Abstract
Biological soft tissues manipulation, including conventional (mechanical) and nonconventional (laser, waterjet and ultrasonic) processes, is critically required in most surgical innervations. However, the soft tissues, with their nature of anisotropic and viscoelastic mechanical properties, and high biological and heat sensitivities, are difficult to manipulated. Moreover, the mechanical and thermal induced damage on the surface and surrounding tissue during the surgery can impair the proliferative phase of healing. Thus, understanding the manipulation mechanism and the resulted surface damage is of importance to the community. In recent years, more and more scholars carried out researches on soft biological tissue cutting in order to improve the cutting performance of surgical instruments and reduce the surgery induced tissue damage. However, there is a lack of compressive review that focused on the recent advances in soft biological tissue manipulating technologies. Hence, this review paper attempts to provide an informative literature survey of the state-of-the-art of soft tissue manipulation processes in surgery. This is achieved by exploring and recollecting the different soft tissue manipulation techniques currently used, including mechanical, laser, waterjet and ultrasonic cutting and advanced anastomosis and reconstruction processes, with highlighting their governing removal mechanisms as well as the surface and subsurface damages.
Similar content being viewed by others
1 Introduction
Soft biological tissues manipulation is critically required in most surgical innervations, whereby the surgeons need to use the mechanical or nonconventional cutting devices to precisely cut, remove, hemostasis and suture the tissues. In general, the development of surgical methods and instruments for soft tissue cutting promoted and improved each other. Figure 1 had shown the history of soft biological tissue cutting. The method of mechanical cutting has undergone a long development process. The circumcision with sharpened stones dated to the Paleolithic and Neolithic periods was recorded as the earliest elective procedures [1]. Up to date, a variety of mechanical cutting instruments, such as general surgical instruments, minimally invasive surgical instruments, interventional surgical instruments, and microsurgical instruments, can be used to cut biological soft tissues during surgery to achieve different clinical treatment effects. These can be simply divided into incising, puncture and shearing. On the other hand, the nonconventional cutting has also been developed very rapidly in the past 40 years. This includes laser, waterjet and ultrasonic cutting, electrocautery and radiofrequency ablation, etc. The cutting mechanism of soft biological tissue can be significantly different with various cutting methods.
The processing technologies of soft biological tissue have also significantly attracted the attention of clinical medicine and engineering science. Biological soft tissue has multiple layers of complex components, anisotropic and viscoelastic mechanical properties, and sensitive biological activity, hence it is difficult to handle. In recent years, more and more scholars have carried out researches on soft biological tissue cutting in order to improve the cutting performance of surgical instruments and reduce the surgery induced tissue damage. In the mechanical cutting process, the force of soft biological tissue is complicated and of importance for understanding the cutting mechanism. Since surgical robots are becoming more and more commonly used for general surgical operations, cutting force modeling becomes critical to improve the safety and force feedback of surgical robots, as well as for evaluating biological tissue damages. The cutting force produced by the deformation, fracture and friction between the biological soft tissue and the tool was affected by factors such as the cutting method, the structure of the surgical instrument, and the cutting parameters. On the other hand, different from the cutting of engineering materials, there are many biological injuries not sensitive to cutting force, such as tissue fluid backflow, tissue bleeding, etc. Therefore, the cutting damage of soft biological tissues needed to consider the systemic influence of the living body. Moreover, with the development of the surgery technology, surgeons and patients hope to reduce surgical trauma through the use of advanced surgical instruments and surgical methods, while there is also an increasing demand for improving the quality of life after surgery. Methods such as innovation tool structure, assisted cutting technology, and nontraditional manipulation methods are also becoming more popular soft biological tissues cutting with their advantages such as high efficiency, low tissue damage and fast healing. However, currently there is no critical review of the state-of-the-art in soft biological tissue manipulation which can support the wide scientific and application prospect. Hence, this article will review the most advanced researches of soft tissues manipulation with focuses on cutting mechanism, tissue residual damage, devices development and process optimization methods.
2 Characteristics of Soft Biological Tissues
2.1 Definition and Structure
Soft biological tissue is defined as body tissue that is not hardened or calcified in anatomy [2], including connective tissue, muscle tissue, epithelial tissue and nervous tissue. Most of the objects in surgical cutting are organs that are composed of a variety of soft biological tissues, such as skin, blood vessel, liver, muscle and brain, etc. In the point of view of material composition, Soft biological tissue is composed of cells and extracellular matrix. Extracellular matrix is a complex network composed of a variety of macromolecules around cells, including collagen fibers, elastic fibers, and reticular fibers. The reinforced structures of soft biological tissue are obtained by the combination and arrangement of these fibers in different directions. Figure 2 shows the reinforced structure of soft biological tissue. The reinforced structure includes network reinforced structure, multi-unit reinforced structure, reinforced structure of multi-layer elastic membrane and reinforced structure of multi-layer fiber bundle. The reinforced structures contribute the residual stress, viscoelasticity and anisotropy of biological soft tissue, and hence influences the cutting process. The connective tissue is the main component of reinforced structure and plays important roles in the generate and propagation of microcracks during surgical cutting processes [3].
Reinforced structure of soft biological tissue: (a) Skin: a network reinforced structure composed of elastic fibers and collagen fibers; (b) Liver: a multi-unit reinforced structure with densely distributed vascular network and connective tissue; (c) Artery: a reinforced structure of multi-layer elastic membrane; (d) Muscle: a reinforced structure of multi-layer fiber bundle
When manipulating the soft biological tissues, it should consider to maintain the original histological structure as the destruction of the histological structure of soft tissues will lead to disorders of biological functions. For instance, in the process of cutting or suturing, the unrecoverable deformation and distortion of blood vessels may cause vasospasm, blood flow disorder and other problems. The thermal sensitivity of cells inside the soft tissue or their surrounding tissue also is an important factor that should be taken into consideration in their manipulation process. Specifically, as cells contain a large number of biological macromolecules such as proteins the excessive heat will denature these biological macromolecules and cause cell necrosis and rupture. Therefore, these cells must remain free of damage and below a threshold of the temperature to ensure that they can perform their important functions normally after surgery.
2.2 Fracture Mechanisms
Understanding the fracture mechanics of soft biological tissues, including the propagation of microcracks and the evaluation of fracture toughness, can be helpful when investigate their cutting mechanism. The cutting behavior of soft tissues is ultimately the growth of cracks. It is a difficult task to study the crack propagation of soft tissues due to their high viscoelasticity. Therefore, the microcrack propagation are investigated below.
Biological soft tissue contains a large amount of collagen fibers, muscle fibers and other proteins. Rupture of hydrogen bonds is the basic expansion mechanism the of individual protein molecules at the ultrascale. As shown in Figure 3(a)‒(b), The unfolding wave can destroy these hydrogen bonds, leading to loss of the helical structure [4].
Propagation mode of cracks in the process of soft tissue rupture under tension: (a, b) Visualization of the atomistic-scale unfolding process of the coiled-coil protein structure [4]; (c‒e) Crack propagation in the skin and annulus fibrosus [5, 6]; (f, g) Crack propagation in the adipose tissue; (h, i) Crack propagation in the ligaments and tendons [7]; (j) Progressive layer delamination of skin undergoing tensile deformation[5]
The propagation mode of cracks in the rupture process of soft tissue under tension which can support the understanding of their mechanical cutting mechanism. As shown in Figure 3(c)‒(e), collagen reorganization happened during the crack propagation of a prenotched biological tissue. Aligned and straightened fibers ahead of the crack tip can prevent crack propagation by alleviating the concentration of stresses near the tip [5]. The same fracture mode of annulus fibrosus were observed (Figure 3(d)), but Sabouri et al. [6] believed that the crack should grow along the initial crack direction. The propagation of cracks in adipose tissue is different. The crack was along the border of the reinforced basement membrane and the fat cells had ruptured [6]. Gregory et al. [7] observed that the crack appeared in the direction of the fiber but not in the direction of the initial crack, as shown in Figure 3(h), (i). Gregory proposed these ligaments and tendons were able to completely resist any further crack propagation of an initial tear, regardless of fiber orientation or applied loading condition. This layered fracture mode was also applicable to skin fractures, as shown in Figure 3(j).
Among the three fracture modes, the crack propagation of soft tissue is like fiber-reinforced material. Although the fiber resisted the fracture of soft tissue, the crack propagated along the lower strength matrix. Although the crack propagation of the material played an important role in the excision of hard materials, there is no relevant literature report in soft tissue cutting. In addition, the fracture toughness measured through mechanical experiments is also different from the fracture toughness in the actual cutting process. This article will also discuss this issue in Section 3. Therefore, the relationship between soft tissue fracture mechanics and soft tissue cutting is unclear.
It is worth noting that soft biological tissue is a material of which its property can be largely varied through age [8], water content [9], osmolarity and hydration [10] and loading conditions (e.g., holding force [11], velocity [12], fatigue [13]). Because soft tissue has complex individual differences, it is difficult to make breakthrough progress in the research on soft biological tissue cutting.
2.3 Thermal Properties
Cutting soft tissue mainly includes mechanical cutting and energy cutting methods. The principle of the energy cutting method is to increase the temperature of the tissue through the action of energy, so as to achieve the purpose of cutting the tissue and hemostasis.
The energy cutting method generate thermal cutting to tumors by using energy sources, such as radiofrequency, laser, focused ultrasound and microwaves. These energy cutting methods require precise control of energy to cut the lesion area without affecting healthy tissue, but it is difficult to achieve the precise control of energy in the clinical practices [15]. Due to excessive energy, complications such as burns to neighboring biological tissue often happened. On the contrary, the lesions are often not completely cleared due to insufficient energy [16].
Therefore, the energy cutting method needs to control the temperature of the soft tissue. High temperature will cause damage to surrounding tissues, such as carbonization, while low temperature will not achieve good cutting effect and hemostatic ability.
Heat capacity and thermal conductivity are two important parameters that affect the energy cutting method and thermal damage. Figure 4 shows the heat capacity and thermal conductivity of different soft tissues [14]. Adipose tissue has the lowest thermal conductivity and heat capacity. Heat conductivity, heat capacity and density are strongly influenced by the water content of the tissue. Furthermore, heat and thermal conductivity depending on the status of the tissue (in vivo & in vitro) and the effective heat capacities of in vivo tissues are up to three times higher than the in vitro ones [17].
The thermal properties of soft biological tissue [14]
3 Mechanical Cutting of Soft Tissues
Mechanical cutting soft tissue has a long development history, and has now become a mature method in surgery [18]. Mechanical cutting methods in surgery can be divided into incising, puncture and shearing, as shown in Table 1 [19]. Different cutting methods usually use different surgical tools to achieve the purpose of surgery which are used to excise, separate, inject, and suck soft tissues under various anatomical conditions.
Specifically, surgical blades are normally used to remove or separate soft tissues by incising soft tissues. These include disposable scalpel blades, ophthalmic scalpels, diaphragm knives and other knife instruments, which often have sharp cutting edge that can easily cut soft tissues and make low trauma. Typical applications include opening skin in surgical operations [23], removing diseased tissue from an organ [20], separating blood vessel or nerve in neurosurgery [24], etc. Medical needles are mainly used to insert soft tissues to form channels through the tissue layer to achieve drug injection, tissue sampling, device implantation and other functions, including puncture needles, injection needles, and microneedles. Medical needle instruments are delicate and long, resulting in small surgical wounds but also easy to deform. Typical applications include tissue acquisition by-fine needle biopsy [25], drug injection into subcutaneous tissue [26], and repairing soft tissue with suture delivery device [27]. Soft tissue shearing is to cut and trim free damaged tissue through two scissors blades of medical scissors including surgical scissors, minimally invasive shaver and punches. The two blades of the surgical scissors have a clamping effect on the soft tissue, so the tissue trimming effect is relatively smooth. Typical applications include end-to-side anastomosis in free-tissue transfer [28], trimming the ruptured meniscus with arthroscopic punch [29].
The methods of mechanical cutting soft tissue have differences in the structural features, operating methods and functions of the tools. This section mainly focuses on the cutting mechanism of soft biological tissue, the damage of biological tissue and cutting tools, and the optimization of cutting tools.
3.1 Cutting Mechanisms
3.1.1 Cutting Process and Force
Recently, various models of the human upper-limb anatomy have been derived. The biomechanical models of the arm that stand for precise anatomical models including muscles, tendons and bones are too complex to be utilized in mechanical design of an anthropomorphic robot arm. From the view of the mechanism, we should set up a more practicable model for easy and effective realization.
The researches on the cutting mechanism of soft tissues mainly focused on the observation of the process of soft tissues cutting and cutting forces analysis. Most of researches were carried on puncturing soft tissue with needle. The process of cutting soft tissue usually can be divided into three phases, including deformation stage, rupture stage and cutting stage [11], while sometimes an extra extraction stage cab also be considered for measuring the friction force [30]. During the cutting process, the cutting force F is usually divided into stiffness force Fs and friction force f [31]. The friction force and stiffness force are considered be important indicators of cutting performance, particularly for a sharp cutting tool [32]. Eventually the energy conversion in different cutting process of the soft tissue [11, 31, 33]: (I) in the deformation stage, the tool squeezes the soft tissue to deform, and the work done by the tool is converted into the elastic potential energy of the soft tissue; (II) in the rupture stage, the elastic potential energy stored in part of the soft tissue is converted into the rupture energy of the soft tissue, and the crack propagates; (III) in the cutting stage, the microscopic cyclic cutting of soft tissue continues to occur deform and fracture. The cutting tool overcomes friction and deformation force, and the work of external force is converted into friction work and rupture energy, while the elastic potential energy of soft tissue is relatively Stable.
Since the needle can be deflected, part of the work done by the external force will also be converted into the deformation energy of the needle [34]. On the contrary, during the shearing process the deformation of the fracture area is basically stable, and the friction between the blades is the key factor affecting the cutting force [35, 36].
The left side of Figure 5 shows the cutting force curve under the three mechanical cutting methods (i.e., incising, puncturing, shearing). There are differences in the cutting force of different mechanical cutting in the different stage of cutting soft tissue. In the deformation stage, the typical J-shaped curve shows elasticity deformation of soft tissue [37], but the cutting force characteristics in the rupture and cutting stages are different.
When incising soft tissue, the cracks rapidly expand at the tip area of the soft tissue, and the cutting force drops sharply [38, 39]. During incising porcine ascending aorta tissue and porcine liver, the fluctuations of the cutting force reflected that the soft tissue is constantly deformed and ruptured [33, 39]. Such mechanical phenomena of cell destruction may be related to the multi-layered complex structure of soft tissues, such as fiber breakage in blood vessel walls or rupture of local cell clusters in the liver. The contact area related to the friction force has two increasing stages and stabilizing stage during the incising process, so the cutting phases are subdivided into cutting phase 1 and 2 [40]. Therefore, Hu et al. [38] found that the friction force increased in cutting phase 1 (Figure 5(a)).
When puncturing soft tissue, it is easy to observe a short decrease in cutting force (Phase bc in Figure 5(b)), which may be caused by the rupture of the biofilm on the surface of the soft biological tissue [41, 42]. After the surface ruptures, the soft tissue continues to deform until it starts cutting. Due to the changes in friction force and stiffness force, the puncture process can be divided into cutting phase 1 (Puncturing) and cutting phase 2 (Punctured). It is worth noting that the friction force is increasing in cutting phase 1, while the cutting force is unchanged [43]. If the needle is inserted in the tissue and does not move, the cutting force composed of static friction force and stiffness force would be smaller because of the viscoelastic properties of soft tissue [44].
When shearing soft tissue, the cutting force of the scissors remains relatively stable until completed [35, 45]. Although relatively few literatures focused on the shearing of soft tissues, it can still be seen from the cutting process that the blade of scissor continuedly clamped the soft tissues, indicating that the fracture during the shearing process is stable. In addition, the friction between the blades of the scissor is an important factor affecting the cutting force [35].
3.1.2 Mathematical Model and Fracture Toughness
For understanding the interaction between cutting tool and soft biological tissue, researchers have carried out the work of the force modeling. Among them, there are a number of the researches on modeling the force of needle inserted soft tissue.
The stiffness force is mainly caused by the deformation of soft tissues. The models of stiffness force include nonlinear spring model [46], quasi-static model [47,48,49], exponential model [50, 51], contact model [45, 52, 53], modified Kelvin model [54], modified linear electrostatic model [55], Hunt-Crossley model [42] and linear Kelvin–Voigt model [56]. A stiffness force model was obtained, which changed in a non-linear manner based on the Hunt-Crossley model [42]. This model could well match the deformation force caused by the needle insertion. However, this model of stiffness force requires high integrity and huge workload. The nonlinear spring model was used to describe the stiffness force of the tissue before cutting [46]. Nonlinear spring model includes different material properties but has higher root mean square error than the quasi-static model and exponential model [47, 50, 51]. Quasi-static model and exponential model could capture the local effects but used only for corresponding conditions of tool, material and cutting parameters. The exponential function needs excessive calculation and is unsuitable for real time force control. The contact model has been presented the mechanical properties and deformation of the skin and considered the viscoelasticity and the elastic properties of the soft tissue during needle cutting [53]. However, the contact model is not suitable for real-time force control due to the inability to achieve fast online calculations [45, 52, 53]. The modified Kelvin model was used to predict the relationship between rupture force and needle velocity which was confirmed by experiments with porcine cardiac tissue [54]. Fung et al. [56] formulated a two-dimensional quasi-linear viscoelastic constitutive equation for blood vessels, using the pseudo-elasticity concept. In general, the mechanical constitutive equation of biomaterials is the core of the modeling of stiffness force that can describe the deformation of soft tissue during cutting.
The cutting force is the force necessary to pass through the tissue. This can be obtained by subtracting the estimated friction force from the total force [43, 54]. The cutting forces are different in the tissue and phantom at various insertion speeds, cutting depth and the rake angle [52, 57, 58]. The method of elemental cutting tool was used to establish a cutting force model based on the rake angle and inclination angle of the tool, and achieved good results [58, 59]. The ‘slice/push ratio’ was proposed and used to illustrate the influence of different cutting motion on cutting force and found that that the greater the ‘slice/push ratio’, the lower the cutting forces [60]. The ‘slice/push ratio’ is effective to help a deeper understanding of soft tissue cutting under complex movements.
The friction force is produced by the relative movement of the cut surface to the tool surface. The models of friction force included modified Karnopp friction model [31, 47, 61], Coulomb friction model [53], modified Winkler based model [55], Fourier series based model [31], Elasto-Plastic model [62, 63], relative velocity model [64] and damping based model [65]. The dynamic friction and static friction could be reflected in the modified Karnopp friction model which captured the effects of the Stribeck effect and Dahl model in biological material. The Modified Winkler based model could reflected the force distribution but affected by relative velocity easily [55]. Compared with the Fourier series-based model, the Elasto-Plastic model and Relative velocity model need to obtain the needle-tissue relative velocity [31, 62, 63]. Damping based model and Modified Winkler based model can easily calculate the friction force from the total measured force or the final cutting length.
Soft biological tissue fracture is considered to be a continuous crack propagation, which could be described by energy-based approaches [44]. Fracture toughness is a key parameter that describes the ability of a material to resist the propagation of pre-existing cracks which often measured using uniaxial stretching or tearing test [5, 66,67,68]. Cutting fracture toughness is popularized to indicate the ability of soft tissues to resist cutting [69], defined by [30, 69]:
where W is the work done, U is the strain energy and A is the crack area. When the J-integral is larger than the material crack resistance Jc, the crack begins to propagate. Though fracture toughness and cutting fracture toughness shared the similarity of inducing material failure at a crack tip, the cutting energies and tearing energies of soft biological tissue cannot be quantitatively related to one another [19]. Table 2 shows the fracture toughness and cutting fracture toughness of soft tissue. The cutting fracture toughness is much smaller than the fracture toughness of the material itself. The reason for this phenomenon is worthy of further study.
Cutting fracture toughness can effectively evaluate the resistance to mechanical cutting of soft tissues and the performance of the tool. However, cutting fracture toughness is not a material constant, which is affected by the cutting parameters, tool structure and the material inhomogeneity and inconsistency [79,80,81]. Barnett et al. [80] proposed that cutting fracture toughness was almost irrelevant to the cutting speed of inserting soft biological tissue and was positively related to the diameter of the needle. Khadem et al. [82] defined the cutting force as the traction force to open up the crack as shown in Figure 6 rather than parallel to the cutting direction. Liu et al. [52] proposed a modified model based and an energy-based maximum stiffness force model to describe the variation in the stiffness force of the needle and to predict the surface rupture.
Crack propagation in tissue cutting: (a) Needle tip-tissue interface during cutting represented by a force distribution with magnitude of Fc (δ is the crack-tip-opening displacement, a is the needle tip length, da is the incremental crack growth); (b) Modelling finite compliance and viscoelastic behavior of tissue using a linear solid model element (δT =0 is the displacement of the tissue sufficiently far from the crack surface) [82]
3.1.3 Computational Models
Accurately predicting the cutting force through the computational models is the key challenge because of the uncertainty of the parameters of soft biological tissue and cutting parameters. The model of finite element analysis (FEA) had been used in soft biological tissue cutting, which included the node-separation models and the cohesive zone models.
The node-separation models were used to simulate the crack propagation at the tip during slicing pig liver with a piece of scalpel [30, 33]. The model used the “local effective modulus” as fracture parameter of the tool-nodal interface without involving fracture mechanics. During the simulation process, the nodes broke sequentially as the blade advances. Therefore, the cutting force obtained from this model was purely generated by the elastic deformation of the tissue and was fluctuating caused by the crack propagation. This model did not involve element failure so that had higher computational efficiency, but was difficult to apply to tools with more complex structures. In addition, the cutting interaction must be limited to the nodes. Gokgol et al. [70] proposed a node-separation model based on the cutting fracture toughness. During the penetration simulation, the difference between the total work and the fracture energy provided the viscoelastic energy of soft biological tissue. If viscoelastic energy is larger than the fracture energy, the constraint on the node in contact with the cutting edge is released to open a crack. Ultimate strain was used to define element failure in some researches. When simulated the needle insertion into brain tissue, Singh et al. [83] used a maximum shear strain of 0.05 as the element failure.
In the cohesive zone models, fracture toughness was used to be described the separation of soft tissue, which was defined as surface energy of the crack area in the cohesive zone. Therefore, the cohesive zone method essentially has the same mechanism as the mathematical model based on fracture mechanics. The cohesive zone model was applied to simulate crack propagation during soft tissue puncturing. It was found that the cutting forces were sensitive to the rupture toughness [84, 85]. Tai et al. [86] used the cohesive zone model to simulating hollow needle puncturing to a PVC-based phantom tissue but did not complete the simulation. Due to the large elastic deformation of soft tissue, the cutting edge moves to the outside of the cohesive zone area so that the simulation cannot continue. Obtained from the simulation of needle insertion into gelatine phantom in the cohesive zone models, the results showed a successful insertion and good agreement with the experimental data [87].
In the existing simulation work, in order to improve the calculation efficiency, the composite structure, viscoelasticity and anisotropy of the soft tissue are not considered, which will affect the accuracy of the simulation structure. Cohesive element parameters were determined in a different way by Misra et al. [84, 85], Tai et al. [86] and Oldfield et al. [87]. Choosing the right parameters is very important for soft tissue cutting simulation, especially the parameter that indicates the ability of soft tissue to resist cutting and breaking.
3.1.4 Influence Factor of Cutting Force
In this section, the influence factors of the tool-tissue interaction force during the cutting process have been reviewed, which can be divided into three parts: tool properties, tissue characteristics and cutting parameters. In order to better compare and analyze the influence on cutting force of different mechanical cutting methods, the common tool geometry models based on metal cutting theory (Shown in Figure 7) has been established [45, 88,89,90]. The tool properties and cutting parameters of different surgical tools have been uniformly defined which were summarized and discussed in Table 3. While most of these statistical researches were studied on cutting soft tissues with needles and blade, there were few works about shearing soft tissues and they have not been involved in this review.
Tool properties include the diameter of needle, bevel angle, inclination angle, normal rake angle. and edge number. the diameter of needle, bevel angle, and edge number have the positive effects on the related forces and the normal rake angle has the negative effect on the forces. There is no clear relationship between inclination angle and cutting force.
Tissue characteristics include materials type, experimental pretreatment and holding force. It is worth noting that the influence of material type and experimental pretreatment on force are different. The experimental materials included biological tissues, hydrogels, or silica gels. Although they have similar mechanical properties, it is still unknown whether they have the same cutting mechanical properties due to the complex structure of biomaterials. In addition to the material, the state of the soft tissue is also an important factor affecting the cutting performance. Hu et al. [39] studied the cutting force and cutting toughness of biological soft tissue under different initial tensile forces, and found that the greater the initial tensile force, the smaller the tissue cutting force and fracture toughness. There are obvious differences in the tissue state of in vivo and in vitro tissue, which should be considered when conducting researches on soft tissue cutting.
Cutting parameters include velocity, rotational speed, vibration speed, cutting depth, slice-push ratio. There is no doubt that cutting depth, slice-push ratio and cutting force were positively correlated, rotational speed, vibration speed and force are negatively correlated. When the cutting speed increased, the stiffness force decreases. There are differences in the influence of cutting speed on friction and cutting force. There is no clear relationship between cutting speed and friction force or cutting force. The reason is not yet known.
3.2 Damage of Soft Biological Tissue and Cutting Tool
3.2.1 Damage of Soft Biological Tissue
In clinical surgery, mechanical cutting soft tissue will leave a cut surface that will be sutured or heal on its own. Damage to the cut surface will affect the healing of the soft tissue, function recovery, and even organ regeneration [110]. Compared with bone cutting [111] and energy cutting methods, the damage caused by mechanical cutting soft biological tissue is slight [112, 113]. Because incising, puncturing and shearing soft tissues are usually at low-speed, precise, and micro-cutting. Cutting force is not necessarily an evaluation parameter for soft tissue damage [114, 115], because soft tissue is a viscoelastic material that can undergo large deformations. Therefore, there are relatively few literatures on soft tissue damage caused by mechanical cutting. However, the inherent damage caused by mechanical cutting methods is worth noting, including histological damage [116,117,118], hemorrhage [117], tissue deformation/ displacement [117, 119], wound size [44] and tissue fluid return [117]. Of course, accidental injuries caused by clinical misuse are not discussed, although they often occur [120].
Histological damage caused by mechanical cutting of soft tissue is an important factor affecting tissue function. As shown in Figure 8(a)‒(c) and (f), the histological damage included cell apoptosis, cell separation, torn fiber tissue, deformation, tissue fracture, etc. Erdim et al. [116] used needles for liposuction and found that adipocyte cells would undergo apoptosis. The smaller the needle for liposuction, the higher the apoptosis rate of adipocyte cells [121]. In addition, there was obvious tissue damage and insufficient production of progenitor cells in the aspirated adipose tissue [118]. As shown in Figure 8(c), the capillary network and cells of the adipose tissue were partially destroyed. Compared with incising adipose tissue, aspirated adipose tissue lacked large vascular structure and showed a large number of small lipid droplets (ruptured fat cells) and dead space (dead cell). As shown in Figure 8(f), several forms of blood vessel damage happened when needle insertion, including fluid displacement, vessel rupture, and severing, tugging, and dragging of microvasculature [119]. Flow back along a needle track (backflow) can be a problem during direct infusion, e.g., convection-enhanced delivery, of drugs into soft tissues such as brain. insertion rate-dependent damage and changes in pre-stress were found to directly contribute to the extent of backflow [117]. Although the damage of mechanical cutting soft tissue is relatively small, it is still necessary to pay great attention to the histological damage when mechanical cutting important soft tissues such as brain tissue, eyes, blood vessels, etc.
Cutting damage of soft biological tissue: (a) Adipocytes apoptosis [116]; (b) Needle tissue damage at the hole surface [117]; (c) Structure damage of living adipose tissue after aspirated and excised [118]; (d) Backflow along the needle track in the rat brain [117]; (e) Surface damage of fresh porcine liver inserted with needles [44]; (f) Blood vessel damage accompanied needle insertion, including fluid displacement, vessel rupture, and severing, tugging, and dragging of microvasculature [119]
Hemorrhage is the most common tissue damage, especially in incising soft tissue. When cutting soft tissue, the capillaries in the soft tissue will be severed and bleeding. Hemorrhage and blood vessel damage caused by mechanical cutting would affect the surgical field of vision [122, 123] and cause thrombus formation [124, 125]. As shown in Figure 8(b), when needle inserted into brain, bleeding happen in the external capsule [117]. Bleeding is the main shortcoming of the mechanical cutting soft tissue. One of the reasons why energy tools such as laser knives, high-frequency electric knives, and ultrasonic knives can replace mechanical cutting methods in some fields is that they can reduce the amount of bleeding [113, 126, 127].
Mechanical cutting soft tissue will inevitably leave a cut surface that is wound. The shape and size of the wound directly affected the healing effect of the tissue. Figure 8(e) showed the shape and size of the soft tissue after puncture [44]. Generally, the smaller the size of the crack, the faster the healing. Barnett et al. [80] found that the size of the wound was related to the diameter of the puncture needle, but not equal to the diameter of the needle. Due to the viscoelasticity and large deformation of soft tissue, the crack did not open after mechanical cutting. Inserting the needle under bidirectional rotation seemed to minimize deflection and prevented the entanglement of the tissue and the expansion of the hole created by the needle path [128]. The crack shows different shapes after penetrating fresh porcine liver with a Franseen tip, standard bevel tip, and short bevel biopsy needle. It is worth noting that the T-shaped crack mouth is very difficult to heal. When trimming soft tissues, the incision of the soft tissues is neat and can reduce the occurrence of complications.
The research on soft tissue damage is still in the observational stage, and there is no related literature to study the mechanism of how the tool causes soft tissue damage.
3.2.2 Failure of Surgical Instruments
The failure of the mechanical cutting tool for soft tissue will affect the efficiency and cutting quality of the operation, and even cause medical accidents. The failure of medical tools mainly caused by manufacturing process and cutting process. In fact, new medical cutting tools may also have quality problems, which are currently difficult to supervise. The investigation report showed that more than 50% of unqualified products had quality problems such as cracks, burrs, corrosion, failure of cutting action, etc. [129]. Once surgical instruments are put into use, the factors influencing their behavior are many and individual inconspicuous effects can add up and trigger something serious, such as corrosion leading to an unexpected break. As shown in Figure 9, the failure modes of surgical instruments include fracture, deformation, wear, corrosion, tissue adhesion, etc.
Failure of surgical instruments in cutting soft tissue: (a) Intradiscal deeply seated broken surgical blade [130]; (b) Deformation and wear of surgical blade after incising gingival tissue [138]; (c-1) Rust stain on a pair of scissors; (c-2) Pitting corrosion on a retractor[139]; (d) Critical factors for lath martensite failure upon cutting hair [132]; (e) Failure of stainless steel surgical scissor and sucker subjected to multiple use/processing: (e-1) Extensive scratching, black staining including pits in metal (Areas of thick biofilm, many of the pits have biofilm associated with them); (e-2) Large metal defects with continuous biofilm and contaminating soil [135]; (f) Failure of commercial blade and MGTF coated blade; (f-2) Scratches and burrs; (f-4,6) coating peeled off [140]
The fracture failure of the surgical instruments brought a huge iatrogenic risk to the medical operations. One risk accompanying with Lumbar discectomy is breaking of the surgical scalpel during discectomy shown in the Figure 9(a) [130]. In arthroscopic surgery, the gill axis of the punch may break, leaving the scissors head in the joint cavity [131]. Carbon steel blade eventually become blunt after shaving even though the blade was about 50 times harder than the hair. The increase in the corner radius of the tip and the brittle fracture of the cutting edge were thought to be responsible for the blunt blade. The fracture of cutting edge caused by the combination of out-of-plane bending, microstructural heterogeneity, and asperities, which originated at the hair-edge asperity interface and created chipping that dulled a blade faster than other processes [132].
Figure 9(b) showed the deformation and wear of surgical blade after incising gingival tissue. The blades, needles or scissors blades are usually relatively soft and may be deformed greatly during mechanical cutting soft tissues, especially the needles [133]. The deformation control of the surgical instruments was the core technology of precision treatment, reviewed in Ref. [134]. During the cutting process, biological components such as biological cells, severed fibers and blood can. adhere to the surface of the surgical instruments, i.e., tissue adhesion. The surgical scissors and sucker were found to have a large amount of tissue adhesion, as shown in Figure 9(e) [135]. It is almost impossible to completely remove residual protein from surgical instruments by disinfection [136]. Blades with high surface roughness affected the faster recovery of structure and function after peripheral nerve transection [110]. Liu et al. [137] proposed a cutting durability decay model based on sharpness index and a failure model of surgical blade when cutting soft tissue. Tissue adhesion wear is the main failure of surgical blade in soft tissue cutting which caused the deterioration of the sharpness and surface roughness, and eventually causes the failure of the cutting tool.
Rust and corrosion of metal surgical instruments after high-temperature sterilization is a common phenomenon, as shown in Figure 9(c). Although the coating technology can improve the wear resistance and rust resistance of surgical instruments, the peeling of the coating is also its disadvantage. As shown in Figure 9(f), the coating of surgical blade began to peel off after cut 25 cm length.
So far, there has not been an in-depth study on the failure mechanism of surgical instruments cutting soft biological tissues, the law of life, and the methods of improving cutting life.
3.3 Tool Optimization
Soft tissue has a complex multi-layer structure, anisotropic viscoelastic mechanical properties, and easily damaged biological activity. Due to the complexity of the human body system and the diversity of surgical methods, the manufacturing and design techniques of surgical instruments are changing day by day. As shown in Figure 10, the optimization research of surgical instruments can be divided into three parts: innovative tool structure, microstructure on edge and surface and assisted cutting technology, to sort out the current situation of surgical instruments for soft tissue processing.
3.3.1 Innovative Tool Structure
After obtaining the general geometric model of surgical instruments [45, 88,89,90], the structure parameters of various instruments can be unified. It is a traditional effective method to optimize these structure parameters according to the cutting characteristics of soft tissues. Table 3 summarized the influence of structure parameters such as diameter, bevel angle, inclination angle, normal rake angle and edge number on the force of soft tissue cutting. Choosing the right structure parameters would reduce the cutting force and improve the efficiency of surgical cutting. At the same time, optimizing the structure parameters could reduce tissue damage during cutting. Determining the most advantageous size of liposuction cannula and injection needles in terms of adipocyte viability could help to increase fat graft survival [116]. When puncturing brain, faster insertion of sharp surgical instruments resulted in lower mean effective strain damage [119]. The cracks caused by the needle with the three cutting edges made the wound more difficult to heal [44]. When cutting the vitreous body of the eye, the cutting efficiency with a double-edged blade was higher, and it did not significantly affect the liquid pressure in the vitreous body [142]. The optimization of the structure parameters is an effective optimization method, but it needs to be based on a large amount of reasonable experimental data, and the workload is very large.
Surgical instruments include blades and handles. The handle is an operating part for the surgery to perform the functions of the surgical instrument. A comfortable and convenient handle can reduce the difficulty and risk of surgery. Ergonomics can help improve the design of tool handles and there is room for improvement in the ergonomics of the handle [154]. Martin [155] designed an ergonomic scalpel handles with optimized weight and balance (Figure 11(a)). Gonzalez et al. [156, 157] optimized the handle size of a laparoscopic grasper tool shown in Figure 11(b), which was based on the relationship between the size of the surgeon's hand and the perceived optimal diameter of the handle from an ergonomic point of view. A new handle for laparoscopic surgical instruments is proposed by Sancibrian et al. [158]. The opening and closing of the handle were performed by the thumb through the lever located on the top of the handle ((Figure 11(c))). Although the number of relevant literature is relatively small, the improvement of the handle based on ergonomics is always in progress.
Multifunctional surgical instruments are mainly used in minimally invasive surgery such as arthroscopic surgery and laparoscopic surgery. Use of endoscopic staplers that had cutting function and suture function reduced hemostasis-related complications, reduced hospital costs and improved efficiency in VATS lobectomy [159]. Suture Passer was used in repairing the meniscus which had the functions of holding, puncturing, and recovering sutures. When repairing the meniscus, suture passer will be used. It has the functions of holding, puncturing, and recovering sutures [160]. The multifunction surgical instrument has a great advantage in improving the efficiency of surgery, which perform multiple operations with one instrument.
3.3.2 Microstructure on Edge and Surface
The strategies for optimizing the cutting structure and surface of the blade mainly include bionics, surface texture, and microblade/microneedle. The bionic structure applied to the cutting tool is an effective method to reduce the cutting force of biological tissue. There was a 10%‒25% decrease in the insertion force for insertions into bovine brain, and a 35%‒45% reduction in the insertion force for insertions into bovine liver using the proposed bioinspired needles with specially designed barbs [161]. Izumi et al. [147] proposed the combined silicon microneedles comprising a central straight needle and two outer jagged needles imitating the mosquito’ s proboscis shown in Figure 12(b). However, the silicon needle is not safe enough on the human body owing to its brittleness. Scali et al. [162] designed a needle that advanced through straight and curved trajectories in a soft substrate without being pushed, without buckling, and without the need of axial rotation. Lu et al. improved the cutting performance of the scalpel by imitating the micro-structures of miscanthus leaves shown in Figure 12(d). Compared to the unprocessed commercial scalpel, the micro-serrated scalpels could significantly reduce the cutting force and actual cutting depth. The bionic structure can sometimes achieve particularly excellent results, but the mechanism of its various structures needs to be further studied.
The surgical instruments can obtain some useful surface functions after surface treatment technology, such as anti-adhesion, wear reduction, and antibacterial. The surface treatment technology of medical device had been reviewed in Refs. [163, 164], so this paper did not introduce it. It is worth noting that surface treatment technology has not been widely used in surgical instruments because it is difficult to produce effective microstructures on curved surfaces.
The microblade on the tool performed micro-cutting on the soft tissue, resulting in lower force, more stability, and less tissue damage. Zhang et al. [150] designed a micro/nano-particle decorated metal wire for cutting soft tissue under lower cutting force. Giovannini et al. [145] manufactured micro serrations on the cutting edge by using a picosecond laser and achieved a reduction of 20%‒30% in the insertion forces. Micro-blade knives are now mainly combined with high-frequency vibration to improve cutting efficiency.
3.3.3 Assisted Cutting Technology
Traditional surgical instruments for mechanically cutting soft tissues are being combined with some assisted technologies to improve the effect of surgical treatment, such as vibration assisted technology, low pressure suction assisted and drug assisted technology. Vibration-assisted technology refers to the use of high-frequency vibration to drive surgical instruments to reciprocate cutting soft tissues. It is especially suitable for surgical instruments with microblade structures. Vibration-assisted technology is the most mature application in orthopedic swing saws. In recent years, vibration-assisted technology has also been applied to soft tissue cutting. Vibration assisted technology promotes the development of soft tissue micro-cutting technology.
When processing soft biological tissues, especially free or ruptured soft tissues, it is difficult for the tool to cut the soft tissues neatly, resulting in tearing and scratching. Low pressure suction assisted technology effectively solves this problem. The soft tissue is cut neatly under the action of negative pressure suction. A side-cutting aspiration device for endoscopic and microscopic tumor removal was presented [165], which allowed for tumor removal without injury to adjacent neuro vascular structures. The multifunctional nature of the instrument (suction, scissors, and dissectors) minimizes multiple exchanges, facilitating tumor resection through these minimal access corridors. Neuronavigation-guided resection of tumors with a variable-suction tissue resection device resulted in gross-total or near-total resection [166]. Low pressure suction assisted technology has a broad application prospect in soft tissue cutting.
Drug-assisted technology combines the advantages of devices and drugs. In thrombectomy, the heparin coating on the surface of the thrombus grinding head can effectively reduce the risk of blood clotting. Microneedles can deliver small amounts of drugs under the skin to achieve precise drug treatment. It is believed that drug-assisted surgical instruments will be an important research direction in the future.
4 Nonconventional Cutting of Soft Tissue
4.1 Laser Ablation of Soft Tissues
Laser was first used as a surgical tool shortly after its invention [167] and now has been applied in widely application in manipulating soft tissue, including tissue cutting, ablation, soldering for oral cavity, This includes different lasers, e.g., CO2, diode, and Er: YAG laser, as well as different pulse durations, e.g., continuous wave laser, microsecond, nanosecond and picosecond pulse laser. Compared to the conventional mechanical cutting process (Figure 13(b)) with producing shear forces which exceed the elastic limit of the tissue, the laser cutting technology (Figure 13(c) and (d)) can significantly reduce the mechanical trauma while automatically sterilize and coagulate the tissue surface and reduce the bacteremia with a faster healing response [168]. In this section, a detailed review of laser cutting of soft tissue will be presented.
Schematics of cutting mechanism of soft tissue: (a) Wavelength-dependent water absorption spectrum of laser energy [169]; (b) The mechanical scalpel cutting by shearing; (c) Conventional long pulse lasers cut by depositing heat with melting or burning; (d) Short pulse laser by vaporization and ionization [173]
4.1.1 Laser Ablation Mechanism of Soft Tissues
Lasers, when used for direct cutting, can manipulate the biological tissues from macroscale (e.g., organs) to microscale (e.g., single cell) with a high energy intensity beam focused onto the tissue surface, i.e., high spatial resolution. While most soft tissues are dominated by water (55%‒99%) and collagen (0‒35%) by mass, the water-laser interaction and laser energy distribution in tissues plays a significant role in the soft tissue ablation. As shown in Figure 13(a), the laser-water interaction mainly depends on the laser wavelength. Specifically, the absorption/penetration depth in water for the CO2 laser wavelength (10, 600 nm) is 0.01 mm while the diode laser wavelengths (800‒1000 nm) can reach a thousand times greater than that for CO2 laser [169]. In this process, the mechanism of laser-tissue interactions can be categorized as thermal, photochemical, plasma induced, photo-mechanical, or a combination mechanism whereby the laser energy can be effectively absorbed by soft tissues [170]. These occurrences depend on the laser parameters such as wavelength, pulse duration, and pulse fluence (i.e., pulse energy per unit area) [171]. As shown in Figure 13(c) and (d), depending on the difference of pulse duration, two main ablation mechanisms can be defined, i.e., thermal induced and plasma induced ablations. The main laser ablation mechanism of soft tissues can be surmised as below.
Thermal induced ablation mechanism: This usually occurs when apply the long pulse laser ablation (i.e., > nanosecond) or continuous wave laser ablation. In this process the tissue will absorb the photon energy and lead to melting, vaporization and explosion, as shown in Figure 14(a). Specifically, as the water inside the tissues has the lowest phase transition temperature it normally undergoes phase explosions whereby the hot pressurized vapour can remove the tissue and form a ablation plume [172]. As shown in Figure 14(b), this ablation mechanism requires high energy density to vaporize the material hence leads to an explosion feature on the ablated surface and severe thermal damage around it. Moreover, the expansion of the ablation plume into the surrounding air also leads to a generation of shockwave that can introduce the mechanical influence of the subsurface. Hence, this high energy induced thermal effect and shock wave can introduce tissue damages that extend well beyond the ablation zone. In general, in conventional surgical lasers, having pulse durations longer than nanoseconds, can impair the proliferative phase of healing due to the severe cell damage in the surrounding tissue.
Plasma induced ablation mechanism: When an ultrashort duration pulse laser is applied, on one hand, the high peak power of pulse laser can cause multiphoton ionization in tissue whereby the thermalization is mainly depended on recombination processes and electron-phonon inelastic collisions within 10‒100 ps range at nanometer (molecular) dimensions; on the other hand, due to the short pulse duration, e.g., < picosecond, the heated water inside the tissue can rapidly eject the tissue from the surface without diffuse the heat energy into the surrounding tissue, as shown in Figure 15. In this case, by achieving superheating on picoseconds timescales, the nucleation sites occurs in the molecular dimensions during the ablation phase transition, e.g., under nanometer scale, which can avoid the cavitation and associated shockwave induced damage that has been one of the major stumbling blocks in using lasers for surgery [173]. Hence, compared to long pulse ablation, during the ultrashort pulses ablation, e.g., picosecond timescale, the water inside the tissue can be superheated and eject the tissue faster than the energies that can diffuse to the surroundings area [173], and leads to a minimal damage remaining in the adjacent tissue, as shown in Figure 15.
Surface damage in Descemet’s membrane in the cornea produced by (a) 6-ns laser pulses of 1064-nm wavelength and (b) by 30-ps laser pulses [175]
4.1.2 Surface and Subsurface Damage in Laser Ablation of Soft Tissues
The extent of surface damage in laser cutting of soft biological tissue is of importance to protect the patient from long time wound healing [171]. The surface damage in general is related to the laser parameters such as power, wavelength, cutting speed and pulse duration. In general, the laser cutting mainly cause thermal damage at an approximately uniform thickness surrounding the ablation area, as shown in Figure 16. This is normally presented as a combined phenomenon including: coagulation with cell swelling, degeneration to a liquid state with loss of cell structures (colliquation), and carbonization caused by tissue overheating [176].
Histological specimen cut by a 445-nm semiconductor laser: (a) Cell swelling and (b) Colliquation [176] (×200)
While the surface damage mechanism of laser cutting of soft tissue is mainly governed by the thermal effect, the short pulse laser can significantly reduce the surface damage due to the fast cutting process (e.g., short pulse duration) that thermal energy cannot penetrate into the surrounding tissues. Saeid et al. investigated the surface damage mouse skin from picosecond laser (short pulse), conventional surgical long pulse laser and mechanical scalpel cutting method [171]. As shown in Figure 17, it is revealed that the surrounding zone of short pulse laser cut tissue is intact while the surface damage of long pulse laser can reach up to 800 µm away from the ablated edge with burning and deforming the surrounding tissue. The mechanical scalpel, however, damages the surface by shearing the tissue and exposing individual cells, and eventually causes dissociation of extracellular matrix fibers up to 400 mm further from the edge.
On the other hand, while the soft tissues show a various absorption ability under different wavelengths of the lasers, their laser ablation surface damages can also show a significant difference [176]. Jiang et al. investigated the laser ablation of porcine bladders with both blue laser (450 nm wavelength) and green laser (532 nm wavelength). It is founded that while the blue green lasers show a similar considerable vaporization and coagulation mechanism, the green laser demonstrated a low tissue penetration compared with blue laser at different cutting speeds (sweeping speed 0.5–2.0 mm/s; working distance 1–3 mm), as shown in Figure 18. This demonstrates that the blue laser can interact with tissues more precisely and has a low thermal damage to adjacent tissues [177].
Histological images of porcine bladder tissue treated with blue laser and green laser at 30 W and at stage speed of 0.5–2 mm/s and 1 mm working distance [177]
4.1.3 Laser Ablation Surgery Devices
The surgical laser cutting/ablation devices have already been well developed and applied in many surgery occasions, and with different lasers, e.g., CO2, diode, and Er: YAG laser, as well as different durations, e.g., continuous wave laser, microsecond, nanosecond and picosecond pulse laser, and various wavelengths. Normally the diode laser requires less energy compared to CO2 and Er: YAG lasers due to the higher water absorption spectrum. In general, the lasers can be used as a beam scalpel for cutting the soft tissue without contacting the tissue, e.g., work distance at 1‒3 mm (at high energy density), or coagulate the tissue at a higher working distance, e.g., 6‒9 mm (at low energy density), as shown in Figure 19. This can be applied either manually by the surgeons or automatically through the robot- and computer assisted systems which can provide the accessibility of teleoperation, i.e., a non-contact endoscopic surgery solution, as shown in Figure 19(b). On the other hand, while the soft tissues demonstrate weak laser abortion capability at non-contact operation, to increase the cutting efficiency of lasers in the surgery, special tips (e.g., hot tip, metal probe, converter and cork-initiated tip) can are mounted at the distal end of optical fiber delivering laser radiation to biological tissue, as shown in Figure 19(c).
4.2 Waterjet Technology in Soft Biological Tissue Manipulation
Waterjet machining was first introduced to the industry as a new cutting tool in the early 1970s after the publication of Norman Franz’s patent [183]. It is used for cutting a wide range of materials [184]. Using a waterjet to prepare soft tissue is already applied in various surgical fields, with several advantages of this technique, e.g., less intraoperative haemorrhage and no thermal damage compared to other common methods [185].
4.2.1 Application and Principle of the Waterjet Processing of Soft Tissues
(1) Waterjet application in soft tissue surgery
Papachristou and Barters [184, 186]were the first who described the usage of the jet in medicine. Four patients had a liver resection through physiologically saline (Figure 20(a) [187]). They concluded that the usage of the waterjet led to the reduction of blood loss during the surgery. Thereafter, the application of waterjet in liver surgery continues to expand, Yoshie Une et al. [188, 189] and Baer et al. [190] applied the waterjet to remove the cirrhosis, hepatocellular carcinoma, and solid hepatic tumours, the preliminary results suggest that the exposed elastic intrahepatic vessels are spared injury. Rau et al. [191] applied the waterjet dissection technique in open (309 patients) and in laparoscopic (41 patients) liver surgery, the results suggested that it was possible to reduce the blood loss, the Pringle and resection time in comparison to CUSA and blunt dissection.
Waterjet technology was also applied in surgery of other internal organs. It was used in animal renal surgery by Hubert et al. [197], Basting et al. [198], and Alireza Moinzadeh et al. [199], all the results demonstrated that the waterjet resection tissue was dissected effectively avoiding damage to the vascular structures. Encouragingly, Basting et al. [192] reported about first clinical experiences with waterjet resection device in kidney surgery. It has been shown to reduce the risk of blood loss significantly, and the higher magnification shows a microscopically normal glomerulus in direct contact to the zone of vacuolization (Figure 20(b)). It was also applied in laparoscopic cholecystectomy [200], testis-Cancer dissection [201], and Spleen Resection [193] (Figure 20(c)), where all the results show the small damage to the tissue by the waterjet, and reflects the gratifying application value.
In addition to its application in internal organs, waterjet technology was also applied in surgery of brain tumour and wound treatment. In brain tumor surgery, the waterjet has been applied by Piek et al. [202], Oertel et al. [203], enabled tumour debulking by aspiration and more important precise separation of tumour and brain parenchyma. The waterjet was used to separate the galea and the dura mater by Christoph et al. [194] (Figure 20(d)), and the experimental and clinical data shows that waterjet separation of dura mater, dural substitute, and galea can be performed with a high level of safety to avoid dural tears. The waterjet technology applied in wound treatment was reported by Nadi Atalla [195] (Figure 20(e)), Vanwijck et al. [204], Rennekampff et al. [205], and Raffi Gurunluoglu [196], it has been shown hydro surgery has the main advantages its tissue selectivity and its high percentage of successful engraftment after immediate skin grafting.
(2) Principle of waterjet soft tissue surgery
According to Shekarriz Hodjat et al. [200], the principle of waterjet dissection in organs can be explained in Figure 21(a) (the schematic of the anatomical principle of gallbladder waterjet [200]). The application of water around and below the serosal layer causes the sub-serosal space to expand and form a surgical plane. During the dissection of the organs and tissues, the thin and high-pressure water flow selectively separates the surrounding connective tissue components from the blood vessels and organs. After removal of the organ, the remaining organization and around organs were almost free of bleeding and subsequent hemostasis was not necessary. Furthermore, continuous water flow allowed to clean the tissue debris and allowed a clear view for the operator.
Figure 21(b) (Raffi Gurunluoglu [196]) shown the principle of waterjet application in wound treatment. Sterile saline flows to the electric console, where it is pressurized. The sterile saline is accelerated to high speed and forced under high pressure into an angled, sterile and disposable handpiece. The saline stream passes through the hole at the end of the handpiece and is directed backwards and immediately sucked into the collecting tube. This creates a local Venturi effect, which allows to grasp, cut and remove the target tissue at the same time. When the hole is held more obliquely, a softer suction effect is obtained.
4.2.2 Potential Complications and Risks of Waterjet Surgery
The tissue selectivity of waterjet resulted in an improved anatomic dissection and decreased the complication rate. It uses sensitivity to the pressure of sterile saline to resect tissue. It allows control of a flow directly into the saline, directly into the workspace. The sensitivity of the saline jet varies in dependence on the type of shared tissue (nerve tissue, ligaments, blood vessels, etc.).
However, there are still some risks, for instance, the accumulation of water may obstruct the view of the wound ground, hindering surgeons from evaluating their progress; the high-pressure waterjet disperses pathogens and debris into the air, potentially creating a risk of infection for the operating surgeon [206]. For the clinical application of waterjet technology in soft biological tissue, further methods and experiments are needed to improve this.
4.2.3 Waterjet Surgery Device
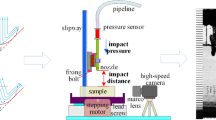
A waterjet device usually consists of three parts (Figure 22(a)): a pressure unit, a flexible connective unit, a handpiece and needle nozzles [207]. For different surgeries, the requirement of the surgeries, different handpieces were developed (Figure 22(e)‒(g) [185, 201, 207]), which could generate a coherent straight or helically turned waterjet. A handpiece applicator with an integrated suction tube was also designed to clear the surgery area (Figure 22(h))[186]. Further to provide the operators with a good view, the touch screen display was equipped and integrated into the operating system (Figure 22(b)) [208]. To reduce the infection risk of operators, the robotic system was developed (Figure 22(c) and (d)) for conducting the surgeries and cleaning the wound debridement, and the experimental results are encouraging. The waterjet technology in the medical area is moving towards automation.
4.3 Ultrasonic Cutting of Soft Tissues
The ultrasonic instruments were used to cutting and emulsify soft biological tissue in surgery [209]. Ultrasonic surgical instruments are safer and more effective than other instruments, which have the advantage of the precise control, accurate and high quality cut, reduced cutting forces, limited debris, reduced bleeding and avoidance of surrounding tissue damage [210]. Recently, Wang et al. developed a novel ultrasonic cutting system incorporating in a microtome for histology. The introduction of ultrasound-assisted cutting in the microtome can help tp obtain high-quality ultra-thin tissue sections [211].
4.3.1 Ultrasonic Effects of Soft Tissues
Ultrasonic cutting of soft tissue was often used in cataract surgery. The mechanism of ultrasonic cutting were both mechanical action and cavitation at the tip of the ultrasonic tool [212]. And the thermal effects and generation of microcurrents may occurred at the cellular level [213]. The mechanical effect was used to cutting soft tissue, while the thermal effect of ultrasound is mainly to coagulate the soft tissue. Therefore, the mechanical and thermal effects should be balanced for better cutting soft tissues and less tissue damage [214]. It was reported that the ultrasonic effects in soft tissues were predominantly related to mechanical impact of direct shear, pressure wave components and acoustic streaming [215,216,217]. The main effects of ultrasonic vibration on soft tissue are mechanical shearing effect, thermal effect and acoustic cavitation effect, and the secondary effects include streaming.
4.3.2 Cutting Mechanism of Ultrasonic Surgery
In the applications of ultrasonic cutting, blades/tips are made in different shapes for plunge and shear cuttings. Generally, a cutting blade operating at shear cutting mode is used in most surgical applications.
In the ultrasonic cutting process (shear cutting mode), the blade is vibrated at a frequency of \({f}_{1}\) and an amplitude of A with a clearance angle of \({\theta }_{1}\). The trajectory of the blade to the sample block can be expressed
where z direction and x direction are parallel and perpendicular to the cutting direction respectively and \({v}_{1}\) is the speed of the block moving.
The relative motion speed of blade to the block is
The cutting can be divided into separating type and non-separating type. When vz > 0, the blade is separated from the section and the block in each cycle of the vibration called the separating type UAC. When vz < 0, the blade is contacting with the section and the block in each vibration cycle called non-separating type UAC.
Figure 23 provides a schematic illustration of the trajectory. In each cycle of the vibration, the blade starts to cut the block at the point of 1 and a section is generated. Then, the blade is reaching to the lowest vibration point 2, where the horizontal and vertical vibration speeds are zero. From the point 2 to 3, there are contact between the section and the blade due to vz < 0. But the rake face of the blade starts to moving upward in the vertical direction. The friction direction between the blade rake face and the section is reversed as compared with it in conventional cutting. This reverse of the friction could assist to pull out the section away from the block. Due to the rake face of the blade pulls the chip upward, a more straighten less curled section could be formed. The blade separates from the section at point 3, when vz > 0 at the blade retraction [211].
Thus, the ultrasonically cutting could reduce cutting forces, form smooth sections and promote the section removal [211].
4.3.3 Ultrasonic Surgery Devices
The ultrasonic surgical instrument were used to precise cutting soft tissue [218] and improved haemostasis [219, 220]. The hemostatic effect during ultrasonic cutting of soft tissue is of great significance in minimally invasive surgery [221]. There are two main types of ultrasonic instruments used for soft tissue cutting or ablation including ultrasonic aspirators (Figure 24(a)) and ultrasonically activated dissecting and coagulation devices with sharper tips operating (Figure 24(b)). The ultrasonic aspirators are used to removing cataracts in the eye and debulking solid tissues for neurosurgeries. The ultrasonically activated dissecting and coagulation instruments were applied to directly cutting and coagulating tissue in general or laparoscopic surgery. The novel ultrasonic system used for microtome cutting is also illustrated below to show the recent advances of ultrasonic cutting (Figure 24(c)).
5 Anastomosis and Reconstruction
Anastomosis is a surgical procedure to rebuild normal physiological structure and functions. In the past, the most common technique is needle-based suturing and sutures for biological tissues anastomosis. However, this method needs high operational skills and long operation time, which also increase risks of intraoperative infection.
Nowadays mechanical stapling is widely used in surgery with various staplers to connect soft biological tissues, such as stomach and intestine, etc. In this anastomotic process, tissues are compressed in two jaws, and connected by titanium staples, and then the healing process carries on gradually with acute inflammatory phase, and ultimately remodeling or maturation phase.
Recently powered stapler has been developed for robotic surgery. Wang et al. [223] designed a powered stapler with simplified mechanical structure and optimized current procedure, and the evaluated performances were proved that this novel stapler could control pressure transmission which helps to reduce excessive mechanical damage. However, the titanium staples remain inside tissues after surgical operation, which may cause inevitably inflammatory action and severely influence on tissue recovery.
The energy-based anastomotic technology without additional material intervention attracts increasing attentions, such as radiofrequency tissue welding technology. Due to tissue bio-impedance, heat can be accumulated with higher energy and longer welding time. The heat-induced collagen biological chemical reactions could help to create a uniform anastomosis. Due to no additional material leaving in body, energy-based welding exhibits significant advantages of non-rejection and non-inflammation, which can promote healing process after operation. However, tissue welding technology has not been matured yet in clinical applications, more research is needed to increase biomechanical strength in welding zones and reduce excessive thermal damages.
The energy controlling, closed-loop control system with electrical impedance signal as feedback, and welding electrode optimization are the key technology for anastomosis success with satisfied biomechanical strength. Winter et al. [224] designed a welding electrode with end-to-end type to anastomosis colons and explored the effect of welding parameters. Inspired by this electrode, Zhao et al. [225] developed and compared two “end-to-end” electrodes, and found the electrodes with reciprocating concave–convex configuration exhibits larger and faster thermal diffusion profiles than that with smooth surface (Shown in Figure 25). Furthermore, it is generally believed that heating is required for separated tissues to obtain strong and reliable bonding. However, exceeding high temperature may cause thermal biological damage in tissues. To better control thermal diffusion and excessive thermal damage, Tu et al. [226] developed a control algorithm for energy-induced tissue welding, which provides more options in welding parameters to achieve satisfied anastomosis with controllable thermal performance.
(a) Welding electrode with smooth surface (A1‒A3) and electrode with reciprocating concave–convex configuration (B1‒B3); (b) HE photographs of intestinal tissues with the effect of CC electrodes (top and right bottom) and S electrodes (left bottom) [225]
Ideal anastomosis technology will help tissues to reconnect and recover its philological functions, leaving no additional materials inside. It is still under development to realize such a dreaming technology with improved biomaterials, mechanical design and control algorithms. The fundamental research in biophysical and biochemical properties are the keys to deep understanding anastomosis process, in particular the theoretical research of micro-structure and micro-mechanism of tissue welding, which will be a great help to design better anastomotic devices and achieve better clinical performances.
Structural and functional reconstruction is key parameter to assess the post-surgery efficacy after anastomosis, which is also a major consideration on the anastomotic technology selection. Due to almost all thermal, mechanical, and electric-based anastomotic technologies have to damage the health tissue to get the desire the results. For example, the thermal welding is inevitable to cause the cell dysfunction or stenosis sometime due to the temperature elevation. The needle-based suturing forces the tip to penetrate the tissue, which leaves a scar. The electric-based technology also induces the cell dysfunction when the electricity passes through the tissue.
The evaluation of the structural and functional reconstruction in clinically has three main factors, which are blood supply, tissue viability, and function recovery. The blood supply can bring the nutrition for the cell growth, which can increase the metabolism. Tissue viability is a growing speciality that primarily considers all aspects of skin and soft tissue wounds including acute surgical wounds, pressure ulcers and all forms of leg ulceration. The microstructural of the tissue is also an indicator for the reconstruction.
6 Conclusions
Biological soft tissues manipulation, including mechanical or nonconventional processes, is critically required in most surgical innervations. The soft tissues are with high viscoelasticity, anisotropy, heat sensitivity and regeneration, and contain high percentage liquids inside the structure, which are in general difficult to manipulated. Thus, understanding the manipulation processes and the resulted surface damage mechanism is of importance to the community. This paper reviews the state-of-the-art of soft tissue manipulation processes in surgery, including mechanical, laser and waterjet cutting and advanced anastomosis and reconstruction process. It can be concluded that while the most widely applied conventional mechanical cutting and anastomosis processes are of high maturity, the nonconventional manipulations processes such as laser and waterjet cutting, energy-based anastomotic and thermal welding are also drawing increasing attention. The major conclusion can be summarized.
-
(1)
There are differences in the composition and structure of different soft tissues, which will have an important impact on soft tissue manipulating. The distribution of elastic fibers and connective tissue in the soft tissue affects the crack propagation and cutting of the soft tissue. The interface between cells or the direction perpendicular to the fiber is the direction of microcracks’ initiation and propagation. Soft tissues are more sensitive to thermal damage and have certain differences among species.
-
(2)
The current research mainly focuses on soft biological tissue deformation, cutting force modeling and tissue damage in soft tissue mechanical cutting, especially puncturing skin and liver with a needle. The cutting force model contributes to the mechanical feedback of the surgical robot system and the virtual reality surgical training system. However, it is worth noting that the relationship between the cutting behaviors and microstructure of soft biological tissues, and the crack propagation in soft biological tissues are still seldom studied. There is a lack of scientific explanation of soft biological tissue cutting behavior.
-
(3)
In most researches, mechanical cutting of soft tissues causes relatively little damage, but there are still biological tissue damages, such as apoptosis, hemorrhage, tissue loss, and deformation. At present, the research on soft tissue damage is still in the observational stage, and there is no related literature to study the mechanism of how the tool causes soft tissue damage. In addition, the impact on the biological body should be considered during the mechanical cutting process, such as the backflow of liquid along the puncture path of the brain tissue. The microstructure of the cutting edge after cutting soft tissues has been observed to break and wear, but the wear mechanism and factors of the cutting edge after cutting soft biological tissues are still unclear. The wear of the blade is an important factor that affects the reusability of surgical instruments.
-
(4)
Many researches on tool optimization for mechanical cutting of soft tissues have been carried out from the aspects of innovative tool structure, microstructure on edge and surface and assisted cutting technology, which obtained smaller cutting force, higher efficiency or lower tissue damage. However, the principle of tool optimization needs to be studied deeply but is often overlooked.
-
(5)
In laser ablation of soft tissues, the surface damage normally presents as a combined phenomenon including: coagulation with cell swelling, degeneration to a liquid state with loss of cell structures (colliquation), and carbonization caused by tissue overheating. These are induced by two main cutting mechanisms of laser cutting of soft tissues. That is, in long pulse and continuous wave laser cutting the ablation mechanism is mainly induced by thermal effect whereby a high surface and subsurface damage can be found due to the accumulated high thermal energy as well as the shockwave introduced during the water explosion. In ultrashort pulse laser ablation of soft tissue the material removal mechanism is mainly occupied by plasma induced ablation, whereby the water inside the tissue can be superheated and eject the tissue faster than the energies that can diffuse to the surroundings area, and hence leads to a minimum surface damage of the tissue.
-
(6)
The ultrasonic cutting instruments simplifies difficult operations, minimizes intraoperative blood loss, reduces tissue damage, improves surgical vision with absence of smoke, and reduces cost and time by combining grasping, cutting and coagulation into an integrated tool set and intraoperative equipment replacement. The main effects of soft tissue to ultrasonic vibration are mechanical shearing effect, thermal effect and acoustic cavitation, while secondary effect includes streaming.
-
(7)
Anastomosis and reconstruction are important surgical procedures to rebuild normal physiological structure and functions. Although mechanical stapling is widely used to connect soft biological tissues, energy-based anastomotic technology, such as radiofrequency tissue welding technology, which can be operated without additional material intervention, also attracts increasing attentions. Moreover, due to no additional material leaving in body, energy-based welding exhibits significant advantages of non-rejection and non-inflammation, which can promote healing process after operation.
References
J Rezaian, F Forouzanfar. Consideration on Trephinated skull in the Ŝahre-e Sukte (Burnt City) in Sistan. Journal of Research on History of Medicine, 2012, 1, https://rhm.sums.ac.ir/article_42868_5cbaecf64c968c4dbc16a9a58bafef58.pdf.
Merriam-Webster.com, ed. Medical definition of soft tissue. Medical Dictionary, https://www.merriam-webster.com/medical/soft tissue.
M Gridi-Papp. Human Anatomy, OpenStax CNX, 2018, http://cnx.org/contents/c29920ff-cf11-4c44-a140-046fb0e6e1f0@2.57.
M J Buehler, T Ackbarow. Fracture mechanics of protein materials. Materials Today, 2007, 10: 46–58.
A Pissarenko, W Yang, H Quan, et al. The toughness of porcine skin: Quantitative measurements and microstructural characterization. Journal of the Mechanical Behavior of Biomedical Materials, 2020, 109: 103848.
P Sabouri, A Hashemi. Influence of crack length and anatomical location on the fracture toughness of annulus fibrosus. Medical Engineering & Physics, 2021, 88: 1–8.
G A Von Forell, P S Hyoung, A E Bowden. Failure modes and fracture toughness in partially torn ligaments and tendons. Journal of the Mechanical Behavior of Biomedical Materials, 2014, 35: 77–84.
B Gogly, G Godeau, D Septier, et al. Measurement of the amounts of elastic fibers in the skin and temporal arteries of healthy aged individuals by automated image analysis. Gerontology, 1998, 44: 318–323.
S Bose, S Li, E Mele, et al. Fracture behaviour of collagen: Effect of environment. Procedia Structural Integrity, 2020, 28: 843–849.
K Bircher, R Merluzzi, A Wahlsten, et al. Influence of osmolarity and hydration on the tear resistance of the human amniotic membrane. Journal of Biomechanics, 2020, 98: 109419.
Z Hu, W Sun, B Zhang. Characterization of aortic tissue cutting process: experimental investigation using porcine ascending aorta. Journal of the Mechanical Behavior of Biomedical Materials, 2013, 18: 81–89.
Y Qi, J Caillard, R Long. Fracture toughness of soft materials with rate-independent hysteresis. Journal of the Mechanics and Physics of Solids, 2018, 118: 341–364.
B Chu, E Gaillard, R Mongrain, et al. Characterization of fracture toughness exhaustion in pig aorta. Journal of the Mechanical Behavior of Biomedical Materials, 2013, 17: 126–136.
PA Hasgall, F Di Gennaro, C Baumgartner, et al. IT'IS Database for thermal and electromagnetic parameters of biological tissues. Version 4.1, Feb 22, 2022, DOI: https://doi.org/10.13099/VIP21000-04-1.
H P Kok, A N T J Kotte, J Crezee. Planning, optimisation and evaluation of hyperthermia treatments. International Journal of Hyperthermia, 2017, 33: 593–607.
J Zhang, J Hills, Y Zhong, et al. Modeling of soft tissue thermal damage based on GPU acceleration. Computer Assisted Surgery, 2019, 24: 5–12.
K Giering, O Minet, I Lamprecht, et al. Review of thermal properties of biological tissues. Proceedings of SPIE - The International Society for Optical Engineering. 1995: 45–65.
Jason B Brill, Evan K Harrison, Michael J Sise, et al. The history of the scalpel: From flint to zirconium-coated steel. The American College of Surgeons (ACS) Clinical Congress 2017, 2017: 13–17.
Z Liu, C Wang, Z Chen, et al. The advance of surgical blades in cutting soft biological tissue: A review. The International Journal of Advanced Manufacturing Technology, 2021.
S Luo, X Hu, C Wang, et al. Diamond application in medical field. Diamond & Abrasives Engineering, 2018, 38: 1–7.
G Wang. Endarterectomy for restenosis after internal carotid artery stenting. n.d., http://www.zzsqy.com.cn/OfficeNewsDetail-211.html.
Meniscal CinchTM All-Inside Repair. n.d., https://www.arthrex.com/knee/meniscal-cinch-allinside-repair/resources.
S Chandra, I Podder, M Chatterjee, et al. Anatomy and applications of the #15 scalpel blade and its variations. Journal of Cutaneous and Aesthetic Surgery, 2018, 11: 79.
S K R Rai, C Mancarella, A H Goel. Brain tumor interface dissection technique with surgical blade from laboratory to neurosurgical operating room. World Neurosurgery, 2017, 100: 601–606.
T W James, T H Baron. A comprehensive review of endoscopic ultrasound core biopsy needles. Expert Review of Medical Devices, 2018, 15: 127–135.
H Kim, H Park, S J Lee. Effective method for drug injection into subcutaneous tissue. Scientific Reports, 2017, 7: 1–11.
M Fujii, T Furumatsu, Y Kodama, et al. A novel suture technique using the FasT-Fix combined with Ultrabraid for pullout repair of the medial meniscus posterior root tear. European Journal of Orthopaedic Surgery & Traumatology, 2017, 27: 559–562.
J-H Chung, S-M Sohn, H-J You, et al. Use of a biopsy punch for end-to-side anastomosis in free-tissue transfer. Journal of Plastic Surgery and Hand Surgery, 2020, 54: 215–219.
Z Chen, Y Zhang, C Wang, et al. Understanding the cutting mechanisms of composite structured soft tissues. International Journal of Machine Tools and Manufacture, 2021, 161: 103685.
T Chanthasopeephan, J P Desai, A C W Lau. Determining fracture characteristics in scalpel cutting of soft tissue. First IEEE/RAS-EMBS Int. Conf. Biomed. Robot. Biomechatronics, IEEE, 2006: 899–904.
T Yang, H Yin, X Zhao, et al. Interaction modeling and simulation of a flexible needle insertion into soft tissues, ISR/Robotik 2014; 41st Int. Symp. Robot., 2014: 1–6.
J Pan, J Bai, X Zhao, et al. Real‐time haptic manipulation and cutting of hybrid soft tissue models by extended position‐based dynamics. Computer Animation and Virtual Worlds, 2015, 26: 321–335.
T Chanthasopeephan, J P Desai, A C W Lau. Measuring forces in liver cutting: New equipment and experimental results. Annals of Biomedical Engineering, 2003, 31: 1372–1382.
Y Xu, Q Zhang, G Liu. Cutting performance orthogonal test of single plane puncture biopsy needle based on puncture force. AIP Conf. Proc., AIP Publishing LLC, 2017: 30016.
M Mahvash, L M Voo, D Kim, et al. Modeling the forces of cutting with scissors. IEEE Transactions on Biomedical Engineering, 2008, 55: 848–856.
K T Miura, R U Gobithaasan, S Usuki. The polar-aesthetic curve and its applications to scissors design. Computer-Aided Design and Applications, 2015, 12: 431–438.
K Kendall, K N G Fuller. J-shaped stress/strain curves and crack resistance of biological materials. Journal of Physics D: Applied Physics, 1987, 20: 1596.
H Zhongwei. Experiment and theory research on cutting mechanism of biological soft tissue. Changsha: Hunan University, 2011. (in Chinese)
Z Hu, B Zhang, W Sun. Cutting characteristics of biological soft tissues. CIRP Annals - Manufacturing Technology, 2012, 61: 135–138.
C T McCarthy, M Hussey, M D Gilchrist. On the sharpness of straight edge blades in cutting soft solids: Part I–indentation experiments. Engineering Fracture Mechanics, 2007, 74: 2205–2224.
M Mahvash, P E Dupont. Mechanics of dynamic needle insertion into a biological material. IEEE Transactions on Biomedical Engineering, 2009, 57: 934–943.
L Barbé, B Bayle, M de Mathelin, et al. Needle insertions modeling: identifiability and limitations. Biomedical Signal Processing and Control, 2007, 2: 191–198.
J T Hing, A D Brooks, J P Desai. Reality-based needle insertion simulation for haptic feedback in prostate brachytherapy. Proc. 2006 IEEE Int. Conf. Robot. Autom., IEEE, 2006: 619–624.
T Azar, V Hayward. Estimation of the fracture toughness of soft tissue from needle insertion. Int. Symp. Biomed. Simul., Springer, 2008: 166–175.
T Yang, L Xiong, J Zhang, et al. Modeling cutting force of laparoscopic scissors. Int. Conf. Biomed. Eng. Informatics, 2010.
C Simone. Modeling of needle insertion forces for percutaneous therapies. Citeseer, 2002.
A M Okamura, C Simone, M D O’leary. Force modeling for needle insertion into soft tissue. IEEE Transactions on Biomedical Engineering, 2004, 51: 1707–1716.
D d’Aulignac, R Balaniuk, C Laugier. A haptic interface for a virtual exam of the human thigh. Proc. 2000 ICRA. Millenn. Conf. IEEE Int. Conf. Robot. Autom. Symp. Proc. (Cat. No. 00CH37065), IEEE, 2000: 2452–2457.
X Bao, W Li, M Lu, et al. Experiment study on puncture force between MIS suture needle and soft tissue. Biosurface and Biotribology, 2016, 2: 49–58.
T L de Jong, L H Pluymen, D J van Gerwen, et al. PVA matches human liver in needle-tissue interaction. Journal of the Mechanical Behavior of Biomedical Materials, 2017, 69: 223–228.
W Maurel. 3D modeling of the human upper limb including the biomechanics of joints, muscles and soft tissues. English Journal, 1999.
W Liu, Z Yang, P Li, et al. Mechanics of tissue rupture during needle insertion in transverse isotropic soft tissue. Medical & Biological Engineering & Computing, 2019, 57: 1353–1366.
S Jiang, P Li, Y Yu, et al. Experimental study of needle–tissue interaction forces: Effect of needle geometries, insertion methods and tissue characteristics. Journal of Biomechanics, 2014, 47: 3344–3353.
M Mahvash, P E Dupont. Fast needle insertion to minimize tissue deformation and damage. 2009 IEEE Int. Conf. Robot. Autom., 2009: 3097–3102.
S P DiMaio, S E Salcudean. Needle insertion modeling and simulation. IEEE Transactions on Robotics and Automation, 2003, 19: 864–875.
Y C Fung. Mechanical properties and active remodeling of blood vessels. Biomechanics, 1993.
J R Crouch, C M Schneider, J Wainer, et al. A velocity-dependent model for needle insertion in soft tissue. Int. Conf. Med. Image Comput. Comput. Interv., Springer, 2005: 624–632.
L Y Gao, Q H Zhang, M Liu. Orthogonal cutting of biological soft tissue with single cutting edge. Appl. Mech. Mater., Trans. Tech. Publ., 2012: 283–287.
J Z Moore, K Malukhin, A J Shih, et al. Hollow needle tissue insertion force model. CIRP Annals, 2011, 60: 157–160.
A G Atkins, X Xu, G Jeronimidis. Cutting, by ‘pressing and slicing,’of thin floppy slices of materials illustrated by experiments on cheddar cheese and salami. Journal of Materials Science, 2004, 39: 2761–2766.
C Richard, M R Cutkosky, K Maclean. Friction identification for haptic display. 1999.
H Yang, P X Liu, J Zhang. Modelling of needle insertion forces for surgical simulation. IEEE Int. Conf. Mechatronics Autom. 2005, IEEE, 2005: 592–595.
P Dupont, B Armstrong, V Hayward. Elasto-plastic friction model: contact compliance and stiction. Proc. 2000 Am. Control Conf. ACC (IEEE Cat. No. 00CH36334), IEEE, 2000: 1072–1077.
Y Kobayashi, T Sato, M G Fujie. Modeling of friction force based on relative velocity between liver tissue and needle for needle insertion simulation. 2009 Annu. Int. Conf. IEEE Eng. Med. Biol. Soc., IEEE, 2009: 5274–5278.
C Simone, A M Okamura. Modeling of needle insertion forces for robot-assisted percutaneous therapy. Proc. 2002 IEEE Int. Conf. Robot. Autom. (Cat. No. 02CH37292), IEEE, 2002: 2085–2091.
K Comley, N A Fleck. The toughness of adipose tissue: measurements and physical basis. Journal of Biomechanics, 2010, 43: 1823–1826.
B Takabi, B L Tai. A review of cutting mechanics and modeling techniques for biological materials. Medical Engineering & Physics, 2017, 45: 1–14.
P P Purslow. Measurement of the fracture toughness of extensible connective tissues. Journal of Materials Science, 1983, 18: 3591–3598.
N K Simha, C S Carlson, J L Lewis. Evaluation of fracture toughness of cartilage by micropenetration. Journal of Materials Science: Materials in Medicine, 2004, 15: 631–639.
C Gokgol, C Basdogan, D Canadinc. Estimation of fracture toughness of liver tissue: Experiments and validation. Medical Engineering & Physics, 2012, 34: 882–891.
A C Barnett, L Tan, J Barrett, et al. Needle cutting of skin simulants. ASME 2016 11th Int. Manuf. Sci. Eng. Conf., 2016.
B P Pereira, P W Lucas, T Swee-Hin. Ranking the fracture toughness of thin mammalian soft tissues using the scissors cutting test. Journal of Biomechanics, 1997, 30: 91–94.
P P Purslow. Positional variations in fracture toughness, stiffness and strength of descending thoracic pig aorta. Journal of Biomechanics, 1983, 16: 947–953.
D Taylor, N O’Mara, E Ryan, et al. The fracture toughness of soft tissues. Journal of the Mechanical Behavior of Biomedical Materials, 2012, 6: 139–147.
C T Koh, K Tonsomboon, M L Oyen. Fracture toughness of human amniotic membranes. Interface Focus, 2019, 9: 20190012.
K Tonsomboon, C T Koh, M L Oyen. Time-dependent fracture toughness of cornea. Journal of the Mechanical Behavior of Biomedical Materials, 2014, 34: 116–123.
M V Chin-Purcell, J L Lewis, T R Oegema, et al. Fracture of articular cartilage, 1996.
A K Miri, L X Chen, R Mongrain, et al. Fracture toughness of vocal fold tissue: A preliminary study. Journal of Voice, 2016, 30: 251–254.
O A Shergold, N A Fleck. Mechanisms of deep penetration of soft solids, with application to the injection and wounding of skin. Proceedings of the Royal Society of London, Series A: Mathematical, Physical and Engineering Sciences, 2004, 460: 3037–3058.
A C Barnett, Y-S Lee, J Z Moore. Fracture mechanics model of needle cutting tissue. Journal of Manufacturing Science and Engineering, 2015, 138, doi:https://doi.org/10.1115/1.4030374.
K Bircher, M Zündel, M Pensalfini, et al. Tear resistance of soft collagenous tissues. Nature Communications, 2019, 10: 792.
M Khadem, C Rossa, R S Sloboda, et al. Mechanics of tissue cutting during needle insertion in biological tissue. IEEE Robotics and Automation Letters, 2016, 1: 800–807.
S Singh, M-C Lo, V B Damodaran, et al. Modeling the insertion mechanics of flexible neural probes coated with sacrificial polymers for optimizing probe design. Sensors, 2016, 16: 330.
S Misra, K B Reed, A S Douglas, et al. Needle-tissue interaction forces for bevel-tip steerable needles. 2008 2nd IEEE RAS EMBS Int. Conf. Biomed. Robot. Biomechatronics, IEEE, 2008: 224–231.
S Misra, K J Macura, K T Ramesh, et al. The importance of organ geometry and boundary constraints for planning of medical interventions. Medical Engineering & Physics, 2009, 31: 195–206.
B L Tai, Y Wang, A J Shih. Cutting force of hollow needle insertion in soft tissue. Int. Manuf. Sci. Eng. Conf., American Society of Mechanical Engineers, 2013: V001T01A007.
M Oldfield, D Dini, G Giordano, et al. Detailed finite element modelling of deep needle insertions into a soft tissue phantom using a cohesive approach. Computer Methods in Biomechanics and Biomedical Engineering, 2013, 16: 530–543.
P P Shetty, R W Hatton, A C Barnett, et al. Modeling the cutting edge geometry of scalpel blades. Proceedings of the Institution of Mechanical Engineers, Part B: Journal of Engineering Manufacture, 2015, 231: 65–72.
J Z Moore, Q Zhang, C S Mcgill, et al. Modeling of the plane needle cutting edge rake and inclination angles for biopsy. Journal of Manufacturing Science & Engineering, 2010, 132: 635–645.
A G Atkins, X Xu. Slicing of soft flexible solids with industrial applications. International Journal of Mechanical Sciences, 2005, 47: 479–492.
M D O’Leary, C Simone, T Washio, et al. Robotic needle insertion: Effects of friction and needle geometry. 2003 IEEE Int. Conf. Robot. Autom. (Cat. No. 03CH37422), IEEE, 2003: 1774–1780.
J L Westbrook, D R Uncles, B T Sitzman, et al. Comparison of the force required for dural puncture with different spinal needles and subsequent leakage of cerebrospinal fluid. Anesthesia and Analgesia, 1994, 79: 769–772.
M Giovannini, H Ren, J Cao, et al. Study on design and cutting parameters of rotating needles for core biopsy. Journal of the Mechanical Behavior of Biomedical Materials, 2018, 86: 43–54.
J Z Moore, A J Shih, P McLaughlin, et al. Blade oblique cutting of tissue for investigation of biopsy needle insertion. Trans. NAMRI/SME, 2009, 37: 49–56.
Y Wang, R K Chen, B L Tai, et al. Optimal needle design for minimal insertion force and bevel length. Medical Engineering & Physics, 2014, 36: 1093–1100.
K Sorouri, D J Podolsky, A M Q Wang, et al. Utilization of a robotic mount to determine the force required to cut palatal tissue. Journal of the Mechanical Behavior of Biomedical Materials, 2018, 86: 433–439.
A Spagnoli, R Brighenti, M Terzano, et al. Cutting resistance of soft materials: Effects of blade inclination and friction. Theoretical and Applied Fracture Mechanics, 2019, 101: 200–206.
D Wang, Q Song, Z Liu, et al. Cutting characteristics of porcine tenderloin tissue along tangential direction of surface. The International Journal of Advanced Manufacturing Technology, 2018, 98: 17–27.
F Casanova, P R Carney, M Sarntinoranont. In vivo evaluation of needle force and friction stress during insertion at varying insertion speed into the brain. Journal of Neuroscience Methods, 2014, 237: 79–89.
P Han, K Ehmann. Study of the effect of cannula rotation on tissue cutting for needle biopsy. Medical Engineering & Physics, 2013, 35: 1584–1590.
L L Holton. Force models for needle insertion created from measured needle puncture data. Studies in Health Technology and Informatics, 2001, 81: 180–186.
Y Kobayashi, A Onishi, T Hoshi, et al. Development and validation of a viscoelastic and nonlinear liver model for needle insertion. International Journal of Computer Assisted Radiology and Surgery, 2009, 4: 53–63.
A A Elagha, A H Kim, O Kocaturk, et al. Blunt atrial transseptal puncture using excimer laser in swine. Catheterization and Cardiovascular Interventions, 2007, 70: 585–590.
M J Oldfield, D Dini, T Jaiswal, et al. The significance of rate dependency in blade insertions into a gelatin soft tissue phantom. Tribology International, 2013, 63: 226–234.
J T Hing, A D Brooks, J P Desai. A biplanar fluoroscopic approach for the measurement, modeling, and simulation of needle and soft-tissue interaction. Medical Image Analysis, 2007, 11: 62–78.
N Abolhassani, R Patel, M Moallem. Trajectory generation for robotic needle insertion in soft tissue. 26th Annu. Int. Conf. IEEE Eng. Med. Biol. Soc., IEEE, 2004: 2730–2733.
M A Meltsner, N J Ferrier, B R Thomadsen. Observations on rotating needle insertions using a brachytherapy robot. Physics in Medicine & Biology, 2007, 52: 6027.
I Khalaji, M Hadavand, A Asadian, et al. Analysis of needle-tissue friction during vibration-assisted needle insertion. 2013 IEEE/RSJ Int. Conf. Intell. Robot. Syst., IEEE, 2013: 4099–4104.
L Tan, X M Qin, H C Zhang, et al. Penetration force in biopsy under condition of biomimetic vibration. Appl. Mech. Mater., Trans. Tech. Publ., 2015: 436–440.
H Prescher, M X Ling, V Bigdelle, et al. Scalpel edge roughness affects post-transection peripheral nerve regeneration. Surgery Open Science, 2021, 4: 1–6.
L Shu, S Li, M Terashima, et al. A novel self-centring drill bit design for low-trauma bone drilling. International Journal of Machine Tools and Manufacture, 2020, 154: 103568.
M Sabah, A N Aldabagh, A S Mahmood. Histological assessment for healing of intraoral surgical wounds produced by Diode Laser 940 nm versus surgical scalpel blade (an in vivo study). Al-Rafidain Dental Journal, 2020, 20: 233–243.
J E Scott, E A Swanson, J Cooley, et al. Healing of canine skin incisions made with monopolar electrosurgery versus scalpel blade. Veterinary Surgery, 2017, 46: 520–529.
C-L Lin, Y-A Huang. Simultaneously reducing cutting force and tissue damage in needle insertion with rotation. IEEE Transactions on Biomedical Engineering, 2020, 67: 3195–3202.
F A Urrea, F Casanova, G A Orozco, et al. Evaluation of the friction coefficient, the radial stress, and the damage work during needle insertions into agarose gels. Journal of the Mechanical Behavior of Biomedical Materials, 2016, 56: 98–105.
M Erdim, E Tezel, A Numanoglu, et al. The effects of the size of liposuction cannula on adipocyte survival and the optimum temperature for fat graft storage: An experimental study. Journal of Plastic, Reconstructive & Aesthetic Surgery, 2009, 62: 1210–1214.
F Casanova, P R Carney, M Sarntinoranont. Effect of needle insertion speed on tissue injury, stress, and backflow distribution for convection-enhanced delivery in the rat brain. PLOS ONE, 2014, 9: e94919.
H Eto, H Suga, D Matsumoto, et al. Characterization of structure and cellular components of aspirated and excised adipose tissue. Plastic and Reconstructive Surgery, 2009, 124: 1087–1097.
C S Bjornsson, S J Oh, Y A Al-Kofahi, et al. Effects of insertion conditions on tissue strain and vascular damage during neuroprosthetic device insertion. Journal of Neural Engineering, 2006, 3: 196.
M Seredyka-Burduk, P K Burduk, et al. Ophthalmic complications of endoscopic sinus surgery. Brazilian Journal of Otorhinolaryngology, 2017, 83: 318–323.
I B James, D A Bourne, G DiBernardo, et al. The architecture of fat grafting II: Impact of cannula diameter. Plastic and Reconstructive Surgery, 2018, 142: 1219–1225.
R Valentine, P Wormald. Controlling the surgical field during a large endoscopic vascular injury. The Laryngoscope, 2011, 121: 562–566.
F Richter, S Shen, F Liu, et al. Autonomous robotic suction to clear the surgical field for hemostasis using image-based blood flow detection. IEEE Robotics and Automation Letters, 2021, 6: 1383–1390.
R Acland.Thrombus formation in microvascular surgery: an experimental study of the effects of surgical trauma. Surgery, 1973, 73: 766–771.
F May, J Krupka, M Fries, et al. FXII a inhibitor rHA‐Infestin‐4: Safe thromboprotection in experimental venous, arterial and foreign surface‐induced thrombosis. British Journal of Haematology, 2016, 173: 769–778.
A Ismail, A I Abushouk, A Elmaraezy, et al. Cutting electrocautery versus scalpel for surgical incisions: a systematic review and meta-analysis. Journal of Surgical Research, 2017, 220: 147–163.
W Lin, Y Dai, J Niu, et al. Scalpel can achieve better clinical outcomes compared with electric cautery in primary total knee arthroplasty: A comparison study. BMC Musculoskeletal Disorders, 2020, 21: 1–6.
R Tsumura, Y Takishita, Y Fukushima, et al. Histological evaluation of tissue damage caused by rotational needle insertion. 2016 38th Annu. Int. Conf. IEEE Eng. Med. Biol. Soc., 2016: 5120–5123.
T Brophy, P D Srodon, C Briggs, et al. Quality of surgical instruments. The Annals of The Royal College of Surgeons of England, 2006, 88: 390–393.
A Rahimizadeh, K Haddadi. Is transforaminal retrieval of intradiscal deeply seated broken surgical knife blade all time pars sparing? A case report. International Journal of Surgery Case Reports, 2016, 19: 131–133.
Z Chen, C Wang, W Jiang, et al. A review on surgical instruments of knee arthroscopic debridement and total hip arthroplasty. Procedia CIRP, 2017, 65: 291–298.
G Roscioli, S M Taheri-Mousavi, C C Tasan. How hair deforms steel. Science, 2020, 369: 689–694.
Y Jiang, Q Song, F Gao, et al. Needle deformation in the process of puncture surgery: Experiment and simulation. Procedia CIRP, 2020, 89: 270–276.
P Li, Z Yang, S Jiang. Needle-tissue interactive mechanism and steering control in image-guided robot-assisted minimally invasive surgery: A review. Medical & Biological Engineering & Computing, 2018, 56: 931–949.
D de Melo Costa, L K de Oliveira Lopes, A F V Tipple, et al. Evaluation of stainless steel surgical instruments subjected to multiple use/processing. Infection, Disease & Health, 2018, 23: 3–9.
Y Saito, H Yasuhara, S Murakoshi, et al. Challenging residual contamination of instruments for robotic surgery in Japan. Infection Control & Hospital Epidemiology, 2017, 38: 143–146.
Z Liu, H Chen, J Sui, et al. Failure behavior and influence of surgical tool edges in soft tissue cutting. Journal of Manufacturing Processes, 2021, 68: 69–78.
A D Caballero, A T Dunoyer, R H Ligardo, et al. Deformation of scalpel blades after incision of gingival tissue in pig mandibles. An ex vivo study. Revista Odontológica Mexicana, 2017, 21(3): e168-e174.
Y Xu. Safety evaluation of surgical instruments. University of Dundee, 2017.
P H Tsai, T H Li, K T Hsu, et al. Effect of coating thickness on the cutting sharpness and durability of Zr-based metallic glass thin film coated surgical blades. Thin Solid Films, 2016, 618: 36–41.
N J van de Berg, T L de Jong, D J van Gerwen, et al. The influence of tip shape on bending force during needle insertion. Scientific Reports, 2017, 7: 40477.
T Rossi, G Querzoli, G Angelini, et al. Hydraulic resistance of vitreous cutters: the impact of blade design and cut rate. Translational Vision Science & Technology, 2016, 5: 1.
M D Greenwood, L K Seibold, N M Radcliffe, et al. Goniotomy with a single-use dual blade: short-term results. Journal of Cataract & Refractive Surgery, 2017, 43: 1197–1201.
J Lu, X Wang, Y Huang, et al. Fabrication and cutting performance of bionic micro-serrated scalpels based on the miscanthus leaves. Tribology International, 2020, 145: 106162.
M Giovannini, H Ren, X Wang, et al. Tissue cutting with microserrated biopsy punches. Journal of Micro and Nano-Manufacturing, 2017, 5: 41004.
J Lu, X Wang, C Ma, et al. Influence of blended textures generated by laser surface texturing on friction behavior in soft tissue cutting. Tribology International, 2019, 134: 1–6.
H Izumi, T Yajima, S Aoyagi, et al. Combined harpoonlike jagged microneedles imitating Mosquito’s proboscis and its insertion experiment with vibration. IEEJ Transactions on Electrical and Electronic Engineering, 2008, 3: 425–431.
M Giovannini, K Ehmann. Vibrational cutting of soft tissue with micro-serrated surgical scalpels. Procedia CIRP, 2016, 45: 199–202.
A Burke, M Ross, S Shaidani, et al. Design of improved surgical scalpel handles with optimized grips, 2020.
W Zhang, L Feng, F Wu, et al. Micro/nano-particle decorated metal wire for cutting soft matter. Nanotechnology, 2016, 27: 355708.
X Yi, C Wang, X Yu, et al. Chitosan/zinc nitrate microneedles for bacterial biofilm eradication. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 2020, 109(6): 911‒920.
A McConville, C Hegarty, J Davis. Mini-Review: Assessing the potential impact of microneedle technologies on home healthcare applications. Medicines (Basel, Switzerland), 2018, 5: 50.
L-R Jong. Calf reduction by partial resection of gastrocnemius using a suction-assisted cartilage shaver. Plastic and Reconstructive Surgery, 2020, 145: 734e-743e.
L Santos-Carreras, M Hagen, R Gassert, et al. Survey on surgical instrument handle design: ergonomics and acceptance. Surgical Innovation, 2012, 19: 50–59.
O Martin, R Schiebel, P T Truc Pham, et al. Designing of ergonomic scalpel handles with optimized weight and balance. Worcester Polytechnic Institute, 2020.
A G Gonzalez, D R Salgado, L G Moruno. Optimisation of a laparoscopic tool handle dimension based on ergonomic analysis. International Journal of Industrial Ergonomics, 2015, 48: 16–24.
A G González, J Barrios-Muriel, F Romero-Sánchez, et al. Ergonomic assessment of a new hand tool design for laparoscopic surgery based on surgeons’ muscular activity. Applied Ergonomics, 2020, 88: 103161.
R Sancibrian, C Redondo-Figuero, M C Gutierrez-Diez, et al. Ergonomic evaluation and performance of a new handle for laparoscopic tools in surgery. Applied Ergonomics, 2020, 89: 103210.
S Y Park, D J Kim, C Mo Nam, et al. Clinical and economic benefits associated with the use of powered and tissue-specific endoscopic staplers among the patients undergoing thoracoscopic lobectomy for lung cancer. Journal of Medical Economics, 2019, 22: 1274–1280.
G S DiFelice, J P van der List. Arthroscopic primary repair of proximal anterior cruciate ligament tears. Arthroscopy Techniques, 2016, 5: e1057–e1061.
M Sahlabadi, S Khodaei, K Jezler, et al. Insertion mechanics of bioinspired needles into soft tissues. Minimally Invasive Therapy & Allied Technologies, 2018, 27: 284–291.
M Scali, T P Pusch, P Breedveld, et al. Ovipositor-inspired steerable needle: design and preliminary experimental evaluation. Bioinspiration & Biomimetics, 2017, 13: 16006.
J Gallo, M Holinka, C S Moucha. Antibacterial surface treatment for orthopaedic implants. International Journal of Molecular Sciences, 2014, 15: 13849–13880.
H B Skinner. Current diagnosis & treatment in orthopedics. Stamford, 2006.
N McLaughlin, L F S Ditzel Filho, D M Prevedello, et al. Side-cutting aspiration device for endoscopic and microscopic tumor removal. Journal of Neurological Surgery. Part B, Skull Base, 2012, 73: 11.
R A Packer, S McGrath. Onscreen‐guided resection of extra‐axial and intra‐axial forebrain masses through registration of a variable‐suction tissue resection device with a neuronavigation system. Veterinary Surgery, 2020, 49: 676–684.
L R Solon, R Aronson, G Gould. Physiological implications of laser beams. Science, 1961, 134: 1506–1508. http://www.jstor.org/stable/1707987.
R Bhandari, K Singla, S V Sandhu, et al. Soft tissue applications of lasers: A review. International Journal of Dental Research, 2014, 2(1), doi:https://doi.org/10.14419/ijdr.v2i1.1720.
B P Vitruk, D Ph. Soft tissue cutting with CO2, Diode Lasers, 2012.
K Grattan. Laser–tissue interactions: fundamentals and applications. 2nd ed. Springer, Berlin, 2002.
K Franjic, M L Cowan, D Kraemer, et al. Laser selective cutting of biological tissues by impulsive heat deposition through ultrafast vibrational excitations. Optics Express, 2009, 17: 22937.
A Vogel, V Venugopalan. Mechanisms of pulsed laser ablation of biological tissues. Chemical Reviews, 2003, 103: 577–644.
S Amini-Nik, D Kraemer, M L Cowan, et al. Ultrafast mid-IR laser scalpel: Protein signals of the fundamental limits to minimally invasive surgery. PLoS ONE, 2010, 5: 1–6.
A Vogel, P Schmidt, B Flucke. Minimization of thermomechanical side effects and increase of ablation efficiency in IR ablation by use of multiply Q-switched laser pulses. Proc. SPIE, 2002.
A Vogel, M R Capon, M N Asiyo-Vogel, et al. Intraocular photodisruption with picosecond and nanosecond laser pulses: Tissue effects in cornea, lens, and retina. Investigative Ophthalmology & Visual Science, 1994, 35: 3032–3044.
A Braun, M Kettner, M Berthold, et al. Efficiency of soft tissue incision with a novel 445-nm semiconductor laser. Lasers in Medical Science, 2018, 33: 27–33.
D L Jiang, Z Yang, G X Liu, et al. A novel 450-nm blue laser system for surgical applications: efficacy of specific laser-tissue interactions in bladder soft tissue. Lasers in Medical Science, 2019, 34: 807–813.
K Wuertz, P Vitruk. Superpulse 10, 600 nm CO2 laser revision of lingual frenum previously released with a Diode hot glass tip. 2017: 2–4.
M C Kong, D Axinte, W Voice. Aspects of material removal mechanism in plain waterjet milling on gamma titanium aluminide. Journal of Materials Processing Technology, 2010, 210: 573–584.
D A Axinte. Approach into the use of probabilistic neural networks for automated classification of tool malfunctions in broaching. International Journal of Machine Tools and Manufacture, 2006, 46: 1445–1448.
D Kundrat, R Graesslin, A Schoob, et al. Preclinical performance evaluation of a robotic endoscope for non-contact laser surgery. Annals of Biomedical Engineering, 2021, 49: 585–600.
A V Belikov, A V Skrypnik. Soft tissue cutting efficiency by 980 nm laser with carbon-, erbium-, and titanium-doped optothermal fiber converters. Lasers in Surgery and Medicine, 2019, 51: 185–200.
J S Shim. Finite element analysis of skin injuries by water jet cutting. Public Health, 2007, 125, http://scholar.google.com/scholar?hl=en&btnG=Search&q=intitle:Some+Contributions+on+MIMO+Radar#0.
P Hreha, S Hloch, D Magurová, et al. Water jet technology used in medicine. Tehnicki Vjesnik, 2010, 17: 237–240.
T Bahls, F A Fröhlich, A Hellings, et al. Extending the capability of using a waterjet in surgical interventions by the use of robotics. IEEE Transactions on Biomedical Engineering, 2017, 64: 284–294.
D N Papachristou, R Barters. Resection of the liver with a water jet. British Journal of Surgery, 1982, 69: 93–94.
D Axinte, F Boud, J Penny, et al. Broaching of Ti-6-4 – Detection of workpiece surface anomalies on dovetail slots through process monitoring. CIRP Annals, 2005, 54: 87–90.
Y Hata, F Sasaki, H Takahashi, et al. Liver resection in children, using a water-jet. Journal of Pediatric Surgery. 1994, 29: 648–650.
Y Une, J Uchino, T Horie, et al. Liver resection using a water jet. Cancer Chemotherapy and Pharmacology, 1989, 23(Suppl): 74–77.
H U Baer, S C Stain, T Guastella, et al. Hepatic resection using a water jet dissector. HPB Surgery : A World Journal of Hepatic, Pancreatic and Biliary Surgery, 1993, 6: 189–198.
H G Rau, A P Duessel, S Wurzbacher. The use of water-jet dissection in open and laparoscopic liver resection. HPB, 2008, 10: 275–280.
R F Basting, S Corvin, C Antwerpen, et al. Use of water jet resection in renal surgery: Early clinical experiences. European Urology, 2000, 38: 104–107.
W Li, M Preuss, P J Withers, et al. Characterisation of residual stresses in machined surfaces of a high strength Nickel-Base superalloy. Materials Science Forum, 2006, 524–525: 587–592.
C A Tschan, E J Hermann, W Wagner, et al. Waterjet dissection in pediatric cranioplasty: Technical note. Journal of Neurosurgery: Pediatrics PED, 2010, 5: 243–249.
K Kolluru, D Axinte. Coupled interaction of dynamic responses of tool and workpiece in thin wall milling. Journal of Materials Processing Technology, 2013, 213: 1565–1574.
R Gurunluoglu. Experiences with waterjet hydrosurgery system in wound debridement. World Journal of Emergency Surgery, 2007, 2: 10.
J Hubert, E Mourey, J M Suty, et al. Water-jet dissection in renal surgery: Experimental study of a new device in the pig. Urological Research, 1996, 24: 355–359.
R F Basting, N Djakovic, P Widmann. Use of water jet resection in organ-sparing kidney surgery. Journal of Endourology, 2000, 14: 501–505.
M Alireza, H Waleed, S Massimiliano, et al. Water jet assisted laparoscopic partial nephrectomy without hilar clamping in the calf model. Journal of Urology, 2005, 174: 317–321.
H Shekarriz, B Shekarriz, P Kujath, et al. Hydro-Jet-assisted laparoscopic cholecystectomy: A prospective randomized clinical study. Surgery, 2003, 133: 635–640.
S Corvin, W Sturm, E Schlatter, et al. Laparoscopic retroperitoneal lymph-node dissection with the waterjet is technically feasible and safe in testis-cancer patient. Journal of Endourology, 2005, 19: 823–826.
J Piek, C Wille, R Warzok, et al. Waterjet dissection of the brain: experimental and first clinical results: Technical note. Journal of Neurosurgery, 1998, 89: 861–864.
J Oertel, M R Gaab, J Piek. Waterjet resection of brain metastases – first clinical results with 10 patients. European Journal of Surgical Oncology (EJSO), 2003, 29: 407–414.
R Vanwijck, L Kaba, S Boland, et al. Immediate skin grafting of sub-acute and chronic wounds debrided by hydrosurgery. Journal of Plastic, Reconstructive & Aesthetic Surgery, 2010, 63: 544–549.
M Tenenhaus, D Bhavsar, H-O Rennekampff.Treatment of deep partial thickness and indeterminate depth facial burn wounds with water–jet debridement and a biosynthetic dressing. Injury, 2007, 38: S38–S44.
D S Schoeb, J Klodmann, D Schlager, et al. Robotic waterjet wound debridement – Workflow adaption for clinical application and systematic evaluation of a novel technology. PLOS ONE, 2018, 13: e0204315. https://doi.org/https://doi.org/10.1371/journal.pone.0204315.
A J A Terzis, G Nowak, O Rentzsch, et al. A new system for cutting brain tissue preserving vessels: Water jet cutting. British Journal of Neurosurgery, 1989, 3: 361–366.
A Schneider, H Feussner. Classical (Open) Surgery - ScienceDirect. Biomedical Engineering in Gastrointestinal Surgery, 2017: 221–267.
C D Kelman. Phaco-emulsification and aspiration. A new technique of cataract removal. A preliminary report. American Journal of Ophthalmology, 1967, 64: 23–35.
HARMONIC ACE® +7 Shears with Advanced Hemostasis. n.d.,. https://www.jnjmedicaldevices.com/en-EMEA/product/harmonic-ace7-shears-advanced-hemostasis.
D Wang, A Roy, V V Silberschmidt. Production of high-quality extremely-thin histological sections by ultrasonically assisted cutting. Journal of Materials Processing Technology, 2020, 276: 116403.
M E Schafer, B Arbisser. Quantification of acoustic exposure during cataract surgery. IEEE Ultrason. Symp. 2004, 2004, 3: 1828-1831.
U Rosenschein, J J Bernstein, E DiSegni, et al. Experimental ultrasonic angioplasty: disruption of atherosclerotic plaques and thrombi in vitro and arterial recanalization in vivo. Journal of the American College of Cardiology, 1990, 15: 711–717.
J F Amaral. The experimental development of an ultrasonically activated scalpel for laparoscopic use. Surgical Laparoscopy & Endoscopy, 1994, 4: 92–99.
L J Bond, W W Cimino. Physics of ultrasonic surgery using tissue fragmentation: Part II. Ultrasound in Medicine & Biology, 1996, 22: 101–117.
W W Cimino, L J Bond. Physics of ultrasonic surgery using tissue fragmentation: Part I. Ultrasound in Medicine & Biology, 1996, 22: 89–100.
B S Khambay, A D Walmsley. Investigations into the use of an ultrasonic chisel to cut bone. Part 2: Cutting ability. Journal of Dentistry, 2000, 28: 39–44.
T Kinoshita, E Kanehira, K Omura, et al. Experimental study on heat production by a 23.5-kHz ultrasonically activated device for endoscopic surgery. Surgical Endoscopy, 1999, 13: 621–625.
K M Kadesky, B Schopf, J F Magee, et al. Proximity injury by the ultrasonically activated scalpel during dissection. Journal of Pediatric Surgery, 1997, 32: 878–879.
B J O’Daly, E Morris, G P Gavin, et al. High-power low-frequency ultrasound: A review of tissue dissection and ablation in medicine and surgery. Journal of Materials Processing Technology, 2008, 200: 38–58.
J Lowry. Ultrasonic Energy for Cutting, Coagulation and Dissection. Annals of the Royal College of Surgeons of England, 2006, 88(4): 426.
SONOPET® Ultrasonic Aspirator. n.d.,. https://neurosurgical.stryker.com/products/sonopet-ultrasonic-aspirator.
H Wang, W Ge, C Liu, et al. Design and performance evaluation of a powered stapler for gastrointestinal anastomosis. Minimally Invasive Therapy & Allied Technologies, 2021: 1–8.
H Winter, C Holmer, H-J Buhr, et al. Pilot study of bipolar radiofrequency-induced anastomotic thermofusion–exploration of therapy parameters ex vivo. International Journal of Colorectal Disease, 2009, 25: 129.
L Zhao, C Song, Z Wang, et al. Novel concave–convex electrode for colonic anastomoses by radiofrequency thermo-fusion. Surgical Endoscopy, 2015, 29: 1809–1816.
L Tu, Y Zhou, C Song, et al. Preliminary study of a control algorithm for radio-frequency-induced intestinal tissue fusion. International Journal of Hyperthermia. 2019, 36: 1296–1305.
Acknowledgements
Not applicable.
Funding
Supported by National Natural Science Foundation of China (Grant Nos. 51735003, 51805091) and Guangdong Provincial Natural Science Foundation of China (Grant No. 2018A030313713).
Author information
Authors and Affiliations
Contributions
ZHL and ZRL led the writing of the manuscript. ZHL, ZRL, DW, HNL, CLS and YL wrote this manuscript. ZRL and CYW generated the concept of the manuscript, provided supervision and methodology, and reviewed and edited the manuscript. All authors read and approved the final manuscript.
Authors’ Information
Zhihua Liu, born in 1994, is currently a PhD candidate at Guangdong Provincial Key Laboratory of Minimally Invasive Surgical Instruments and Manufacturing Technology, Guangdong University of Technology, China. He received his bachelor degree from Guangdong University of Technology, China, in 2017. His research interests include soft biological tissue manipulating technologies and design of advanced medical devices.
Zhirong Liao, born in 1987, is an associate professor at University of Nottingham, UK. He received his BSc, MSc, PhD degree, from Harbin Institute of Technology, China, in 2010, 2012 and 2017 respectively. His research interests include machining of difficult-to-cut materials, surface integrity, laser and waterjet machining, biomanufacturing, sensing and signal processing.
Dong Wang is a lecturer in Engineering & Entrepreneurship at University of Exeter, UK, and a research consultant at Imperial College London, UK. He received his PhD degree in Mechanical Engineering from University of Glasgow, UK. His research focuses on three core themes crossing over medical instrumentation, biomechanics and advanced manufacturing. His multi-disciplinary research was developed through collaborations with consultants/surgeons, academics and industrial partners, involving developing laboratory prototypes and taking them through commercialisation, clinical validation and human testing to make a real-world impact. Website: https://emps.exeter.ac.uk/engineering/staff/dw562.
Chengyong Wang, born in 1964, is currently a professor and the Director of Guangdong Provincial Key Laboratory of Minimally Invasive Surgical Instruments and Manufacturing Technology, Guangdong University of Technology, China. He received his PhD from Dalian University of Technology, China, in 1989. His research interests include machining of difficult-to-cut materials, micro-drilling of PCB composites,coated tool design and preparation, medical instruments design and manufacturing, laser machining and 3D printing.
Chengli Song, born in 1968, is currently a professor at School of Medical Instrument and Food Engineering, University of Shanghai for Science and Technology, China. He received his PhD degree from Cardiff University, UK, in 2000. His research interests include minimally invasive devices, medical robot and biomechanics.
Haonan Li is an associate professor at University of Nottingham Ningbo, China. He is Research Affiliate of The International Academy for Production Engineering CIRP. He received his PhD degree from Northeastern University, China. His current research interests focus on advanced and intelligent manufacturing and automation. Until June 2021, he has published 52 SCI-indexed articles on top journals in Manufacturing Engineering (42 out of 52 in JCR Q1 journals) with the total citations of 1050 including 6 ESI highly-cited papers and 2 ESI hot paper.
Yao Liu, born in 1990, is currently an associate professor at Shanxi Key Laboratory of Advanced Manufacturing Technology, North University of China. He received his PhD degree from Donghua University, China, in 2018 and jointed North University of China in 2019. His research interests are biomedical manufacturing and design, including atherectomy, clot cutting, 3D printing of orthosis, bone drilling, and wearable sensor.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing financial interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, Z., Liao, Z., Wang, D. et al. Recent Advances in Soft Biological Tissue Manipulating Technologies. Chin. J. Mech. Eng. 35, 89 (2022). https://doi.org/10.1186/s10033-022-00767-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10033-022-00767-4