to healthy controls as indicated in Fig. 3B.
Significant quadratic differences between verum and sham tDCS group revealed by TBM. Overlay of statistical parametric maps (uncorrected p < 0.001) shows a significant quadratic difference in the left lingual gyrus (A). The color bar indicates the t-score. Comparison of the statistical parametric maps (uncorrected p < 0.005, for illustrative purposes) of the cross-sectional cluster (in yellow) and the longitudinal cluster (in red) reveals that the regions of both clusters in the left lingual gyrus overlap (B)
Extracting the individual values of each subject at every time point in this cluster and plotting the difference in volumetric GM changes between tDCS and sham patients reveals that these dynamic longitudinal GM changes in the lingual gyrus effectively parallel the development of the clinical effect after tDCS treatment (see Fig. 4).
Clinical and structural changes following tDCS treatment. Change in monthly migraine days depicted as difference in days between verum and sham tDCS group (A). Change in GM volume depicted as difference in percent between verum and sham tDCS group (B). Negative values represent less migraine days, respectively lower GM volume in the verum tDCS group compared to the sham tDCS group. All values are normalized to the baseline measurement
Correlation of migraine days and GM changes
The associations between longitudinal GM changes in the lingual gyrus and the development of the number of migraine days after tDCS treatment did not show any significant correlation between these two parameters in this patient cohort.
Discussion
This study investigated structural differences between EM and healthy controls and the impact of a 28-day treatment period with occipital tDCS on brain structure, evaluated approximately at 50 and 170 days after initiation of the treatment. The cross-sectional analysis of this study reveals two major findings. First, episodic migraine patients show distinct volumetric alterations in occipital cortical areas, located in the left lingual gyrus, when comparing them with a healthy control population. Second, these volumetric alterations are not related to the severity of migraine at baseline and the duration of the disease in this study cohort. Investigating these volumetric changes in episodic migraineurs longitudinally after a 28-day period of daily occipital tDCS treatment, we can draw two important conclusions. First, the observed cortical alterations between episodic migraineurs and healthy controls located in the left lingual gyrus are dynamic and show volumetric decreases over time in tDCS treated patients compared to sham treated patients. Second, these dynamic changes in the lingual gyrus are not correlated with the change in monthly migraine days in this study cohort.
Our observation of altered cortical structure in occipital areas is in line with the findings of a large study that compared migraineurs with aura (n = 333) and healthy controls and found increased cortical thickness in the left lingual gyrus [41]. Similarities between the study populations comprise the high percentage of migraineurs with aura in our study. Likewise, several previous studies could demonstrate altered cortical thickness and cortical volume in visual areas [42,43,44,45,46,47]. Some of these studies found decreases in cortical thickness respectively cortical volume of migraineurs, while in other studies migraineurs possessed increased cortical thickness respectively cortical volume compared to healthy controls. It should also be mentioned that some studies could not replicate these findings [48,49,50,51,52]. However, there are certain aspects to consider when comparing the results of these different studies. First, the cortical volume in VBM assessments is a mixed measure of cortical surface area, cortical folding as well as cortical thickness [53]. Second, migraine is a disorder with a great variance of clinical phenotypes and its structural alterations with respect to several parameters such as presence of aura, attack frequency, age, phase of the migraine cycle, the lateralization of migraine attacks and the presence of interictal photosensitivity [45, 46, 54,55,56,57,58,59,60]. These factors introduce further variability and thus limit the comparability among many studies. Nevertheless, the consistent observation of alterations in occipital areas suggests that this region might indeed be affected by structural changes in migraine.
There is strong evidence of the involvement of visual areas in the pathophysiology of migraine in ways of altered visual processing, which might explain the frequent presence of visual symptoms such as photophobia and visual aura [61]. Previous studies demonstrated altered cortical excitability [22, 23, 26] as well as functional changes including altered functional activity upon visual stimuli and functional connectivity in visual areas [62,63,64,65,66]. Furthermore, many studies found a strong link between cortical spreading depression, a wave of depolarization starting in the occipital lobe, and visual aura, as well as the involvement of cortical spreading depression in migraine attacks without aura [67,68,69]. The observed structural differences in visual areas might represent structural correlates of these functional changes that have been reported in migraineurs. However, the direction of the association between the functional and structural findings has yet to be elucidated. It is still unclear, whether these structural alterations represent a predisposition or abnormalities that lead to the development of migraine disease or if they occur because of recurring migraine attacks.
We did not detect any significant correlations of the observed structural cortical alterations with clinical baseline parameters representing disease severity such as the number of monthly migraine days and migraine intensity, and the number of years that the patients have suffered from this disease. One possible explanation is that the structural alterations do not depend on disease severity but instead represent an intrinsic trait that is not modified by the severity or duration of migraine. However, these findings should be interpreted with caution due to the relatively small size of the study population.
Tracking the observed structural differences over time after a 28-day treatment period with occipital tDCS, we observed a dynamic in the cortical volume that seems to parallel the change in disease severity represented by a reduction in monthly migraine days. Notably, these longitudinal changes were located in the same area as the observed structural differences between migraineurs and healthy controls, suggesting that these alterations are plastic and seem to respond to tDCS treatment. Whether these changes are mainly induced by the tDCS treatment or reflect the improvement of migraine symptoms still needs to be elucidated. Until today, only very few studies have assessed longitudinal structural changes in migraine populations [70]. These studies, however, could show longitudinal dynamic cortical volumetric changes over one respectively four years as well as changes during the migraine cycle, speaking to the potential for plasticity of these structural alterations in migraineurs [28, 29, 57]. Another study tracked the morphometric changes in chronic migraineurs following sphenopalatine ganglion block over six weeks and similarly to our results found a change in cortical and subcortical morphology with improving migraine symptoms [30].
This finding might have implications for migraine treatment and future interventional trials, as we could demonstrate that the cortical alterations are not permanent and fixed but rather plastic and can be modified by successful migraine treatment. Tracking the morphological cortical changes post-treatment might lead to the discovery of new biomarkers for the assessment of treatment response.
Although both, local cortical volume and monthly migraine days decreased after tDCS treatment compared to sham treated patients, we did not detect significant longitudinal correlations between these two measures. However, two important factors limit the interpretability of this assessment. First, the study population was relatively small as already mentioned above. Second, due to the exploratory nature of this study the structural MRI examinations were scheduled and performed at time points that missed the period of maximal clinical effect size. The first follow-up was performed just before the clinical effect had completely evolved (in T3 and T4) and the second follow-up after the effect had already vanished. These two factors might explain the lack of significant association between the structural and clinical changes and they should be considered in future studies.
Further limitations of this study are the remaining heterogeneity of the study population by including both patients with and without aura (i.e. no subgroup analysis for the effect tDCS on aura presence was possible, as the number of patients with and without aura was too low. Further, the aura type in patients with aura was not examined), a rather large spectrum of disease duration, and the lack of information about the timing of the MRI examinations during the patients’ migraine cycle, hence no correction for ictal/interictal variability was possible. Finally, we did not collect data on the usual headache lateralization and side. Thus, we do not know if any potential lateralization could be related to the observed dominant findings in the left hemisphere.
Conclusions
Our results support the presence of structural changes in visual areas in episodic migraineurs. Whether these structural changes represent the substrate for migraine development or result due to recurring migraine attacks still needs to be elucidated. Furthermore, while the number of monthly migraine days decreased after tDCS treatment compared to sham treated patients, the cortical volume decreased as well. This paves the way for the development of new imaging biomarkers based on measuring structural volumetric changes for tracking the treatment effect of specific migraine therapies.
Availability of data and materials
The datasets presented in this article are not readily available due to Swiss law, the researchers must assess whether the use of the data and coded datasets are within the primary scope of the informed consent. Data is only available upon request and after the researchers have reviewed the purpose of the inquiry. Requests to access the datasets should be directed to Dr. Lars Michels, lars.michels@usz.ch.
Abbreviations
- CE:
-
Cluster extent
- CSF:
-
Cerebrospinal fluid
- DARTEL:
-
Diffeomorphic Anatomical Registration using Exponentiated Lie algebra
- EM:
-
Episodic migraineur
- FDR:
-
False discovery rate
- FUP:
-
Follow-up
- FWE:
-
Family-wise error
- GM:
-
Grey matter
- HADS-D:
-
Hospital Anxiety and Depression Scale, Depression subscale
- ICHD-3:
-
International Classification of Headache Disorders, 3rd edition
- MNI:
-
Montreal Neurological Institute
- MPRAGE:
-
Magnetization prepared rapid gradient echo
- MRI:
-
Magnetic resonance imaging
- MwA:
-
Migraineur with aura
- MwoA:
-
Migraineur without aura
- TBM:
-
Tensor-based morphometry
- tDCS:
-
Transcranial direct current stimulation
- TIV:
-
Total intracranial volume
- VBM:
-
Voxel-based morphometry
- WM:
-
White matter
References
Burch RC, Buse DC, Lipton RB (2019) Migraine: epidemiology, burden, and comorbidity. Neurol Clin 37(4):631–649. https://doi.org/10.1016/j.ncl.2019.06.001
GBD (2016) Headache collaborators (2018) global, regional, and national burden of migraine and tension-type headache, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol 17(11):954–976. https://doi.org/10.1016/s1474-4422(18)30322-3
Abu Bakar N, Tanprawate S, Lambru G, Torkamani M, Jahanshahi M, Matharu M (2016) Quality of life in primary headache disorders: a review. Cephalalgia 36(1):67–91. https://doi.org/10.1177/0333102415580099
Buse D, Manack A, Serrano D, Reed M, Varon S, Turkel C, Lipton R (2012) Headache impact of chronic and episodic migraine: results from the American Migraine Prevalence and Prevention study. Headache 52(1):3–17. https://doi.org/10.1111/j.1526-4610.2011.02046.x
Blumenfeld AM, Varon SF, Wilcox TK, Buse DC, Kawata AK, Manack A, Goadsby PJ, Lipton RB (2011) Disability, HRQoL and resource use among chronic and episodic migraineurs: results from the international burden of migraine study (IBMS). Cephalalgia 31(3):301–315. https://doi.org/10.1177/0333102410381145
Scher AI, Bigal ME, Lipton RB (2005) Comorbidity of migraine. Curr Opin Neurol 18(3):305–310. https://doi.org/10.1097/01.wco.0000169750.52406.a2
Minen MT, Begasse De Dhaem O, Kroon Van Diest A, Powers S, Schwedt TJ, Lipton R, Silbersweig D (2016) Migraine and its psychiatric comorbidities. J Neurol Neurosurg Psychiatry 87(7):741–749. https://doi.org/10.1136/jnnp-2015-312233
Baigi K, Stewart WF (2015) Headache and migraine: a leading cause of absenteeism. Handb Clin Neurol 131:447–463. https://doi.org/10.1016/b978-0-444-62627-1.00025-1
Vargas BB (2018) Acute treatment of migraine. Continuum (Minneap Minn) 24 (4, headache):1032-1051. 24(4):1032–1051. https://doi.org/10.1212/con.0000000000000639
Schwedt TJ (2018) Preventive therapy of migraine. Continuum (Minneap Minn) 24(4):1052–1065. https://doi.org/10.1212/con.0000000000000635
Viganò A, D'Elia TS, Sava SL, Auvé M, De Pasqua V, Colosimo A, Di Piero V, Schoenen J, Magis D (2013) Transcranial direct current stimulation (tDCS) of the visual cortex: a proof-of-concept study based on interictal electrophysiological abnormalities in migraine. J Headache Pain 14(1):23. https://doi.org/10.1186/1129-2377-14-23
Rocha S, Melo L, Boudoux C, Foerster Á, Araújo D, Monte-Silva K (2015) Transcranial direct current stimulation in the prophylactic treatment of migraine based on interictal visual cortex excitability abnormalities: a pilot randomized controlled trial. J Neurol Sci 349(1–2):33–39. https://doi.org/10.1016/j.jns.2014.12.018
Antal A, Kriener N, Lang N, Boros K, Paulus W (2011) Cathodal transcranial direct current stimulation of the visual cortex in the prophylactic treatment of migraine. Cephalalgia 31(7):820–828. https://doi.org/10.1177/0333102411399349
Ahdab R, Mansour AG, Khazen G, El-Khoury C, Sabbouh TM, Salem M, Yamak W, Ayache SS, Riachi N (2019) Cathodal transcranial direct current stimulation of the occipital cortex in episodic migraine: a randomized sham-controlled crossover study. J Clin Med 9(1). https://doi.org/10.3390/jcm9010060
Dasilva AF, Mendonca ME, Zaghi S, Lopes M, Dossantos MF, Spierings EL, Bajwa Z, Datta A, Bikson M, Fregni F (2012) tDCS-induced analgesia and electrical fields in pain-related neural networks in chronic migraine. Headache 52(8):1283–1295. https://doi.org/10.1111/j.1526-4610.2012.02141.x
Andrade SM, de Brito Aranha REL, de Oliveira EA, de Mendonça C, Martins WKN, Alves NT, Fernández-Calvo B (2017) Transcranial direct current stimulation over the primary motor vs prefrontal cortex in refractory chronic migraine: a pilot randomized controlled trial. J Neurol Sci 378:225–232. https://doi.org/10.1016/j.jns.2017.05.007
Chase HW, Boudewyn MA, Carter CS, Phillips ML (2020) Transcranial direct current stimulation: a roadmap for research, from mechanism of action to clinical implementation. Mol Psychiatry 25(2):397–407. https://doi.org/10.1038/s41380-019-0499-9
Bindman LJ, Lippold OC, Redfearn JW (1964) The action of brief polarizing currents on the cerebral cortex of the rat (1) during current flow and (2) in the production of LONG-lasting after-effects. J Physiol 172(3):369–382. https://doi.org/10.1113/jphysiol.1964.sp007425
Antal A, Nitsche MA, Paulus W (2006) Transcranial direct current stimulation and the visual cortex. Brain Res Bull 68(6):459–463. https://doi.org/10.1016/j.brainresbull.2005.10.006
Antal A, Kincses TZ, Nitsche MA, Bartfai O, Paulus W (2004) Excitability changes induced in the human primary visual cortex by transcranial direct current stimulation: direct electrophysiological evidence. Invest Ophthalmol Vis Sci 45(2):702–707. https://doi.org/10.1167/iovs.03-0688
Nitsche MA, Paulus W (2000) Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 527 Pt 3(Pt 3):633–639. https://doi.org/10.1111/j.1469-7793.2000.t01-1-00633.x
Bohotin V, Fumal A, Vandenheede M, Bohotin C, Schoenen J (2003) Excitability of visual V1-V2 and motor cortices to single transcranial magnetic stimuli in migraine: a reappraisal using a figure-of-eight coil. Cephalalgia 23(4):264–270. https://doi.org/10.1046/j.1468-2982.2003.00475.x
Mulleners WM, Chronicle EP, Palmer JE, Koehler PJ, Vredeveld JW (2001) Visual cortex excitability in migraine with and without aura. Headache 41(6):565–572. https://doi.org/10.1046/j.1526-4610.2001.041006565.x
Coppola G, Schoenen J (2012) Cortical excitability in chronic migraine. Curr Pain Headache Rep 16(1):93–100. https://doi.org/10.1007/s11916-011-0231-1
Coppola G, Pierelli F, Schoenen J (2007) Is the cerebral cortex hyperexcitable or hyperresponsive in migraine? Cephalalgia 27(12):1427–1439. https://doi.org/10.1111/j.1468-2982.2007.01500.x
Chen WT, Wang SJ, Fuh JL, Lin CP, Ko YC, Lin YY (2011) Persistent ictal-like visual cortical excitability in chronic migraine. Pain 152(2):254–258. https://doi.org/10.1016/j.pain.2010.08.047
Chong CD, Schwedt TJ, Dodick DW (2016) Migraine: what imaging reveals. Curr Neurol Neurosci Rep 16(7):64. https://doi.org/10.1007/s11910-016-0662-5
Messina R, Rocca MA, Colombo B, Pagani E, Falini A, Goadsby PJ, Filippi M (2018) Gray matter volume modifications in migraine: a cross-sectional and longitudinal study. Neurology 91(3):e280–e292. https://doi.org/10.1212/wnl.0000000000005819
Liu J, Lan L, Li G, Yan X, Nan J, Xiong S, Yin Q, von Deneen KM, Gong Q, Liang F, Qin W, Tian J (2013) Migraine-related gray matter and white matter changes at a 1-year follow-up evaluation. J Pain 14(12):1703–1708. https://doi.org/10.1016/j.jpain.2013.08.013
Newman-Norlund RD, Rorden C, Maleki N, Patel M, Cheng B, Androulakis XM (2020) Cortical and subcortical changes following sphenopalatine ganglion blocks in chronic migraine with medication overuse headache: a preliminary longitudinal study. Womens Midlife Health 6(1):7. https://doi.org/10.1186/s40695-020-00055-y
Headache Classification Committee of the International Headache Society (IHS) (2018) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 38(1):1–211. https://doi.org/10.1177/0333102417738202
Pohl H, Moisa M, Jung HH, Brenner K, Aschmann J, Riederer F, Ruff CC, Schoenen J, Luechinger R, Widmer L, Petersen JA, Gantenbein AR, Sandor PS, Michels L (2020) Long-term effects of self-administered transcranial direct current stimulation in episodic migraine prevention: results of a randomized controlled trial. Neuromodulation 24(5):890–898. https://doi.org/10.1111/ner.13292
Ashburner J, Friston KJ (2000) Voxel-based morphometry--the methods. Neuroimage 11(6 Pt 1):805–821. https://doi.org/10.1006/nimg.2000.0582
Ashburner J, Friston KJ (2005) Unified segmentation. Neuroimage 26(3):839–851. https://doi.org/10.1016/j.neuroimage.2005.02.018
Ashburner J (2007) A fast diffeomorphic image registration algorithm. Neuroimage 38(1):95–113. https://doi.org/10.1016/j.neuroimage.2007.07.007
Guillaume B, Hua X, Thompson PM, Waldorp L, Nichols TE (2014) Fast and accurate modelling of longitudinal and repeated measures neuroimaging data. Neuroimage 94:287–302. https://doi.org/10.1016/j.neuroimage.2014.03.029
Shajahan PM, Glabus MF, Gooding PA, Shah PJ, Ebmeier KP (1999) Reduced cortical excitability in depression. Impaired post-exercise motor facilitation with transcranial magnetic stimulation. Br J Psychiatry 174(5):449–454. https://doi.org/10.1192/bjp.174.5.449
Croarkin PE, Nakonezny PA, Lewis CP, Zaccariello MJ, Huxsahl JE, Husain MM, Kennard BD, Emslie GJ, Daskalakis ZJ (2014) Developmental aspects of cortical excitability and inhibition in depressed and healthy youth: an exploratory study. Front Hum Neurosci 8:669. https://doi.org/10.3389/fnhum.2014.00669
Concerto C, Lanza G, Cantone M, Pennisi M, Giordano D, Spampinato C, Ricceri R, Pennisi G, Aguglia E, Bella R (2013) Different patterns of cortical excitability in major depression and vascular depression: a transcranial magnetic stimulation study. BMC Psychiatry 13(1):300. https://doi.org/10.1186/1471-244x-13-300
Salustri C, Tecchio F, Zappasodi F, Bevacqua G, Fontana M, Ercolani M, Milazzo D, Squitti R, Rossini PM (2007) Cortical excitability and rest activity properties in patients with depression. J Psychiatry Neurosci 32(4):259–266
Gaist D, Hougaard A, Garde E, Reislev NL, Wiwie R, Iversen P, Madsen CG, Blaabjerg M, Nielsen HH, Krøigård T, Østergaard K, Kyvik KO, Hjelmborg J, Madsen K, Siebner HR, Ashina M (2018) Migraine with visual aura associated with thicker visual cortex. Brain 141(3):776–785. https://doi.org/10.1093/brain/awx382
Palm-Meinders IH, Arkink EB, Koppen H, Amlal S, Terwindt GM, Launer LJ, van Buchem MA, Ferrari MD, Kruit MC (2017) Volumetric brain changes in migraineurs from the general population. Neurology 89(20):2066–2074. https://doi.org/10.1212/wnl.0000000000004640
Granziera C, DaSilva AF, Snyder J, Tuch DS, Hadjikhani N (2006) Anatomical alterations of the visual motion processing network in migraine with and without aura. PLoS Med 3(10):e402. https://doi.org/10.1371/journal.pmed.0030402
Messina R, Rocca MA, Colombo B, Valsasina P, Horsfield MA, Copetti M, Falini A, Comi G, Filippi M (2013) Cortical abnormalities in patients with migraine: a surface-based analysis. Radiology 268(1):170–180. https://doi.org/10.1148/radiol.13122004
Magon S, May A, Stankewitz A, Goadsby PJ, Schankin C, Ashina M, Amin FM, Seifert CL, Mallar Chakravarty M, Müller J, Sprenger T (2019) Cortical abnormalities in episodic migraine: a multi-center 3T MRI study. Cephalalgia 39(5):665–673. https://doi.org/10.1177/0333102418795163
Bonanno L, Lo Buono V, De Salvo S, Ruvolo C, Torre V, Bramanti P, Marino S, Corallo F (2020) Brain morphologic abnormalities in migraine patients: an observational study. J Headache Pain 21(1):39. https://doi.org/10.1186/s10194-020-01109-2
Jin C, Yuan K, Zhao L, Zhao L, Yu D, von Deneen KM, Zhang M, Qin W, Sun W, Tian J (2013) Structural and functional abnormalities in migraine patients without aura. NMR Biomed 26(1):58–64. https://doi.org/10.1002/nbm.2819
Matharu MS, Good CD, May A, Bahra A, Goadsby PJ (2003) No change in the structure of the brain in migraine: a voxel-based morphometric study. Eur J Neurol 10(1):53–57. https://doi.org/10.1046/j.1468-1331.2003.00510.x
Mehnert J, Schulte L, May A (2020) No grey matter alterations in longitudinal data of migraine patients. Brain 143(11):e93. https://doi.org/10.1093/brain/awaa300
Sheng L, Zhao P, Ma H, Yuan C, Zhong J, Dai Z, Pan P (2020) A lack of consistent brain grey matter alterations in migraine. Brain 143(6):e45. https://doi.org/10.1093/brain/awaa123
Wang HZ, Wang WH, Shi HC, Yuan CH (2020) Is there a reliable brain morphological signature for migraine? J Headache Pain 21(1):89. https://doi.org/10.1186/s10194-020-01158-7
Datta R, Detre JA, Aguirre GK, Cucchiara B (2011) Absence of changes in cortical thickness in patients with migraine. Cephalalgia 31(14):1452–1458. https://doi.org/10.1177/0333102411421025
Hutton C, Draganski B, Ashburner J, Weiskopf N (2009) A comparison between voxel-based cortical thickness and voxel-based morphometry in normal aging. Neuroimage 48(2):371–380. https://doi.org/10.1016/j.neuroimage.2009.06.043
Valfrè W, Rainero I, Bergui M, Pinessi L (2008) Voxel-based morphometry reveals gray matter abnormalities in migraine. Headache 48(1):109–117. https://doi.org/10.1111/j.1526-4610.2007.00723.x
Planchuelo-Gómez Á, García-Azorín D, Guerrero ÁL, Rodríguez M, Aja-Fernández S, de Luis-García R (2020) Gray matter structural alterations in chronic and episodic migraine: a morphometric magnetic resonance imaging study. Pain Med 21(11):2997–3011. https://doi.org/10.1093/pm/pnaa271
Coppola G, Tinelli E, Lepre C, Iacovelli E, Di Lorenzo C, Di Lorenzo G, Serrao M, Pauri F, Fiermonte G, Bianco F, Pierelli F (2014) Dynamic changes in thalamic microstructure of migraine without aura patients: a diffusion tensor magnetic resonance imaging study. Eur J Neurol 21(2):287–e213. https://doi.org/10.1111/ene.12296
Coppola G, Di Renzo A, Tinelli E, Iacovelli E, Lepre C, Di Lorenzo C, Di Lorenzo G, Di Lenola D, Parisi V, Serrao M, Pauri F, Fiermonte G, Bianco F, Pierelli F (2015) Evidence for brain morphometric changes during the migraine cycle: a magnetic resonance-based morphometry study. Cephalalgia 35(9):783–791. https://doi.org/10.1177/0333102414559732
Hougaard A, Amin FM, Hoffmann MB, Larsson HB, Magon S, Sprenger T, Ashina M (2015) Structural gray matter abnormalities in migraine relate to headache lateralization, but not aura. Cephalalgia 35(1):3–9. https://doi.org/10.1177/0333102414532378
Chong CD, Starling AJ, Schwedt TJ (2016) Interictal photosensitivity associates with altered brain structure in patients with episodic migraine. Cephalalgia 36(6):526–533. https://doi.org/10.1177/0333102415606080
Schmitz N, Admiraal-Behloul F, Arkink EB, Kruit MC, Schoonman GG, Ferrari MD, van Buchem MA (2008) Attack frequency and disease duration as indicators for brain damage in migraine. Headache 48(7):1044–1055. https://doi.org/10.1111/j.1526-4610.2008.01133.x
Puledda F, Ffytche D, O'Daly O, Goadsby PJ (2019) Imaging the visual network in the migraine Spectrum. Front Neurol 10:1325. https://doi.org/10.3389/fneur.2019.01325
Schwedt TJ, Chiang CC, Chong CD, Dodick DW (2015) Functional MRI of migraine. Lancet Neurol 14(1):81–91. https://doi.org/10.1016/s1474-4422(14)70193-0
Michels L, Villanueva J, O'Gorman R, Muthuraman M, Koirala N, Büchler R, Gantenbein AR, Sandor PS, Luechinger R, Kollias S, Riederer F (2019) Interictal Hyperperfusion in the higher visual cortex in patients with episodic migraine. Headache 59(10):1808–1820. https://doi.org/10.1111/head.13646
Lo Buono V, Bonanno L, Corallo F, Pisani LR, Lo Presti R, Grugno R, Di Lorenzo G, Bramanti P, Marino S (2017) Functional connectivity and cognitive impairment in migraine with and without aura. J Headache Pain 18(1):72. https://doi.org/10.1186/s10194-017-0782-6
Li Z, Zhou J, Lan L, Cheng S, Sun R, Gong Q, Wintermark M, Zeng F, Liang F (2020) Concurrent brain structural and functional alterations in patients with migraine without aura: an fMRI study. J Headache Pain 21(1):141. https://doi.org/10.1186/s10194-020-01203-5
Tedeschi G, Russo A, Conte F, Corbo D, Caiazzo G, Giordano A, Conforti R, Esposito F, Tessitore A (2016) Increased interictal visual network connectivity in patients with migraine with aura. Cephalalgia 36(2):139–147. https://doi.org/10.1177/0333102415584360
Hadjikhani N, Sanchez Del Rio M, Wu O, Schwartz D, Bakker D, Fischl B, Kwong KK, Cutrer FM, Rosen BR, Tootell RB, Sorensen AG, Moskowitz MA (2001) Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A 98(8):4687–4692. https://doi.org/10.1073/pnas.071582498
Chen SP, Ayata C (2016) Spreading depression in primary and secondary headache disorders. Curr Pain Headache Rep 20(7):44. https://doi.org/10.1007/s11916-016-0574-8
Noseda R, Burstein R (2013) Migraine pathophysiology: anatomy of the trigeminovascular pathway and associated neurological symptoms, CSD, sensitization and modulation of pain. Pain 154(Suppl 1):S44–S53. https://doi.org/10.1016/j.pain.2013.07.021
Masson R, Demarquay G, Meunier D, Lévêque Y, Hannoun S, Bidet-Caulet A, Caclin A (2021) Is migraine associated to brain anatomical alterations? New data and coordinate-based Meta-analysis. Brain Topogr 34(3):384–401. https://doi.org/10.1007/s10548-021-00824-6
Acknowledgements
Not applicable.
Funding
Heiko Pohl is financially supported by the Werner Dessauer Foundation. Patrick Freund is funded by a SNF Eccellenza Professorial Fellowship grant (PCEFP3_181362 / 1).
Author information
Authors and Affiliations
Contributions
AG, PS, FR, PF, RL, LM participated in the conception and design of the work. SS, PF, HP, and LM were involved in the elaboration of the methodology and the data analysis. All authors participated in the interpretation of the data. SS drafted the manuscript which all authors critically reviewed. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Stated in Participants and Exclusion/Inclusion criteria section of Methods: “All participants gave their informed consent and the study was approved by the local ethics committee.”
Consent for publication
Not applicable.
Competing interests
Pohl received speaker fees from TEVA Pharmaceuticals and honoraria from Eli Lilly. Franz Riederer has received speaker honoraria from Burgerstein Foundation, Lilly, Teva, and Novartis. Dr. Sandor reports personal fees from Novartis, Teva, Lilly, Almirall, outside the submitted work. Simon Schading, Dr. Gantenbein, Dr. Luechinger, Dr. Freund, and Dr. Michels have no conflicts of interest to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Schading, S., Pohl, H., Gantenbein, A. et al. Tracking tDCS induced grey matter changes in episodic migraine: a randomized controlled trial. J Headache Pain 22, 139 (2021). https://doi.org/10.1186/s10194-021-01347-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10194-021-01347-y